MicroRNAs: Biogenesis, Functions and Potential Biomarkers for Early Screening, Prognosis and Therapeutic Molecular Monitoring of Nasopharyngeal Carcinoma
Abstract
1. Introduction
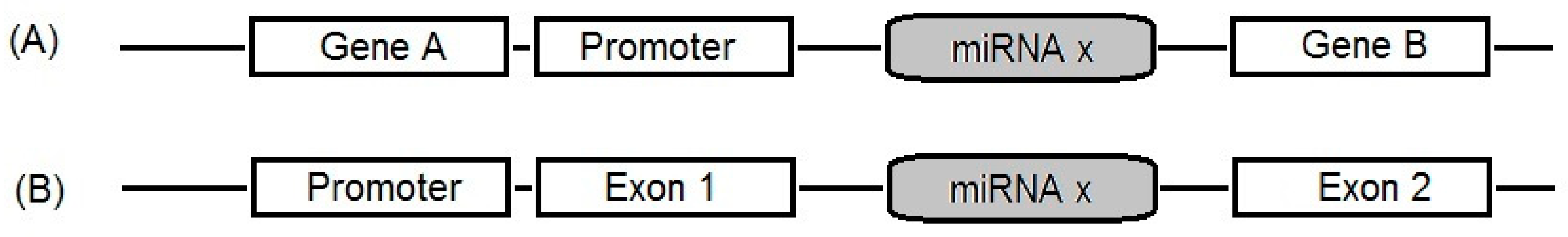
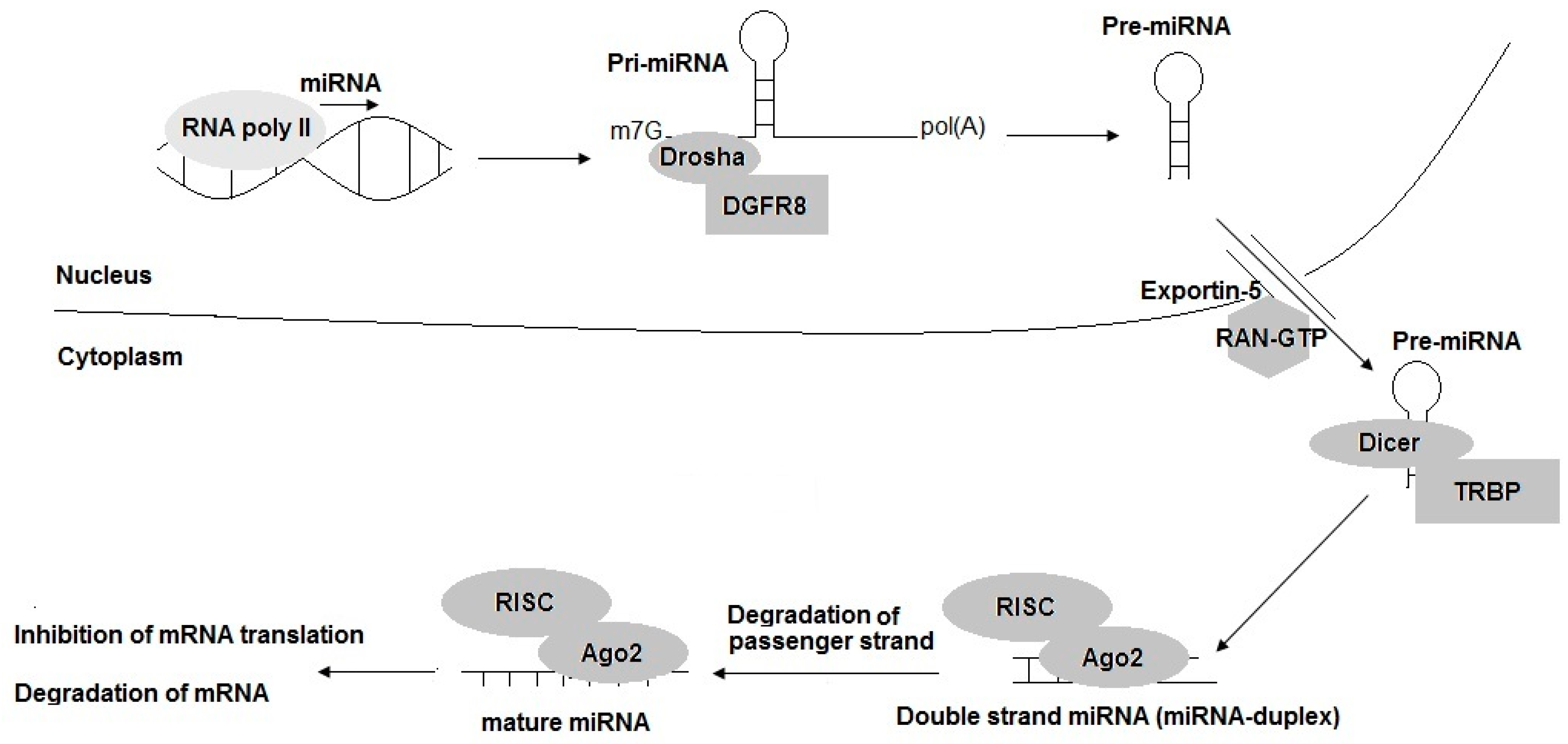
2. Brief Understanding of miRNAs Biogenesis
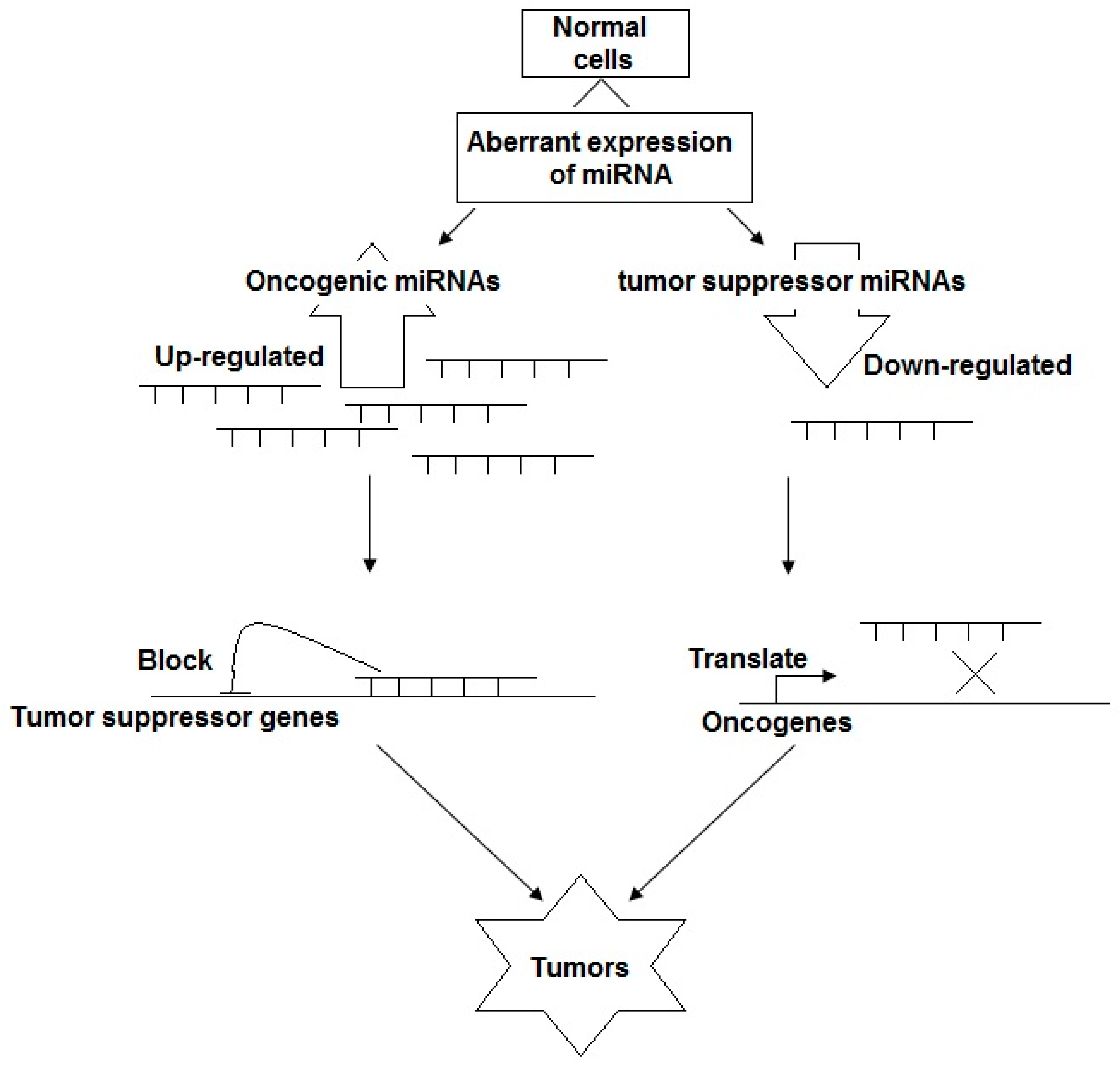
3. The Roles of miRNAs in NPC Tumorigenesis: Potential Biomarkers for NPC Early Screening, Prognosis, and Therapy
3.1. miRNAs as Tumor Suppressor Genes
3.2. miRNAs as Oncogenes
4. Circulating miRNA: Potential Values in Nasopharyngeal Carcinoma Early Screening, Prognosis and Cancer Treatment
5. miRNAs as Novel Molecular Targets for NPC Therapies
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Li, K.; Zhu, X.; Li, L.; Ning, R.; Liang, Z.; Zeng, F.; Su, F.; Huang, S.; Yang, X.; Qu, S. Identification of non-invasive biomarkers for predicting the radiosensitivity of nasopharyngeal carcinoma from serum microRNAs. Sci. Rep. 2020, 10, 5161. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Bushati, N.; Cohen, S.M. microRNA functions. Annu. Rev. Cell Dev. Biol. 2007, 23, 175–205. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Stahlhut Espinosa, C.E.; Slack, F.J. The role of microRNAs in cancer. Yale J. Biol. Med. 2006, 79, 131–140. [Google Scholar]
- Si, W.; Shen, J.; Zheng, H.; Fan, W. The role and mechanisms of action of microRNAs in cancer drug resistance. Clin. Epigenet. 2019, 11, 25. [Google Scholar] [CrossRef]
- Li, M.; Wang, C.; Yu, B.; Zhang, X.; Shi, F.; Liu, X. Diagnostic value of RASSF1A methylation for breast cancer: A meta-analysis. Biosci. Rep. 2019, 39, BSR20190923. [Google Scholar] [CrossRef]
- Tabuchi, K.; Nakayama, M.; Nishimura, B.; Hayashi, K.; Hara, A. Early detection of nasopharyngeal carcinoma. Int. J. Otolaryngol. 2011, 2011, 638058. [Google Scholar] [CrossRef]
- Felekkis, K.; Touvana, E.; Stefanou, C.; Deltas, C. microRNAs: A newly described class of encoded molecules that play a role in health and disease. Hippokratia 2010, 14, 236–240. [Google Scholar]
- Wong, L.L.; Wang, J.; Liew, O.W.; Richards, A.M.; Chen, Y.T. MicroRNA and Heart Failure. Int. J. Mol. Sci. 2016, 17, 502. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Hagedorn, C.H.; Cullen, B.R. Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. RNA 2004, 10, 1957–1966. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, M.; Han, J.; Yeom, K.H.; Lee, S.; Baek, S.H.; Kim, V.N. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004, 23, 4051–4060. [Google Scholar] [CrossRef] [PubMed]
- Lao, D.T.; Truong, K.P.; Le, H.A.T. miRNA-141 as the Biomarker for Human Cancers. AJPRHC 2018, 10, 42–49. [Google Scholar] [CrossRef]
- Peng, Y.; Croce, C.M. The role of MicroRNAs in human cancer. Signal Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Ahn, C.; Han, J.; Choi, H.; Kim, J.; Yim, J.; Lee, J.; Provost, P.; Rådmark, O.; Kim, S.; et al. The nuclear RNase III Drosha initiates microRNA processing. Nature 2003, 425, 415–419. [Google Scholar] [CrossRef]
- Kim, V.N.; Han, J.; Siomi, M.C. Biogenesis of small RNAs in animals. Nat. Rev. Mol. Cell Biol. 2009, 10, 126–139. [Google Scholar] [CrossRef]
- Inui, M.; Martello, G.; Piccolo, S. MicroRNA control of signal transduction. Nat. Rev. Mol. Cell Biol. 2010, 11, 252–263. [Google Scholar] [CrossRef]
- Witkos, T.M.; Koscianska, E.; Krzyzosiak, W.J. Practical Aspects of microRNA Target Prediction. Curr. Mol. Med. 2011, 11, 93–109. [Google Scholar] [CrossRef]
- Sengupta, S.; den Boon, J.A.; Chen, I.H.; Newton, M.A.; Stanhope, S.A.; Cheng, Y.J.; Chen, C.J.; Hildesheim, A.; Sugden, B.; Ahlquist, P. MicroRNA 29c is down-regulated in nasopharyngeal carcinomas, up-regulating mRNAs encoding extracellular matrix proteins. Proc. Natl. Acad. Sci. USA 2008, 105, 5874–5878. [Google Scholar] [CrossRef]
- Bruce, J.P.; Liu, F.F. MicroRNAs in nasopharyngeal carcinoma. Chin. J. Cancer 2014, 33, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Spence, T.; Bruce, J.; Yip, K.W.; Liu, F.F. MicroRNAs in nasopharyngeal carcinoma. Chin. Clin. Oncol. 2016, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Shenouda, S.K.; Alahari, S.K. MicroRNA function in cancer: Oncogene or a tumor suppressor? Cancer Metastasis Rev. 2009, 28, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Tang, X.; Tang, F. The role of microRNAs in nasopharyngeal carcinoma. Tumour Biol. 2015, 36, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.S.; Man, O.Y.; Tsang, C.M.; Tsao, S.W.; Tsang, R.K.; Chan, J.Y.; Ho, W.K.; Wei, W.I.; To, V.S. MicroRNA let-7 suppresses nasopharyngeal carcinoma cells proliferation through downregulating c-Myc expression. J. Cancer Res. Clin. Oncol. 2011, 137, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wu, K.; Yang, Z.; Wu, A. High-mobility group A2 overexpression is an unfavorable prognostic biomarker for nasopharyngeal carcinoma patients. Mol. Cell Biochem. 2015, 409, 155–162. [Google Scholar] [CrossRef]
- Wu, A.; Wu, K.; Li, J.; Mo, Y.; Lin, Y.; Wang, Y.; Shen, X.; Li, S.; Li, L.; Yang, Z. Let-7a inhibits migration, invasion and epithelial-mesenchymal transition by targeting HMGA2 in nasopharyngeal carcinoma. J. Transl. Med. 2015, 13, 105. [Google Scholar] [CrossRef]
- Cai, K.; Wan, Y.; Sun, G.; Shi, L.; Bao, X.; Wang, Z. Let-7a inhibits proliferation and induces apoptosis by targeting EZH2 in nasopharyngeal carcinoma cells. Oncol. Rep. 2012, 28, 2101–2106. [Google Scholar] [CrossRef]
- Lu, J.; Luo, H.; Liu, X.; Peng, Y.; Zhang, B.; Wang, L.; Xu, X.; Peng, X.; Li, G.; Tian, W.; et al. miR-9 targets CXCR4 and functions as a potential tumor suppressor in nasopharyngeal carcinoma. Carcinogenesis 2014, 35, 554–563. [Google Scholar] [CrossRef]
- Lu, J.; Xu, X.; Liu, X.; Peng, Y.; Zhang, B.; Wang, L.; Luo, H.; Peng, X.; Li, G.; Tian, W.; et al. Predictive value of miR-9 as a potential biomarker for nasopharyngeal carcinoma metastasis. Br. J. Cancer 2014, 110, 392–398. [Google Scholar] [CrossRef]
- Gao, F.; Zhao, Z.L.; Zhao, W.T.; Fan, Q.R.; Wang, S.C.; Li, J.; Zhang, Y.Q.; Shi, J.W.; Lin, X.L.; Yang, S.; et al. miR-9 modulates the expression of interferon-regulated genes and MHC class I molecules in human nasopharyngeal carcinoma cells. Biochem. Biophys. Res. Commun. 2013, 431, 610–616. [Google Scholar] [CrossRef]
- Sam, C.K.; Brooks, L.A.; Niedobitek, G.; Young, L.S.; Prasad, U.; Rickinson, A.B. Analysis of Epstein-Barr virus infection in nasopharyngeal biopsies from a group at high risk of nasopharyngeal carcinoma. Int. J. Cancer 1993, 53, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Tang, L.L.; Sun, Y.; Cui, R.X.; Wang, H.Y.; Huang, B.J.; He, Q.M.; Jiang, W.; Ma, J. MiR-29c suppresses invasion and metastasis by targeting TIAM1 in nasopharyngeal carcinoma. Cancer Lett. 2013, 329, 181–188. [Google Scholar] [CrossRef]
- He, Q.; Ren, X.; Chen, J.; Li, Y.; Tang, X.; Wen, X.; Yang, X.; Zhang, J.; Wang, Y.; Ma, J.; et al. miR-16 targets fibroblast growth factor 2 to inhibit NPC cell proliferation and invasion via PI3K/AKT and MAPK signaling pathways. Oncotarget 2016, 7, 3047–3058. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Zhang, Y.; Zhao, M.; Li, Q.; Chen, R.; Long, X.; Fang, W.; Liu, Z. miR-16 induction after CDK4 knockdown is mediated by c-Myc suppression and inhibits cell growth as well as sensitizes nasopharyngeal carcinoma cells to chemotherapy. Tumour Biol. 2016, 37, 2425–2433. [Google Scholar] [CrossRef] [PubMed]
- Alajez, N.M.; Shi, W.; Hui, A.B.; Bruce, J.; Lenarduzzi, M.; Ito, E.; Yue, S.; O’Sullivan, B.; Liu, F.F. Enhancer of Zeste homolog 2 (EZH2) is overexpressed in recurrent nasopharyngeal carcinoma and is regulated by miR-26a, miR-101, and miR-98. Cell Death Dis. 2010, 1, e85. [Google Scholar] [CrossRef]
- Yu, L.; Lu, J.; Zhang, B.; Liu, X.; Wang, L.; Li, S.Y.; Peng, X.H.; Xu, X.; Tian, W.D.; Li, X.P. miR-26a inhibits invasion and metastasis of nasopharyngeal cancer by targeting EZH2. Oncol. Lett. 2013, 5, 1223–1228. [Google Scholar] [CrossRef]
- Wang, H.Y.; Li, Y.Y.; Fu, S.; Wang, X.P.; Huang, M.Y.; Zhang, X.; Shao, Q.; Deng, L.; Zeng, M.S.; Zeng, Y.X.; et al. MicroRNA-30a promotes invasiveness and metastasis in vitro and in vivo through epithelial-mesenchymal transition and results in poor survival of nasopharyngeal carcinoma patients. Exp. Biol. Med. 2014, 239, 891–898. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Ge, X.; Jia, L.; Zhang, Z.; Fang, R.; Yang, J.; Liu, J.; Peng, S.; Zhou, M.; et al. MiR-34b-3 and miR-449a inhibit malignant progression of nasopharyngeal carcinoma by targeting lactate dehydrogenase A. Oncotarget 2016, 23, 54838–54851. [Google Scholar] [CrossRef]
- Li, Y.Q.; Ren, X.Y.; He, Q.M.; Xu, Y.F.; Tang, X.R.; Sun, Y.; Zeng, M.S.; Kang, T.B.; Liu, N.; Ma, J. MiR-34c suppresses tumor growth and metastasis in nasopharyngeal carcinoma by targeting MET. Cell Death Dis. 2015, 22, e1618. [Google Scholar] [CrossRef][Green Version]
- Peng, X.H.; Huang, H.R.; Lu, J.; Liu, X.; Zhao, F.P.; Zhang, B.; Lin, S.X.; Wang, L.; Chen, H.H.; Xu, X.; et al. MiR-124 suppresses tumor growth and metastasis by targeting Foxq1 in nasopharyngeal carcinoma. Mol. Cancer 2014, 13, 186. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lv, X.B.; Wang, X.P.; Sang, Y.; Xu, S.; Hu, K.; Wu, M.; Liang, Y.; Liu, P.; Tang, J.; et al. MiR-138 suppressed nasopharyngeal carcinoma growth and tumorigenesis by targeting the CCND1 oncogene. Cell Cycle 2012, 11, 2495–2506. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Qi, X.; Li, J.; Zhou, C.; Lv, C.; Tian, M. MiR-142-3p Suppresses SOCS6 Expression and Promotes Cell Proliferation in Nasopharyngeal Carcinoma. Cell Physiol. Biochem. 2015, 36, 1743–1752. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, Q.; Wen, X.; Hong, X.; Yang, X.; Tang, X.; Zhang, P.; Lei, Y.; Sun, Y.; Zhang, J.; et al. EZH2-DNMT1-mediated epigenetic silencing of miR-142-3p promotes metastasis through targeting ZEB2 in nasopharyngeal carcinoma. Cell Death Differ. 2019, 26, 1089–1106. [Google Scholar] [CrossRef]
- Wang, L.; Tian, W.D.; Xu, X.; Nie, B.; Lu, J.; Liu, X.; Zhang, B.; Dong, Q.; Sunwoo, J.B.; Li, G.; et al. Epstein-Barr virus nuclear antigen 1 (EBNA1) protein induction of epithelial-mesenchymal transition in nasopharyngeal carcinoma cells. Cancer 2014, 120, 363–372. [Google Scholar] [CrossRef]
- Yang, X.; Ni, W.; Lei, K. miR-200b suppresses cell growth, migration and invasion by targeting Notch1 in nasopharyngeal carcinoma. Cell Physiol. Biochem. 2013, 32, 1288–1298. [Google Scholar] [CrossRef]
- Ma, L.; Deng, X.; Wu, M.; Zhang, G.; Huang, J. Down-regulation of miRNA-204 by LMP-1 enhances CDC42 activity and facilitates invasion of EBV-associated nasopharyngeal carcinoma cells. FEBS Lett. 2014, 588, 1562–1570. [Google Scholar] [CrossRef]
- Deng, M.; Tang, H.; Zhou, Y.; Zhou, M.; Xiong, W.; Zheng, Y.; Ye, Q.; Zeng, X.; Liao, Q.; Guo, X.; et al. miR-216b suppresses tumor growth and invasion by targeting KRAS in nasopharyngeal carcinoma. J. Cell Sci. 2011, 124 Pt 17, 2997–3005. [Google Scholar] [CrossRef]
- Deng, M.; Liu, J.F.; Gu, Y.X.; Zheng, G.P.; He, Z.M. miR-216b suppresses cell proliferation and invasion by targeting PKCα in nasopharyngeal carcinoma cells. Zhonghua Zhong Liu Za Zhi 2013, 35, 645–650. [Google Scholar]
- Alajez, N.M.; Lenarduzzi, M.; Ito, E.; Hui, A.B.; Shi, W.; Bruce, J.; Yue, S.; Huang, S.H.; Xu, W.; Waldron, J.; et al. MiR-218 suppresses nasopharyngeal cancer progression through downregulation of survivin and the SLIT2-ROBO1 pathway. Cancer Res. 2011, 71, 2381–2391. [Google Scholar] [CrossRef]
- Gao, L.; Xiong, X. MiR-223 inhibits the proliferation, invasion and EMT of nasopharyngeal carcinoma cells by targeting SSRP1. Int. J. Clin. Exp. Pathol. 2018, 11, 4374–4384. [Google Scholar]
- Yang, W.; Lan, X.; Li, D.; Li, T.; Lu, S. MiR-223 targeting MAFB suppresses proliferation and migration of nasopharyngeal carcinoma cells. BMC Cancer 2015, 15, 461. [Google Scholar] [CrossRef]
- Hui, A.B.; Bruce, J.P.; Alajez, N.M.; Shi, W.; Yue, S.; Perez-Ordonez, B.; Xu, W.; O’Sullivan, B.; Waldron, J.; Cummings, B.; et al. Significance of dysregulated metadherin and microRNA-375 in head and neck cancer. Clin Cancer Res. 2011, 17, 7539–7550. [Google Scholar] [CrossRef]
- Hou, J.; Yan, J.; Ren, X.Y.; Zhu, K.; Du, X.Y.; Li, J.J.; Xu, M. Long noncoding RNA ROR1-AS1 induces tumor metastasis and epithelial-mesenchymal transition by sponging miR-375 in nasopharyngeal carcinoma. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 174–180. [Google Scholar] [CrossRef]
- Liu, N.; Jiang, N.; Guo, R.; Jiang, W.; He, Q.M.; Xu, Y.F.; Li, Y.Q.; Tang, L.L.; Mao, Y.P.; Sun, Y.; et al. MiR-451 inhibits cell growth and invasion by targeting MIF and is associated with survival in nasopharyngeal carcinoma. Mol. Cancer 2013, 12, 123. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Gao, R.; Yu, Y.; Kaul, Z.; Wang, J.; Kalra, R.S.; Zhang, Z.; Kaul, S.C.; Wadhwa, R. Tumor suppressor activity of miR-451: Identification of CARF as a new target. Sci. Rep. 2018, 8, 375. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, N.; Vijayarathna, S.; Jothy, S.L.; Oon, C.E.; Chen, Y.; Kanwar, J.R.; Sasidharan, S. MicroRNAs: Biogenesis, roles for carcinogenesis and as potential biomarkers for cancer diagnosis and prognosis. Asian Pac. J. Cancer Prev. 2014, 15, 7489–7497. [Google Scholar] [CrossRef] [PubMed]
- Ørom, U.A.; Nielsen, F.C.; Lund, A.H. MicroRNA-10a binds the 5’UTR of ribosomal protein mRNAs and enhances their translation. Mol. Cell 2008, 30, 460–471. [Google Scholar] [CrossRef]
- Sun, X.J.; Liu, H.; Zhang, P.; Zhang, X.D.; Jiang, Z.W.; Jiang, C.C. miR-10b promotes migration and invasion in nasopharyngeal carcinoma cells. Asian Pac. J. Cancer Prev. 2013, 14, 5533–5537. [Google Scholar] [CrossRef]
- Allaya, N.; Khabir, A.; Sallemi-Boudawara, T.; Sellami, N.; Daoud, J.; Ghorbel, A.; Frikha, M.; Gargouri, A.; Mokdad-Gargouri, R.; Ayadi, W. Over-expression of miR-10b in NPC patients: Correlation with LMP1 and Twist1. Tumour Biol. 2015, 36, 3807–3814. [Google Scholar] [CrossRef]
- Chen, C.; Lu, Z.; Yang, J.; Hao, W.; Qin, Y.; Wang, H.; Xie, C.; Xie, R. MiR-17-5p promotes cancer cell proliferation and tumorigenesis in nasopharyngeal carcinoma by targeting p21. Cancer Med. 2016, 5, 3489–3499. [Google Scholar] [CrossRef]
- Hu, Z.; Zhou, S.; Luo, H.; Ji, M.; Zheng, J.; Huang, F.; Wang, F. miRNA-17 promotes nasopharyngeal carcinoma radioresistance by targeting PTEN/AKT. Int. J. Clin. Exp. Pathol. 2019, 12, 229–240. [Google Scholar]
- Luo, Z.; Dai, Y.; Zhang, L.; Jiang, C.; Li, Z.; Yang, J.; McCarthy, J.B.; She, X.; Zhang, W.; Ma, J.; et al. miR-18a promotes malignant progression by impairing microRNA biogenesis in nasopharyngeal carcinoma. Carcinogenesis 2013, 34, 415–425. [Google Scholar] [CrossRef]
- Mai, S.; Xiao, R.; Shi, L.; Zhou, X.; Yang, T.; Zhang, M.; Weng, N.; Zhao, X.; Wang, R.; Liu, J.; et al. MicroRNA-18a promotes cancer progression through SMG1 suppression and mTOR pathway activation in nasopharyngeal carcinoma. Cell Death Dis. 2019, 10, 819. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Bian, G.; Pan, Y.; Han, X.; Sun, Y.; Wang, Y.; Shen, G.; Cheng, M.; Fang, X.; Hu, S. MiR-20a-5p promotes radio-resistance by targeting Rab27B in nasopharyngeal cancer cells. Cancer Cell Int. 2017, 17, 32. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yan, L.; Zhang, W.; Wang, H.; Chen, W.; Hu, N.; Ou, H. miR-21 inhibitor suppresses proliferation and migration of nasopharyngeal carcinoma cells through down-regulation of BCL2 expression. Int. J. Clin. Exp. Pathol. 2014, 7, 3478–3487. [Google Scholar]
- Ou, H.; Li, Y.; Kang, M. Activation of miR-21 by STAT3 induces proliferation and suppresses apoptosis in nasopharyngeal carcinoma by targeting PTEN gene. PLoS ONE 2014, 9, e109929. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, H.; Guo, X.; Wang, Z.; Liang, S.; Dang, C. IGF-I Induces Epithelial-to-Mesenchymal Transition via the IGF-IR-Src-MicroRNA-30a-E-Cadherin Pathway in Nasopharyngeal Carcinoma Cells. Oncol. Res. 2016, 24, 225–231. [Google Scholar] [CrossRef]
- Lyu, X.; Fang, W.; Cai, L.; Zheng, H.; Ye, Y.; Zhang, L.; Li, J.; Peng, H.; Cho, W.C.; Wang, E.; et al. TGFβR2 is a major target of miR-93 in nasopharyngeal carcinoma aggressiveness. Mol. Cancer 2014, 13, 51. [Google Scholar] [CrossRef]
- Xu, Y.F.; Mao, Y.P.; Li, Y.Q.; Ren, X.Y.; He, Q.M.; Tang, X.R.; Sun, Y.; Liu, N.; Ma, J. MicroRNA-93 promotes cell growth and invasion in nasopharyngeal carcinoma by targeting disabled homolog-2. Cancer Lett. 2015, 363, 146–155. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Z. miR-93 enhances cell proliferation by directly targeting CDKN1A in nasopharyngeal carcinoma. Oncol. Lett. 2018, 15, 1723–1727. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, W.; Li, S.; Yang, Q.; Hu, J.; Zeng, N.; Gao, C. MicroRNA 141 represses nasopharyngeal carcinoma growth through inhibiting BMI1. Oncol Lett. 2018, 16, 6479–6487. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, R.; Wang, H.; Luo, Y.; Wang, X.; Niu, W.; Zhou, Y.; Wen, Q.; Fan, S.; Li, X.; et al. miR-141 is involved in BRD7-mediated cell proliferation and tumor formation through suppression of the PTEN/AKT pathway in nasopharyngeal carcinoma. Cell Death Dis. 2016, 7, e2156. [Google Scholar] [CrossRef] [PubMed]
- Lao, T.D.; Nguyen, T.V.; Nguyen, D.H.; Nguyen, M.T.; Nguyen, C.H.; Le, T.H.A. miR-141 is up-regulated in biopsies from Vietnamese patients with nasopharyngeal carcinoma. Braz. Oral Res. 2018, 32, e126. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Ho-Fun Lee, V.; Wong, A.M.; Kwong, D.L.; Zhu, Y.H.; Dong, S.S.; Kong, K.L.; Chen, J.; Tsao, S.W.; Guan, X.Y.; et al. MicroRNA-144 promotes cell proliferation, migration and invasion in nasopharyngeal carcinoma through repression of PTEN. Carcinogenesis 2013, 34, 454–463. [Google Scholar] [CrossRef]
- Song, L.; Chen, L.; Luan, Q.; Kong, Q. miR-144-3p facilitates nasopharyngeal carcinoma via crosstalk with PTEN. J. Cell Physiol. 2019, 234, 17912–17924. [Google Scholar] [CrossRef]
- Wu, C.W.; Wang, S.G.; Lin, M.L.; Chen, S.S. Downregulation of miR-144 by triptolide enhanced p85α-PTEN complex formation causing S phase arrest of human nasopharyngeal carcinoma cells. Eur. J. Pharmacol. 2019, 855, 137–148. [Google Scholar] [CrossRef]
- Kong, Y.G.; Cui, M.; Chen, S.M.; Xu, Y.; Xu, Y.; Tao, Z.Z. LncRNA-LINC00460 facilitates nasopharyngeal carcinoma tumorigenesis through sponging miR-149-5p to up-regulate IL6. Gene 2018, 639, 77–84. [Google Scholar] [CrossRef]
- Luo, Z.; Zhang, L.; Li, Z.; Jiang, C.; Dai, Y.; Liu, X.; Zheng, Y.; Yu, H.; Xiang, J.; Li, G. miR-149 promotes epithelial-mesenchymal transition and invasion in nasopharyngeal carcinoma cells. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2011, 36, 604–609. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, Y.; Sun, Y.; Zheng, J.; Zhu, D. MiR-155 up-regulation by LMP1 DNA contributes to increased nasopharyngeal carcinoma cell proliferation and migration. Eur. Arch. Otorhinolaryngol. 2014, 271, 1939–1945. [Google Scholar] [CrossRef]
- Zuo, W.N.; Zhu, H.; Li, L.P.; Jin, A.Y.; Wang, H.Q. MiR-155 promotes proliferation and inhibits apoptosis of nasopharyngeal carcinoma cells through targeting PTEN-PI3K/AKT pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 7935–7942. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Xie, D.L.; Dai, X.Y. Down-regulation of miR-155 promotes apoptosis of nasopharyngeal carcinoma CNE-1 cells by targeting PI3K/AKT-FOXO3a signaling. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 7391–7398. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Wu, S.; Zhao, R.; Deng, Q. MiR-205 promotes proliferation, migration and invasion of nasopharyngeal carcinoma cells by activation of AKT signalling. J. Int. Med. Res. 2016, 44, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Nie, G.; Duan, H.; Li, X.; Yu, Z.; Luo, L.; Lu, R.; Ji, Z.; Zhang, W. MicroRNA 205 promotes the tumorigenesis of nasopharyngeal carcinoma through targeting tumor protein p53-inducible nuclear protein 1. Mol. Med. Rep. 2015, 12, 5715–5722. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, Z.C.; Li, Y.Y.; Wang, H.Y.; Fu, S.; Wang, X.P.; Zeng, M.S.; Zeng, Y.X.; Shao, J.Y. Knockdown of miR-214 promotes apoptosis and inhibits cell proliferation in nasopharyngeal carcinoma. PLoS ONE 2014, 9, e86149. [Google Scholar] [CrossRef]
- Deng, M.; Ye, Q.; Qin, Z.; Zheng, Y.; He, W.; Tang, H.; Zhou, Y.; Xiong, W.; Zhou, M.; Li, X.; et al. miR-214 promotes tumorigenesis by targeting lactotransferrin in nasopharyngeal carcinoma. Tumour Biol. 2013, 34, 1793–1800. [Google Scholar] [CrossRef]
- Yu, B.L.; Peng, X.H.; Zhao, F.P.; Liu, X.; Lu, J.; Wang, L.; Li, G.; Chen, H.H.; Li, X.P. MicroRNA-378 functions as an onco-miR in nasopharyngeal carcinoma by repressing TOB2 expression. Int. J. Oncol. 2014, 44, 1215–1222. [Google Scholar] [CrossRef]
- Chen, L.; Tang, Y.; Wang, J.; Yan, Z.; Xu, R. miR-421 induces cell proliferation and apoptosis resistance in human nasopharyngeal carcinoma via downregulation of FOXO4. Biochem. Biophys. Res. Commun. 2013, 435, 745–750. [Google Scholar] [CrossRef]
- Zhao, L.; Tang, M.; Hu, Z.; Yan, B.; Pi, W.; Li, Z.; Zhang, J.; Zhang, L.; Jiang, W.; Li, G.; et al. miR-504 mediated down-regulation of nuclear respiratory factor 1 leads to radio-resistance in nasopharyngeal carcinoma. Oncotarget 2015, 6, 15995–16018. [Google Scholar] [CrossRef]
- Liang, S.; Zhang, N.; Deng, Y.; Chen, L.; Zhang, Y.; Zheng, Z.; Luo, W.; Lv, Z.; Li, S.; Xun, T. Increased Serum Level of MicroRNA-663 Is Correlated with Poor Prognosis of Patients with Nasopharyngeal Carcinoma. Dis. Markers 2016, 2016, 7648215. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, F.; Du, Z.; Xiang, Y. Up-regulation of serum miR-744 predicts poor prognosis in patients with nasopharyngeal carcinoma. Int. J. Clin. Exp. Med. 2015, 8, 13296–13302. [Google Scholar] [PubMed]
- Mo, X.; Yin, W.; Huang, Y.; Guo, W.; Zhou, M.; Ye, H. Expression of miR-3182 and EBV-miR-BART8-3p in nasopharyngeal carcinoma is correlated with distant metastasis. Int. J. Clin. Exp. Pathol. 2018, 11, 3134–3140. [Google Scholar] [PubMed]
- Ma, R.; Jiang, T.; Kang, X. Circulating microRNAs in cancer: Origin, function and application. J. Exp. Clin. Cancer Res. 2012, 31, 38. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.P.; Ramphul, E.; Howard, L.; Gallagher, W.M.; Malone, C.; Kerin, M.J.; Dwyer, R.M. Circulating MicroRNAs in Cancer. Methods Mol. Biol. 2017, 1509, 123–139. [Google Scholar]
- Cufaro, M.C.; Pieragostino, D.; Lanuti, P.; Rossi, C.; Cicalini, I.; Federici, L.; De Laurenzi, V.; Del Boccio, P. Extracellular Vesicles and Their Potential Use in Monitoring Cancer Progression and Therapy: The Contribution of Proteomics. J. Oncol. 2019, 2019, 1639854. [Google Scholar] [CrossRef]
- Sohel, M.H. Extracellular/Circulating MicroRNAs: Release Mechanisms, Functions and Challenges. Achiev. Life Sci. 2016, 10, 175–186. [Google Scholar] [CrossRef]
- Xu, X.; Lu, J.; Wang, F.; Liu, X.; Peng, X.; Yu, B.; Zhao, F.; Li, X. Dynamic Changes in Plasma MicroRNAs Have Potential Predictive Values in Monitoring Recurrence and Metastasis of Nasopharyngeal Carcinoma. Biomed. Res. Int. 2018, 7329195. [Google Scholar] [CrossRef]
- Wen, W.; Mai, S.J.; Lin, H.X.; Zhang, M.Y.; Huang, J.L.; Hua, X.; Lin, C.; Long, Z.Q.; Lu, Z.J.; Sun, X.Q.; et al. Identification of two microRNA signatures in whole blood as novel biomarkers for diagnosis of nasopharyngeal carcinoma. J. Transl. Med. 2019, 17, 186. [Google Scholar] [CrossRef]
- Cui, M.; Wang, H.; Yao, X.; Zhang, D.; Xie, Y.; Cui, R.; Zhang, X. Circulating MicroRNAs in Cancer: Potential and Challenge. Front. Genet. 2019, 10, 626. [Google Scholar] [CrossRef]
- Chim, S.S.; Shing, T.K.; Hung, E.C.; Leung, T.Y.; Lau, T.K.; Chiu, R.W.; Lo, Y.M. Detection and characterization of placental microRNAs in maternal plasma. Clin. Chem. 2008, 54, 482–490. [Google Scholar] [CrossRef]
- Lawrie, C.H.; Gal, S.; Dunlop, H.M.; Pushkaran, B.; Liggins, A.P.; Pulford, K.; Banham, A.H.; Pezzella, F.; Boultwood, J.; Wainscoat, J.S.; et al. Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. Br. J. Haematol. 2008, 141, 672–675. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef] [PubMed]
- Lodes, M.J.; Caraballo, M.; Suciu, D.; Munro, S.; Kumar, A.; Anderson, B. Detection of cancer with serum miRNAs on an oligonucleotide microarray. PLoS ONE 2009, 4, e6229. [Google Scholar] [CrossRef] [PubMed]
- Resnick, K.E.; Alder, H.; Hagan, J.P.; Richardson, D.L.; Croce, C.M.; Cohn, D.E. The detection of differentially expressed microRNAs from the serum of ovarian cancer patients using a novel real-time PCR platform. Gynecol. Oncol. 2009, 112, 55–59. [Google Scholar] [CrossRef]
- Zeng, X.; Xiang, J.; Wu, M.; Xiong, W.; Tang, H.; Deng, M.; Li, X.; Liao, Q.; Su, B.; Luo, Z.; et al. Circulating miR-17, miR-20a, miR-29c, and miR-223 combined as non-invasive biomarkers in nasopharyngeal carcinoma. PLoS ONE 2012, 7, e46367. [Google Scholar] [CrossRef]
- Yi, S.J.; Liu, P.; Chen, B.L.; Ou-Yang, L.; Xiong, W.M.; Su, J.P. Circulating miR-31-5p may be a potential diagnostic biomarker in nasopharyngeal carcinoma. Neoplasma 2019, 66, 825–829. [Google Scholar] [CrossRef]
- Lu, J.; Liu, Q.H.; Wang, F.; Tan, J.J.; Deng, Y.Q.; Peng, X.H.; Liu, X.; Zhang, B.; Xu, X.; Li, X.P. Exosomal miR-9 inhibits angiogenesis by targeting MDK and regulating PDK/AKT pathway in nasopharyngeal carcinoma. J. Exp. Clin. Cancer Res. 2018, 13, 147. [Google Scholar] [CrossRef]
- Ye, S.B.; Zhang, H.; Cai, T.T.; Liu, Y.N.; Ni, J.J.; He, J.; Peng, J.Y.; Chen, Q.Y.; Mo, H.Y.; Jun-Cui Zhang, X.S.; et al. Exosomal miR-24-3p impedes T-cell function by targeting FGF11 and serves as a potential prognostic biomarker for nasopharyngeal carcinoma. J. Pathol. 2016, 240, 329–340. [Google Scholar] [CrossRef]
- Bao, L.; You, B.; Shi, S.; Shan, Y.; Zhang, Q.; Yue, H.; Zhang, J.; Zhang, W.; Shi, Y.; Liu, Y.; et al. Metastasis-associated miR-23a from nasopharyngeal carcinoma-derived exosomes mediates angiogenesis by repressing a novel target gene TSGA10. Oncogene 2018, 37, 2873–2889. [Google Scholar] [CrossRef]
- Wan, F.Z.; Chen, K.H.; Sun, Y.C.; Chen, X.C.; Liang, R.B.; Chen, L.; Zhu, X.D. Exosomes overexpressing miR-34c inhibit malignant behavior and reverse the radioresistance of nasopharyngeal carcinoma. J. Transl. Med. 2020, 18, 12. [Google Scholar] [CrossRef]
- Kang, M.; Xiao, J.; Wang, J.; Zhou, P.; Wei, T.; Zhao, T.; Wang, R. MiR-24 enhances radiosensitivity in nasopharyngeal carcinoma by targeting SP1. Cancer Med. 2016, 5, 1163–1173. [Google Scholar] [CrossRef] [PubMed]

| miRNAs | Functions and Target Genes |
|---|---|
| Let-7 family (a, -b, -d, -e, -g, and -i) | Promoting the cell proliferation and apoptosis, the epithelial mesenchymal transition by directly regulating the MYC [25], high-mobility group A2 (HMGA2) [26,27], and enhancer of zeste homolog 2 (EZH2) [28]. |
| hsa-miR-9 | Inhibiting the nasopharyngeal tumor cell proliferation, migratory and invasion by targeting the 3′ untranslated region of chemokine (C-X-C motif) receptor 4 (CXCR4) through activation of the Mitogen-activated protein kinase (MAPK) pathway [29,30]; modulating the immune response through targeting numerous interferon-regulated genes: FI44L, IFI27, PSB9_HUMAN, PSMB8, IRF5, PSMB10, IFIT2, TRAIL, IFIT1, PSB8_HUMAN, IRF1, B2M and GBP1, MHC class I molecules and interleukin (IL)-related genes: IL20RB, GALT, IL7, IL1B, IL11, IL1F8, IL1A, IL6 and IL7R [31]. |
| hsa-miR-16 | Inhibiting the NPC cell proliferation, migration, invasion, metastatic colonization by targeting fibroblast growth factor 2 (FGF2) via phosphoinositide-3-kinase/AKT (PI3K/AKT) and mitogen-activated protein kinase (MAPK) signaling pathways [32]. Additionally, Knocking-down CDK4 leads to the induction of miR-16, resulting in inhibiting the cell growth [33]. |
| hsa-miR-26 | Suppressing the growth of NPC cell as well as the formation of colony by inducing G1 cell-cycle arrest. Additionally, hsa-miR-26 targets the CDK inhibitors p14 (ARF), p21 (CIP1), zeste homolog 2 (EZH2), T cell lymphoma invasion and metastasis 1 (TIAM1), leading to decrease oncogenic properties of migration, invasion, and cell survival [34,35]. |
| hsa-miR-29c | Suppressing the NPC cells migration and invasion through the hsa-miR-29c/TIAM1 pathway to inhibit the translation of T cell lymphoma invasion and metastasis (TIAM1) [36,37], as well as targeting the multiple mRNAs, which encoded extracellular matrix proteins, includes seven collagens, laminin γ1, fibrillin, and secreted protein, acidic, cysteine-rich (SPARC) [20]. Additionally, miR-29c can involve to numerous pathways to suppress the proliferation, survival, and motility of NPC cells [21]. |
| hsa-miR-30a | Regulating the invasion and metastasis of nasopharyngeal cancer through epithelial-mesenchymal transition by inhibiting the E-cadherin via targeting ‘-untranslated region (3′-UTR) of E-cadherin [38]. |
| hsa-miR-34b, hsa-miR-449a | Inhibiting the nasopharyngeal malignancy progression through targeting lactate dehydrogenase A (LDHA) [39]. |
| hsa-miR-34c | Suppressing the growth and metastasis of NPC tumor by targeting MET proto-oncogene (MET) through the pathway of hsa-miR-34c/MET pathway [40]. |
| hsa-miR-98, hsa-miR-101 | Inhibiting the cellular processes, including cell differentiation, development as well as apoptosis through targeting the expression EZH2 [34]. |
| hsa-miR-124 | Inhibited cell growth, migration and invasion by repressing Foxq1 expression [41]. |
| hsa-miR-138 | Suppressing cell proliferation, colony formation and nasopharyngeal tumorigenesis by knocking-down the expression of Cyclin D1 (CCND1) [42]. |
| hsa-miR-142 | Suppressing NPC cell proliferation, invasion and metastasis by directly binding to 3′-UTR region of suppressor of cytokine signaling 6 (SOCS6) [43]. Additionally, the hypermethylation of DNMT1 leading to the silence of hsa-miR-142 also promotes the metastasis through targeting zinc finger E-box binding homeobox 2 (ZEB2) [44]. |
| hsa-miR-200b | Inhibiting the NPC cell growth, migration, and invasion. EBV-encoded EB nuclear antigen 1 (EBNA-1) suppresses the expression of hsa-miR-200b, results in upregulating zinc finger E-box binding homeobox 1 and 2 (ZEB1, ZEB2) [38]. Additionally, Notch1 was identified as the direct target gene of hsa-miR-200b [45]. |
| hsa-miR-204 | Inhibiting the invasion of NPC. Latent membrane protein 1 (LMP-1) of Epstein-Barr Virus suppresses the expression of miR-204 through activating Stat-3 and enhances cell division cycle 42 (CDC42) to enhance the NPC invasion [46]. |
| hsa-miR-216b | Inhibiting the NPC cell proliferation, invasion and cell growth by targeting KRAS through the inhibition of the KRAS-related AKT and ERK pathways [47], as well as binding to the 3′-untranslated region (UTR) of PKCα [48]. |
| hsa-miR-218 | Targeting to enhancer of zeste homolog 2 (EZH2) to inhibit the differentiation, development, and apoptosis [34]. Additionally, causing the significant toxicity in NPC leads to the inhibition of NPC cell growth via the SLIT-ROBO pathway [49]. |
| hsa-miR-223 | Inhibiting the proliferation, invasion and epithelial-mesenchymal transition by reducing the expression of structure-specific recognition protein (SSRP1) [50] and MAF BZIP Transcription Factor B (MAFB) [51]. |
| hsa-miR-375 | Reducing the cell migration, invasion and tumor formation by targeting the expression of metadherin (MTDH) [52]. Additionally, ROR1-AS1 could act as a sponge for hsa-miR-375 and promote cell migration and invasion by inducing EMT process in NPC [53]. |
| hsa-miR-451 | Suppressing the cell growth and invasion targeting MIF through hsa-miR-451/MIF pathway [54]. Collaborator of ARF (CARF) was also identified as the target of hsa-miR-451 [55]. |
| miRNAs | Functions and Target Genes |
|---|---|
| hsa-miR-10a | Fascinating the ability of nasopharyngeal cell transformation via the positively control protein synthesis by stimulating ribosomal protein mRNA translation and ribosome biogenesis [57]. |
| hsa-miR-10b | Promoting the nasopharyngeal carcinoma cells migration and invasion, which related genes, including E-cadherin, Vimentin, and MMP-9, were identified [58]. Additionally, over-expression of hsa-miR-10b also correlated with LMP-1 and Twist1 through the regulation of LMP1/Twist1 pathway in NPC malignancy [59]. |
| hsa-miR-17 | Promoting the proliferation of nasopharyngeal cell by targeting p21 [60]. Additionally, the over-expression of hsa-miR-17 directly suppresses the expression of PTEN, which is a key regulator of AKT phosphorylation, resulting in enhancing the radio-resistance of NPC via the PTEN/AKT pathway [61]. |
| hsa-miR-18a | Promoting the nasopharyngeal malignant progression by widespread downregulation of the miRNome and regulating Dicer1 expression [62]. Moreover, hsa-miR-18a promotes the progression of NPC through miR-18a/SMG1/mTOR pathway [63]. |
| hsa-miR-20a | Promoting the radio-resistance by targeting Rab27B [64]. |
| hsa-miR-21 | Promoting migration and proliferation by inhibiting the B cell CLL/lymphoma 2 (BCL2) expression [65]. Additionally, the expression of STAT3 activates the hsa-miR-21, resulting in inducing the nasopharyngeal carcinoma cell proliferation and suppressing the cell apoptosis through targeting PTEN gene (PTEN/AKT pathway) [66]. |
| hsa-miR-30a | Increasing the nasopharyngeal carcinoma cell invasion and metastasis through epithelial-mesenchymal transition by targeting E-cadherin gene via GF-IR-Src-MicroRNA-30a-E-Cadherin Pathway [67,68]. |
| hsa-miR-93 | Promoting cell proliferation, invasion and metastasis via the inhibition of transforming growth factor-β receptor II (TGFβRII) through the attenuation of Smad-dependent TGF-β signaling and the activation of PI3K/Akt pathway [69] and disabled homolog-2 (DAB2) [70]. Additionally, hsa-miR-93 enhances the cell proliferation by directly targeting expression of CDKN1A gene [71]. |
| hsa-miR-141 | Increasing the cell growth, migration, invasion as well as the regulation of cell cycle, and reducing the cell apoptosis through inhibiting BMI1 via hsa-miR-141/BMI1 signaling axis [72], and increasing the tumor formation by targeting phosphatase and tensin homolog (PTEN) via BRD7/hsa-miR-141/PTEN/AKT pathway [73,74]. |
| hsa-miR-144 | Promoting the cell proliferation, migration, invasion through the repression of PTEN to active PI3K/Akt pathway [75,76]. Additionally, downregulation of hsa-miR-144 by triptolide enhanced the formation of p85α-PTEN complex, resulting in causing the S phase arrest of NPC cells [77]. |
| hsa-miR-149 | Promoting the epithelial-mesenchymal transition, nasopharyngeal cell mobility and invasion was facilitated by LncRNA-LINC00460 through sponging hsa-miR-149-5p [78,79]. |
| hsa-miR-155 | Stimulating the NPC proliferation, invasion, migration and colony formation by presence of LMP-1 [80]. Moreover, hsa-miR-155 promoting the cell proliferation and inhibiting apoptosis through targeting PTEN/PI3K/Akt pathway [81] and PI3K/AKT-FOXO3a pathway [82]. |
| hsa-miR-205 | Promoting the nasopharyngeal carcinoma cells’ proliferation, migration and invasion through regulating the PTEN and AKT signaling. Additionally, over-expression of hsa-miR-205 resulted in the down-regulation of E-cadherin and up-regulation of Snail proteins, led to the NPC cell proliferation, invasion [83]. The repression of apoptosis and stimulation of cell proliferation were also through targeting tumor protein p53-inducible nuclear protein 1 [84]. |
| hsa-miR-214 | Promoting the cell proliferation and repressing the cell apoptosis by targeting Bim (Bcl-2-interacting mediator of cell death) [85]. Additionally, the targeting of hsa-miR-214 is responsible for downregulating LTF in the NPC specimens [86]. |
| hsa-miR-378 | Promoting the nasopharyngeal cancer cell proliferation, colony formation, and invasion and migration by downregulating the Transducer of ERBB2 (TOB2) expression [87]. |
| hsa-miR-421 | Stimulating the cell proliferation and apoptosis resistance via downregulating the expression of FOXO4 [88]. |
| hsa-miR-504 | Inducing the radio-resistance in NPC cells through directly downregulating the NRF1 expression [89]. |
| hsa-miR-663 | Promoting the nasopharyngeal malignant progression through the targeting EEF1A2 and HSPG2 [90]. |
| hsa-miR-774 | Promoting the nasopharyngeal malignant progression via the regulation of TGF-beta and cyclin B1 [91]. |
| hsa-miR-3182 | Associated with the distant metastasis of NPC. The infection of EBV promotes the NPC progression through the disrupting miR-3182 [92]. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lao, T.D.; Le, T.A.H. MicroRNAs: Biogenesis, Functions and Potential Biomarkers for Early Screening, Prognosis and Therapeutic Molecular Monitoring of Nasopharyngeal Carcinoma. Processes 2020, 8, 966. https://doi.org/10.3390/pr8080966
Lao TD, Le TAH. MicroRNAs: Biogenesis, Functions and Potential Biomarkers for Early Screening, Prognosis and Therapeutic Molecular Monitoring of Nasopharyngeal Carcinoma. Processes. 2020; 8(8):966. https://doi.org/10.3390/pr8080966
Chicago/Turabian StyleLao, Thuan Duc, and Thuy Ai Huyen Le. 2020. "MicroRNAs: Biogenesis, Functions and Potential Biomarkers for Early Screening, Prognosis and Therapeutic Molecular Monitoring of Nasopharyngeal Carcinoma" Processes 8, no. 8: 966. https://doi.org/10.3390/pr8080966
APA StyleLao, T. D., & Le, T. A. H. (2020). MicroRNAs: Biogenesis, Functions and Potential Biomarkers for Early Screening, Prognosis and Therapeutic Molecular Monitoring of Nasopharyngeal Carcinoma. Processes, 8(8), 966. https://doi.org/10.3390/pr8080966





