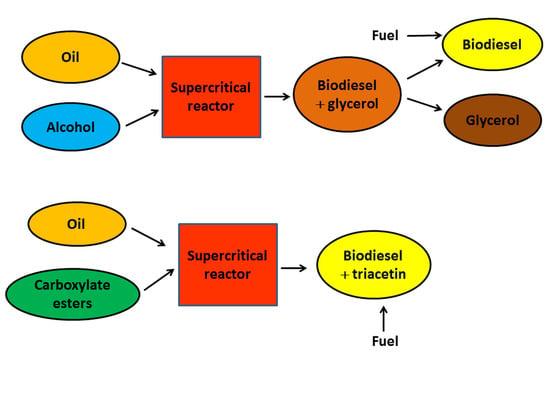
Noncatalytic Biodiesel Synthesis under Supercritical Conditions
Abstract
1. Introduction
2. Noncatalytic Supercritical Transesterification of Triglycerides Using Short-Chain Alcohols
2.1. Influence of Alcohol to Oil Molar Ratio on the Biodiesel Yield
2.2. Influence of Pressure and Temperature on the Product Yield
2.3. Influence of Process Duration on Ester Yield
2.4. Influence of Raw Material on Transesterification Effectiveness
2.5. Thermal Stability of Fatty Acid Alkyl Esters
3. Noncatalytic Interesterification with Carboxylate Esters of Low Molecular Weight under Supercritical Conditions
3.1. Influence of the Carboxylate Esters to Oil Molar Ratio on Biodiesel Yield
3.2. Influence of Pressure and Temperature on the Process Effectiveness
3.3. Influence of Process Duration on the Product Yield
3.4. Influence of Raw Material on the Effectiveness of Interesterification
3.5. Thermal Stability of Biofuel Produced by Interesterification in Supercritical Conditions
3.6. Process Economics for Supercritical Transesterification
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Demirbas, A. Biodiesel from waste cooking oil via base-catalytic and supercritical methanol transesterification. Energy Convers. Manag. 2009, 50, 923–927. [Google Scholar] [CrossRef]
- Sendzikiene, E.; Makareviciene, V.; Janulis, P.; Makareviciute, D. Biodegradability of biodiesel fuel of animal and vegetable origin. Eur. J. Lipid Sci. Technol. 2007, 109, 493–497. [Google Scholar] [CrossRef]
- Yee, K.F.; Tan, K.T.; Abdullah, A.Z.; Lee, K.T. Life cycle assessment of palm biodiesel: Revealing facts and benefits for sustainability. Appl. Energy 2009, 86, 189–196. [Google Scholar] [CrossRef]
- Marchetti, J.M. Biodiesel Production Technologies; Nova Publishers: New York, NY, USA, 2010. [Google Scholar]
- Garcia-Martin, J.F.; Barrios, C.C.; Ales-Alvarez, F.J.; Dominiguez-Saez, A.; Alvarez-Mateos, P. Biodiesel production from waste cooking oil in a oscillatory flow reactor. Performance as a fuel on a TDI diesel engine. Renew. Energy 2018, 125, 546–556. [Google Scholar] [CrossRef]
- Mittelbach, M.; Trathnigg, B. Kinetics of alkaline catalyzed methanolisis of sunflower oil. Fat Sci. Technol. 1990, 92, 145–148. [Google Scholar]
- Lotero, E.; Liu, Y.; Lopez, D.E.; Suwannakarn, K.; Bruce, D.A.; Goodwin, J.G. Synthesis of biodiesel via acid catalysis. Ind. Eng. Chem. Res. 2005, 44, 5353–5363. [Google Scholar] [CrossRef]
- Sendzikiene, E.; Makareviciene, V.; Janulis, P.; Kitrys, S. Kinetics of free fatty acids esterification with methanol in the production of biodiesel fuel. Eur. J. Lipid Sci. Technol. 2004, 106, 831–836. [Google Scholar] [CrossRef]
- Sendzikiene, E.; Sinkuniene, D.; Kazanceva, I.; Kazancev, K. Optimization of low quality rapeseed oil transesterification with butanol by applying the response surface methodology. Renew. Energy 2016, 87, 266–272. [Google Scholar] [CrossRef]
- Di Serio, M.; Cozzolino, M.; Giordao, M.; Tesser, R.; Patrono, P.; Santacesaria, E. From homogeneous to heterogeneous catalysts in biodiesel production. Ind. Eng. Chem. Res. 2007, 46, 6379–6384. [Google Scholar] [CrossRef]
- Buasri, A.; Rochanakit, K.; Wongvitvichot, W.; Masa-ard, U.; Loryuenyong, V. The application of calcium oxide and magnesium oxide from natural dolomitic rock for biodiesel synthesis. Energy Procedia 2015, 79, 562–566. [Google Scholar] [CrossRef]
- Long, Y.D.; Fang, Z.; Su, T.C.; Yang, Q. Co-production of biodiesel and hydrogen from rapeseed and Jatropha oils with sodium silicate and Ni catalysts. Appl. Energy 2014, 113, 1819–1825. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, S.; Yang, R.; Yan, Y. Biodiesel production from vegetable oil using heterogenous acid and alkali catalyst. Fuel 2010, 89, 2939–2944. [Google Scholar] [CrossRef]
- Tan, K.T.; Lee, K.T.; Mohamed, A.R. Production of FAME by palm oil transesterification via supercritical methanol technology. Biomass Bioenergy 2009, 33, 1096–1099. [Google Scholar] [CrossRef]
- García-Martínez, N.; Andreo-Martínez, P.; Quesada-Medina, J.; de los Ríos, A.P.; Chica, A.; Beneito-Ruiz, R.; Caratala-Abril, J. Optimization of non-catalytic transesterification of tobacco (Nicotiana tabacum) seed oil using supercritical methanol to biodiesel production. Energy Convers. Manag. 2017, 131, 99–108. [Google Scholar] [CrossRef]
- Olivares-Carrillo, P.; Quesada-Medina, J. Synthesis of biodiesel from soybean oil using supercritical methanol in a one-step catalyst-free process in batch reactor. J. Supercrit. Fluids 2011, 58, 378–384. [Google Scholar] [CrossRef]
- Manuale, D.L.; Torres, G.C.; Vera, C.R.; Yori, J.C. Study of an energy-integrated biodiesel production process using supercritical methanol and a low-cost feedstock. Fuel Process. Technol. 2015, 140, 252–261. [Google Scholar] [CrossRef]
- Knez, Ž.; Markočič, E.; Leitgeb, M.; Primožič, M.; Knez Hrnčič, M.; Škerget, M. Industrial applications of supercritical fluids: A review. Energy 2014, 77, 235–243. [Google Scholar] [CrossRef]
- Sawangkeaw, R.; Bunyakiat, K.; Ngamprasertsith, S. A review of laboratory-scale research on lipid conversion to biodiesel with supercritical methanol (2001–2009). J. Supercrit. Fluids 2010, 55, 1–13. [Google Scholar] [CrossRef]
- Aransiola, E.F.; Ojumu, T.V.; Oyekola, O.O.; Madzimbamuto, T.F.; Ikhu-Omoregbe, D.I.O. A review of current technology for biodiesel production: State of the art. Biomass Bioenergy 2014, 61, 276–297. [Google Scholar] [CrossRef]
- Bunyakiat, K.; Makmee, S.; Sawangkeaw, R.; Ngamprasertsith, S. Continuous production of biodiesel via transesterification from vegetable oils in supercritical methanol. Energy Fuels 2006, 20, 812–817. [Google Scholar] [CrossRef]
- Tan, K.T.; Gui, M.M.; Lee, K.T.; Mohamed, A.R. An optimized study of methanol and ethanol in supercritical alcohol technology for biodiesel production. J. Supercrit. Fluid 2010, 53, 82–87. [Google Scholar] [CrossRef]
- Sakdasri, W.; Sawangkeaw, R.; Ngamprasertsith, S. Continuous production of biofuel from refined and used palm olein oil with supercritical methanol at a low molar ratio. Energy Convers. Manag. 2015, 103, 934–942. [Google Scholar] [CrossRef]
- Román-Figueroa, C.; Olivares-Carrillo, P.; Paneque, M.L.; Palacios-Nereo, F.J.; Quesada-Medina, J. High-yield production of biodiesel by non-catalytic supercritical methanol transesterification of crude castor oil (Ricinus communis). Energy 2016, 107, 165–171. [Google Scholar] [CrossRef]
- Varma, M.N.; Madras, G. Synthesis of biodiesel from castor oil and linseed oil in supercritical fluids. Ind. Eng. Chem. Res. 2006, 46, 1–6. [Google Scholar] [CrossRef]
- He, H.; Wang, T.; Zhu, S. Continuous production of biodiesel fuel from vegetable oil using supercritical methanol process. Fuel 2007, 86, 442–447. [Google Scholar] [CrossRef]
- Quesada-Medina, J.; Olivares-Carrillo, P. Evidence of thermal decomposition of fatty acid methyl esters during the synthesis of biodiesel with supercritical methanol. J. Supercrit. Fluid 2011, 56, 56–63. [Google Scholar] [CrossRef]
- Madras, G.; Kolluru, C.; Kumar, R. Synthesis of biodiesel in supercritical fluids. Fuel 2004, 83, 2029–2033. [Google Scholar] [CrossRef]
- Demirbas, A. Biodiesel from vegetable oils via transesterification in supercritical methanol. Energy Convers. Manag. 2002, 43, 2349–2356. [Google Scholar] [CrossRef]
- Demirbas, A. Biodiesel production from vegetable oils via catalytic and non-catalytic supercritical methanol transesterification methods. Prog. Energy Combust. Sci. 2005, 31, 466–487. [Google Scholar] [CrossRef]
- Farobie, O.; Matsumura, Y. A comparative study of biodiesel production using methanol, ethanol, and tert-butyl methyl ether (MTBE) under supercritical conditions. Bioresour. Technol. 2015, 191, 306–311. [Google Scholar] [CrossRef]
- Lee, S.; Posarac, D.; Ellis, N. An experimental investigation of biodiesel synthesis from waste canola oil using supercritical methanol. Fuel 2012, 91, 229–237. [Google Scholar] [CrossRef]
- Goembira, F.; Matsuura, K.; Saka, S. Biodiesel production from rapeseed oil by various supercritical carboxylate esters. Fuel 2012, 97, 373–378. [Google Scholar] [CrossRef]
- Kusdiana, D.; Saka, S. Kinetics of transesterification in rapeseed oil to biodiesel fuel as treated in supercritical methanol. Fuel 2001, 80, 693–698. [Google Scholar] [CrossRef]
- Niza, N.; Tan, K.; Ahmad, Z.; Lee, K. Comparison and optimisation of biodiesel production from Jatropha curcas oil using supercritical methyl acetate and methanol. Chem. Pap. 2011, 65, 721–729. [Google Scholar] [CrossRef]
- Lamba, N.; Modak, J.M.; Madras, G. Fatty acid methyl esters synthesis from non-edible vegetable oils using supercritical methanol and methyl tert-butylether. Energy Convers. Manag. 2017, 138, 77–83. [Google Scholar] [CrossRef]
- Ghoreishi, S.M.; Moein, P. Biodiesel synthesis from waste vegetable oil via transesterification reaction in supercritical methanol. J. Supercrit. Fluids 2013, 76, 24–31. [Google Scholar] [CrossRef]
- Lamba, N.; Gupta, K.; Modak, J.M.; Madras, G. Biodiesel synthesis from Calophyllum inophyllum oil with different supercritical fluids. Bioresour. Technol. 2017, 241, 767–774. [Google Scholar] [CrossRef]
- Nan, Y.; Liu, J.; Lin, R.; Tavlarides, L.L. Production of biodiesel from microalgae oil (Chlorella protothecoides) by non-catalytic transesterification in supercritical methanol and ethanol: Process optimization. J. Supercrit. Fluids 2015, 97, 174–182. [Google Scholar] [CrossRef]
- Marulanda, V.F.; Anitescu, G.; Tavlarides, L.L. Biodiesel Fuels through a Continuous Flow Process of Chicken Fat Supercritical Transesterification. Energy Fuels 2010, 24, 253–260. [Google Scholar] [CrossRef]
- Vieitez, I.; da Silva, C.; Borges, G.R.; Corazza, F.C.; Oliveira, J.V.; Grompone, M.A.; Jachmanian, I. Continuous production of soybean biodiesel in supercritical ethanol water mixtures. Energy Fuels 2008, 22, 2805–2809. [Google Scholar] [CrossRef]
- Silva, C.; Weschenfelder, T.A.; Rovani, S.; Corazza, F.C.; Corazza, M.L.; Dariva, C.; Oliveira, J.V. Continuous production of fatty acid ethyl esters from soybean oil in compressed ethanol. Ind. Eng. Chem. Res. 2007, 46, 5304–5309. [Google Scholar] [CrossRef]
- Rade, L.L.; Arvelos, S.; de Souza Barrozo, M.A.; Romanielo, L.L.; Watanabe, E.O.; Hori, C.E. Evaluation of the use of degummed soybean oil and supercritical ethanol for non-catalytic biodiesel production. J. Supercrit. Fluids 2015, 105, 21–28. [Google Scholar] [CrossRef]
- Conceicao, M.M.; Candeia, R.A.; Silva, F.C.; Bezerra, A.F.; Fernandes, V.J., Jr.; Souza, A.G. Thermoanalytical characterization of castor oil biodiesel. Renew. Sustain. Energy Rev. 2007, 11, 964–975. [Google Scholar] [CrossRef]
- Vieitez, I.; Silva, C.; Alkimim, I.; Borges, G.R.; Castilhos, F.; Oliveira, J.V.; Grompone, M.A.; Jachmanián, I. Stability of ethyl esters from soybean oil exposed to high temperatures in supercritical ethanol. J. Supercrit. Fluids 2011, 56, 265–270. [Google Scholar] [CrossRef]
- Silva, C.; de Castilhos, F.; Oliveira, J.V.; Filho, L.C. Continuous production of soybean biodiesel with compressed ethanol in a microtube reactor. Fuel Process. Technol. 2010, 91, 1274–1281. [Google Scholar] [CrossRef]
- Imahara, H.; Minami, E.; Hari, S.; Saka, S. Thermal stability of biodiesel in supercritical methanol. Fuel 2008, 87, 1–6. [Google Scholar] [CrossRef]
- Ayoub, M.; Abdullah, A.Z. Critical review on the current scenario and significance of crude glycerol resulting from biodiesel industry towards more sustainable renewable energy industry. Renew. Sustain. Energy Rev. 2012, 16, 2671–2686. [Google Scholar] [CrossRef]
- Tan, K.T.; Lee, K.T.; Mohamed, A.R. Prospect on non-catalytic supercritical methyl acetate process in biodiesel production. Fuel Process. Technol. 2011, 92, 1905–1909. [Google Scholar] [CrossRef]
- Alavianmehr, M.M.; El-Shaikh, M.; Akbari, F.; Behjatmanesh-Ardakani, R. A new equation of state for modeling thermodynamic properties of some fatty acids alkyl esters, methyl ester-based biodiesels and their blends. Fluid Phase Equilibria 2017, 442, 53–61. [Google Scholar] [CrossRef]
- Casas, A.; Ramos, M.J.; Pérez, A. New trends in biodiesel production: Chemical interesterification of sunflower oil with methyl acetate. Biomass Bioenergy 2011, 35, 1702–1709. [Google Scholar] [CrossRef]
- Saka, S.; Isayama, Y. A new process for catalyst-free production of biodiesel using supercritical methyl acetate. Fuel 2009, 88, 1307–1313. [Google Scholar] [CrossRef]
- Goembira, F.; Saka, S. Optimization of biodiesel production by supercritical methyl acetate. Bioresour. Technol. 2013, 241, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Usai, E.M.; Gualdi, E.; Solinas, V.; Battistel, E. Simultaneous enzymatic synthesis of FAME and triacetyl glycerol from triglycerides and methyl acetate. Bioresour. Technol. 2010, 101, 7707–7712. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.T.; Lee, K.T.; Mohamed, A.R. A glycerol-free process to produce biodiesel by supercritical methyl acetate technology: An optimization study via response surface methodology. Bioresour. Technol. 2010, 101, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Campanelli, P.; Banchero, M.; Manna, L. Synthesis of biodiesel from edible, non-edible and waste cooking oils via supercritical methyl acetate transesterification. Fuel 2010, 89, 3675–3682. [Google Scholar] [CrossRef]
- Goembira, F.; Saka, S. Factors Affecting Biodiesel Yield in Interesterification of Rapeseed Oil by Supercritical Methyl Acetate; Zero-Carbon Energy Kyoto 2011; Springer: Tokyo, Japan, 2012; pp. 147–152. [Google Scholar]
- Farobie, O.; Matsumura, Y. Continuous production of biodiesel under supercritical methyl acetate conditions: Experimental investigation and kinetic model. Bioresour. Technol. 2017, 241, 720–725. [Google Scholar] [CrossRef]
- Doná, G.; Cardozo-Filho, L.; Silva, C.; Castilhos, F. Biodiesel production using supercritical methyl acetate in a tubular packed bedr eactor. Fuel Process. Technol. 2013, 10, 605–610. [Google Scholar] [CrossRef]
- Visioli, L.J.; Trentini, C.P.; Castilhos, F.; Silva, C. Esters production in continuous reactor from macauba pulp oil using methyl acetate in pressurized conditions. J. Supercrit. Fluids 2018, 140, 238–247. [Google Scholar] [CrossRef]
- Goembira, F.; Saka, S. Advanced supercritical Methyl acetate method for biodiesel production from Pongamia pinnata oil. Renew. Energy 2015, 83, 1245–1249. [Google Scholar] [CrossRef]
- Patil, P.D.; Reddy, H.; Muppaneni, T.; Deng, S. Biodiesel fuel production from algal lipids using supercritical methyl acetate (glycerin-free) technology. Fuel 2017, 195, 201–207. [Google Scholar] [CrossRef]
- Sootchiewcharn, N.; Attanatho, L.; Reubroycharoen, P. Biodiesel Production from Refined Palm Oil using Supercritical Ethyl Acetate in A Microreactor. Energy Procedia 2015, 79, 697–703. [Google Scholar] [CrossRef]
- Komintarachat, C.; Sawangkeaw, R.; Ngamprasertsith, S. Continuous production of palm biofuel under supercritical ethyl acetate. Energy Convers. Manag. 2015, 93, 332–338. [Google Scholar] [CrossRef]
- Kasteren, J.M.N.; Nisworo, A.P. A process model to estimate the cost of industrial scale biodiesel production from waste cooking oil by supercritical transesterification. Resour. Conserv. Recycl. 2007, 50, 442–458. [Google Scholar] [CrossRef]
- Micic, R.D.; Tomic, M.D.; Kiss, F.E.; Nikolic-Djoric, E.B.; Simikic, M.D. Influence of reaction conditions and type of alcohol on biodiesel yields and process economics of supercritical transesterification. Energy Convers. Manag. 2014, 86, 717–726. [Google Scholar] [CrossRef]
| Raw Material | Molar Ratio Alcohol/Oil, mol/mol | Pressure, Mpa | Temperature, °C | Duration, min | Yield, w% | References |
|---|---|---|---|---|---|---|
| Coconut oil | 42:1 | 19 | 350 | 6.67 | 95 | [21] |
| Palm kernel oil | 42:1 | 19 | 350 | 667 | 96 | [21] |
| Palm oil | 40:1 | 15–25 | 372 | 16 | 81.5 | [22] |
| Refined palm oil | 12:1 | 15 | 400 | 18–20 | 90 ester content | [23] |
| 99 conversion | ||||||
| Used Palm oil | 12:1 | 15 | 400 | 18–20 | 80 ester content | [23] |
| Crude castor oil | 43:1 | 21 | 300 | 90 | 96.5 | [24] |
| Castor oil | 40:1 | 20 | 350 | 40 | 100 conversion | [25] |
| Linseed oil | 40:1 | 20 | 350 | 40 | 100 conversion | [25] |
| Soybean oil | 40:1 | 35 | 310 | 25 | 77 | [26] |
| Soybean oil | 43:1 | 35 | 325 | 60 | 84 | [16,27] |
| Sunflower oil | 40:1 | 20 | 400 | 30 | 96 | [28] |
| Cottonseed oil | 41:1 | 8 | 240 | 10 | 95 | [29,30] |
| Hazelnut kernel oil | 41:1 | 8 | 240 | 5 | 95 | [29] |
| Canola oil Canola oil | 40:1 | 20 | 350 | 10 | 100 | [31] |
| 40:1 | 20 | 400 | 3 | 100 | [31] | |
| Waste canola oil | 2:1 w/w | 10 | 270 | 45 | 102 | [32] |
| Rapeseed oil | 42:1 | 43 | 350 | 6 | 97.7 | [33] |
| Rapeseed oil | 42:1 | 19 | 350 | 4 | 95 | [34] |
| Jatropha curcas oil | 44:1 | 358 | 27 | 89.4 | [35] | |
| Neem oil | 50:1 | 30 | 425 | 15 | 83 | [36] |
| Mahua oil | 10 | 99 | ||||
| Waste vegetable oil | 33.8:1 | 23.1 | 271.1 | 20.4 | 95.27 | [37] |
| Tobacco (Nicotiana tabacum) seed oil | 43:1 | 26 | 300 | 90 | 92.8 ± 2.1 | [15] |
| Calophyllum inophyllum oil | 40:1 | 30 | 400 | 30 | 80 | [38] |
| Chlorella protothecoides oil | 19:1 | 15.2 | 320 | 31 | 90.8 | [39] |
| Chicken fat | 6:1 | 41.1 | 400 | 6 | 88 | [40] |
| Raw Material | Molar Ratio Alcohol/Oil, mol/mol | Pressure, Mpa | Temperature, °C | Duration, min | Yield, w% | References |
|---|---|---|---|---|---|---|
| Soybean oil | 40:1 | 20 | 350 | n.d. | 77.5 | [41] |
| Canola oil | 40:1 | 20 | 350 | 30 | 100 | [31] |
| Canola oil | 40:1 | 20 | 400 | 10 | 100 | [31] |
| Soybean oil | 40:1 | 20 | 350 | 15 | n.d. | [42] |
| Soybean oil | 15:1 | n.d. | 320 | 50 | 62.5 | [43] |
| Sunflower oil | 40:1 | 20 | 350 | 40 | ~100 | [28] |
| Sunflower oil | 40:1 | 20 | 400 | 30 | ~99 | [28] |
| Palm oil | 33:1 | 15–25 | 349 | 29 | 79.2 | [22] |
| Castor oil | 40:1 | 20 | 350 | 40 | ~100 | [25] |
| Linseed oil | 40:1 | 20 | 350 | 40 | conversion | [25] |
| Chlorella protothecoides oil | 33:1 | 17 | 340 | 35 | 87.8 | [39] |
| Raw Material | Interesterifica Tion Agent | Molar Ratio mol/mol | Pressure, Mpa | Temperature, °C | Duration, min | FAME Yield, w% | References |
|---|---|---|---|---|---|---|---|
| Palm oil | Methyl acetate | 30:1 | n.d. | 399 | 59 | 97.6 | [55] |
| Palm oi | Methyl acetate | 30:1 | 22 | 400 | 60 | 99 | [49] |
| Palm oil | Methyl acetate | 42:1 | 20 | 300 | 45 | 95.0 | [53] |
| Jatropha cucrcas oil | Methyl acetate | 50:1 | 400 | 32 | 71.9 | [35] | |
| Jatropha curcas oil | Methyl acetate Methyl acetate | 42:1 | 20 | 345 | 50 | 100 | [56] |
| Soybean oil | |||||||
| Sunflower seed oil | |||||||
| Waste soybean oil | |||||||
| Rapeseed oil | Methyl acetate | 42:1 | 20 | 350 | 45 | 96.7 | [57] |
| Rapeseed oil | Methyl acetate | 42:1 | 17.8 | 350 | 45 | 97.7 | [33] |
| Rapeseed oil | Methyl acetate | 42:1 | 20 | 350 | 45 | 97.0 | [52] |
| Olein acid | Methyl acetate | 42:1 | 20 | 350 | 20 | 91.0 | |
| Canola oil | Methyl acetate | 40:1 | 20 | 380 | 10 | 80.0 | [58] |
| Macauba oil | Methyl acetate | 5:1 w/w | n.d. | 325 | 45 | 83.0 | [59] |
| Macauba pulp oil | Methyl acetate | 1:1 | 20 | 325 | 40 | 96.7 | [60] |
| Soybean oil | Methyl acetate | 5:1 w/w | n.d. | 350 | 45 | 44.0 | [59] |
| Pongamia pinata oil | Methyl acetate | 42:1 | 20 | 300 | 45 | 96.6 | [61] |
| Nanochloropsis Salina sp. oil | Methyl acetate | 40:1 | 10 | 310 | 60 | 70.0 | [62] |
| Calophyllum inophyllum oil | Methyl acetate | 40:1 | 30 | 400 | 30 | 70.0 | [38] |
| Palm oil | Ethyl acetate | 50:1 | 20 | 350 | 20 | 78.3 | [63] |
| Palm oil | Ethyl acetate + 10% water | 30:1 | 16 | 380 | 42.4 | 90.9 | [64] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makareviciene, V.; Sendzikiene, E. Noncatalytic Biodiesel Synthesis under Supercritical Conditions. Processes 2021, 9, 138. https://doi.org/10.3390/pr9010138
Makareviciene V, Sendzikiene E. Noncatalytic Biodiesel Synthesis under Supercritical Conditions. Processes. 2021; 9(1):138. https://doi.org/10.3390/pr9010138
Chicago/Turabian StyleMakareviciene, Violeta, and Egle Sendzikiene. 2021. "Noncatalytic Biodiesel Synthesis under Supercritical Conditions" Processes 9, no. 1: 138. https://doi.org/10.3390/pr9010138
APA StyleMakareviciene, V., & Sendzikiene, E. (2021). Noncatalytic Biodiesel Synthesis under Supercritical Conditions. Processes, 9(1), 138. https://doi.org/10.3390/pr9010138