Liposomes: From Bangham to Supercritical Fluids
Abstract
:1. Introduction
2. Liposomes: From History to Modern Times
3. Methods of Production
3.1. Conventional Methods
3.2. Supercritical-Assisted Methods
4. Characterization of Liposomes
5. Natural and Artificial Release Mechanisms
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DS | Delivery Systems |
| DDS | Drug Delivery Systems |
| LOM | Lipid Oxygen Containing Microparticles |
| SUV | Single Unilamellar Vesicles |
| MUV | Medium Unilamellar Vesicles |
| LUV | Large Unilamellar Vesicles |
| GUV | Giant Unilamellar Vesicles |
| OLV | Oligo Lamellar Vesicles |
| MLV | Multi Lamellar Vesicles |
| MVV | Multi Vesicular Vesicles |
| NTA | Nanoparticle Tracking Analysis |
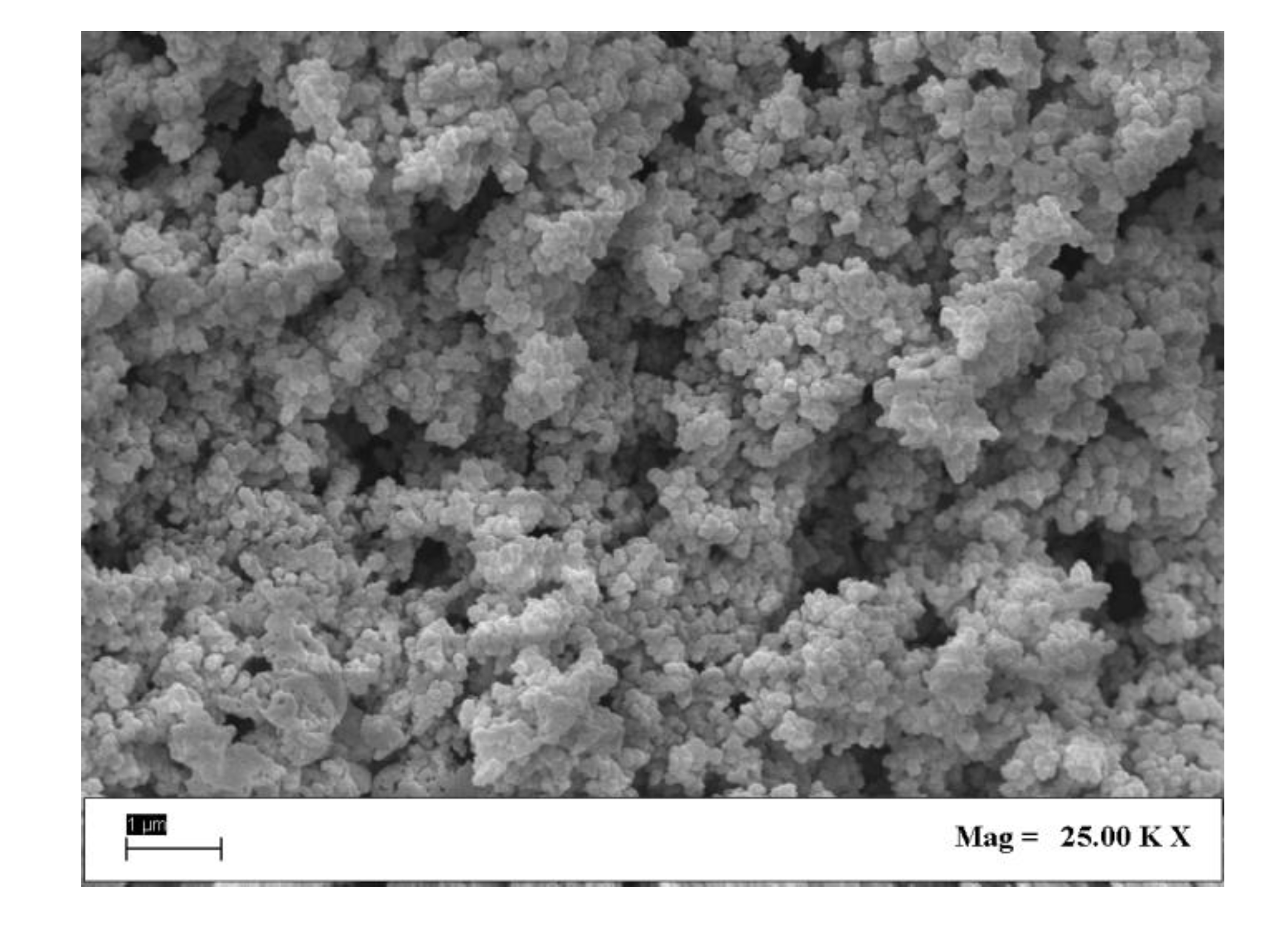
| SEM | Scanning Electron Microscope |
| EE | Encapsulation Efficiency |
| UV-Vis | Ultraviolet-Visible |
| GI | Gastrointestinal Tract |
| HGN | Hollow Gold Nanoshells |
| Mag | Magnification |
| PEG | Polyethylene Glycol |
| TLH | Thin Layer Hydration |
| RPE | Reverse Phase Evaporation |
| DESAM | Depressurization of an Expanded Solution into an Aqueous Medium |
| EI | Ethanol Injection |
| W/O | Water in Oil emulsion |
| SCF | SuperCritical Fluids |
| SCRPE | SuperCritical Reverse Phase Evaporation |
| SAS | Superctitical Anti-Solvent |
| SuperLip | Supercritical-Assisted Liposome Formation |
| RESS | Rapid Expansion of a Supercritical Solution |
| PGSS | Particles from Gas-Saturated Solutions |
| PDI | Polydispersity Index |
References
- Martinho, N. Recent Advances in Drug Delivery Systems. J. Biomater. Nanobiotechnol. 2011, 02, 510–526. [Google Scholar] [CrossRef] [Green Version]
- Kaur, I.P.; Bhandari, R.; Bhandari, S.; Kakkar, V. Potential of solid lipid nanoparticles in brain targeting. J. Control. Release 2008, 127, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Lu, G.W.; Gao, P. Emulsions and Microemulsions for Topical and Transdermal Drug Delivery. In Handbook of Non-Invasive Drug Delivery Systems; William Andrew Publishing: Norwich, NY, USA, 2010; pp. 59–94. [Google Scholar]
- Peetla, C.; Stine, A.; Labhasetwar, V. Biophysical Interactions with Model Lipid Membranes: Applications in Drug Discovery and Drug Delivery. Mol. Pharm. 2009, 6, 1264–1276. [Google Scholar] [CrossRef]
- Jain, N.K.; Mishra, V.; Mehra, N.K. Targeted drug delivery to macrophages. Expert Opin. Drug Deliv. 2013, 10, 353–367. [Google Scholar] [CrossRef]
- Dufès, C.; Uchegbu, I.F.; Schätzlein, A.G. Dendrimers in gene delivery. Adv. Drug Deliv. Rev. 2005, 57, 2177–2202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaheen, S.M.; Shakil Ahmed, F.; Hossen, M.N.; Ahmed, M.; Amran, M.S.; Ul-Islam, M. Liposome as a carrier for advanced drug delivery. Pak. J. Biol. Sci. 2006, 9, 1181–1191. [Google Scholar]
- Lian, T.; Ho, R.J.Y. Trends and developments in liposome drug delivery systems. J. Pharm. Sci. 2001, 90, 667–680. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Zhang, T.; Wang, C.; Huang, Z.; Luo, X.; Deng, Y. A review on phospholipids and their main applications in drug delivery systems. Asian J. Pharm. Sci. 2015, 10, 81–98. [Google Scholar] [CrossRef]
- Bangham, A.D. Review of Lasic, Liposomes: From Physics to Applications. Biophys. J. 1994, 67, 1358–1359. [Google Scholar] [CrossRef] [Green Version]
- Perkins, W.R.; Minchey, S.R.; Ahl, P.L.; Janoff, A.S. The determination of liposome captured volume. Chem. Phys. Lipids 1993, 64, 197–217. [Google Scholar] [CrossRef]
- Abed, N.; Couvreur, P. Nanocarriers for antibiotics: A promising solution to treat intracellular bacterial infections. Int. J. Antimicrob. Agents 2014, 43, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Colletier, J.-P.; Chaize, B.; Winterhalter, M.; Fournier, D. Protein encapsulation in liposomes: Efficiency depends on interactions between protein and phospholipid bilayer. BMC Biotechnol. 2002, 2, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crommelin, D.J.A.; van Rensen, A.J.M.L.; Wauben, M.H.M.; Storm, G. Liposomes in autoimmune diseases: Selected applications in immunotherapy and inflammation detection. J. Control. Release 1999, 62, 245–251. [Google Scholar] [CrossRef]
- Barani, H.; Montazer, M. A Review on Applications of Liposomes in Textile Processing. J. Liposome Res. 2008, 18, 249–262. [Google Scholar] [CrossRef]
- Can, S.; Giuseppina, S.; Vladimir, P.T. Recent advances in siRNA delivery. Biomol. Concepts 2015, 6, 321–341. [Google Scholar]
- Paiva-Martins, F.; Gordon, M.H.; Gameiro, P. Activity and location of olive oil phenolic antioxidants in liposomes. Chem. Phys. Lipids 2003, 124, 23–36. [Google Scholar] [CrossRef]
- Keller, B.C. Liposomes in Nutrition. Trends Food Sci. Technol. 2001, 12, 25–31. [Google Scholar] [CrossRef]
- Kluczyk, D.; Matwijczuk, A.; Górecki, A.; Karpińska, M.M.; Szymanek, M.; Niewiadomy, A.; Gagoś, M. Molecular Organization of Dipalmitoylphosphatidylcholine Bilayers Containing Bioactive Compounds 4-(5-Heptyl-1,3,4-thiadiazol-2-yl) Benzene-1,3-diol and 4-(5-Methyl-1,3,4-thiadiazol-2-yl) Benzene-1,3-diols. J. Phys. Chem. B 2016, 120, 12047–12063. [Google Scholar] [CrossRef]
- Tila, D.; Ghasemi, S.; Yazdani-Arazi, S.N.; Ghanbarzadeh, S. Functional liposomes in the cancer-targeted drug delivery. J. Biomater. Appl. 2015, 30, 3–16. [Google Scholar] [CrossRef]
- Lasic, D.D. Novel applications of liposomes. Trends Biotechnol. 1998, 16, 307–321. [Google Scholar] [CrossRef]
- Gibbs, B.F.; Kermasha, S.; Alli, I.; Mulligan, C.N. Encapsulation in the food industry: A review. Int. J. Food Sci. Nutr. 1999, 50, 213–224. [Google Scholar] [PubMed]
- Ozkan, G.; Franco, P.; De Marco, I.; Xiao, J.; Capanoglu, E. A review of microencapsulation methods for food antioxidants: Principles, advantages, drawbacks and applications. Food Chem. 2019, 272, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Laouini, A.; Jaafar-Maalej, C.; Blouza, I.; Sfar, S.; Charcosset, C.; Fessi, H. Preparation, Characterization and Applications of Liposomes: State of the Art. J. Colloid Sci. Biotechnol. 2012, 1, 147–168. [Google Scholar] [CrossRef]
- Voinea, M.; Simionescu, M. Designing of ‘intelligent’ liposomes for efficient delivery of drugs. J. Cell. Mol. Med. 2002, 6, 465–474. [Google Scholar] [CrossRef] [Green Version]
- da Silva Malheiros, P.; Daroit, D.J.; Brandelli, A. Food applications of liposome-encapsulated antimicrobial peptides. Trends Food Sci. Technol. 2010, 21, 284–292. [Google Scholar] [CrossRef]
- Kaur, I.P.; Kakkar, S. Topical delivery of antifungal agents. Expert Opin. Drug Deliv. 2010, 7, 1303–1327. [Google Scholar] [CrossRef]
- Sharma, D.; Aara, A.; Ali, E.; Trivedi, L. An Updated Review On:Liposomes as Drug Delivery System. Pharmatutor 2018, 6, 50–62. [Google Scholar] [CrossRef]
- Karanth, H.; Murthy, R.S.R. pH-Sensitive liposomes-principle and application in cancer therapy. J. Pharm. Pharmacol. 2007, 59, 469–483. [Google Scholar] [CrossRef]
- Anwekar, H.; Patel, S.; Singhai, A. Liposome-as Drug Carriers. Int. J. Pharm. Life Sci. 2011, 2, 945–951. [Google Scholar]
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomed. 2006, 1, 297–315. [Google Scholar]
- Oussoren, C.; Storm, G. Liposomes to target the lymphatics by subcutaneous administration. Adv. Drug Deliv. Rev. 2001, 50, 143–156. [Google Scholar] [CrossRef]
- Ahmed, K.S.; Hussein, S.A.; Ali, A.H.; Korma, S.A.; Lipeng, Q.; Jinghua, C. Liposome: Composition, characterisation, preparation, and recent innovation in clinical applications. J. Drug Target. 2019, 27, 742–761. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, B.B.; Griffiths, G.; Hansen, H.J. Specific binding of sterically stabilized anti-B-cell immunoliposomes and cytotoxicity of entrapped doxorubicin. Int. J. Pharm. 2000, 205, 101–108. [Google Scholar] [CrossRef]
- Dutta, S.; Moses, J.A.; Anandharamakrishnan, C. Encapsulation of Nutraceutical Ingredients in Liposomes and Their Potential for Cancer Treatment. Nutr. Cancer 2018, 70, 1184–1198. [Google Scholar] [CrossRef]
- Illes, B.; Wuttke, S.; Engelke, H. Liposome-Coated Iron Fumarate Metal-Organic Framework Nanoparticles for Combination Therapy. Nanomaterials 2017, 7, 351. [Google Scholar] [CrossRef]
- Tao, Z.; Ghoroghchian, P.P. Microparticle, nanoparticle, and stem cell-based oxygen carriers as advanced blood substitutes. Trends Biotechnol. 2014, 32, 466–473. [Google Scholar] [CrossRef]
- Bangham, A.D. Surrogate cells or Trojan horses. The discovery of liposomes. BioEssays News Rev. Mol. Cell. Dev. Biol. 1995, 17, 1081–1088. [Google Scholar] [CrossRef]
- Meure, L.A.; Foster, N.R.; Dehghani, F. Conventional and Dense Gas Techniques for the Production of Liposomes: A Review. AAPS PharmSciTech 2008, 9, 798. [Google Scholar] [CrossRef] [Green Version]
- Gregoriadis, G. Immunological adjuvants: A role for liposomes. Immunol. Today 1990, 11, 89–97. [Google Scholar] [CrossRef]
- Gregoriadis, G.; Wills, E.J.; Swain, C.P.; Tavill, A.S. Drug-carrier potential of liposomes in cancer chemotherapy. Lancet 1974, 1, 1313–1316. [Google Scholar] [CrossRef]
- Sopyan, I.; Gozali, D. A Review: A Novel of Efforts to Enhance Liposome Stability as Drug Delivery Approach. Syst. Rev. Pharm. 2020, 11, 555–562. [Google Scholar]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bangham, A.D. Liposomes and the physico-chemical basis of unconsciousness. FASEB J. 2005, 19, 1766–1768. [Google Scholar] [CrossRef]
- Patil, Y.P.; Jadhav, S. Novel methods for liposome preparation. Chem. Phys. Lipids 2014, 177, 8–18. [Google Scholar] [CrossRef]
- Herman, E.H.; Rahman, A.; Ferrans, V.J.; Vick, J.A.; Schein, P.S. Prevention of Chronic Doxorubicin Cardiotoxicity in Beagles by Liposomal Encapsulation. Cancer Res. 1983, 43, 5427–5432. [Google Scholar]
- Balazsovits, J.A.E.; Mayer, L.D.; Bally, M.B.; Cullis, P.R.; McDonell, M.; Ginsberg, R.S.; Falk, R.E. Analysis of the effect of liposome encapsulation on the vesicant properties, acute and cardiac toxicities, and antitumor efficacy of doxorubicin. Cancer Chemother. Pharmacol. 1989, 23, 81–86. [Google Scholar] [CrossRef]
- Bally, M.B.; Nayar, R.; Masin, D.; Hope, M.J.; Cullis, P.R.; Mayer, L.D. Liposomes with entrapped doxorubicin exhibit extended blood residence times. Biochim. Biophys. Acta (BBA) Biomembr. 1990, 1023, 133–139. [Google Scholar] [CrossRef]
- Fu, M.; Tang, W.; Liu, J.-J.; Gong, X.-Q.; Kong, L.; Yao, X.-M.; Jing, M.; Cai, F.-Y.; Li, X.-T.; Ju, R.-J. Combination of targeted daunorubicin liposomes and targeted emodin liposomes for treatment of invasive breast cancer. J. Drug Target. 2020, 28, 245–258. [Google Scholar] [CrossRef]
- Laouini, A.; Jaafar-Maalej, C.; Sfar, S.; Charcosset, C.; Fessi, H. Liposome preparation using a hollow fiber membrane contactor--application to spironolactone encapsulation. Int. J. Pharm. 2011, 415, 53–61. [Google Scholar] [CrossRef]
- Liu, Y.; Kim, Y.J.; Siriwon, N.; Rohrs, J.A.; Yu, Z.; Wanga, P. Combination drug delivery via multilamellar vesicles enables targeting of tumor cells and tumor vasculature. Biotechnol. Bioeng. 2018, 115, 1403–1415. [Google Scholar] [CrossRef] [PubMed]
- Barenholz, Y.; Bombelli, C.; Bonicelli, M.G.; di Profio, P.; Giansanti, L.; Mancini, G.; Pascale, F. Influence of lipid composition on the thermotropic behavior and size distribution of mixed cationic liposomes. J. Colloid Interface Sci. 2011, 356, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Walde, P.; Cosentino, K.; Engel, H.; Stano, P. Giant Vesicles: Preparations and Applications. ChemBioChem 2010, 11, 848–865. [Google Scholar] [CrossRef] [PubMed]
- Guráň, R.; Kominkova, M.; Kopel, P.; Chudobova, D.; Zitka, O.; Adam, V.; Kizek, R. Liposomes as Drug Carriers and Their Characterization Using Different Analytical Methods. Ph.D. Thesis, Mendel University, Brno, Czech Republic, 2013. [Google Scholar]
- Duzgunes, N.; Gregoriadis, G. Introduction: The Origins of Liposomes: Alec Bangham at Babraham. Methods Enzymol. 2005, 391, 1–3. [Google Scholar]
- Gregoriadis, G. Overview of liposomes. J. Antimicrob. Chemother. 1991, 28 (Suppl. B), 39–48. [Google Scholar] [CrossRef]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef]
- Jaafar-Maalej, C.; Diab, R.; Andrieu, V.; Elaissari, A.; Fessi, H. Ethanol injection method for hydrophilic and lipophilic drug-loaded liposome preparation. J. Liposome Res. 2010, 20, 228–243. [Google Scholar] [CrossRef]
- Otake, K.; Imura, T.; Sakai, H.; Abe, M. Development of a New Preparation Method of Liposomes Using Supercritical Carbon Dioxide. Langmuir 2001, 17, 3898–3901. [Google Scholar] [CrossRef]
- Imura, T.; Otake, K.; Hashimoto, S.; Gotoh, T.; Yuasa, M.; Yokoyama, S.; Sakai, H.; Rathman, J.F.; Abe, M. Preparation and physicochemical properties of various soybean lecithin liposomes using supercritical reverse phase evaporation method. Coll. Surf. B Biointerfaces 2003, 27, 133–140. [Google Scholar] [CrossRef]
- Szoka, F.; Papahadjopoulos, D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc. Natl. Acad. Sci. USA 1978, 75, 4194–4198. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Deng, Y. A novel method for the preparation of liposomes: Freeze drying of monophase solutions. J. Pharm. Sci. 2004, 93, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Jahn, A.; Vreeland, W.N.; DeVoe, D.L.; Locascio, L.E.; Gaitan, M. Microfluidic Directed Formation of Liposomes of Controlled Size. Langmuir 2007, 23, 6289–6293. [Google Scholar] [CrossRef] [PubMed]
- Mozafari, M.R. Liposomes: An overview of manufacturing techniques. Cell. Mol. Biol. Lett. 2005, 10, 711–719. [Google Scholar] [PubMed]
- Campardelli, R.; Baldino, L.; Reverchon, E. Supercritical fluids applications in nanomedicine. J. Supercrit. Fluids 2015, 101, 193–214. [Google Scholar] [CrossRef]
- Meure, L.A.; Knott, R.; Foster, N.R.; Dehghani, F. The Depressurization of an Expanded Solution into Aqueous Media for the Bulk Production of Liposomes. Langmuir 2009, 25, 326–337. [Google Scholar] [CrossRef]
- Tsai, W.-C.; Rizvi, S.S.H. Liposomal microencapsulation using the conventional methods and novel supercritical fluid processes. Trends Food Sci. Technol. 2016, 55, 61–71. [Google Scholar] [CrossRef]
- Lesoin, L.; Crampon, C.; Boutin, O.; Badens, E. Preparation of liposomes using the supercritical anti-solvent (SAS) process and comparison with a conventional method. J. Supercrit. Fluids 2011, 57, 162–174. [Google Scholar] [CrossRef]
- Soh, S.H.; Lee, L.Y. Microencapsulation and Nanoencapsulation Using Supercritical Fluid (SCF) Techniques. Pharmaceutics 2019, 11, 21. [Google Scholar] [CrossRef] [Green Version]
- de Paz, E.; Martín, Á.; Cocero, M.J. Formulation of β-carotene with soybean lecithin by PGSS (Particles from Gas Saturated Solutions)-drying. J. Supercrit. Fluids 2012, 72, 125–133. [Google Scholar] [CrossRef]
- Trucillo, P.; Campardelli, R.; Reverchon, E. Supercritical CO2 assisted liposomes formation: Optimization of the lipidic layer for an efficient hydrophilic drug loading. J. CO2 Util. 2017, 18, 181–188. [Google Scholar] [CrossRef]
- Campardelli, R.; Trucillo, P.; Reverchon, E. Supercritical assisted process for the efficient production of liposomes containing antibiotics for ocular delivery. J. CO2 Util. 2018, 25, 235–241. [Google Scholar] [CrossRef]
- Trucillo, P.; Campardelli, R.; Reverchon, E. A versatile supercritical assisted process for the one-shot production of liposomes. J. Supercrit. Fluids 2019, 146, 136–143. [Google Scholar] [CrossRef]
- Trucillo, P.; Campardelli, R.; Scognamiglio, M.; Reverchon, E. Control of liposomes diameter at micrometric and nanometric level using a supercritical assisted technique. J. CO2 Util. 2019, 32, 119–127. [Google Scholar] [CrossRef]
- Trucillo, P.; Campardelli, R.; Reverchon, E. Production of liposomes loaded with antioxidants using a supercritical CO2 assisted process. Powder Technol. 2018, 323, 155–162. [Google Scholar] [CrossRef]
- Woodle, M.C.; Lasic, D.D. Sterically stabilized liposomes. Biochim. Biophys. Acta (BBA) Rev. Biomembr. 1992, 1113, 171–199. [Google Scholar] [CrossRef]
- Gibis, M.; Zeeb, B.; Weiss, J. Formation, characterization, and stability of encapsulated hibiscus extract in multilayered liposomes. Food Hydrocoll. 2014, 38, 28–39. [Google Scholar] [CrossRef]
- Disalvo, E.A.; Bouchet, A.M. Electrophoretic mobility and zeta potential of liposomes due to arginine and polyarginine adsorption. Coll. Surf. A Physicochem. Eng. Asp. 2014, 440, 170–174. [Google Scholar] [CrossRef]
- Lopes, L.B.; Scarpa, M.V.; Silva, G.V.J.; Rodrigues, D.C.; Santilli, C.V.; Oliveira, A.G. Studies on the encapsulation of diclofenac in small unilamellar liposomes of soya phosphatidylcholine. Coll. Surf. B Biointerfaces 2004, 39, 151–158. [Google Scholar] [CrossRef]
- Ball, R.L.; Bajaj, P.; Whitehead, K.A. Achieving long-term stability of lipid nanoparticles: Examining the effect of pH, temperature, and lyophilization. Int. J. Nanomed. 2017, 12, 305–315. [Google Scholar] [CrossRef] [Green Version]
- Rogers, J.A.; Anderson, K.E. The potential of liposomes in oral drug delivery. Crit. Rev. Ther. Drug Carr. Syst. 1998, 15, 421–480. [Google Scholar] [CrossRef]
- Li, X.; Chen, D.; Le, C.; Zhu, C.; Gan, Y.; Hovgaard, L.; Yang, M. Novel mucus-penetrating liposomes as a potential oral drug delivery system: Preparation, in vitro characterization, and enhanced cellular uptake. Int. J. Nanomed. 2011, 6, 3151–3162. [Google Scholar]
- Franzé, S.; Marengo, A.; Stella, B.; Minghetti, P.; Arpicco, S.; Cilurzo, F. Hyaluronan-decorated liposomes as drug delivery systems for cutaneous administration. Int. J. Pharm. 2018, 535, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Doppalapudi, S.; Jain, A.; Chopra, D.K.; Khan, W. Psoralen loaded liposomal nanocarriers for improved skin penetration and efficacy of topical PUVA in psoriasis. Eur. J. Pharm. Sci. 2017, 96, 515–529. [Google Scholar] [CrossRef] [PubMed]
- Oussoren, C.; Zuidema, J.; Crommelin, D.J.A.; Storm, G. Lymphatic uptake and biodistribution of liposomes after subcutaneous injection.: II. Influence of liposomal size, lipid composition and lipid dose. Biochim. Biophys. Acta (BBA) Biomembr. 1997, 1328, 261–272. [Google Scholar] [CrossRef] [Green Version]
- Poste, G.; Fidler, I.J. The pathogenesis of cancer metastasis. Nature 1980, 283, 139–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertrand, N.; Bouvet, C.; Moreau, P.; Leroux, J.-C. Transmembrane pH-Gradient Liposomes To Treat Cardiovascular Drug Intoxication. ACS Nano 2010, 4, 7552–7558. [Google Scholar] [CrossRef]
- Wu, G.; Mikhailovsky, A.; Khant, H.A.; Fu, C.; Chiu, W.; Zasadzinski, J.A. Remotely Triggered Liposome Release by Near-Infrared Light Absorption via Hollow Gold Nanoshells. J. Am. Chem. Soc. 2008, 130, 8175–8177. [Google Scholar] [CrossRef] [Green Version]
- Ravikumar, M.; Modery, C.L.; Wong, T.L.; Sen Gupta, A. Peptide-Decorated Liposomes Promote Arrest and Aggregation of Activated Platelets under Flow on Vascular Injury Relevant Protein Surfaces in Vitro. Biomacromolecules 2012, 13, 1495–1502. [Google Scholar] [CrossRef]
- Papadia, K.; Markoutsa, E.; Antimisiaris, S.G. A simplified method to attach antibodies on liposomes by biotin-streptavidin affinity for rapid and economical screening of targeted liposomes. J. Biomed. Nanotechnol. 2014, 10, 871–876. [Google Scholar] [CrossRef]
- Paleos, C.M.; Tsiourvas, D.; Sideratou, Z. Triphenylphosphonium Decorated Liposomes and Dendritic Polymers: Prospective Second Generation Drug Delivery Systems for Targeting Mitochondria. Mol. Pharm. 2016, 13, 2233–2241. [Google Scholar] [CrossRef]
- Benech, R.-O.; Kheadr, E.E.; Laridi, R.; Lacroix, C.; Fliss, I. Inhibition of Listeria innocua in Cheddar Cheese by Addition of Nisin Z in Liposomes or by In Situ Production in Mixed Culture. Appl. Environ. Microbiol. 2002, 68, 3683–3690. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Dimensions | Lamellarity | ||
|---|---|---|---|
| Name | Size Range [µm] | Name | Number of Layers |
| Single Unilamellar Vesicles (SUV) [50] | 0.02–0.20 | Oligo Lamellar Vesicles [51] (OLV) | <5 |
| Medium Unilamellar Vesicles (MUV) [51,52] | 0.20–0.50 | Multi Lamellar Vesicles [46] (MLV) | 5–20 |
| Large Unilamellar Vesicles (LUV) [53] | 0.50–10 | Multi Vesicular Vesicles inner core disjointed vesicles [52] (MVV) | >50 |
| Giant Unilamellar Vesicles (GUV) [54] | 100–200 | ||
| Other Methods Drawbacks | SuperLip Advantages |
|---|---|
| Micrometric dimensions | Nanometric dimensions |
| High solvent residue | Low solvent residue |
| Low encapsulation efficiencies | Encapsulation efficiencies |
| Post-processing steps | 1-shot production |
| Batch layout | Continuous and replicable |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trucillo, P.; Campardelli, R.; Reverchon, E. Liposomes: From Bangham to Supercritical Fluids. Processes 2020, 8, 1022. https://doi.org/10.3390/pr8091022
Trucillo P, Campardelli R, Reverchon E. Liposomes: From Bangham to Supercritical Fluids. Processes. 2020; 8(9):1022. https://doi.org/10.3390/pr8091022
Chicago/Turabian StyleTrucillo, Paolo, Roberta Campardelli, and Ernesto Reverchon. 2020. "Liposomes: From Bangham to Supercritical Fluids" Processes 8, no. 9: 1022. https://doi.org/10.3390/pr8091022
APA StyleTrucillo, P., Campardelli, R., & Reverchon, E. (2020). Liposomes: From Bangham to Supercritical Fluids. Processes, 8(9), 1022. https://doi.org/10.3390/pr8091022








