Evaluation of Postharvest Processing of Hazelnut Kernel Oil Extraction Using Uniaxial Pressure and Organic Solvent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Moisture Content Determination
2.2. Determination of Percentage Kernel Oil Content
2.3. Heating of Samples
2.4. Compression Tests
2.5. Determination of Peroxide Value (PV) and Free Fatty Acid (FFA)
2.6. Compression Tests Calculated Parameters
2.7. Statistical Analyses
3. Results
3.1. Effects of Speed, Temperature, and Relaxation Time on Responses
3.2. Force–Deformation Curves, Strain, Oil Point Force, and Oil Point Energy
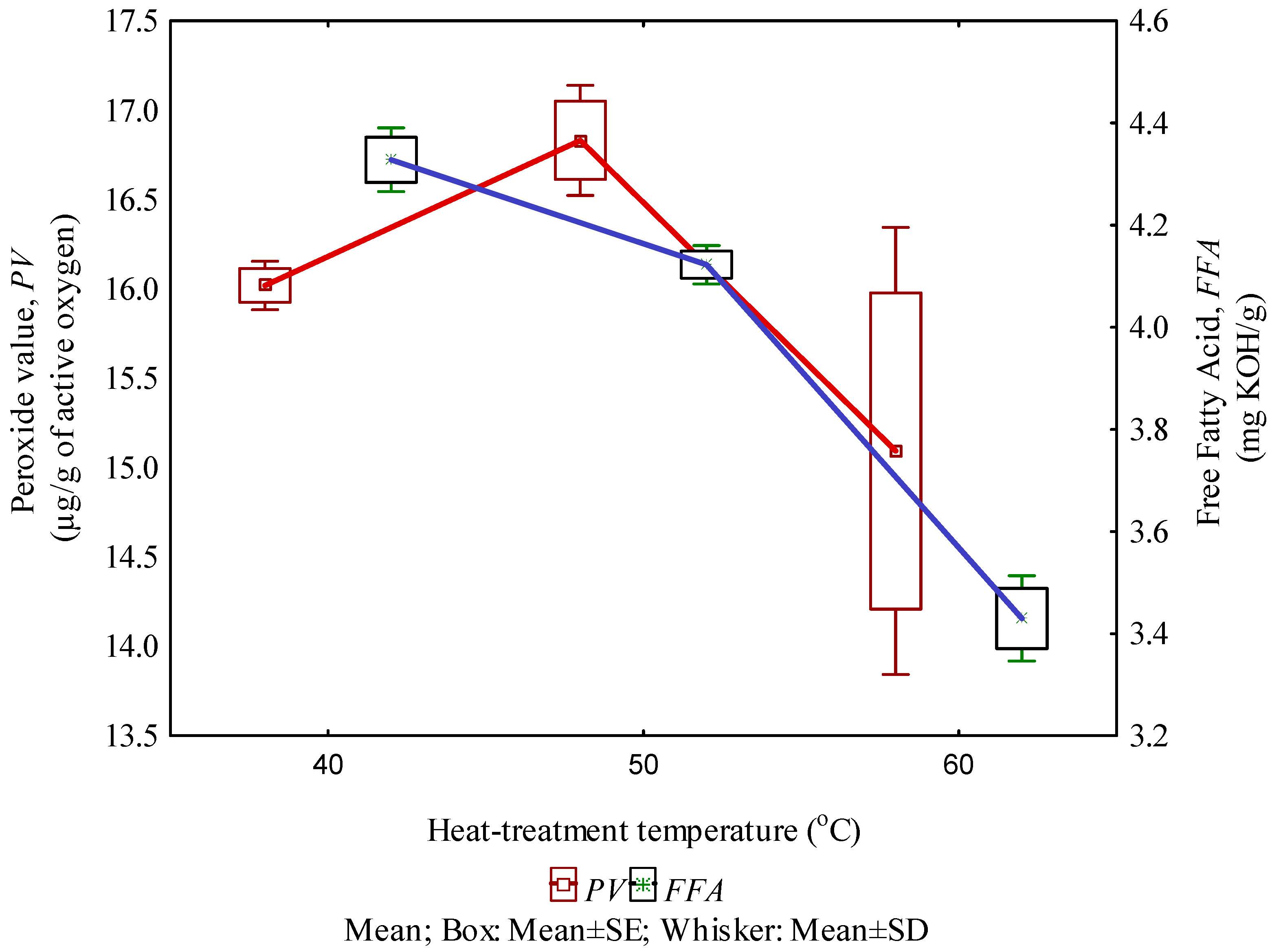
3.3. Peroxide Value and Free Fatty Acid
3.4. ANOVA, Correlation, and Regression Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Guner, M.; Dursun, E.; Dursun, J.G. Mechanical behaviour of hazelnut under compression loading. Biosyst. Eng. 2003, 85, 485–491. [Google Scholar] [CrossRef]
- Delprete, C.; Giacosa, S.; Raviola, E.; Rolle, L.; Sesana, R. Experimental characterization and numerical modeling of the compressive mechanical of hazelnut kernels. J. Food Eng. 2015, 166, 364–369. [Google Scholar] [CrossRef]
- Young, W.J. A Study of Nuts with Special Reference to Microscopic Identification; U.S. Government Printing Office: Washington, DC, USA, 1912.
- Demirkaya, E.; Dal, O.; Yuksel, A. Liquefaction of waste hazelnut shell by using sub-and supercritical solvents as a reaction medium. J. Supercrit Fluids. 2019, 150, 11–20. [Google Scholar] [CrossRef]
- Baran, Y.; Gokce, H.S.; Durmaz, M. Physical and mechanical properties of cement containing regional hazelnut shell ash wastes. J. Clean. Prod. 2020, 259, 1–9. [Google Scholar] [CrossRef]
- FAO. Produccion Avellana. 2016. Available online: http://www.fao.org/faostat/es/#data/QC/visualize (accessed on 20 July 2020).
- Lainas, K.; Alasalvar, C.; Bolling, B.W. Effects of roasting on proanthocyanidin contents of Turkish Tombul hazelnut and its skin. J. Funct. Foods. 2016, 23, 647–653. [Google Scholar] [CrossRef]
- Perez-Armada, L.; Rivas, S.; Gonzalez, B.; Moure, A. Extraction of phenolic compounds from hazelnut shells by green processes. J. Food Eng. 2019, 255, 1–8. [Google Scholar] [CrossRef]
- Ciemniewska-Żytkiewicz, H.; Verardo, V.; Pasini, F.; Bryś, J.; Koczoń, P.; Caboni, M.F. Determination of lipids and phenolic fraction in two hazelnut (Corylus avellana L.) cultivars grown in Poland. Food Chem. 2015, 168, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Pelvan, E.; Alasalvar, C.; Uzman, S. Effects of roasting on the antioxidant status and phenolic profiles of commercial turkish hazelnut varieties (Corylus avellana L.). J. Agric. Food Chem. 2012, 60, 1218–1223. [Google Scholar] [CrossRef] [PubMed]
- Marzocchi, S.; Pasini, F.; Verardo, V.; Ciemniewska-Zytkiewicz, H.; Caboni, M.F.; Romani, S. Effects of different roasting conditions on physical-chemical properties of Polish hazelnuts (Corylus avellana L. Var. Katalonski). LWT Food Sci. Technol. 2017, 77, 440–448. [Google Scholar] [CrossRef]
- Köksal, A.I.; Artik, N.; Simsek, A.; Günes, N. Nutrient composition of hazelnut (Corylus avellana L.) varieties cultivated in Turkey. Food Chem. 2006, 99, 509–515. [Google Scholar] [CrossRef]
- Del Rio, D.; Calani, L.; Dall’Asta, M.; Brighenti, F. Polyphenolic composition of hazelnut skin. J. Agric. Food Chem. 2011, 59, 9935–9941. [Google Scholar] [CrossRef] [PubMed]
- Kara, H.; Orem, A.; Yulug, E.; Yucesan, F.B.; Kerimoglu, G.; Yaman, S.O.; Bodur, A.; Turedi, S.; Alasalvar, C. Hazelnut consumption improves testicular antioxidant function and semen quality in young and old male rats. Food Chem. 2019, 294, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Lu, M.; Eskridge, K.M.; Isom, L.D.; Hanna, A.M. Extraction, identification, and quantification of antioxidant phenolics from hazelnut (Corylus avellana L.) shells. Food Chem. 2018, 244, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Alasalvar, C.; Liyana-Pathirana, C.M. Antioxidant phytochemicals in hazelnut kernel (Corylus avellana L.) and hazelnut byproducts. J. Agric. Food Chem. 2007, 55, 1212–1220. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, J.; Kermani, A.M.; Kouravand, S.; Zarafshan, P. Design, fabrication, and evaluation a laboratory dry-peeling system for hazelnut using infrared radiation. LWT Food Sci. Technol. 2018, 90, 570–576. [Google Scholar] [CrossRef]
- Tas, N.G.; Yilmaz, C.; Gokmen, V. Investigation of serotonin, free and protein-bound tryptophan in Turkish hazelnut varieties and effect of roasting on serotonin content. Food Res. Int. 2019, 120, 865–871. [Google Scholar] [CrossRef]
- Alasalvar, C.; Karamac, M.; Kosinska, A.; Rybarczyk, A.; Shahidi, F.; Amarowicz, R. Antioxidant activity of hazelnut skin phenolics. J. Agric. Food Chem. 2009, 57, 4645–4650. [Google Scholar] [CrossRef]
- Ozdemir, M.; Seyan, F.G.; Bakan, A.K.; Ilter, S.; Ozay, G.; Devres, O. Analysis of internal browning of roasted hazelnuts. Food Chem. 2001, 73, 191–196. [Google Scholar] [CrossRef]
- Burdack-Freitag, A.; Schieberle, P. Changes in the key odorants of Italian hazelnuts (Corylus avellana L. Var. Tonda Romana) induced by roasting. J. Agric. Food Chem. 2010, 58, 6351–6359. [Google Scholar] [CrossRef]
- Belviso, S.; Dal Bello, B.; Giacosa, S.; Bertolino, M.; Ghirardello, D.; Giordano, M.; Rolle, L.; Gerbi, V.; Zeppa, G. Chemical, mechanical and sensory monitoring of hot air-and infrared-roasted hazelnuts (Corylus avellana L.) during nine months of storage. Food Chem. 2017, 217, 398–408. [Google Scholar] [CrossRef]
- Guler, S.K.; Bostan, S.Z.; Con, A.H. Effects of gamma irradiation on chemical and sensory characteristics of natural hazelnut kernels. Postharvest Biol. Technol. 2017, 123, 12–21. [Google Scholar] [CrossRef]
- Ghirardello, D.; Rolle, L.; Zeppa, G. Effects of storage conditions on hazelnut (Corylus avellana L.) textural characteristics. In Proceedings of the VII International Congress of Hazelnuts, Viterbo, Italy, 23 June 2008. [Google Scholar]
- Ghirardello, D.; Contessa, C.; Valentini, N.; Zeppa, G.; Rolle, L.; Gerbi, V.; Botta, R. Effect of storage conditions on chemical and physical characteristics of hazelnut (Corylus avellana L.). Postharvest Biol. Technol. 2013, 81, 37–43. [Google Scholar] [CrossRef]
- Akinoso, R.; Raji, A.O. Physical properties of fruit, nut and kernel of oil palm. Int. Agrophys. 2011, 25, 1–6. [Google Scholar]
- Jahromi, M.K.; Rafiee, S.; Jafari, A.; Bousejin, M.R.G.; Mirasheh, R.; Mohtasebi, S.S. Some physical properties of date fruit (cv. Dairi). Int. Agrophys. 2008, 22, 221–224. [Google Scholar]
- Ozturk, I.; Ercisli, S.; Kara, M. Chosen physical properties of olive cultivars (Olea europaea L.). Int. Agrophys. 2009, 23, 309–312. [Google Scholar]
- Yurtlu, Y.B.; Yesiloglu, E.; Arslanoglu, F. Physical properties of bay laurel seeds. Int. Agrophys. 2010, 24, 325–328. [Google Scholar]
- Ercisli, S.; Ozturk, I.; Kara, M.; Kalkan, F.; Seker, H.; Duyar, O.; Erturk, Y. Physical properties of hazelnuts. Int. Agrophys. 2011, 25, 115–121. [Google Scholar]
- Aydin, C. Physical properties of hazelnuts. Biosyst Eng. 2002, 82, 297–303. [Google Scholar] [CrossRef]
- Ozdemir, F.; Akinci, I. Physical and nutritional properties of four major commercial Turkish hazelnut varieties. J. Food Eng. 2004, 63, 341–347. [Google Scholar] [CrossRef]
- Kibar, H.; Ozturk, T. The effect of moisture content on the physic-mechanical properties of some hazelnut varieties. J. Stored Prod. Res. 2009, 45, 14–18. [Google Scholar] [CrossRef]
- Valentini, N.; Rolle, L.; Stevigny, C.; Zeppa, G. Mechanical behaviour of hazelnuts used for table consumption under compression loading. J. Sci Food Agric. 2006, 86, 1257–1262. [Google Scholar] [CrossRef]
- Giacosa, S.; Belviso, S.; Bertolino, M.; Bello, B.D.; Gerbi, V.; Ghirardello, D.; Giordano, M.; Zeppa, G.; Rolle, L. Hazelnut kernels (Corylus avellana L.) mechanical and acoustic properties determination: Comparison of test speed, compression or shear axis, roasting, and storage condition effect. J. Food Eng. 2016, 173, 59–68. [Google Scholar] [CrossRef]
- Sirisomboon, P.; Kitchaiya, P.; Pholpho, T.; Mahuttanyavanitch, W. Physical and mechanical properties of Jatropha curcas L. fruits, nuts and kernels. Biosyst. Eng. 2007, 97, 201–207. [Google Scholar] [CrossRef]
- Karaj, S.; Muller, J. Determination of physical, mechanical and chemical properties of seeds and kernels of Jatropha curcas L. Ind. Crop. Prod. 2010, 32, 129–138. [Google Scholar] [CrossRef]
- Divisova, M.; Herak, D.; Kabutey, A.; Sigalingging, R.; Svatoňová, T. Deformation curve characteristics of rapeseeds and sunflower seeds under compression loading. Sci. Agric. Bohem. 2014, 45, 180–186. [Google Scholar]
- Herak, D.; Kabutey, A.; Hrabe, P. Oil point determination of Jatropha curcas L. bulk seeds under compression loading. Biosyst. Eng. 2013, 116, 470–477. [Google Scholar] [CrossRef]
- Munson-Mcgee, S.H. D-optimal experimental designs for uniaxial expression. J. Food Process. Eng. 2014, 37, 248–256. [Google Scholar] [CrossRef]
- Herak, D.; Kabutey, A.; Choteborsky, R.; Petru, M.; Sigalingging, R. Mathematical models describing the relaxation behaviour of Jatropha curcas L. bulk seeds under axial compression. Biosyst. Eng. 2015, 131, 77–83. [Google Scholar] [CrossRef]
- Mizera, C.; Herak, D.; Hrabe, P.; Kabutey, A. Mathematical models describing the creep and stress relaxation behaviour of false banana’s fiber (Ensete ventricosum). J. Nat. Fibers. 2019. [Google Scholar] [CrossRef]
- Demirbas, A. Oils from Hazelnut Shell and Hazelnut Kernel Husk for Biodiesel Production. Energ. Sournce Part. A 2008, 30, 1870–1875. [Google Scholar] [CrossRef]
- ISI. Indian Standard Methods for Analysis of Oilseeds, IS:3579; Indian Standard Institute: New Delhi, India, 1966. [Google Scholar]
- Blahovec, J. Agromatereials Study Guide; Czech University of Life Sciences: Prague, Czech Republic, 2008. [Google Scholar]
- Niu, L.; Li, J.; Chen, M.-S.; Xu, Z.-F. Determination of oil contents in Sacha inchi (Plukenetia volubilis) seeds at different developmental stages by two methods: Soxhlet extraction and time-domain nuclear magnetic resonance. Ind Crop. Prod. 2014, 56, 187–190. [Google Scholar] [CrossRef]
- Danlami, J.M.; Arsad, A.; Zaini, M.A.A. Characterization and process optimization of castor oil (Ricinus communis L.) extracted by the Soxhlet method using polar and non-polar solvents. J. Taiwan Inst. Chem Eng. 2015, 47, 99–104. [Google Scholar] [CrossRef]
- Gürdil, G.A.K.; Kabutey, A.; Selvi, K.C.; Hrabě, P.; Herák, D.; Fraňková, A. Investigation of heating and freezing pretreatments on mechanical, chemical and spectral properties of bulk sunflower seeds and oil. Processes 2020, 8, 411. [Google Scholar] [CrossRef] [Green Version]
- Chatepa, L.E.C.; Uluko, H.; Masamba, K. Comparison of oil quality extracted from selected conventional and non conventional sources of vegetable oil from Malawi. Afr. J. Biotechnol. 2019, 18, 171–180. [Google Scholar]
- Perrier, A.; Delsart, C.; Boussetta, N.; Grimi, N.; Citeau, M.; Vorobiev, E. Effect of ultrasound and green solvents addition on the oil extraction efficiency from rapeseed flakes. Ultrason. Sonochem. 2017, 39, 58–65. [Google Scholar] [CrossRef]
- Deli, S.; Masturah, F.; Aris, T.Y.; Nadiah, W.W.A. The effects of physical parameters of the screw press oil expeller on oil yield from Nigella sativa L. Seeds. Int. Food Res. J. 2011, 18, 1367–1373. [Google Scholar]
- Demirel, C.; Kabutey, A.; Herak, D.; Gurdil, G.A.K. Comparison of deformation energy of particular oil-bearing crops. In Proceedings of the 58th ICMD, Prague, Czech Republic, 6–8 September 2017; pp. 60–65. [Google Scholar]
- Gupta, R.K.; Das, S.K. Fracture resistance of sunflower seed and kernel to compressive loading. J. Food Eng. 2000, 46, 1–8. [Google Scholar] [CrossRef]
- StatSoft Inc. (1995). STATISTICA for Windows; StatSoft Inc.: Tulsa, OK, USA, 2013. [Google Scholar]
- Sirisomboon, P.; Kitchaiya, P. Physical properties of Jatropha curcas L. kernels after heat treatment. Biosyst. Eng. 2009, 102, 244–250. [Google Scholar] [CrossRef]
- Baryeh, E.A. Effect of palm oil processing parameters on yield. J. Food Eng. 2001, 48, 1–6. [Google Scholar] [CrossRef]
- Willems, P.; Kuipers, N.J.M.; De Haan, A.B. Hydraulic pressing of oilseeds: Experimental determination and modeling of yield and pressing rates. J. Food Eng. 2008, 89, 8–16. [Google Scholar] [CrossRef]
- Willems, P.; Kuipers, N.J.M.; De Haan, A.B. A consolidation based extruder model to explore GAME process configurations. J. Food Eng. 2009, 90, 238–245. [Google Scholar] [CrossRef]
- Karaj, S.; Muller, J. Optimizing mechanical oil extraction of Jatropha curcas L. seeds with respect to press capacity, oil recovery and energy efficiency. Ind Crop. Prod. 2011, 34, 1010–1016. [Google Scholar] [CrossRef]
- Sahin, S.; Gulum, S. Physical Properties of Foods; Middle East Technical University: Ankara, Turkey, 2006. [Google Scholar]
- Chakespari, A.G.; Rajabipour, A.; Mobli, H. Anisotropic relaxation and creep properties of apple (cv. Shafi Abadi and Golab Kohanz). Adv. J. Food Sci Technol. 2010, 2, 200–205. [Google Scholar]
- Ogunsina, B.S.; Owolarafe, O.K.; Olatunde, G.A. Oil point pressure of cashew (Anacardium occidentale) kernels. Int. Agrophys. 2008, 22, 53–59. [Google Scholar]
- Faborade, M.O.; Favier, J.F. Identification and significance of the oil-point in seed-oil expression. J. Agric. Res. 1996, 65, 335–345. [Google Scholar] [CrossRef]
- Ajibola, O.O.; Okunade, D.A.; Owolarafe, O.K. Oil point pressure of soybean. J. Food Process. Eng. 2002, 25, 407–416. [Google Scholar] [CrossRef]
- Mrema, G.C.; McNulty, P.B. Mathematical model of mechanical oil expression from oilseeds. J. Agric. Eng. Res. 1985, 31, 361–370. [Google Scholar] [CrossRef]
- Christopoulos, M.V.; Tsantili, E. Oil composition in stored walnut cultivars—Quality and nutritional value. Eur. J. Lipid Sci. Technol. 2015, 117, 338–348. [Google Scholar] [CrossRef]
- Maskan, M.; Karataş, Ş. Fatty acid oxidation of pistachio nuts stored under various atmospheric conditions and different temperatures. J. Sci. Food Agric. 1998, 77, 334–340. [Google Scholar] [CrossRef]
- Buransompob, A.; Tang, J.; Mao, R.; Swanson, B.G. Rancidity of walnuts and almonds affected by short time heat treatments for insect control. J. Food Process. Preserv. 2003, 27, 445–464. [Google Scholar] [CrossRef]
- Gecgel, U.; Gumus, T.; Tasan, M.; Daglioglu, O.; Arici, M. Determination of fatty acid composition of γ-irradiated hazelnuts, walnuts, almonds, and pistachios. Radiat. Phys. Chem. 2011, 80, 578–581. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Lipid oxidation and improving the oxidative stability. Chem. Soc. Rev. 2010, 39, 4067–4079. [Google Scholar] [CrossRef] [PubMed]
- Gama, T.; Wallace, H.M.; Trueman, S.J.; Hosseini-Bai, S. Quality and shelf life of tree nuts: A review. Sci. Hortic. 2018, 242, 116–126. [Google Scholar] [CrossRef]
- Phatanayindee, S.; Borompichaichartkul, C.; Srzednicki, G.; Craske, J.; Wootton, M. Changes of chemical and physical quality attributes of macadamia nuts during hybrid drying and processing. Dry. Technol. 2012, 30, 1870–1880. [Google Scholar] [CrossRef]
- Ling, B.; Hou, L.; Li, R.; Wang, S. Thermal treatment and storage condition effects on walnut paste quality associated with enzyme inactivation. Food Sci. Technol. 2014, 59, 786–793. [Google Scholar] [CrossRef]




| * Speed (mm/min) | Deformation (mm) | Oil Yield (%) | Oil Expression Efficiency (%) | Energy (kJ) |
|---|---|---|---|---|
| 4 | 27.75 ± 2.79 | 39.76 ± 0.02 | 61.48 ± 0.04 | 0.21 ± 0.01 |
| 8 | 27.04 ± 1.17 | 37.00 ± 0.51 | 57.22 ± 0.79 | 0.23 ± 0.01 |
| 12 | 28.06 ± 0.74 | 33.83 ± 0.88 | 52.31 ± 1.37 | 0.27 ± 0.04 |
| * Heating Temperature (°C) | Deformation (mm) | Oil Yield (%) | Oil Expression Efficiency (%) | Energy (kJ) |
|---|---|---|---|---|
| 25 | 27.76 ± 0.24 | 36.21 ± 0.04 | 55.99 ± 0.06 | 0.22 ± 0.001 |
| 40 | 30.61 ± 0.50 | 39.83 ± 0.64 | 61.59 ± 0.98 | 0.20 ± 0.001 |
| 50 | 30.41 ± 2.41 | 42.35 ± 0.43 | 65.48 ± 0.67 | 0.21 ± 0.003 |
| 60 | 29.96 ± 2.11 | 42.79 ± 0.24 | 66.16 ± 0.37 | 0.21 ± 0.001 |
| ** Relaxation Time (min) | Oil Yield (%) | Oil Expression Efficiency (%) |
|---|---|---|
| 0 | 36.21 ± 0.04 | 55.99 ± 0.06 |
| 3 | 41.73 ± 0.34 | 64.53 ± 0.52 |
| 6 | 45.08 ± 0.03 | 69.71 ± 0.05 |
| 9 | 46.04 ± 0.96 | 71.21 ± 1.48 |
| 12 | 46.31 ± 0.34 | 71.59 ± 0.53 |
| * Deformation Levels (mm) | Strain (−) | ** Oil Point Force (kN) | ** Oil Point Energy (kJ) |
|---|---|---|---|
| 15 | 0.375 | 3.58 ± 1.13 | 0.03 ± 0.01 |
| 20 | 0.5 a | 6.46 ± 0.08 | 0.06 ± 0.002 |
| 25 | 0.625 b | 10.79 ± 2.62 | 0.09 ± 0.01 |
| * Deformation Levels (mm) | Strain (−) | *** Oil Point Force (kN) | *** Oil Point Energy (kJ) |
|---|---|---|---|
| 15 | 0.375 | 3.22 ± 0.21 | 0.02 ± 0.001 |
| 20 | 0.5 a | 6.21 ± 0.58 | 0.05 ± 0.01 |
| 25 | 0.625 b | 10.61 ± 0.71 | 0.08 ± 0.01 |
| Dependent Variables | R | R2 | F-Value | p-Value |
|---|---|---|---|---|
| Deformation (mm) | 0.09 a | 0.10 a | 0.16 a | >0.05 a,b |
| 0.49 b | 0.49 b | 1.29 b | ||
| Oil yield (%) | −0.99 a | 0.97 a | 50.65 a | <0.05 a,b |
| 0.89 b | 0.99 b | 111.89 b | ||
| Oil expression efficiency (%) | −0.99 a | 0.97 a | 50.65 a | <0.05 a,b |
| 0.89 b | 0.99 b | 111.89 b | ||
| Energy (kJ) | 0.78 a | 0.63 a | 2.53 a | >0.05 a,b |
| 0.22 b | 0.94 b | 19.27 b |
| Dependent Variables | R | R2 | F-Value | p-Value |
|---|---|---|---|---|
| * Oil yield (%) | 0.91 | 0.99 | 157.07 | <0.05 |
| * Oil expression efficiency (%) | 0.91 | 0.99 |
| Dependent Variables | R | R2 | F-Value | F-Value |
|---|---|---|---|---|
| * Oil point force (kN) * Oil point expression efficiency (%) | 0.92 a | 0.87 a | 9.73 a | <0.05 a,b |
| 0.99 b | 0.98 b | 96.03 b | ||
| 0.98 a | 0.99 a | 267.45 a | ||
| 0.99 b | 0.99 b | 1549.86 b | ||
| * Oil point energy (kJ) | 0.97 a | 0.95 a | 29.13 a | |
| 0.99 b | 0.97 b | 56.87 b |
| Dependent variables | R | R2 | F-Value | p-Value |
|---|---|---|---|---|
| Peroxide value, PV (µg/g of active oxygen) | −0.43 | 0.64 | 2.70 | >0.05 |
| Free Fatty Acid, FFA (mg KOH/g) | −0.95 | 0.99 | 108.37 | <0.05 |
| Dependent Variables | Speed, Sp (mm/min) | |||
| Equation | R2 | F-Value | p-Value | |
| Oil yield (%) | 42.79 − 0.74 × Sp | 0.97 | 127.60 | <0.05 |
| Oil expression efficiency (%) Energy (kJ) | 66.17 − 0.15 × Sp | 0.97 | 127.60 | <0.05 |
| 0.18 + 0.01 × Sp | 0.60 | 6.09 | >0.05 | |
| Dependent variables | Heating temperature, Tp (°C) | |||
| Equation | R2 | F-value | p-value | |
| Oil yield (%) | 34.26 + 0.15 × Tp | 0.81 0.81 | 16.78 | <0.05 |
| Oil expression efficiency (%) Energy (kJ) | 52.98 + 0.23 × Tp | 16.78 | <0.05 | |
| 0.20 + 0.0001 × Tp | 0.21 | >0.05 | ||
| Dependent variables | Relaxation time Rt (min) | |||
| Equation | R2 | F-value | p-value | |
| Oil yield (%) | 38.18 + 0.82 × Rt | 0.82 | 37.57 | <0.05 |
| Oil expression efficiency (%) | 59.03 + 1.26 × Rt | 0.82 | 37.57 | |
| Dependent variables | Heating temperature, Tp (°C) | |||
| Equation | R2 | F-value | p-value | |
| Peroxide value, PV (µg/g of active oxygen) | 18.31 − 0.05 × Tp | 0.18 | 0.89 | >0.05 |
| Free Fatty Acid, FFA (mg KOH/g) | 6.21 − 0.04 × Tp | 0.91 | 35.27 | <0.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gürdil, G.A.K.; Kabutey, A.; Selvi, K.Ç.; Mizera, Č.; Herák, D.; Fraňková, A. Evaluation of Postharvest Processing of Hazelnut Kernel Oil Extraction Using Uniaxial Pressure and Organic Solvent. Processes 2020, 8, 957. https://doi.org/10.3390/pr8080957
Gürdil GAK, Kabutey A, Selvi KÇ, Mizera Č, Herák D, Fraňková A. Evaluation of Postharvest Processing of Hazelnut Kernel Oil Extraction Using Uniaxial Pressure and Organic Solvent. Processes. 2020; 8(8):957. https://doi.org/10.3390/pr8080957
Chicago/Turabian StyleGürdil, Gürkan Alp Kağan, Abraham Kabutey, Kemal Çağatay Selvi, Čestmír Mizera, David Herák, and Adéla Fraňková. 2020. "Evaluation of Postharvest Processing of Hazelnut Kernel Oil Extraction Using Uniaxial Pressure and Organic Solvent" Processes 8, no. 8: 957. https://doi.org/10.3390/pr8080957
APA StyleGürdil, G. A. K., Kabutey, A., Selvi, K. Ç., Mizera, Č., Herák, D., & Fraňková, A. (2020). Evaluation of Postharvest Processing of Hazelnut Kernel Oil Extraction Using Uniaxial Pressure and Organic Solvent. Processes, 8(8), 957. https://doi.org/10.3390/pr8080957








