Scale-Up of Self-Regenerating Semi-Batch Adsorption Cycles through Concurrent Adsorption and Reduction of Cr(VI) on Sheep Wool
Abstract
1. Introduction
2. Materials and Instrumentation
2.1. Materials
2.2. Instrumentation
3. Methods
3.1. Sample Preparation
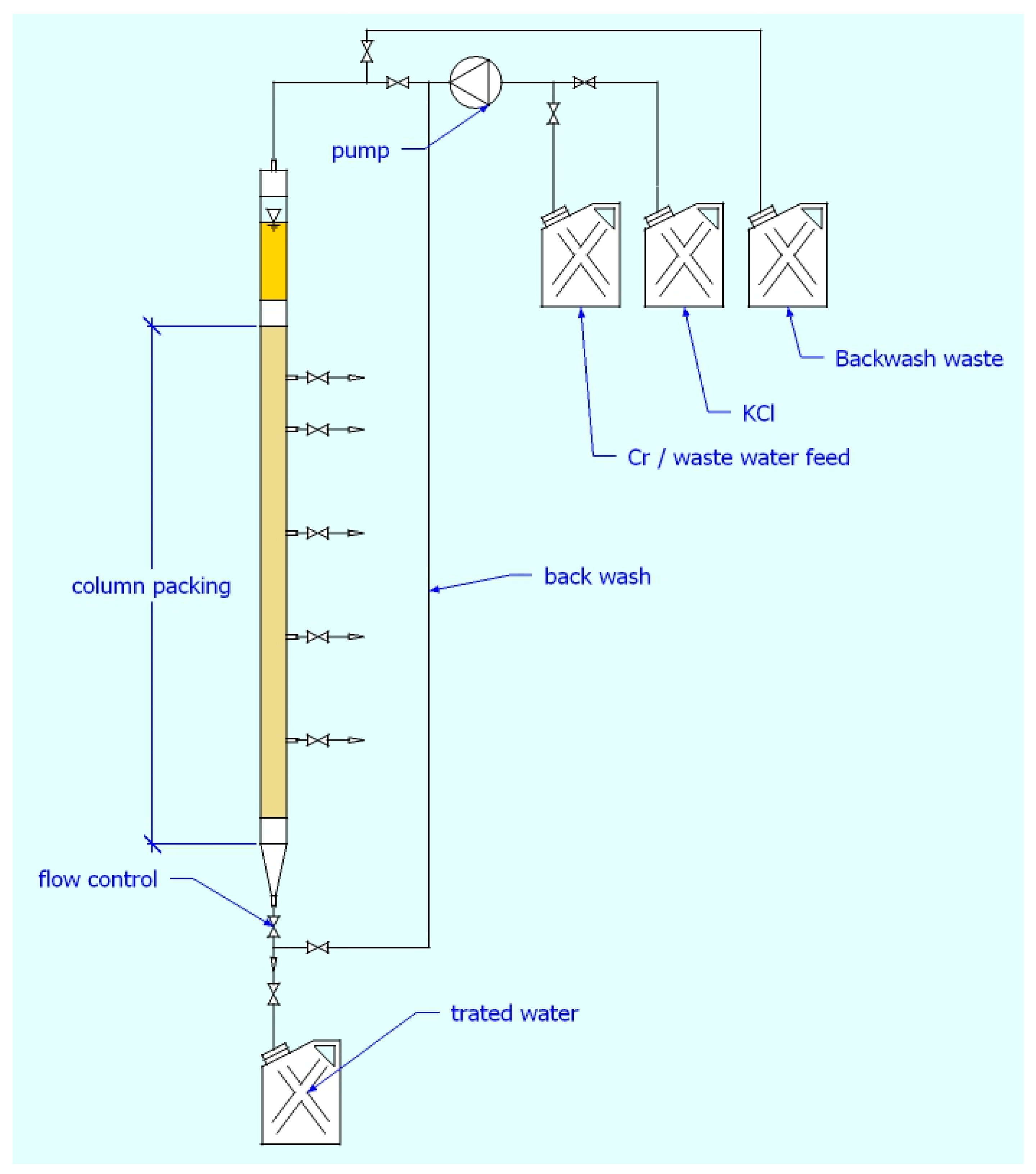
3.2. Continuous Flow Experiments
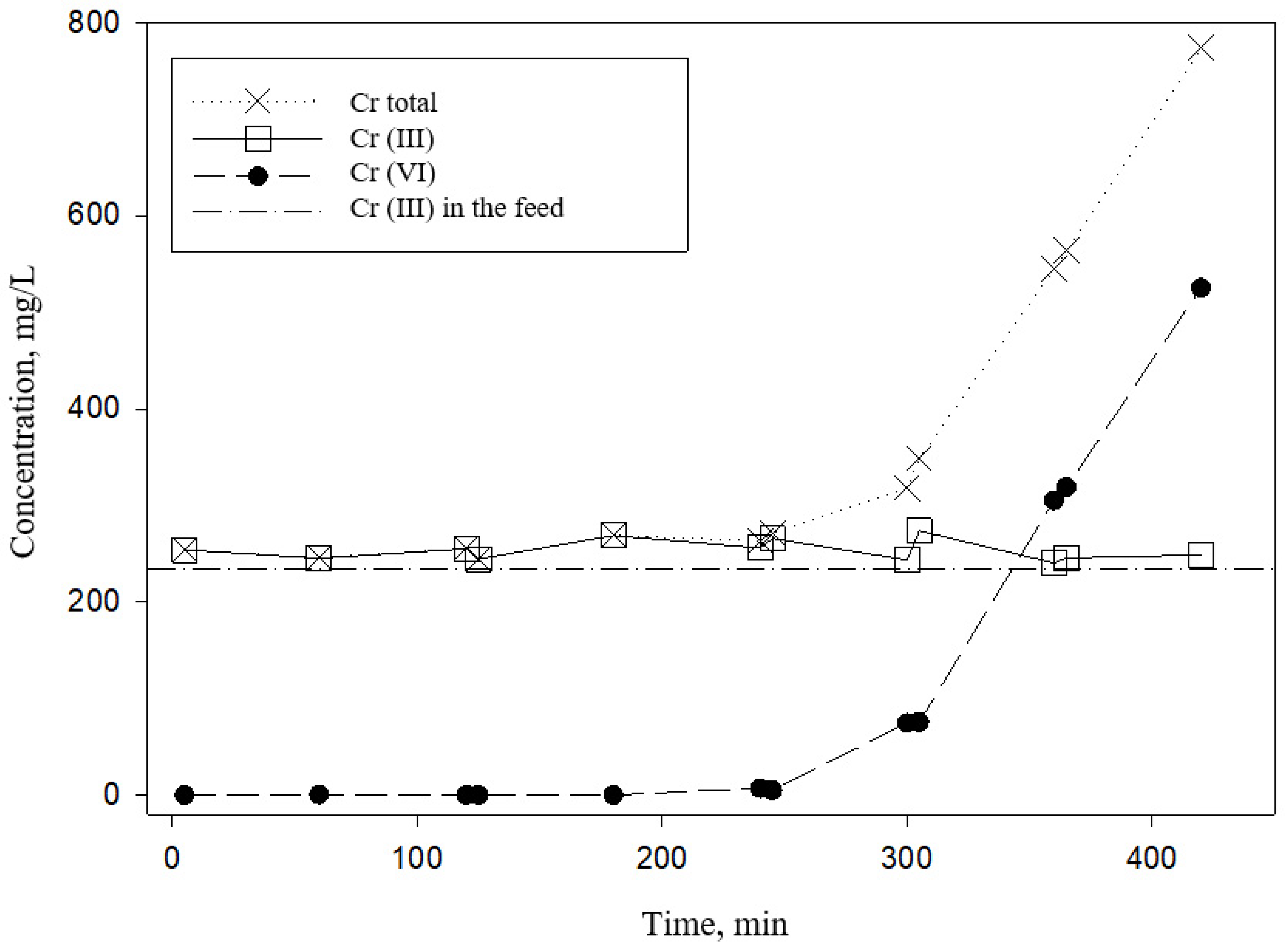
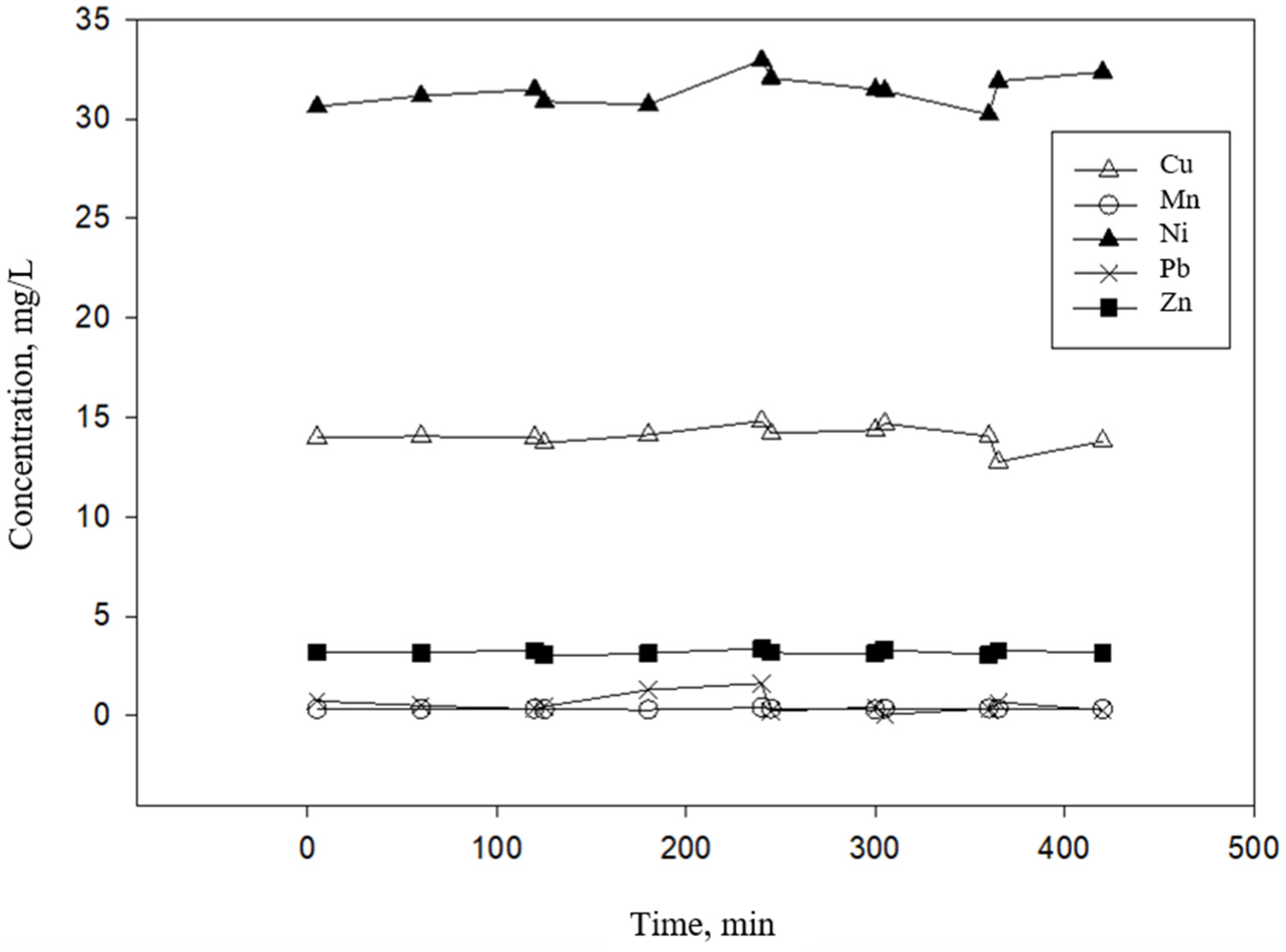
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. In Molecular, Clinical and Environmental Toxicology; Springer: Basel, Switzerland, 2012; Volume 101, pp. 133–164. [Google Scholar] [CrossRef]
- Costa, M.; Klein, C.B. Toxicity and carcinogencity of chromium compounds in humans. Crit. Rev. Toxicol. 2006, 36, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Barnhart, J. Occurrences, uses, and properties of chromium. Regul. Toxicol. Pharmacol. 1997, 1, S3–S7. [Google Scholar] [CrossRef]
- Lunk, H.-J. Discovery, Properties and Applications of Chromium and its Compounds. Chem. Texts 2015, 1, 1–7. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U.P., Jr. Activated carbons and low cost adsorbents for remediation of tri- and hexavalent chromium from water. J. Hazard. Mater. 2006, 177, 762–811. [Google Scholar] [CrossRef] [PubMed]
- Kendig, M.; Jeanjaquet, S.; Addison, R.; Waldrop, J. Role of hexavalent chromium in the inhibition of corrosion of aluminum alloys. Surf. Coat. 2001, 140, 58–66. [Google Scholar] [CrossRef]
- Pavithra, K.G.; Jaikumar, V.; Kumar, M.M.; Sundarrajan, P. A review on cleaner strategies for chromium industrial wastewater: Present research and future perspective. J. Clean. Prod. 2019, 228, 580–593. [Google Scholar] [CrossRef]
- Kimbrough, D.E.; Cohen, Y.; Winer, A.M.; Creelman, L.; Mabuni, C. A critical assessment of chromium in the environment. Crit. Rev. Environ. Sci. Technol. 1999, 29, 1–46. [Google Scholar] [CrossRef]
- Bakshi, A.; Panigrahi, A. A comprehensive review on chromium induced alterations in fresh water fishes. Toxicol. Rep. 2018, 5, 440–447. [Google Scholar] [CrossRef]
- Occupational Safety and Health Administration. Occupational Exposure to Hexavalent Chromium. Final Rule; Federal Register; Occupational Safety and Health Administration: Washington, DC, USA, 2006; pp. 10099–10385.
- Miningwatch Canada. Chromium, chromite min. ferrochrome Prod. In Potential Toxic Effects of Chromium, Chromite Mining and Ferrochrome Production: A Literature Review; MiningWatch Canada: Ottawa, ON, Canada, 2012; pp. 1–42. [Google Scholar]
- Vaiopoulou, E.; Gikas, P. Effects of chromium on activated sludge and on the performance of wastewater treatment plants: A review. Water Res. 2012, 46, 549–570. [Google Scholar] [CrossRef]
- Cavaco, S.A.; Fernandes, S.; Quina, M.J.; Ferreira, L.M.G. Ferreira, Removal of chromium from electroplating industry by ion exchange resins. J. Hazard. Mater. 2007, 144, 634–648. [Google Scholar] [CrossRef]
- Hafiane, A.; Lemordant, D.; Dhahbi, M. Removal of hexavalent chromium by nanofiltration. Desalination 2000, 130, 305–312. [Google Scholar] [CrossRef]
- Benito, Y.; Ruiz, M. Reverse osmosis applied to metal finishing wastewater. Desalination 2002, 142, 229–234. [Google Scholar] [CrossRef]
- Mant, C.; Costa, S.; Williams, J.B.; Tambourgi, E. Phytoremediation of chromium by model constructed wetland. Bioresour. Technol. 2006, 15, 1767–1772. [Google Scholar] [CrossRef] [PubMed]
- Deeb, R.; Kavanaugh, M.; Hawley, E.; Jacobs, J. Treatment technologies for chromium(VI). In Chromium(VI) Handbook; CRC Press: Boca Raton, FLA, USA, 2004. [Google Scholar]
- Henryk, K.; Jaroslaw, C.; Witold, Z. Peat and coconut fiber as biofilters for chromium adsorption from contaminated wastewaters. Environ. Sci. Pollut. Res. Int. 2016, 23, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Zubair, M.; Khosa, M.A.; Song, S.; Ullah, A. Keratin and chitosan biosorbents for wastewater treatment: A review. J. Polym. Environ. 2019, 27, 1389–1403. [Google Scholar] [CrossRef]
- Dakiky, M.; Khamis, M.I.; Manassra, A.; Mer’Eb, M. Selective adsorption of chromium(VI) in industrial wastewater using low-cost abundantly available adsorbents. Adv. Environ. Res. 2002, 6, 533–540. [Google Scholar] [CrossRef]
- Ray, P.; Sabri, M.A.; Ibrahim, T.H.; Khamis, M.I.; Jumean, F.H. Design and optimization of a batch sequential contactor for the removal of chromium(VI) from industrial wastewater using sheep wool as a low-cost adsorbent. Deswater 2018, 113, 109–113. [Google Scholar] [CrossRef]
- Jumean, F.H.; Khamis, M.I.; Sara, Z.A.; AbouRich, M.S. Concurrent removal and reduction of Cr(VI) by wool: Short and long term equilibration studies. Am. J. Anal. Chem. 2015, 6, 47–57. [Google Scholar] [CrossRef]
- Balkaya, N.; Bektas, N. Chromium(VI) sorption from dilute aqueous solutions using wool. Deswater 2012, 3, 43–49. [Google Scholar] [CrossRef]
- Aksu, Z.; Akpinar, D. Competitive biosorption of phenol and chromium(VI) from binary mixtures onto dried anaerobic activated sludge. Biochem. Eng. J. 2001, 7, 183–193. [Google Scholar] [CrossRef]
- Huang, C.P.; Bowers, A.R. Activated carbon process for treatment of wastewaters containing hexavalent chromium. In EPA-600/279-130; US Environmental Protection Agency: Washington, DC, USA, 1979. [Google Scholar]
- Miretzky, P.; Cirelli, A.F. Cr(VI) and Cr(III) removal from aqueous solution by raw and modified lignocellulosic materials: A review. J. Hazard. Mater. 2010, 180, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Hongmei, W.; Xiao, Y.; Guo, Y.; Miao, S.; Chen, Q.; Chen, Z. Functionalization of SBA-15 mesoporous materials with 2-acetylthiophene for adsorption of Cr(III) ions. Microporous Mesoporous Mater. 2020, 292, 109754. [Google Scholar]
- Yarnell, A. Cysteine oxidation. Chem. Eng. News 2009, 87, 38–40. [Google Scholar] [CrossRef]
- Alcock, L.J.; Perkins, M.V.; Chalker, J.M. Chemical methods for mapping cysteine oxidation. Chem. Soc. Rev. 2018, 47, 231–268. [Google Scholar] [CrossRef]
- Poole, L.B. The basics of thiols and cysteines in redox biology and chemistry. Free Radic. Biol. Med. 2015, 80, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Kar, P.; Misra, M. Use of keratin fiber for separation of heavy metals from water. J. Chem. Technol. Biotechnol. 2004, 79, 1313–1319. [Google Scholar] [CrossRef]
- Pradhan, D.; Sukla, L.B.; Sawyer, M.; Rahman, P.K. Recent bioreduction of hexavalent chromium in wastewater treatment: A review. J. Ind. Eng. Chem. 2017, 55, 1–20. [Google Scholar] [CrossRef]
- Silva, B.; Figueiredo, H.; Quintelas, C.; Neves, I.C.; Tavares, T. Improved biosorption for Cr(VI) reduction and removal by Arthrobacter viscosus using zeolite. Int. Biodeterior. Biodegrad. 2012, 74, 116–123. [Google Scholar] [CrossRef]




| Heavy Metal | Concentration, mg/L |
|---|---|
| Cr (Total) | 8.6 × 102 |
| Cr(VI) | 6.2 × 102 |
| Cr(III) | 2.3 × 102 |
| Cu | 14 |
| Mn | 0.30 |
| Ni | 32 |
| Pb | 0.90 |
| Zn | 3.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badrelzaman, M.; Khamis, M.I.; Ibrahim, T.H.; Jumean, F.H. Scale-Up of Self-Regenerating Semi-Batch Adsorption Cycles through Concurrent Adsorption and Reduction of Cr(VI) on Sheep Wool. Processes 2020, 8, 1092. https://doi.org/10.3390/pr8091092
Badrelzaman M, Khamis MI, Ibrahim TH, Jumean FH. Scale-Up of Self-Regenerating Semi-Batch Adsorption Cycles through Concurrent Adsorption and Reduction of Cr(VI) on Sheep Wool. Processes. 2020; 8(9):1092. https://doi.org/10.3390/pr8091092
Chicago/Turabian StyleBadrelzaman, Mohamed, Mustafa I. Khamis, Taleb H. Ibrahim, and Fawwaz H. Jumean. 2020. "Scale-Up of Self-Regenerating Semi-Batch Adsorption Cycles through Concurrent Adsorption and Reduction of Cr(VI) on Sheep Wool" Processes 8, no. 9: 1092. https://doi.org/10.3390/pr8091092
APA StyleBadrelzaman, M., Khamis, M. I., Ibrahim, T. H., & Jumean, F. H. (2020). Scale-Up of Self-Regenerating Semi-Batch Adsorption Cycles through Concurrent Adsorption and Reduction of Cr(VI) on Sheep Wool. Processes, 8(9), 1092. https://doi.org/10.3390/pr8091092







