Short-Chain Polyglycerol Production via Microwave-Assisted Solventless Glycerol Polymerization Process Over Lioh-Modified Aluminium Pillared Clay Catalyst: Parametric Study
Abstract
:1. Introduction
2. Materials and Method
2.1. Materials
2.2. Preparation of Li/AlPC Catalyst
2.3. Catalyst Characterization
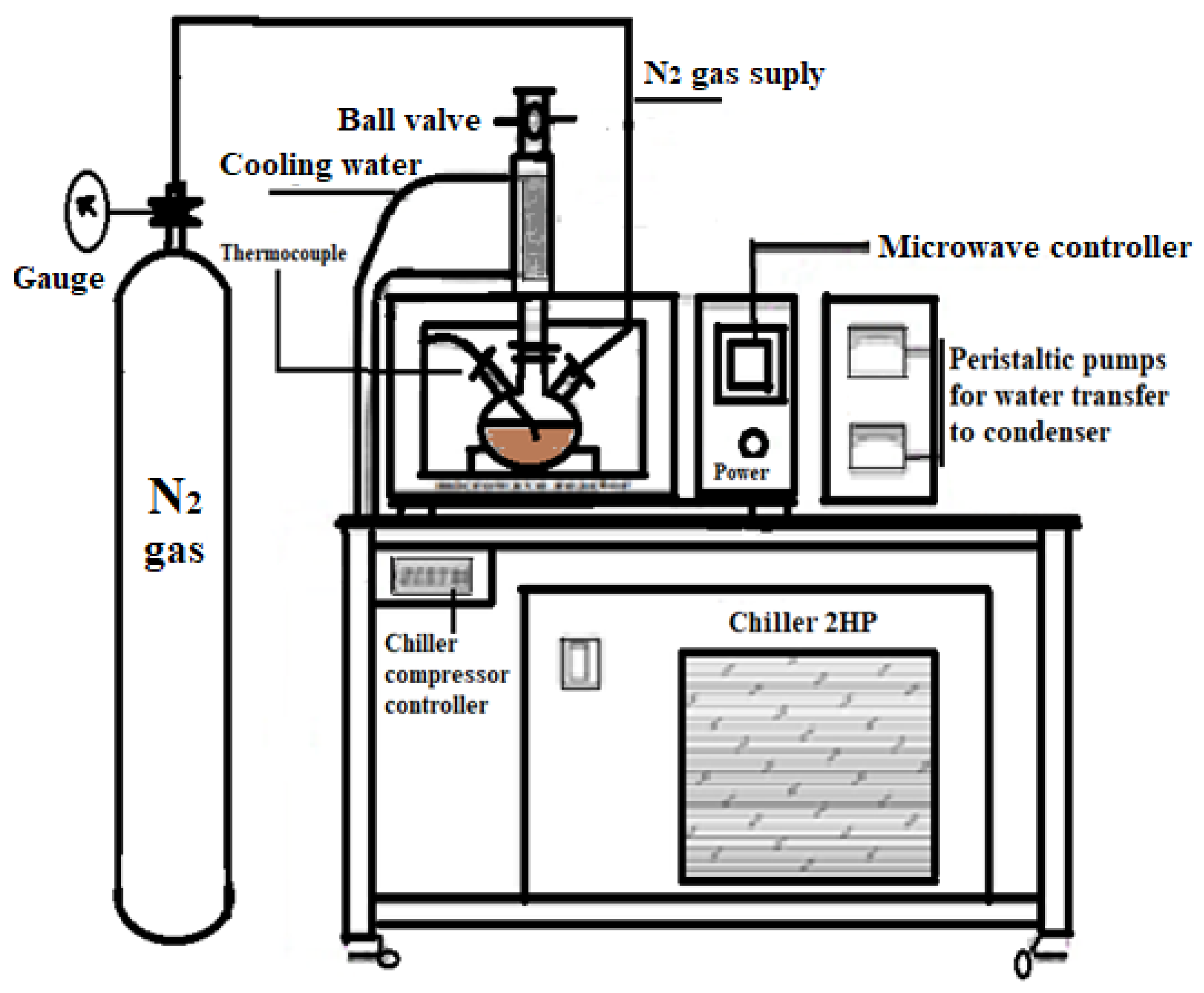
2.4. Glycerol Polymerization Reaction
3. Results and Discussion
3.1. Fresh Catalyst Characterisation
3.1.1. BET Analysis
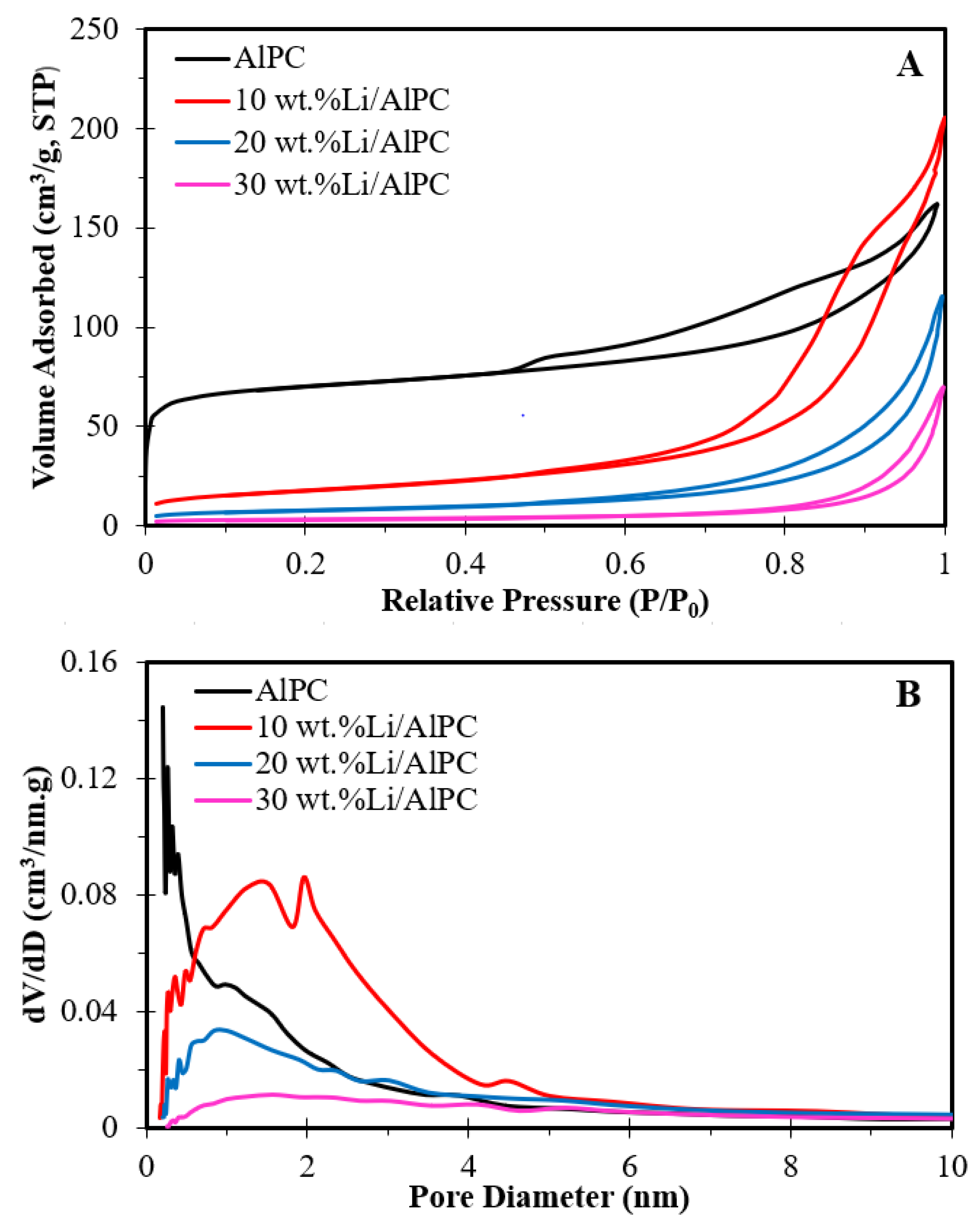
3.1.2. N2 Adsorption–Desorption Analysis
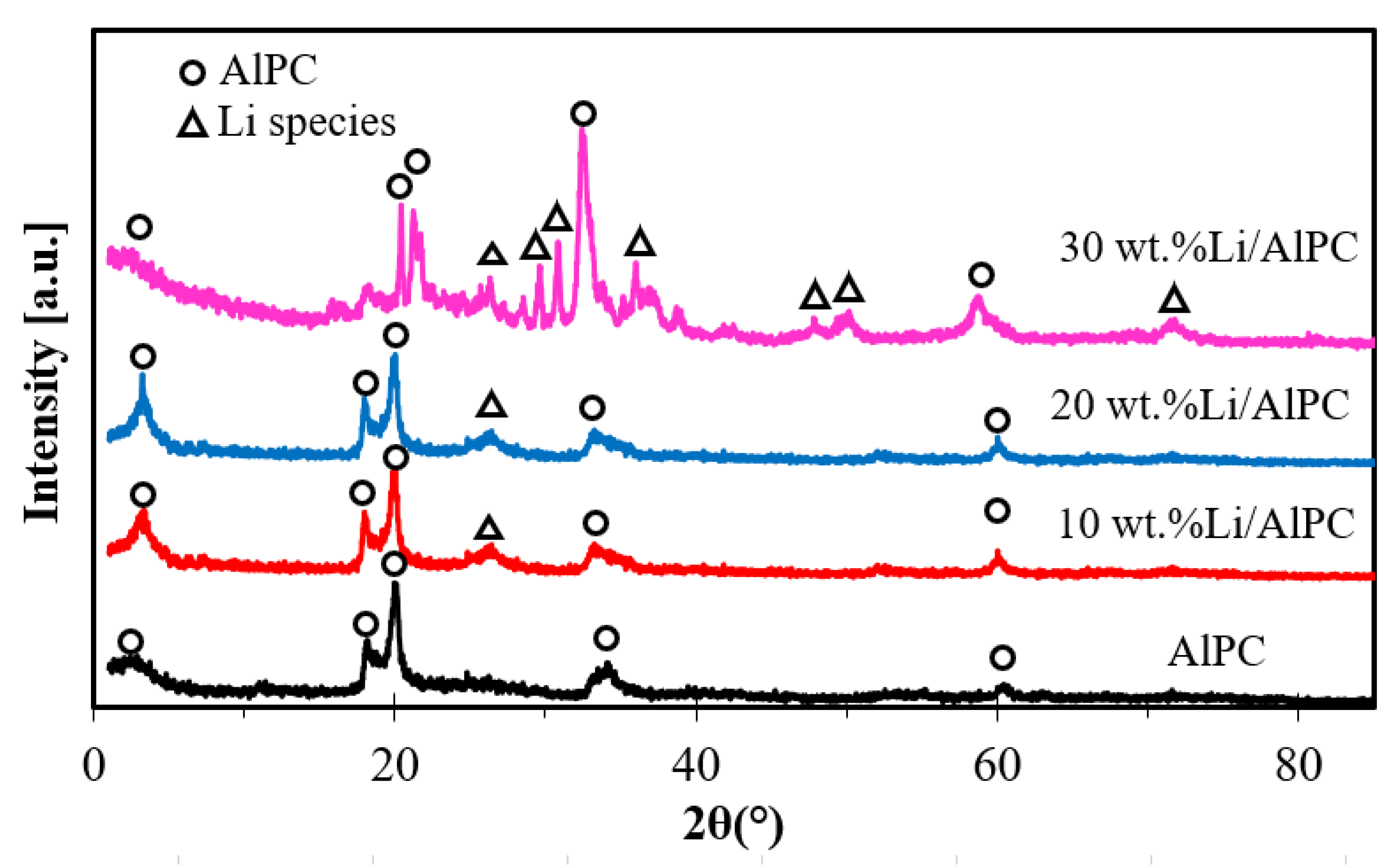
3.1.3. XRD Analysis
3.1.4. SEM Analysis
3.1.5. NH3-TPD Analysis
3.1.6. CO2-TPD Analysis
3.2. Microwave-Assisted Glycerol Polymerization
3.2.1. Effect of Li Loadings on AIPC
3.2.2. Effect of Catalyst Loading
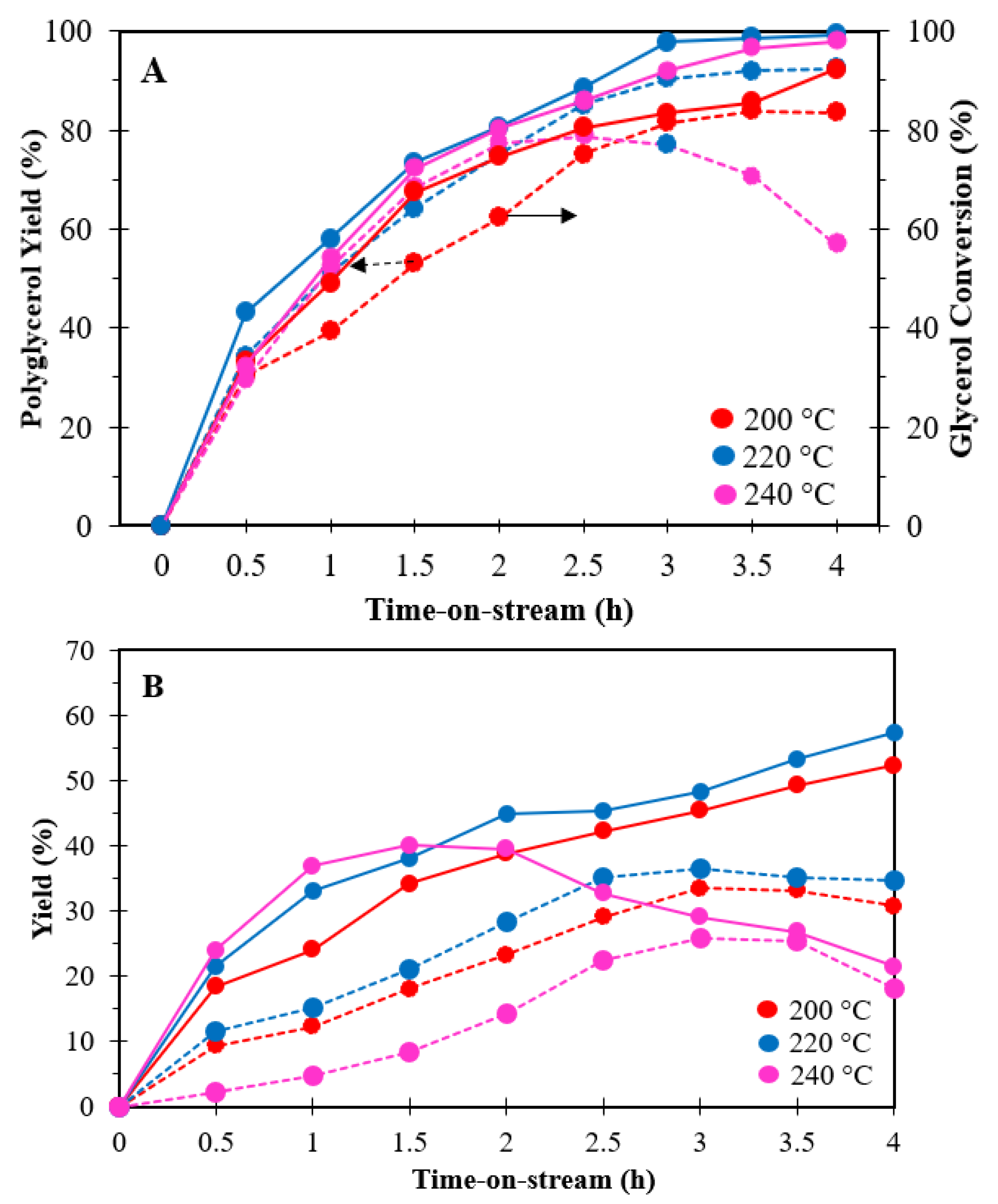
3.2.3. Effect of Reaction Temperature
3.2.4. Effect of Reaction Time
3.3. Spent Catalyst Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martin, A.; Richter, M. Oligomerization of glycerol—A critical review. Eur. J. Lipid Sci. Technol. 2010, 113, 100–117. [Google Scholar] [CrossRef]
- Tan, H.; Aziz, A.A.; Aroua, M. Glycerol production and its applications as a raw material: A review. Renew. Sustain. Energy Rev. 2013, 27, 118–127. [Google Scholar] [CrossRef]
- Liu, X.; Ma, H.; Wu, Y.; Wang, C.; Yang, M.; Yan, P.; Welz-Biermann, U. Esterification of glycerol with acetic acid using double SO3H-functionalized ionic liquids as recoverable catalysts. Green Chem. 2011, 13, 697. [Google Scholar] [CrossRef]
- Sun, D.; Yamada, Y.; Sato, S.; Ueda, W. Glycerol hydrogenolysis into useful C3 chemicals. Appl. Catal. B 2016, 193, 75–92. [Google Scholar] [CrossRef] [Green Version]
- Katryniok, B.; Paul, S.; Bellière-Baca, V.; Rey, P.; Dumeignil, F. Glycerol dehydration to acrolein in the context of new uses of glycerol. Green Chem. 2010, 12, 2079. [Google Scholar] [CrossRef]
- Talebian-Kiakalaieh, A.; Amin, N.A.S.; Najaafi, N.; Tarighi, S. A Review on the Catalytic Acetalization of Bio-renewable Glycerol to Fuel Additives. Front. Chem. 2018, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Ayoub, M.; Khayoon, M.; Abdullah, A.Z. Synthesis of oxygenated fuel additives via the solventless etherification of glycerol. Bioresour. Technol. 2012, 112, 308–312. [Google Scholar] [CrossRef]
- Melero, J.A.; Vicente, G.; Paniagua, M.; Morales, G.; Muñoz, P. Etherification of biodiesel-derived glycerol with ethanol for fuel formulation over sulfonic modified catalysts. Bioresour. Technol. 2012, 103, 142–151. [Google Scholar] [CrossRef]
- Quispe, C.A.; Coronado, C.J.; De Carvalho, J.A. Glycerol: Production, consumption, prices, characterization and new trends in combustion. Renew. Sustain. Energy Rev. 2013, 27, 475–493. [Google Scholar] [CrossRef]
- Chong, C.C.; Aqsha, A.; Ayoub, M.; Sajid, M.; Abdullah, A.Z.; Yusup, S.; Abdullah, B. A review over the role of catalysts for selective short-chain polyglycerol production from biodiesel derived waste glycerol. Environ. Technol. Innov. 2020, 19, 100859. [Google Scholar] [CrossRef]
- Gholami, Z.; Abdullah, A.Z.; Lee, K.T. Heterogeneously catalyzed etherification of glycerol to diglycerol over calcium–lanthanum oxide supported on MCM-41: A heterogeneous basic catalyst. Appl. Catal. A Gen. 2014, 479, 76–86. [Google Scholar] [CrossRef]
- Ayoub, M.; Abdullah, A.Z. Instability of SBA-15 to Strong Base: Effects of LiOH Impregnation on its Surface Characteristics and Mesoporous Structure. J. Appl. Sci. 2011, 11, 3510–3514. [Google Scholar] [CrossRef]
- Jerome, F.; Pouilloux, Y.; Barrault, J. Rational Design of Solid Catalysts for the Selective Use of Glycerol as a Natural Organic Building Block. ChemSusChem. 2008, 1, 586–613. [Google Scholar] [CrossRef] [PubMed]
- Gholami, Z.; Abdullah, A.Z.; Lee, K.T. Glycerol etherification to polyglycerols using Ca1+xAl1−xLaxO3 composite catalysts in a solventless medium. J. Taiwan Inst. Chem. Eng. 2013, 44, 117–122. [Google Scholar] [CrossRef]
- Gonçalves, M.; Castro, C.S.; Oliveira, L.C.; Carvalho, W.A. Green acid catalyst obtained from industrial wastes for glycerol etherification. Fuel Process. Technol. 2015, 138, 695–703. [Google Scholar] [CrossRef]
- Ayoub, M.; Sufian, S.; Hailegiorgis, S.M.; Ullah, S.; Uemura, Y. Conversion of glycerol to polyglycerol over waste duck-bones as a catalyst in solvent free etherification process. Iop Conf. Ser. Mater. Sci. Eng. 2017, 226, 012073. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, M.; Soler, F.C.; Isoda, N.; Carvalho, W.A.; Mandelli, D.; Sepúlveda, J. Glycerol conversion into value-added products in presence of a green recyclable catalyst: Acid black carbon obtained from coffee ground wastes. J. Taiwan Inst. Chem. Eng. 2016, 60, 294–301. [Google Scholar] [CrossRef]
- Ayoub, M.; Abdullah, A.Z. Diglycerol synthesis via solvent-free selective glycerol etherification process over lithium-modified clay catalyst. Chem. Eng. J. 2013, 225, 784–789. [Google Scholar] [CrossRef]
- Ayoub, M.; Abdullah, A.Z. LiOH-modified montmorillonite K-10 as catalyst for selective glycerol etherification to diglycerol. Catal. Commun. 2013, 34, 22–25. [Google Scholar] [CrossRef]
- Estevez, R.; Lopez-Pedrajas, S.; Luna, D.; Bautista, F. Microwave-assisted etherification of glycerol with tert-butyl alcohol over amorphous organosilica-aluminum phosphates. Appl. Catal. B 2017, 213, 42–52. [Google Scholar] [CrossRef]
- Gil, A.; Gandía, L.M.; Vicente, M.A. Recent Advances in the Synthesis and Catalytic Applications of Pillared Clays. Catal. Rev. 2000, 42, 145–212. [Google Scholar] [CrossRef]
- Chong, C.C.; Teh, L.P.; Setiabudi, H.D. Syngas production via CO2 reforming of CH4 over Ni-based SBA-15: Promotional effect of promoters (Ce, Mg, and Zr). Mater. Today Energy 2019, 12, 408–417. [Google Scholar] [CrossRef]
- Mohapatra, P.; Mishra, T.; Parida, K. Pillared Clay as an Effective Catalyst for Low Temperature VOCs Decomposition. Key Eng. Mater. 2013, 571, 71–91. [Google Scholar] [CrossRef]
- Soetaredjo, F.E.; Ayucitra, A.; Ismadji, S.; Maukar, A.L. KOH/bentonite catalysts for transesterification of palm oil to biodiesel. Appl. Clay Sci. 2011, 53, 341–346. [Google Scholar] [CrossRef] [Green Version]
- Chong, C.C.; Setiabudi, H.D.; Jalil, A.A. Dendritic fibrous SBA-15 supported nickel (Ni/DFSBA-15): A sustainable catalyst for hydrogen production. Int. J. Hydrog. Energy 2019, 45, 18533–18548. [Google Scholar] [CrossRef]
- Chong, C.; Bukhari, S.; Cheng, Y.; Setiabudi, H.D.; Jalil, A.; Phalakornkule, C. Robust Ni/Dendritic fibrous SBA-15 (Ni/DFSBA-15) for methane dry reforming: Effect of Ni loadings. Appl. Catal. A Gen. 2019, 584, 117174. [Google Scholar] [CrossRef]
- Ayoub, M.; Inayat, A.; Hailegiorgis, S.M.; Bhat, A.H. Synthesis of alumina based alkaline catalyst for biodiesel-derived glycerol to polyglycerol. J. Adv. Mater. Res. 2016, 1133, 33–37. [Google Scholar] [CrossRef]
- Guo, J.; Nguyen, B.N.; Li, L.; Meador, M.A.B.; Scheiman, D.A.; Cakmak, M. Clay reinforced polyimide/silica hybrid aerogel. J. Mater. Chem. A 2013, 1, 7211–7221. [Google Scholar] [CrossRef]
- Krupskaya, V.V.; Zakusin, S.; Tyupina, E.A.; Dorzhieva, O.; Zhukhlistov, A.P.; Belousov, P.; Timofeeva, M.N. Experimental Study of Montmorillonite Structure and Transformation of Its Properties under Treatment with Inorganic Acid Solutions. Miner. 2017, 7, 49. [Google Scholar] [CrossRef]
- Priya, S.S.; Selvakannan, P.; Chary, K.V.R.; Kantam, M.L.; Bhargava, S.K. Solvent-free microwave-assisted synthesis of solketal from glycerol using transition metal ions promoted mordenite solid acid catalysts. Mol. Catal. 2017, 434, 184–193. [Google Scholar] [CrossRef]
- Surendra, B.; Veerabhadraswamy, M. Microwave assisted synthesis of Schiff base via bioplatform chemical intermediate (HMF) derived from Jatropha deoiled seed cake catalyzed by modified Bentonite clay. Mater. Today Proc. 2017, 4, 11968–11976. [Google Scholar] [CrossRef]
- Ayoub, M.; Ullah, S.; Inayat, A.; Mahmood, S.M. Investigation of Biodiesel-Drive Glycerol Conversion to Polyglycerol over Basic Modified ALPC Catalyst. ARPN J. Eng. Appl. Sci. 2016, 11, 2668–2672. [Google Scholar]
- Bineesh, K.V.; Kim, D.-K.; Kim, M.-I.; Selvaraj, M.; Park, D.-W. Design, synthesis and characterization of vanadia-doped iron-oxide pillared montmorillonite clay for the selective catalyticoxidation of H2S. Dalton Trans. 2011, 40, 3938–3945. [Google Scholar] [CrossRef] [PubMed]
- Sivaiah, M.; Robles-Manuel, S.; Valange, S.; Barrault, J. Recent developments in acid and base-catalyzed etherification of glycerol to polyglycerols. Catal. Today 2012, 198, 305–313. [Google Scholar] [CrossRef]
- Ruppert, A.M.; Meeldijk, J.D.; Kuipers, B.W.M.; Erné, B.H.; Weckhuysen, B.M. Glycerol Etherification over Highly Active CaO-Based Materials: New Mechanistic Aspects and Related Colloidal Particle Formation. Chem. Eur. J. 2008, 14, 2016–2024. [Google Scholar] [CrossRef] [Green Version]
- Muhammad, A.F.S.; Awad, A.; Saidur, R.; Masiran, N.; Salam, A.; Abdullah, B. Recent advances in cleaner hydrogen productions via thermo-catalytic decomposition of methane: Admixture with hydrocarbon. Int. J. Hydrog. Energy 2018, 43, 18713–18734. [Google Scholar] [CrossRef] [Green Version]
- Bookong, P.; Ruchirawat, S.; Boonyarattanakalin, S. Optimization of microwave-assisted etherification of glycerol to polyglycerols by sodium carbonate as catalyst. Chem. Eng. J. 2015, 275, 253–261. [Google Scholar] [CrossRef]






| Sample | Surface Area (m2/g) | Pore Diameter (nm) | Pore Volume (cm3/g) |
|---|---|---|---|
| AlPC | 230 | 4.106 | 0.236 |
| 10 wt.%Li/AlPC | 163.85 | 16.32 | 0.295 |
| 20 wt.%Li/AlPC | 95.48 | 19.21 | 0.309 |
| 30 wt.%Li/AlPC | 11.33 | 35.14 | 0.108 |
| Sample | Acidity (mmol/g) | Basicity (mmol/g) |
|---|---|---|
| AlPC | 0.059 | 0.005 |
| 10% Li/AlPC | 0.48 | 12.67 |
| 20% Li/AlPC | 2.35 | 34.48 |
| 30% Li/AlPC | 2.17 | 45.48 |
| Time (h) | Li Loadings (wt.%) a | Metal Loadings (wt.%) b | Temperature (°C) c | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 10 | 20 | 30 | 2 | 3 | 4 | 200 | 220 | 240 | |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0.5 | 0.32 | 4.52 | 0 | 4.52 | 2.66 | 2.62 | 2.66 | 1.50 | 3.10 |
| 1.0 | 0.36 | 2.04 | 2.45 | 2.04 | 3.00 | 4.71 | 3.00 | 3.00 | 10.71 |
| 1.5 | 1.65 | 2.78 | 3.97 | 2.78 | 1.00 | 7.62 | 1.00 | 5.00 | 19.94 |
| 2.0 | 0.1 | 5.25 | 9.55 | 5.25 | 0.25 | 11.56 | 0.25 | 2.25 | 23.76 |
| 2.5 | 4.00 | 6.85 | 11.52 | 6.85 | 3.73 | 17.39 | 3.73 | 4.73 | 23.55 |
| 3.0 | 8.00 | 5.61 | 16.72 | 5.61 | 2.61 | 17.36 | 2.61 | 5.61 | 22.27 |
| 3.5 | 13.69 | 1.45 | 16.34 | 1.45 | 1.45 | 14.73 | 1.45 | 3.45 | 18.47 |
| 4.0 | 16.054 | 0.57 | 24.02 | 0.57 | 0.57 | 22.46 | 0.57 | 0.57 | 17.20 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sajid, M.; Ayoub, M.; Yusup, S.; Abdullah, B.; Shamsuddin, R.; Bilad, R.; Chong, C.C.; Aqsha, A. Short-Chain Polyglycerol Production via Microwave-Assisted Solventless Glycerol Polymerization Process Over Lioh-Modified Aluminium Pillared Clay Catalyst: Parametric Study. Processes 2020, 8, 1093. https://doi.org/10.3390/pr8091093
Sajid M, Ayoub M, Yusup S, Abdullah B, Shamsuddin R, Bilad R, Chong CC, Aqsha A. Short-Chain Polyglycerol Production via Microwave-Assisted Solventless Glycerol Polymerization Process Over Lioh-Modified Aluminium Pillared Clay Catalyst: Parametric Study. Processes. 2020; 8(9):1093. https://doi.org/10.3390/pr8091093
Chicago/Turabian StyleSajid, Muhammad, Muhammad Ayoub, Suzana Yusup, Bawadi Abdullah, Rashid Shamsuddin, Roil Bilad, Chi Cheng Chong, and Aqsha Aqsha. 2020. "Short-Chain Polyglycerol Production via Microwave-Assisted Solventless Glycerol Polymerization Process Over Lioh-Modified Aluminium Pillared Clay Catalyst: Parametric Study" Processes 8, no. 9: 1093. https://doi.org/10.3390/pr8091093
APA StyleSajid, M., Ayoub, M., Yusup, S., Abdullah, B., Shamsuddin, R., Bilad, R., Chong, C. C., & Aqsha, A. (2020). Short-Chain Polyglycerol Production via Microwave-Assisted Solventless Glycerol Polymerization Process Over Lioh-Modified Aluminium Pillared Clay Catalyst: Parametric Study. Processes, 8(9), 1093. https://doi.org/10.3390/pr8091093









