Effect of Blanching on Enzyme Inactivation, Physicochemical Attributes and Antioxidant Capacity of Hot-Air Dried Pomegranate (Punica granatum L.) Arils (cv. Wonderful)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fruit Material and Processing
Aril Drying Procedure
2.2. Enzyme Extraction
2.2.1. Polyphenol Oxidase (PPO) Activity Determination
2.2.2. Peroxidase Activity (POD) Activity Determination
2.3. Measurement of Physical Properties of Arils
2.3.1. Aril Colour
2.3.2. Aril Texture
2.3.3. Rehydration Capacity
2.4. Extraction of Samples
2.5. Determination of Total Soluble Solids (TSS), Titratable Acidity (TA) and pH
2.6. Phytochemical Properties and Antioxidant Activity
2.6.1. Determination of Total Phenolic Content
2.6.2. Total Anthocyanin Content
2.6.3. Radical-Scavenging Activity
2.6.4. Ferric Ion Reducing Antioxidant Power (FRAP)
2.7. Statistical Analysis
3. Results
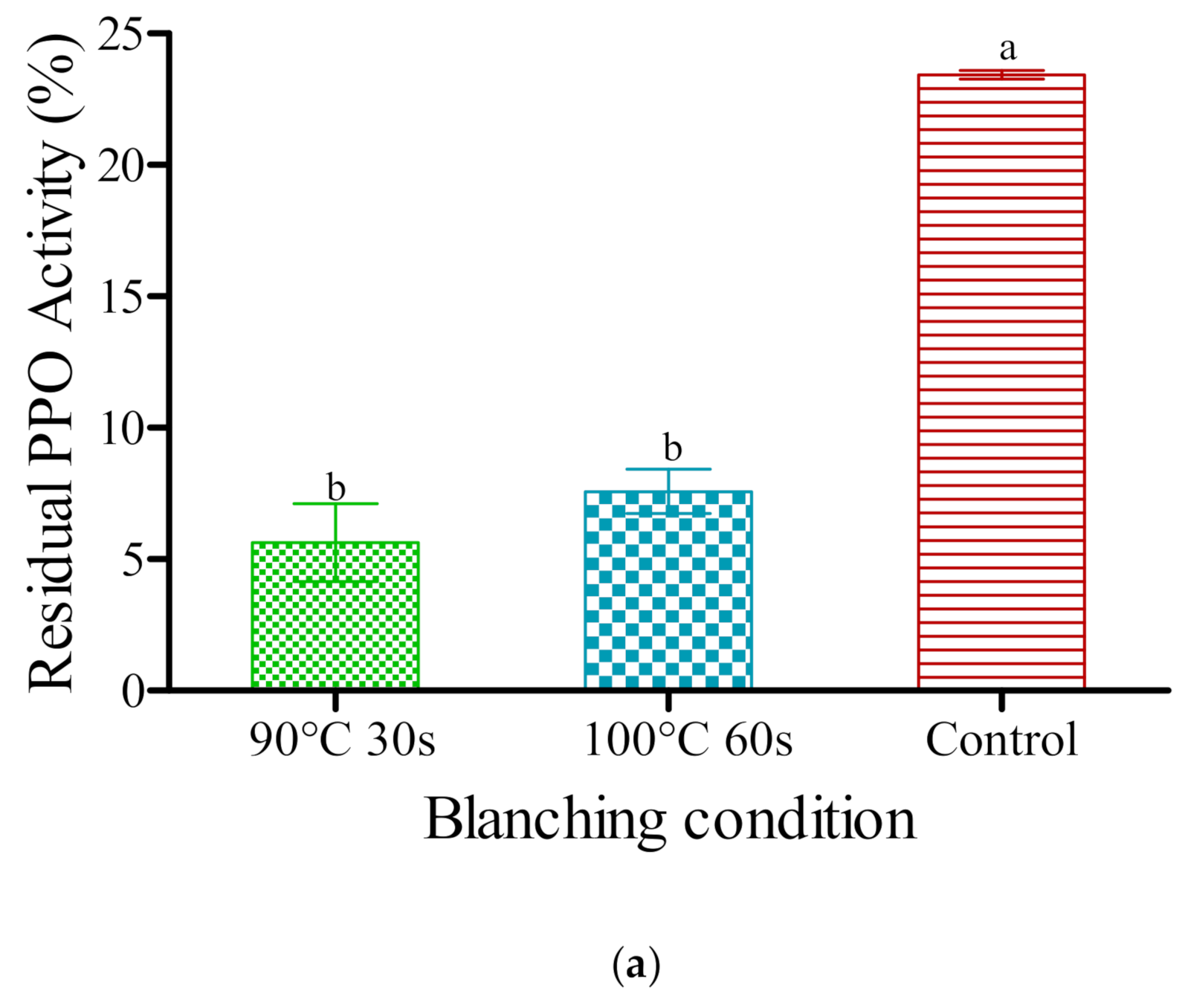
3.1. Influence of Blanching on Enzymes Inactivation
3.2. Colour Change
3.3. Textural Properties
3.4. Rehydration Capacity
3.5. Total Soluble Solids (TSS), Titratable Acidity (TA) and pH
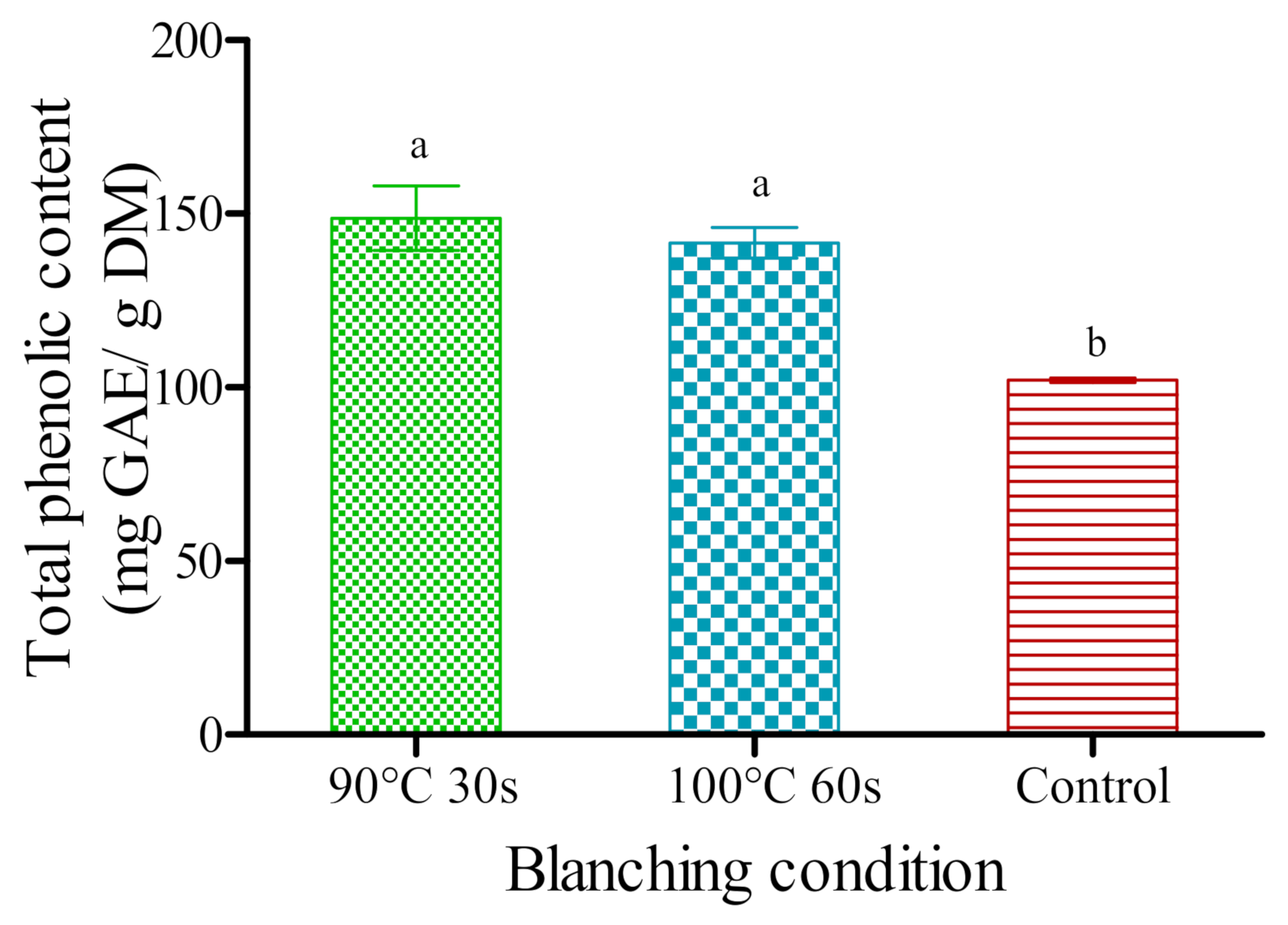
3.6. Total Phenolic Content (TPC) and Total Anthocyanin Content (TAC)
3.7. Antioxidant Capacity
3.8. Multivariate Analysis
3.8.1. Principal Component Analysis
3.8.2. Correlation between Quality Attributes of Dried Arils
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Al-Said, F.A.; Opara, U.L.; Al-Yahyai, R.A. Physico-chemical and textural quality attributes of pomegranate cultivars (Punica granatum) L. grown in the sultanate of Oman. J. Food Eng. 2009, 90, 129–134. [Google Scholar] [CrossRef]
- Holland, D.; Hatib, K.; Bar-Yaakov, I. Pomegranate: Botany, horticulture, breeding. Hortic. Rev. 2009, 35, 127–191. [Google Scholar]
- Fawole, O.A.; Makunga, N.P.; Opara, U.L. Antibacterial, antioxidant and tyrosinase-inhibition activities of pomegranate fruit peel methanolic extract. BMC Complement. Altern. Med. 2012, 12, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pomegranate Association of South Africa (POMASA). Statistics and Information. 2018. Available online: https://www.sapomegranate.co.za/focus-areas/statistics-and-information-2018/ (accessed on 9 November 2019).
- Fawole, O.A.; Opara, U.L. Changes in physical properties, chemical and elemental composition and antioxidant capacity of pomegranate. cv. Ruby fruit at five maturity stages. Sci. Hortic. 2013, 150, 37–46. [Google Scholar] [CrossRef]
- Caleb, O.J.; Opara, U.L.; Witthuhn, C.R. Modified atmosphere packaging of pomegranate fruit and arils: A review. Food Bioprocess Technol. 2012, 5, 15–30. [Google Scholar] [CrossRef]
- Espín, J.C.; García-Conesa, M.T.; Tomás-Barberán, F.A. Nutraceuticals: Facts and fiction. Phytochemistry 2007, 68, 2986–3008. [Google Scholar] [CrossRef] [PubMed]
- Fawole, O.A.; Opara, U.L. Effects of maturity status on biochemical concentration, polyphenol composition and antioxidant capacity of pomegranate fruit arils. cv. Bhagwa. S. Afr. J. Bot. 2013, 85, 23–31. [Google Scholar] [CrossRef] [Green Version]
- Fawole, O.A.; Opara, U.L.; Chen, L. Bio accessibility of total phenolic concentration and antioxidant capacity of pomegranate fruit juice and marc after in vitro digestion. Acta Hortic. 2015, 1079, 285–290. [Google Scholar] [CrossRef]
- Opara, U.L.; Al-Ani, M.R.; Al-Shuaibi, Y.S. Physio-chemical properties, vitamin C content, and antimicrobial properties of pomegranate fruit (Punica granatum L.). Food Bioprocess Technol. 2009, 2, 315–321. [Google Scholar] [CrossRef]
- Lara, I.; Belge, B.; Goulao, L.F. The fruit cuticle as a modulator of postharvest quality. Postharvest Biol. Technol. 2014, 87, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Jahanfar, S.; Fatemian, H.; Hosseini, E.; Asadi, G.H.; Darvish, F. Physicochemical Changes of Impacted Tomatoes with Pendulum Mechanical Forces. IJASR 2011, 2, 39–47. [Google Scholar]
- Zaharan, H.; Fawole, O.A.; Opara, U.L. Investigating bruise susceptibility of pomegranate cultivars during postharvest handling. Afr. J. Rural Dev. 2017, 1, 33–39. [Google Scholar]
- Pomegranate Association of South Africa (POMASA). Pomegranate Industry Statistics. Paarl, South Africa, 2012. Available online: http://hortogro.co.za/porfolio/pomegranates/ (accessed on 24 September 2019).
- Ratti, C. Hot-air and freeze-drying of high-value foods: A review. J. Food Eng. 2001, 49, 311–319. [Google Scholar] [CrossRef]
- Kim, T.H.; Hampton, J.G.; Opara, L.U.; Hardacre, A.K.; Mackay, B.R. Effects of maize grain size, shape and hardness on drying rate and the occurrence of stress cracks. J. Sci. Food Agric. 2002, 82, 1232–1239. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, M.; Adhikari, B.; Mujumdar, A.S. Effects of preparation and drying methods on the antioxidant activity of enzymatically hydrolyzed porcine placenta hydrolysates. Dry Technol. 2013, 31, 1600–1610. [Google Scholar] [CrossRef]
- Alibas, O.I.; Isik, E. Determination of drying parameters in microwave drying of apricot and sweet cherry. In First Stone Fruits Symposium; Ergun, M.E., Burak, M., Eds.; Yalova, Turkey, 2001; pp. 317–328. [Google Scholar]
- Fazaeli, M.; Yousefi, S.; Emam-Djomeh, Z. Investigation on the effects of microwave and conventional heating methods on the phytochemicals of pomegranate (Punica granatum) and black mulberry juices. Int. Food Res. J. 2013, 50, 568–573. [Google Scholar] [CrossRef]
- Adetoro, A.O.; Fawole, O.A.; Opara, U.L. Effects of pretreatment and drying on the quality attributes of fruit. Acta Hortic. 2017, 1201, 1–6. [Google Scholar]
- Thakur, N.S.; Bhat, M.M.; Rana, N.; Joshi, V.K. Standardization of pretreatments for the preparation of dried arils from wild pomegranate. J. Food Sci. Technol. 2010, 47, 620–625. [Google Scholar] [CrossRef] [Green Version]
- Meighani, H.; Ghasemnezhad, M.; Bakshi, D. Evaluation of biochemical composition and enzyme activities in browned arils of pomegranate fruits. Int. J. Hortic. Sci. Technol. 2014, 1, 53–65. [Google Scholar]
- Arendse, E.; Fawole, O.A.; Magwaza, L.S.; Nieuwoudt, H.H.; Opara, U.L. Evaluation of biochemical markers associated with the development of husk scald and the use of diffuse reflectance NIR spectroscopy to predict husk scald in pomegranate fruit. Sci. Hortic. 2018, 232, 240–249. [Google Scholar] [CrossRef]
- Sarpong, F.; Yu, X.; Zhou, C.; Hongpeng, Y.; Bernard, B.; Junwen, U. Influence of anti-browning agent pretreatment on drying kinetics, enzymes inactivation and other qualities of dried banana. Musa ssp. under relative humidity-convective air dryer. J. Food Meas. Charact. 2018, 12, 1229–1241. [Google Scholar] [CrossRef]
- Tembo, L.; Chiteka, Z.A.; Kadzere, I.; Akinnifesi, F.K.; Tagwira, F. Blanching and drying period affect moisture loss and vitamin C content in Ziziphus mauritiana Lamk. Afr. J. Biotechnol. 2008, 7, 3100–3106. [Google Scholar]
- Doymaz, İ. Effect of citric acid and blanching pretreatments on drying and rehydration of Amasya red apples. Food Bioprod. Process. 2010, 88, 124–132. [Google Scholar] [CrossRef]
- Adetoro, A.O.; Tsige, A.A.; Opara, U.L.; Fawole, O.A. Mathematical Modelling of Blanch-Assisted Drying of Pomegranate (Punica granatum) Arils in a Hot-Air Drier. Processes 2020, 8, 611. [Google Scholar] [CrossRef]
- Deylami, M.Z.; Rahman, R.A.; Tan, C.P.; Bakar, J.; Olusegun, L. Effect of blanching on enzyme activity, colour change, anthocyanin stability and extractability of mangosteen pericarp: A kinetic study. J. Food Eng. 2016, 178, 12–19. [Google Scholar] [CrossRef]
- Maté, J.I.; Zwietering, M.; Van’t Riet, K. The effect of blanching on the mechanical and rehydration properties of dried potato slices. Eur. Food Res. Technol. 1999, 209, 343–347. [Google Scholar] [CrossRef]
- Maghoumi, M.; Gómez, P.A.; Mostofi, Y.; Zamani, Z.; Artés-Hernández, F.; Artés, F. Combined effect of heat treatment, UV-C and super atmospheric oxygen packing on phenolics and browning related enzymes of fresh-cut pomegranate arils. Food Sci. Technol. 2013, 54, 389–396. [Google Scholar]
- Doymaz, I. Drying of pomegranate seeds using infrared radiation. Food Sci. Biotechnol. 2012, 21, 1269–1275. [Google Scholar] [CrossRef]
- Jiang, Y.M. Purification and some properties of polyphenol oxidase of longan fruit. Food Chem. 1999, 66, 75–79. [Google Scholar] [CrossRef]
- Shah, H.M.S.; Khan, A.S.; Ali, S. Pre-storage kojic acid application delays pericarp browning and maintains antioxidant activities of litchi fruit. Postharvest Biol. Technol. 2017, 132, 154–161. [Google Scholar] [CrossRef]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.J. Colour measurement and analysis in fresh and processed foods: A review. Food Bioproc. Tech. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Chen, L.; Opara, U.L. Approaches to analysis and modeling texture in fresh and processed foods—A review. J. Food Eng. 2013, 119, 497–507. [Google Scholar] [CrossRef]
- Chen, L.; Opara, U.L. Texture measurement approaches in fresh and processed foods—A review. Food Res. Int. 2013, 51, 823–835. [Google Scholar] [CrossRef]
- Giri, S.K.; Prasad, S. Drying kinetics and rehydration characteristics of microwave-vacuum and convective hot-air dried mushrooms. J. Food Eng. 2007, 78, 512–521. [Google Scholar] [CrossRef]
- Wrolstad, R.E. Colour and pigment analyses in fruit products. In Agricultural Experiment Station; Oregon State University Station Bulletin: Corvallis, OR, USA, 1993; Volume 624. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma. FRAP as a measure of “antioxidant power” the FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Jaiswal, V.; DerMarderosian, A.; Porter, J.R. Anthocyanins and polyphenol oxidase from dried arils of pomegranate (Punica granatum L.). Food Chem. 2010, 118, 11–16. [Google Scholar] [CrossRef]
- Ashebir, D.; Jezik, K.; Weingartemann, H.; Gretzmacher, R. Change in colour and other fruit quality characteristics of tomato cultivars after hot-air drying at low final-moisture content. Int. J. Food Sci. Nutr. 2009, 60, 308–315. [Google Scholar] [CrossRef]
- Vega-Gálvez, A.; Lemus-Mondaca, R.; Bilbao-Sáinz, C.; Fito, P.; Andrés, A. Effect of air drying temperature on the quality of rehydrated dried red bell pepper (Capsicum annuum L.). J. Food Eng. 2008, 85, 42–50. [Google Scholar] [CrossRef]
- Dadali, G.; Demirhan, E.; Özbek, B. Color change kinetics of spinach undergoing microwave drying. Dry Technol. 2007, 25, 1713–1723. [Google Scholar] [CrossRef]
- Horuz, E.; Maskan, M. Hot-air and microwave drying of pomegranate (Punica granatum) arils. J. Food Sci. Technol. 2015, 52, 285–293. [Google Scholar] [CrossRef]
- Adetoro, A.O.; Opara, U.L.; Fawole, O.A. Effect of Hot-Air and Freeze-Drying on the Quality Attributes of Dried Pomegranate (Punica granatum L.) Arils During Long-Term Cold Storage of Whole Fruit. Agriculture 2020, 10, 493. [Google Scholar] [CrossRef]
- Adetoro, A.O.; Opara, U.L.; Fawole, O.A. Effect of Carrier Agents on the Physicochemical and Technofunctional Properties and Antioxidant Capacity of Freeze-Dried Pomegranate Juice (Punica granatum) Powder. Foods 2020, 9, 1388. [Google Scholar] [CrossRef] [PubMed]
- Beveridge, T.; Weintraub, S.E. Effect of blanching pretreatment on color and texture of apple slices at various water activities. Int. Food Res. J. 1995, 28, 83–86. [Google Scholar] [CrossRef]
- Maskan, M. Drying, shrinkage and rehydration characteristics of kiwifruits during hot air and microwave drying. J. Food Eng. 2001, 48, 177–182. [Google Scholar] [CrossRef]
- Piga, A.; Pinna, I.; Özer, K.B.; Agabbio, M.; Aksoy, U. Hot air dehydration of figs (Ficus carica L.): Drying kinetics and quality loss. Int. J. Food Sci. 2004, 39, 793–799. [Google Scholar] [CrossRef]
- Brennand, C.P. Home Drying of Food; Cooperative Extension Service, Utah State University: Logan, UT, USA, 1994; p. 330.
- Workneh, T.S.; Zinash, A.; Woldetsadik, K. Blanching, salting and sun drying of different pumpkin fruit slices. J. Food Sci. Technol. 2014, 51, 3114–3123. [Google Scholar] [CrossRef] [Green Version]
- Jordan, R.; Seelye, R.; Mcglone, A. A sensory-based alternative to brix/acid ratio. J. Food Sci. 2001, 55, 36–44. [Google Scholar]
- Jaya, S.; Das, H. A vacuum drying model for mango pulp. Dry Technol. 2003, 21, 1215–1234. [Google Scholar] [CrossRef]
- Rawson, A.; Patras, A.; Tiwari, B.K.; Noci, F.; Koutchma, T.; Brunton, N. Effect of thermal and non-thermal processing technologies on the bioactive content of exotic fruits and their products: Review of recent advances. Food Res. Int. 2011, 44, 1875–1887. [Google Scholar] [CrossRef]
- Karaaslan, M.; Yilmaz, F.M.; Cesur, O.; Vardin, H.; Ikinci, A.; Coskun Dalgic, A. Drying kinetics and thermal degradation of phenolic compounds and anthocyanins in pomegranate arils dried under vacuum conditions. Int. J. Food Sci. Technol. 2014, 49, 595–605. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.Z.; Yang, X.H.; Mujumdar, A.S.; Zhao, J.H.; Wang, D.; Zhang, Q.; Wang, J.; Gao, Z.J.; Xiao, H.W. Red pepper (Capsicum annuum L.) drying: Effects of different drying methods on drying kinetics, physicochemical properties, antioxidant capacity, and microstructure. Dry Technol. 2018, 36, 893–907. [Google Scholar] [CrossRef]
- Gil, G.I.; Sanchez, R.; Marin, J.G.; Artes, F. Quality changes in pomegranate during ripening and cold storage. Eur. Food Res. Technol. 1996, 202, 481–485. [Google Scholar] [CrossRef]
- Artés, F.; Tudela, J.; Gil, M. Improving the keeping quality of pomegranate fruit by intermittent warming. Eur. Food Res. Technol. 1998, 207, 316–321. [Google Scholar] [CrossRef]
- Sablani, S.S.; Andrews, P.K.; Davies, N.M.; Walters, T.; Saez, H.; Bastarrachea, L. Effects of air and freeze-drying on phytochemical content of conventional and organic berries. Dry Technol. 2011, 29, 205–216. [Google Scholar] [CrossRef]
- Lopez, J.; Vega-Ga’lvez, A.; Torres, M.J.; Lemus-Mondaca, R.; QuispeFuentes, I.; Di Scala, K. Effect of dehydration temperature on physico-chemical properties and antioxidant capacity of goldenberry (Physalis peruviana L.). Chil. J. Agric. Res. 2013, 73, 293–300. [Google Scholar] [CrossRef]
- Süfer, Ö.; Palazoğlu, T.K. Microwave–Vacuum drying of pomegranate arils (Punica granatum L. cv. Hicaznar): Effect on quality and nutrient content. J. Food Process. Preserv. 2019, 43, 14085. [Google Scholar] [CrossRef]
- Nurhuda, H.H.; Maskat, M.Y.; Mamot, S.; Aiq, J.; Aminah, A. Effect of blanching on enzyme and antioxidant activities of rambutan. Nephelium lappaceum. peel. Int. Food Res. J. 2013, 20, 1725–1730. [Google Scholar]
- Arendse, E.; Fawole, O.A.; Opara, U.L. Effects of postharvest storage conditions on phytochemical and radical-scavenging activity of pomegranate fruit (cv. Wonderful). Sci. Hortic. 2014, 169, 125–129. [Google Scholar] [CrossRef]





| Blanching Condition | L* | a* | C* | h° | TCD |
|---|---|---|---|---|---|
| Fresh | 29.4 ± 0.06 a | 18.4 ± 0.25 b | 21.2 ± 0.22 a | 29.8 ± 1.51 a | - |
| Control | 21.4 ± 0.08 c | 20.4 ± 0.08 a | 22.0 ± 0.02 a | 21.9 ± 0.63 b | 8.56 ± 0.16 a |
| 90 °C 30 s | 26.5 ± 0.67 b | 17.6 ± 0.81 b | 18.2 ± 0.85 b | 14.7 ± 1.37 c | 6.77 ± 0.68 b |
| 100 °C 60 s | 24.6 ± 0.56 b | 18.4 ± 0.19 b | 18.9 ± 0.14 b | 13.6 ± 1.83 c | 7.14 ± 0.10 b |
| Blanching Condition | Hardness (N) | Stickiness (−N) |
|---|---|---|
| Fresh | 117.1 ± 1.09 b | - |
| Control | 5633.1 ± 233.8 a | −46.8 ± 3.68 c |
| 90 °C 30 s | 5448.9 ± 221.1 a | −35.1 ± 4.21 b |
| 100 °C 60 s | 5339.7 ± 209.1 a | −23.3 ± 2.69 a |
| Blanching Condition | TSS (°Brix) | TA (% Citric Acid) | TSS/TA | pH | BrimA |
|---|---|---|---|---|---|
| Fresh | 16.1 ± 0.18 d | 1.47 ± 0.06 c | 11.0 ± 0.39 a | 3.22 ± 0.16 c | 13.2 ± 0.13 c |
| Control | 24.9 ± 0.37 a | 4.20 ± 0.06 a | 5.95 ± 0.16 b | 3.39 ± 0.01 bc | 16.6 ± 0.47 a |
| 90 °C 30 s | 21.4 ± 0.10 b | 3.57 ± 0.09 b | 6.01 ± 0.17 b | 3.56 ± 0.01 ab | 14.3 ± 0.26 b |
| 100 °C 60 s | 18.5 ± 0.15 c | 3.40 ± 0.06 b | 5.45 ± 0.09 b | 3.75 ± 0.01 a | 11.7 ± 0.15 d |
| Loadings | F1 | F2 |
|---|---|---|
| L* | 0.981 | 0.196 |
| a* | −0.950 | −0.312 |
| C* | −0.976 | −0.218 |
| h° | −0.995 | 0.098 |
| TCD | −0.975 | −0.224 |
| pH | 0.865 | −0.501 |
| TSS | −0.909 | 0.417 |
| TA | −0.986 | 0.167 |
| TSS/TA | −0.440 | 0.898 |
| BrimA | −0.867 | 0.498 |
| PPO | −0.992 | −0.130 |
| POD | −0.983 | −0.185 |
| TPC | 0.998 | 0.057 |
| TAC | 0.814 | 0.581 |
| RSA | 1.000 | −0.001 |
| FRAP | 0.989 | 0.145 |
| Scores | ||
| 90 °C 30 s | 2.490 | 1.832 |
| 100 °C 60 s | 2.769 | −1.768 |
| Control | −5.258 | −0.064 |
| Variables | L* | a* | C* | h° | TCD | pH | TSS | TA | TSS/TA | BrimA | PPO | POD | TPC | TAC | RSA | FRAP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L* | 1 | |||||||||||||||
| a* | −0.993 | 1 | ||||||||||||||
| C* | −1.000 | 0.995 | 1 | |||||||||||||
| h° | −0.957 | 0.915 | 0.950 | 1 | ||||||||||||
| TCD | −1.000 | 0.996 | 1.000 | 0.948 | 1 | |||||||||||
| pH | 0.750 | −0.666 | −0.735 | −0.910 | −0.731 | 1 | ||||||||||
| TSS | −0.809 | 0.734 | 0.796 | 0.945 | 0.792 | −0.996 | 1 | |||||||||
| TA | −0.934 | 0.885 | 0.926 | 0.998 | 0.923 | −0.937 | 0.966 | 1 | ||||||||
| TSS/TA | −0.255 | 0.139 | 0.234 | 0.526 | 0.228 | −0.831 | 0.775 | 0.584 | 1 | |||||||
| Brim A | −0.753 | 0.669 | 0.738 | 0.912 | 0.734 | −1.000 | 0.996 | 0.938 | 0.829 | 1 | ||||||
| PPO | −0.998 | 0.983 | 0.996 | 0.974 | 0.995 | −0.793 | 0.847 | 0.956 | 0.320 | 0.795 | 1 | |||||
| POD | −1.000 | 0.991 | 0.999 | 0.960 | 0.999 | −0.758 | 0.816 | 0.938 | 0.267 | 0.761 | 0.998 | 1 | ||||
| TPC | 0.990 | −0.966 | −0.987 | −0.988 | −0.986 | 0.835 | −0.884 | −0.975 | −0.388 | −0.838 | −0.997 | −0.992 | 1 | |||
| TAC | 0.912 | −0.955 | −0.921 | −0.754 | −0.924 | 0.413 | −0.498 | −0.705 | 0.163 | −0.417 | −0.883 | −0.907 | 0.846 | 1 | ||
| RSA | 0.980 | −0.950 | −0.976 | −0.995 | −0.974 | 0.866 | −0.909 | −0.986 | −0.441 | −0.868 | −0.991 | −0.983 | 0.998 | 0.814 | 1 | |
| FRAP | 0.999 | −0.985 | −0.997 | −0.971 | −0.997 | 0.784 | −0.839 | −0.951 | −0.306 | −0.786 | −1.000 | −0.999 | 0.996 | 0.890 | 0.989 | 1111 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adetoro, A.O.; Opara, U.L.; Fawole, O.A. Effect of Blanching on Enzyme Inactivation, Physicochemical Attributes and Antioxidant Capacity of Hot-Air Dried Pomegranate (Punica granatum L.) Arils (cv. Wonderful). Processes 2021, 9, 25. https://doi.org/10.3390/pr9010025
Adetoro AO, Opara UL, Fawole OA. Effect of Blanching on Enzyme Inactivation, Physicochemical Attributes and Antioxidant Capacity of Hot-Air Dried Pomegranate (Punica granatum L.) Arils (cv. Wonderful). Processes. 2021; 9(1):25. https://doi.org/10.3390/pr9010025
Chicago/Turabian StyleAdetoro, Adegoke Olusesan, Umezuruike Linus Opara, and Olaniyi Amos Fawole. 2021. "Effect of Blanching on Enzyme Inactivation, Physicochemical Attributes and Antioxidant Capacity of Hot-Air Dried Pomegranate (Punica granatum L.) Arils (cv. Wonderful)" Processes 9, no. 1: 25. https://doi.org/10.3390/pr9010025







