Development and Optimization of Chitosan-Hydroxypropyl Methylcellulose In Situ Gelling Systems for Ophthalmic Delivery of Bupivacaine Hydrochloride
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of In Situ Gelling Systems with Chitosan and HPMC E 5 LV
2.3. Determination of Gelling Capacity for Colloidal Chitosan and HPMC E 5 LV Systems
2.4. Surface Property Analysis for Ophthalmic Systems with In Situ Gelling Based on Chitosan and HPMC E 5 LV
2.4.1. Determination of Contact Angle Values
2.4.2. Evaluation of Surface Tension
2.5. Rheological Studies on Colloidal Systems with In Situ Gelling Based on Chitosan and HPMC E 5 LV
2.6. In Vitro Drug Release Kinetics Studies from Colloidal Systems with Chitosan and HPMC E 5 LV
2.7. Optimization of Colloidal Systems with In Situ Gelling Based on Chitosan and HPMC E 5 LV Using Response Surface Analysis
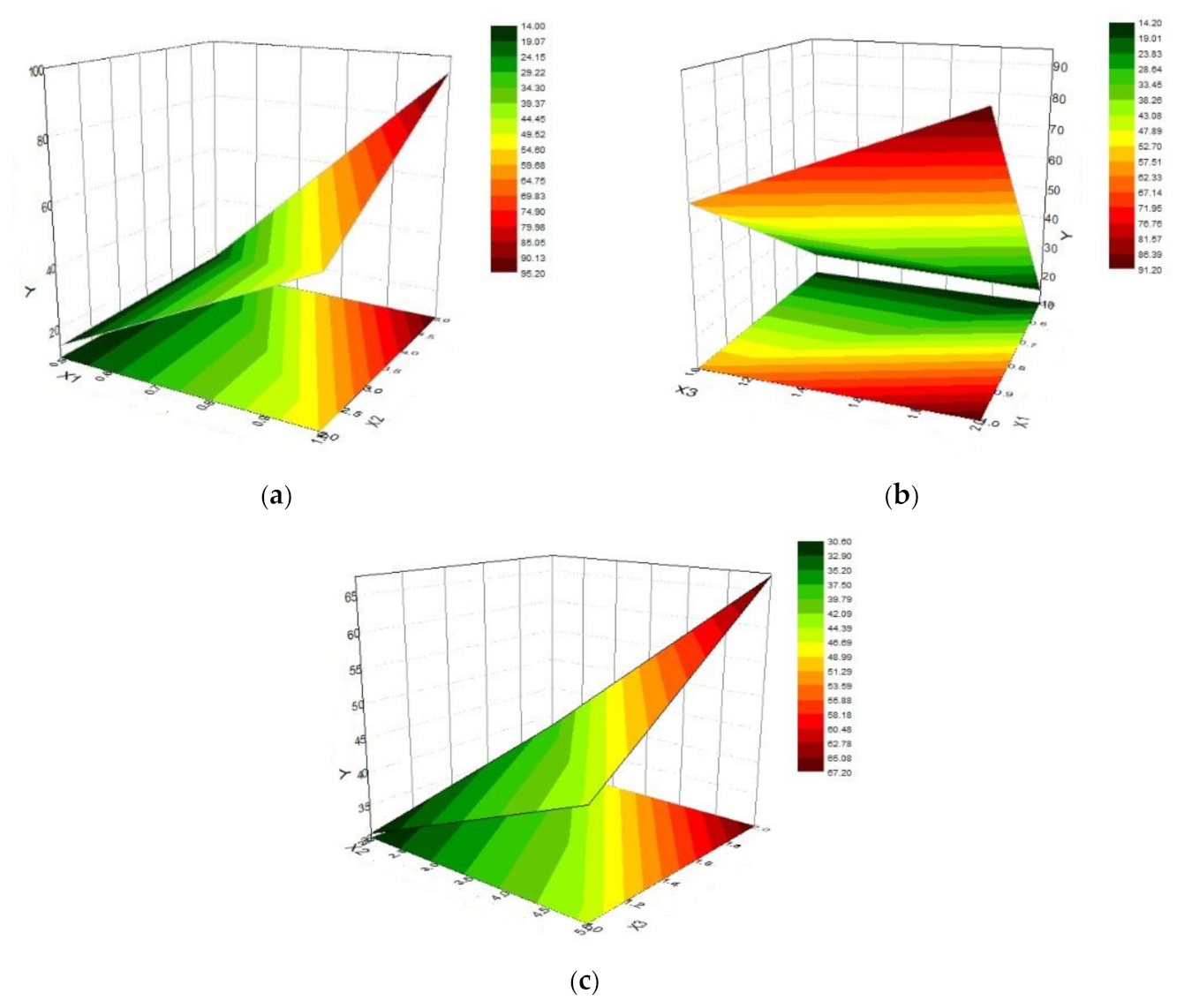
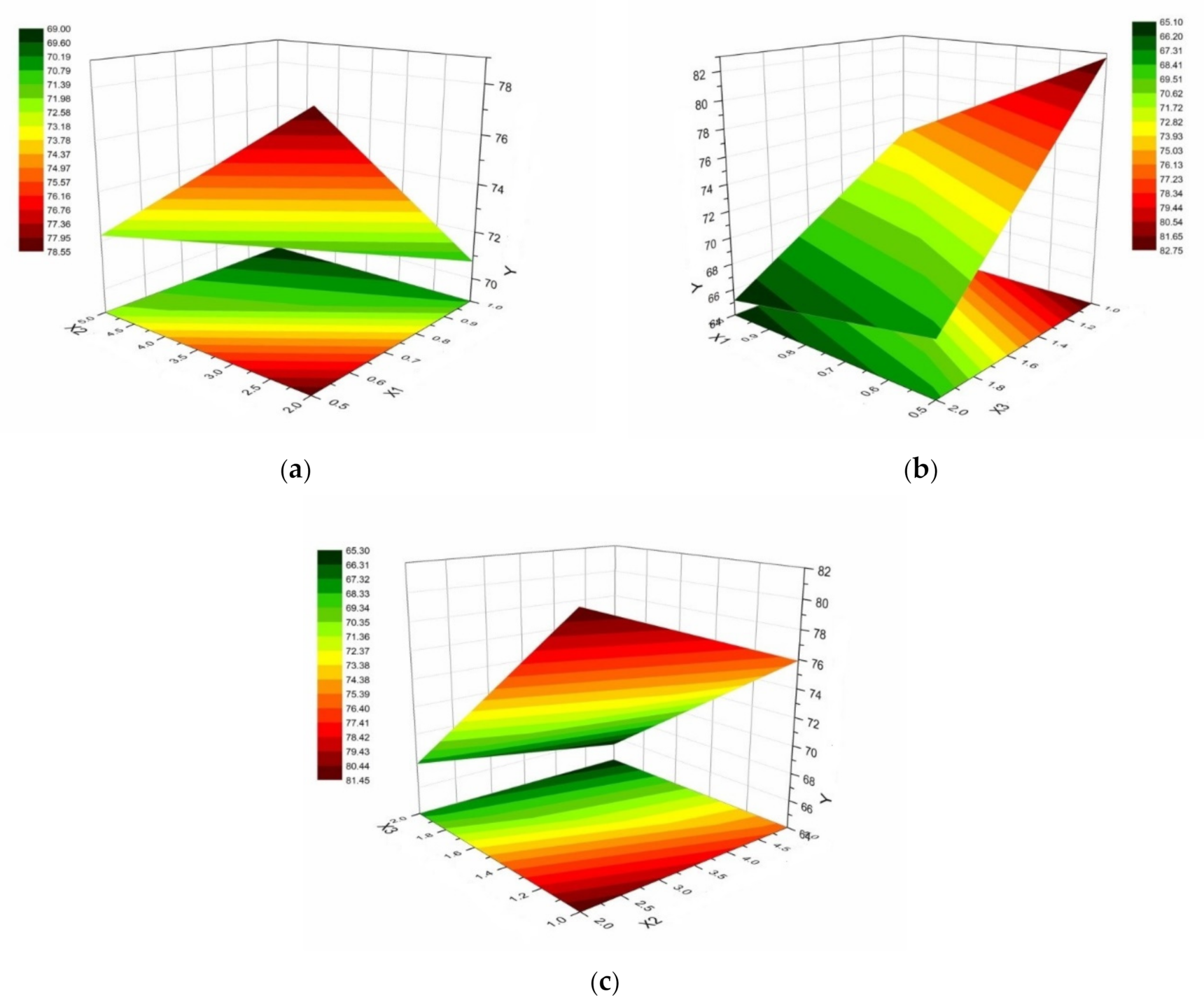
3. Results
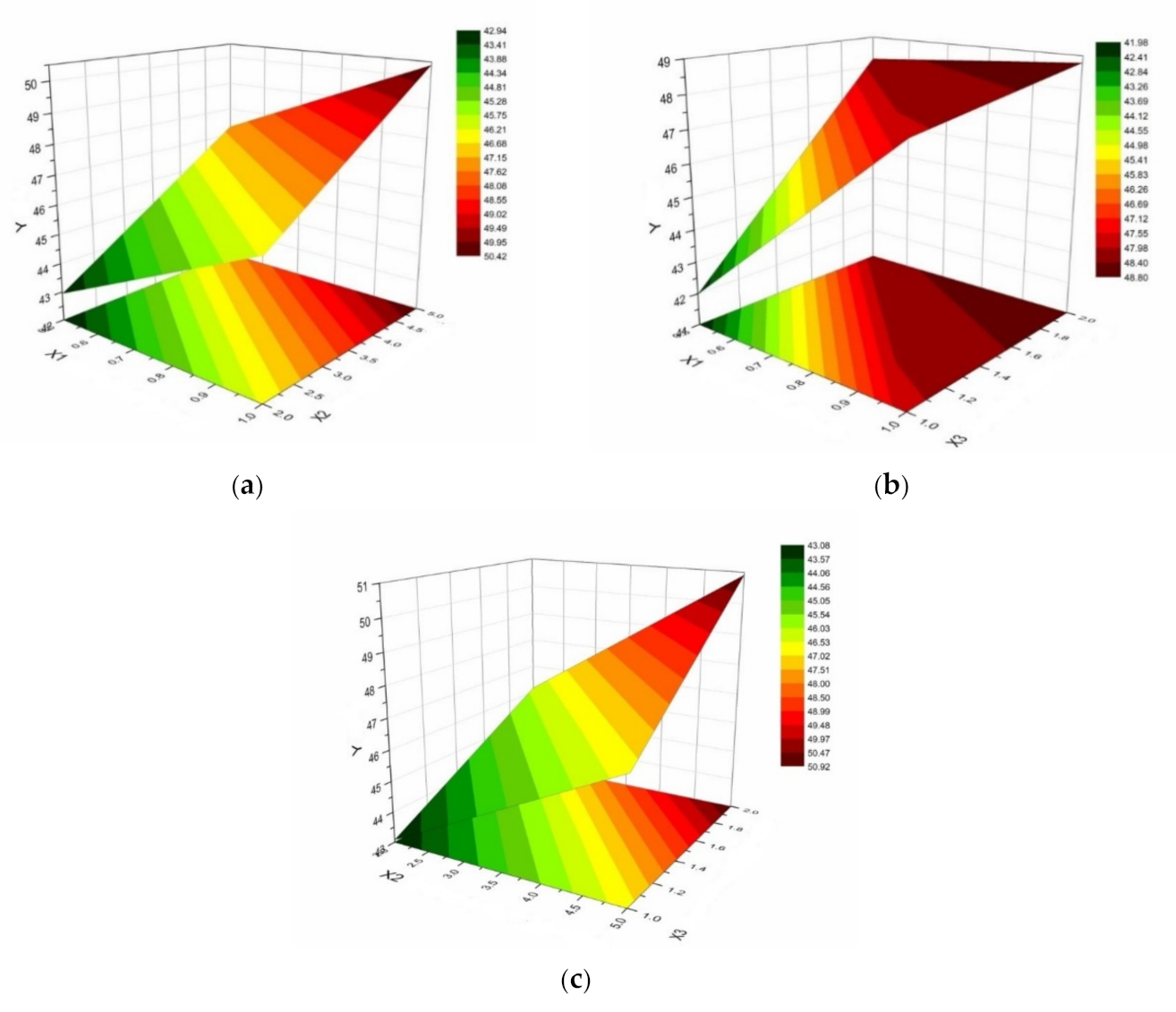
- X1: chitosan concentration (0.5% or 1%);
- X2: HPMC E 5 LV concentration (2% or 5%);
- X3: (chitosan / HPMC ratio of 1/1 or 2/1).
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pai, R.V.; Vavia, P.R. Chitosan oligosaccharide enhances binding of nanostructured lipid carriers to ocular mucins: Effect on ocular disposition. Int. J. Pharm. 2020, 577, 119095. [Google Scholar] [CrossRef]
- Thakkar, R.; Komanduri, N.; Dudhipala, N.; Tripathi, S.; Repka, M.A.; Majumdar, S. Development and optimization of hot-melt extruded moxifloxacin hydrochloride inserts, for ocular applications, using the design of experiments. Int. J. Pharm. 2021, 603, 120676. [Google Scholar] [CrossRef] [PubMed]
- Tundisi, L.L.; Mostaco, G.B.; Carricondo, P.C.; Petri, D.F.S. Hydroxypropyl methylcellulose: Physicochemical properties and ocular drug delivery formulations. Eur. J. Pharm. Sci. 2021, 159, 105736. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Torres-Luna, C.; Azadi, M.; Domszy, R.; Hu, N.; Yang, A.; David, A.E. Evaluation of commercial soft contact lenses for ocular drug delivery: A review. Acta Biomater. 2020, 115, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zuo, Z.; Cheung, C.K.C.; Leung, S.S.Y. Updates on thermosensitive hydrogel for nasal, ocular and cutaneous delivery. Int. J. Pharm. 2019, 559, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Popescu, R.; Ghica, M.V.; Dinu-Pirvu, C.E.; Anuta, V.; Lupuliasa, D.; Popa, L. New Opportunity to Formulate Intranasal Vaccines and Drug Delivery Systems Based on Chitosan. Int. J. Mol. Sci. 2020, 21, 5016. [Google Scholar] [CrossRef]
- Irimia, T.; Dinu-Pirvu, C.E.; Ghica, M.V.; Lupuleasa, D.; Muntean, D.L.; Udeanu, D.I.; Popa, L. Chitosan-Based In Situ Gels for Ocular Delivery of Therapeutics: A State-of-the-Art Review. Mar. Drugs 2018, 16, 373. [Google Scholar] [CrossRef] [Green Version]
- Sami El-banna, F.; Mahfouz, M.E.; Leporatti, S.; El-Kemary, M.; Hanafy, N.A.N. Chitosan as a Natural Copolymer with Unique Properties for the Development of Hydrogels. Appl. Sci. 2019, 9, 2193. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.P.; Hsieh, C.M.; Tsai, T.; Yang, J.C.; Chen, C.T. Optimization and Evaluation of a Chitosan/Hydroxypropyl Methylcellulose Hydrogel Containing Toluidine Blue O for Antimicrobial Photodynamic Inactivation. Int. J. Mol. Sci. 2015, 16, 20859–20872. [Google Scholar] [CrossRef] [Green Version]
- Yusufu, M.; Liu, X.; Zheng, T.; Fan, F.; Xu, J.; Luo, Y. Hydroxypropyl methylcellulose 2% for dry eye prevention during phacoemulsification in senile and diabetic patients. Int. Ophthalmol. 2018, 38, 1261–1273. [Google Scholar] [CrossRef]
- Chen, Y.A.; Hirnschall, N.; Findl, O. Comparison of corneal wetting properties of viscous eye lubricant and balanced salt solution to maintain optical clarity during cataract surgery. J. Cataract Refract. Surg. 2011, 37, 1806–1808. [Google Scholar] [CrossRef]
- El-Sousi, S.; Nacher, A.; Mura, C.; Catalan-Latorre, A.; Merino, V.; Merino-Sanjuan, M.; Diez-Sales, O. Hydroxypropylmethylcellulose films for the ophthalmic delivery of diclofenac sodium. J. Pharm. Pharmacol. 2013, 65, 193–200. [Google Scholar] [CrossRef]
- Sheshala, R.; Ming, N.J.; Kok, Y.Y.; Singh, T.R.R.; Dua, K. Formulation and Characterization of pH Induced in situ Gels Containing Sulfacetamide Sodium for Ocular Drug Delivery: A Combination of Carbopol®/HPMC Polymer. Indian J. Pharm. Educ. Res. 2019, 53, 654–662. [Google Scholar] [CrossRef] [Green Version]
- Sheil, M.; Chambers, M.; Polkinghorne, A.; Sharpe, B. Topical Application of Lidocaine and Bupivacaine to Disbudding Wounds in Dairy Calves: Safety, Toxicology and Wound Healing. Animals 2021, 11, 869. [Google Scholar] [CrossRef]
- Bavli, Y.; Rabie, M.; Fellig, Y.; Nevo, Y.; Barenholz, Y. Liposomal Bupivacaine (Bupigel) Demonstrates Minimal Local Nerve Toxicity in a Rabbit Functional Model. Pharmaceutics 2021, 13, 185. [Google Scholar] [CrossRef]
- Irimia, T. Contributions on Formulation and Preliminary Evaluation of Ocular Colloidal Systems of Chitosan and Poloxamer 407 with Bupivacaine Hydrochloride. Farmacia 2019, 67, 702–708. [Google Scholar] [CrossRef]
- Minto, B.W.; Zanato, L.; Franco, G.G.; Kawamoto, F.Y.K.; Borsaro, C.P.; Pazzini, J.M.; Carvalho, E.R.; Matsui, A. Topical application of lidocaine or bupivacaine in the healing of surgical wounds in dogs. Acta Circ. Bras. 2020, 35, e202000701. [Google Scholar] [CrossRef]
- Goder, D.; Eshkol-Yogev, I.; Matsliah, L.; Lemberger, M.; Harlev, M.; Furer, A.; Zilberman, M.; Egozi, D. In vivo study of the efficacy of bupivacaine-eluting novel soy protein wound dressings in a rat burn model. Burns 2021. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.M.; Macky, T.A.; Samir, M.K. Comparative clinical trial of topical anesthetic agents in cataract surgery: Lidocaine 2% gel, bupivacaine 0.5% drops, and benoxinate 0.4% drops. J. Cataract. Refract. Surg. 2004, 30, 1716–1720. [Google Scholar] [CrossRef]
- Kashyap, A.; Varshney, R.; Titiyal, G.S.; Sinha, A.K. Comparison between ropivacaine and bupivacaine in deep topical fornix nerve block anesthesia in patients undergoing cataract surgery by phacoemulsification. Indian J. Ophthalmol. 2018, 66, 1268–1271. [Google Scholar] [PubMed]
- Oji, E.; Oji, A. Bupivacaine and lignocaine for ophthalmic surgery. Br. J. Ophthalmol. 1987, 71, 66–68. [Google Scholar] [CrossRef] [Green Version]
- Kaewjiaranai, T.; Srisatjaluk, R.L.; Sakdajeyont, W.; Pairuchvej, V.; Wongsirichat, N. The efficiency of topical anesthetics as antimicrobial agents: A review of use in dentistry. J. Dent. Anesth. Pain Med. 2018, 18, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Neuwersch, S.; Köstenberger, M.; Sorschag, S.; Ilias, W.; Likar, R. Antimicrobial Activity of Lidocaine, Bupivacaine, Mepivacaine and Ropivacaine on Staphylococcus epidermidis, Staphylococcus aureus and Bacillus subtilis. Open Pain J. 2017, 10, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Garg, N.K.; Sharma, G.; Singh, B.; Nirbhavane, P.; Tyagi, R.K.; Shukla, R.; Katare, O.P. Quality by Design (QbD)-enabled development of aceclofenac loaded-nano structured lipid carriers (NLCs): An improved dermatokinetic profile for inflammatory disorder(s). Int. J. Pharm. 2017, 517, 413–431. [Google Scholar] [CrossRef]
- Fukuda, I.M.; Pinto, C.F.F.; Moreira, C.d.S.; Saviano, A.M.; Lourenço, F.R. Design of Experiments (DoE) applied to Pharmaceutical and Analytical Quality by Design (QbD). Braz. J. Pharm. Sci. 2018, 54. [Google Scholar] [CrossRef]
- Yolmeh, M.; Jafari, S.M. Applications of Response Surface Methodology in the Food Industry Processes. Food Bioprocess Technol. 2017, 10, 413–433. [Google Scholar] [CrossRef]
- Kumari, M.; Gupta, S.K. Response surface methodological (RSM) approach for optimizing the removal of trihalomethanes (THMs) and its precursor’s by surfactant modified magnetic nanoadsorbents (sMNP)—An endeavor to diminish probable cancer risk. Sci. Rep. 2019, 9, 18339. [Google Scholar] [CrossRef]
- Bajwa, K.; Bishnoi, N.R.; Kirrolia, A.; Gupta, S.; Selvan, S.T. Response surface methodology as a statistical tool for optimization of physio-biochemical cellular components of microalgae Chlorella pyrenoidosa for biodiesel production. Appl. Water Sci. 2019, 9, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Prisada, R.M. Perspectives to Describe Surface Properties of Raw Pharmaceutical Materials. A Fractal Approach on the Wetting of Powders. Farmacia 2020, 68, 354–361. [Google Scholar] [CrossRef]
- Kim, J.; Kumar, Y.S.; Yoo, J.; Kwon, S. Change of Blink Rate in Viewing Virtual Reality with HMD. Symmetry 2018, 10, 400. [Google Scholar] [CrossRef] [Green Version]
- Abusharha, A.A. Changes in blink rate and ocular symptoms during different reading tasks. Clin. Optom. 2017, 9, 133–138. [Google Scholar] [CrossRef] [Green Version]
- Saini, S.; Chawla, J.; Kumar, R.; Kaur, I. Response surface methodology (RSM) for optimization of cadmium ions adsorption using C16-6-16 incorporated mesoporous MCM-41. SN Appl. Sci. 2019, 1, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Dasankoppa, F.; Kujur, S.; Sholapur, H.N.A.; Jamakandi, V. Design, formulation and evaluation of carboxy methyl tamarind based in situ gelling ophthalmic drug delivery system of dorzolamide hydrochloride. Indian J. Health Sci. 2016, 9, 56. [Google Scholar] [CrossRef]
- Ciolacu, D.E.; Nicu, R.; Ciolacu, F. Cellulose-Based Hydrogels as Sustained Drug-Delivery Systems. Materials 2020, 13, 5270. [Google Scholar] [CrossRef] [PubMed]
- Kurniawansyah, I.S.; Rusdiana, T.; Sopyan, I.; Ramoko, H.; Wahab, H.A.; Subarnas, A. In situ ophthalmic gel forming systems of poloxamer 407 and hydroxypropyl methyl cellulose mixtures for sustained ocular delivery of chloramphenicole: Optimization study by factorial design. Heliyon 2020, 6, e05365. [Google Scholar] [CrossRef]
- Bajwa, G.S.; Sammon, C.; Timmins, P.; Melia, C.D. Molecular and mechanical properties of hydroxypropyl methylcellulose solutions during the sol:gel transition. Polymer 2009, 50, 4571–4576. [Google Scholar] [CrossRef]
- Almeida, N.; Rakesh, L.; Zhao, J. Monovalent and divalent salt effects on thermogelation of aqueous hypromellose solutions. Food Hydrocoll. 2014, 36, 323–331. [Google Scholar] [CrossRef]
- Joshi, S.C. Sol-Gel Behavior of Hydroxypropyl Methylcellulose (HPMC) in Ionic Media Including Drug Release. Materials 2011, 4, 1861–1905. [Google Scholar] [CrossRef] [Green Version]
- Dubashynskaya, N.; Poshina, D.; Raik, S.; Urtti, A.; Skorik, Y.A. Polysaccharides in Ocular Drug Delivery. Pharmaceutics 2019, 12, 22. [Google Scholar] [CrossRef] [Green Version]
- Hotujac Grgurević, M.; Juretić, M.; Hafner, A.; Lovrić, J.; Pepić, I. Tear fluid-eye drops compatibility assessment using surface tension. Drug Dev. Ind. Pharm. 2017, 43, 275–282. [Google Scholar] [CrossRef]
- Han, K.; Woghiren, O.E.; Priefer, R. Surface tension examination of various liquid oral, nasal, and ophthalmic dosage forms. Chem. Cent. J. 2016, 10, 31. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Karki, R.; Meena, M.; Prakash, T.; Rajeswari, T.; Goli, D. Reduction in drop size of ophthalmic topical drop preparations and the impact of treatment. J. Adv. Pharm. Technol. Res. 2011, 2, 192–194. [Google Scholar] [PubMed]
- Ghica, M.V.; Hirjau, M.; Lupuleasa, D.; Dinu-Pirvu, C.E. Flow and Thixotropic Parameters for Rheological Characterization of Hydrogels. Molecules 2016, 21, 786. [Google Scholar] [CrossRef]
- Akinosho, H.; Hawkins, S.; Wicker, L. Hydroxypropyl methylcellulose substituent analysis and rheological properties. Carbohydr. Polym. 2013, 98, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Sandri, G.; Bonferoni, M.C.; Rossi, S.; Ferrari, F.; Mori, M.; del Fante, C.; Perotti, C.; Caramella, C. Thermosensitive eyedrops containing platelet lysate for the treatment of corneal ulcers. Int. J. Pharm. 2012, 426, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ghica, M.V.; Kaya, M.G.A.; Dinu-Pirvu, C.E.; Lupuleasa, D.; Udeanu, D.I. Development, Optimization and In Vitro/In Vivo Characterization of Collagen-Dextran Spongious Wound Dressings Loaded with Flufenamic Acid. Molecules 2017, 22, 1552. [Google Scholar] [CrossRef]
- Thitiwichienlert, S.; Saksritawee, K. Postoperative pain control of subtenon bupivacaine injection in adult strabismus surgery: A double-masked randomized trial. Eye South East Asia 2020, 15, 64–67. [Google Scholar] [CrossRef]
- Ghosal, K.; Chandra, A.; Rajabalaya, R.; Chakraborty, S.; Nanda, A. Mathematical modeling of drug release profiles for modified hydrophobic HPMC based gels. Pharmazie 2012, 67, 147–155. [Google Scholar] [PubMed]
- Chanaj-Kaczmarek, J.; Paczkowska, M.; Osmalek, T.; Kapron, B.; Plech, T.; Szymanowska, D.; Karazniewicz-Lada, M.; Kobus-Cisowska, J.; Cielecka-Piontek, J. Hydrogel Delivery System Containing Calendulae flos Lyophilized Extract with Chitosan as a Supporting Strategy for Wound Healing Applications. Pharmaceutics 2020, 12, 634. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Bi, H.; Xie, X.; Guo, J.; Wang, X.; Liu, D. Preparation and evaluation of sinomenine hydrochloride in situ gel for uveitis treatment. Int. Immunopharmacol. 2013, 17, 99–107. [Google Scholar] [CrossRef] [Green Version]
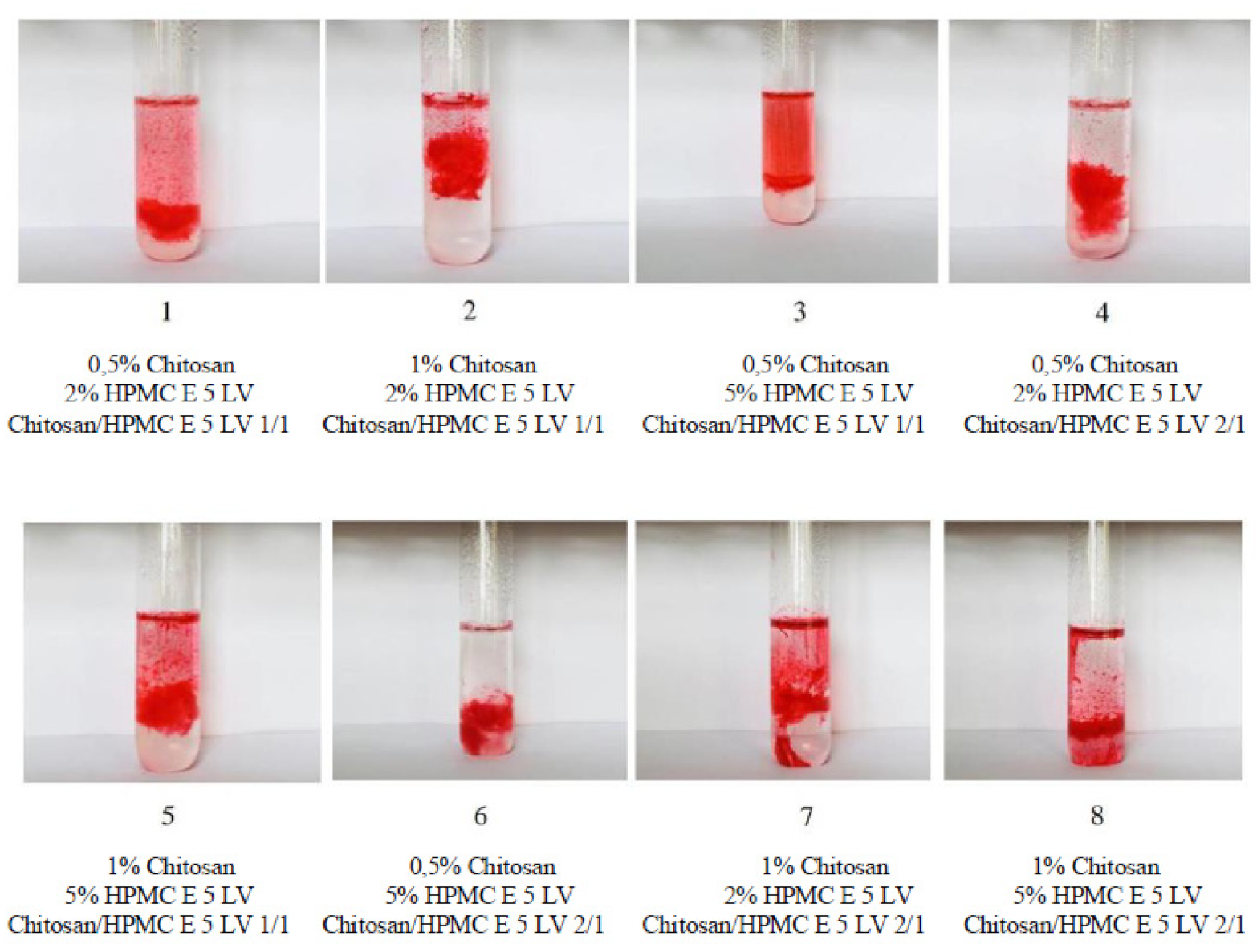
| System | Chitosan X1 % | HPMC X2 % | Chitosan/ HPMC X3 (p:p) | System Volume V (mL) | NaCl w (g) | Bupivacaine Hydrochloride w (g) | NaOH 10% V (mL) | pH |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.50 | 2 | 1:1 | 30 | 0.27 | 0.0757 | 0.49 | 5.35 |
| 2 | 1.00 | 2 | 1:1 | 30 | 0.27 | 0.0760 | 0.50 | 5.42 |
| 3 | 0.50 | 5 | 1:1 | 30 | 0.27 | 0.0757 | 0.53 | 5.38 |
| 4 | 0.50 | 2 | 2:1 | 45 | 0.40 | 0.1143 | 1.05 | 5.48 |
| 5 | 1.00 | 5 | 1:1 | 30 | 0.27 | 0.0764 | 0.34 | 5.42 |
| 6 | 0.50 | 5 | 2:1 | 45 | 0.40 | 0.1153 | 1.07 | 5.47 |
| 7 | 1.00 | 2 | 2:1 | 45 | 0.40 | 0.1146 | 0.87 | 5.37 |
| 8 | 1.00 | 5 | 2:1 | 45 | 0.40 | 0.1168 | 0.89 | 5.47 |
| Factors | Parameters | Levels of Variation | |
|---|---|---|---|
| Lower (−) | Upper (+) | ||
| X1 | Chitosan (%) | 0.5% | 1% |
| X2 | HPMC E 5 LV (%) | 2% | 5% |
| X3 | Chitosan/HPMC E 5 LV ratio | 1/1 | 2/1 |
| System Code | Gelling Capacity |
|---|---|
| 1 | ++ |
| 2 | +++ |
| 3 | ++ |
| 4 | ++ |
| 5 | +++ |
| 6 | ++ |
| 7 | +++ |
| 8 | +++ |
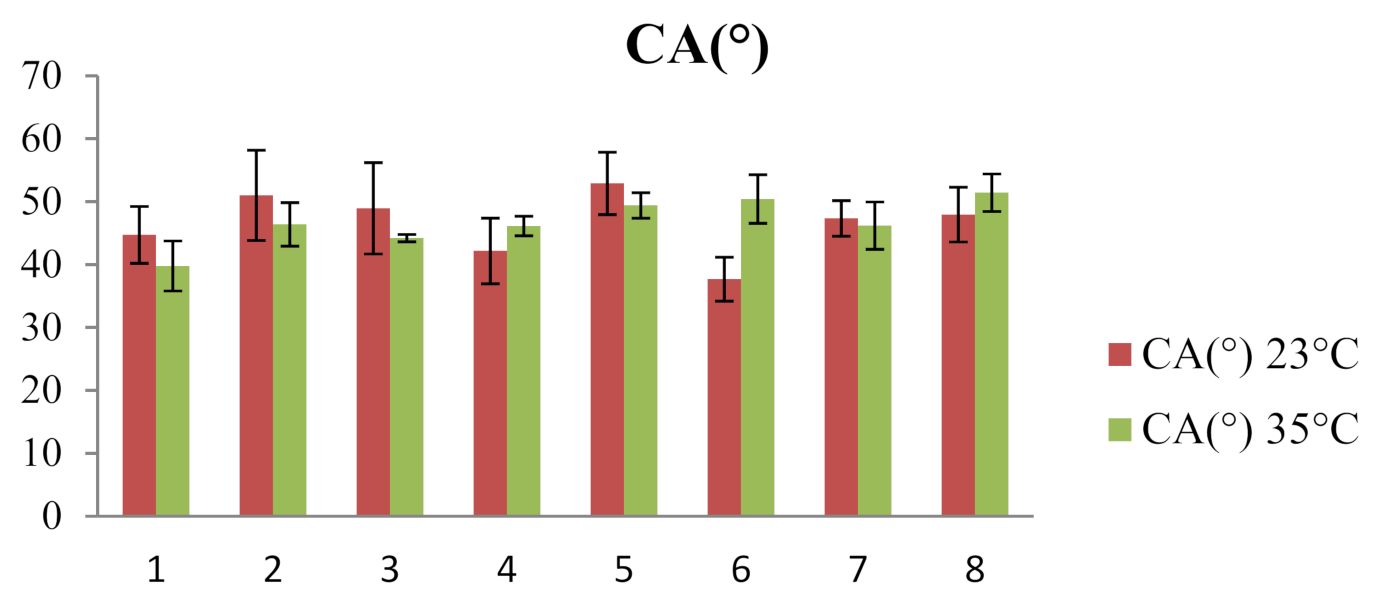
| 23 °C | 35 °C | |||||
|---|---|---|---|---|---|---|
| System | CA(M) (°) | V (µL) | γSL (mN/m) | CA(M) (°) | V (µL) | γSL (mN/m) |
| 1 | 44.70 ± 4.52 | 11.55 ± 2.44 | 30.65 ± 9.81 | 39.77 ± 3.97 | 8.74 ± 0.76 | 28.67 ± 2.71 |
| 2 | 51.00 ± 7.18 | 10.27 ± 1.18 | 35.14 ± 5.86 | 46.39 ± 3.46 | 8.39 ± 0.47 | 31.59 ± 7.34 |
| 3 | 48.94 ± 7.27 | 9.46 ± 1.07 | 33.17 ± 3.40 | 44.19 ± 0.58 | 8.39 ± 0.38 | 31.29 ± 4.26 |
| 4 | 42.16 ± 5.23 | 9.65 ± 0.39 | 39.55 ± 4.67 | 46.11 ± 1.56 | 9.50 ± 0.55 | 30.63 ± 4.23 |
| 5 | 52.89 ± 4.96 | 11.44 ± 1.20 | 31.43 ± 4.21 | 49.39 ± 2.02 | 9.96 ± 0.36 | 36.95 ± 12.87 |
| 6 | 37.67 ± 3.49 | 7.90 ± 1.15 | 28.84 ± 5.16 | 50.41 ± 3.87 | 9.46 ± 1.20 | 30.09 ± 3.37 |
| 7 | 47.33 ± 2.84 | 10.94 ± 1.63 | 31.78 ± 2.93 | 46.18 ± 3.77 | 12.73 ± 1.44 | 29.33 ± 3.30 |
| 8 | 47.94 ± 4.35 | 10.65 ± 0.70 | 43.49 ± 13.81 | 51.42 ± 2.98 | 9.54 ± 0.90 | 29.46 ± 5.96 |
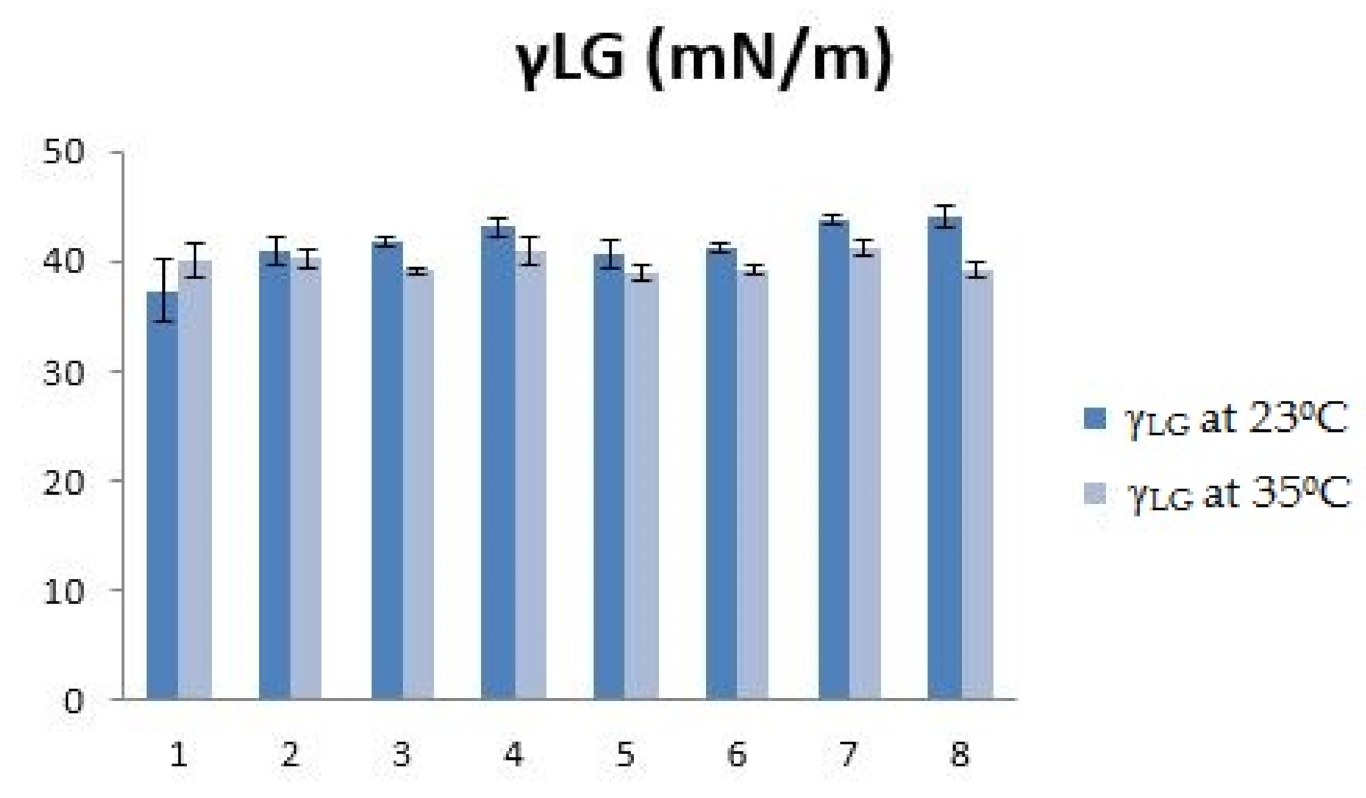
| 23 °C | 35 °C | |||
|---|---|---|---|---|
| System | V (μL) | V (μL) | ||
| 1 | 9.23 ± 0.34 | 37.32 ± 2.79 | 8.97 ± 0.35 | 40.15 ± 1.53 |
| 2 | 10.54 ± 0.54 | 40.83 ± 1.27 | 9.42 ± 0.43 | 40.33 ± 0.82 |
| 3 | 10.32 ± 0.32 | 41.79 ± 0.41 | 9.20 ± 0.19 | 39.10 ± 0.26 |
| 4 | 10.63 ± 0.40 | 43.10 ± 0.90 | 9.06 ± 0.30 | 41.01 ± 1.27 |
| 5 | 10.56 ± 0.22 | 40.68 ± 1.20 | 8.86 ± 0.46 | 38.93 ± 0.64 |
| 6 | 10.21 ± 0.22 | 41.11 ± 0.45 | 9.16 ± 0.16 | 39.34 ± 0.43 |
| 7 | 10.92 ± 0.48 | 43.81 ± 0.50 | 9.52 ± 0.16 | 41.35 ± 0.71 |
| 8 | 10.82 ± 0.43 | 43.99 ± 1.05 | 8.86 ± 0.39 | 39.16 ± 0.64 |
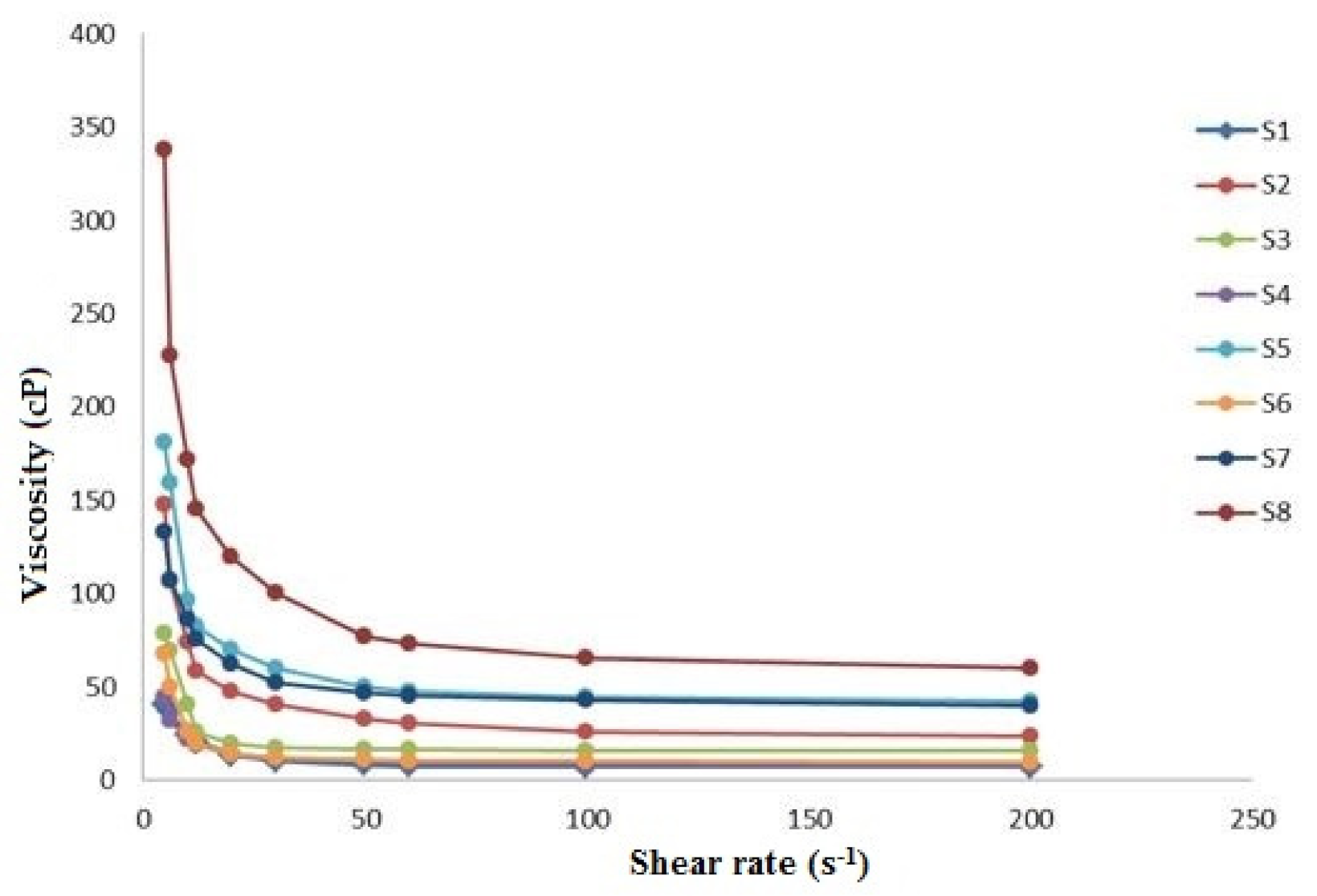
| System | X1 Chitosan (%) | X2 HPMC (%) | X3 Chitosan/HPMC Ratio | Viscosity (cP) |
|---|---|---|---|---|
| 1 | 0.5 | 2 | 1 | 13.72 ± 0.83 |
| 2 | 1.0 | 2 | 1 | 47.60 ± 2.74 |
| 3 | 0.5 | 5 | 1 | 19.52 ± 1.15 |
| 4 | 0.5 | 2 | 2 | 14.28 ± 0.48 |
| 5 | 1.0 | 5 | 1 | 70.36 ± 2.99 |
| 6 | 0.5 | 5 | 2 | 14.44 ± 0.14 |
| 7 | 1.0 | 2 | 2 | 62.32 ± 3.85 |
| 8 | 1.0 | 5 | 2 | 119.87 ± 4.02 |
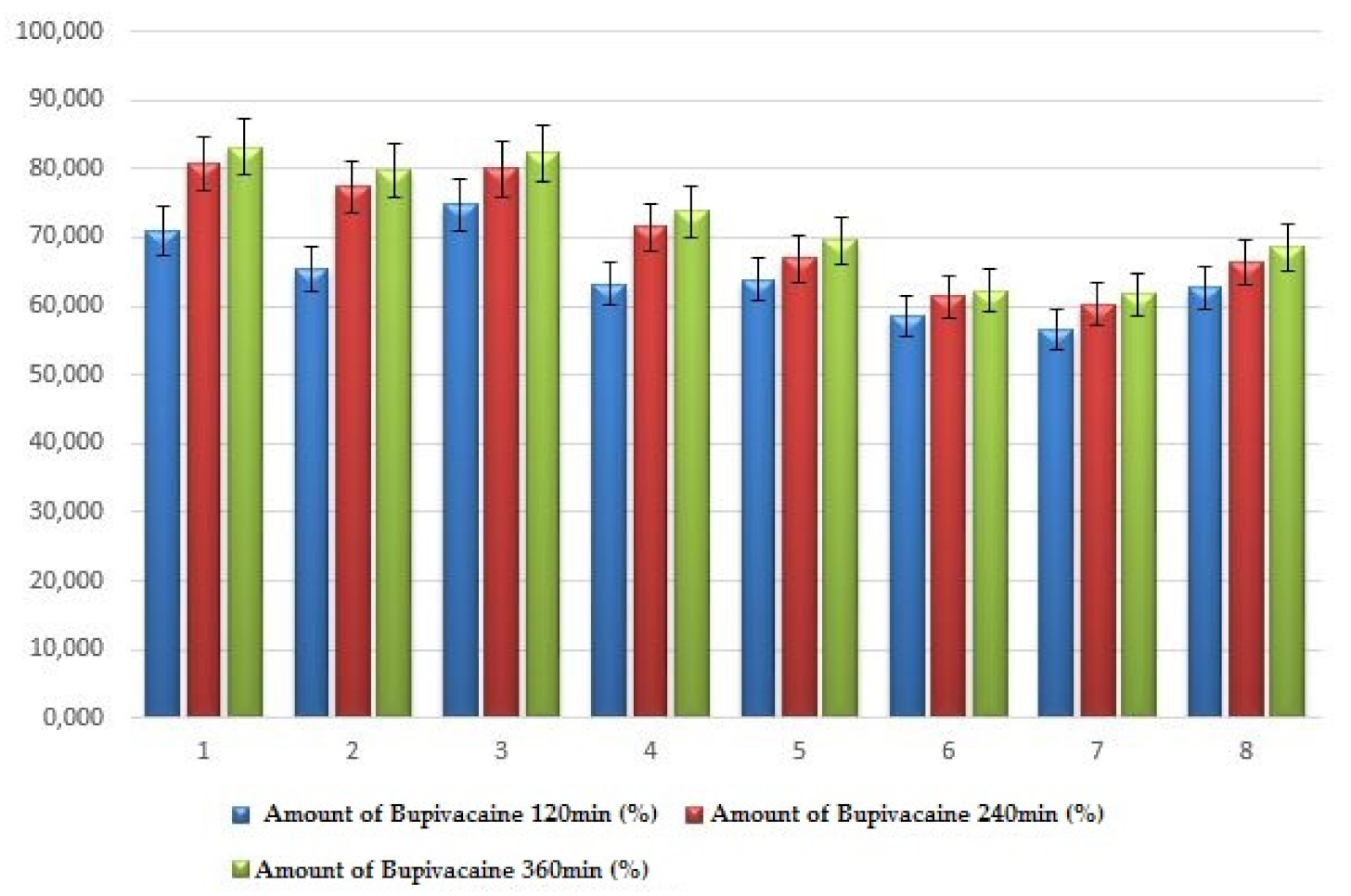
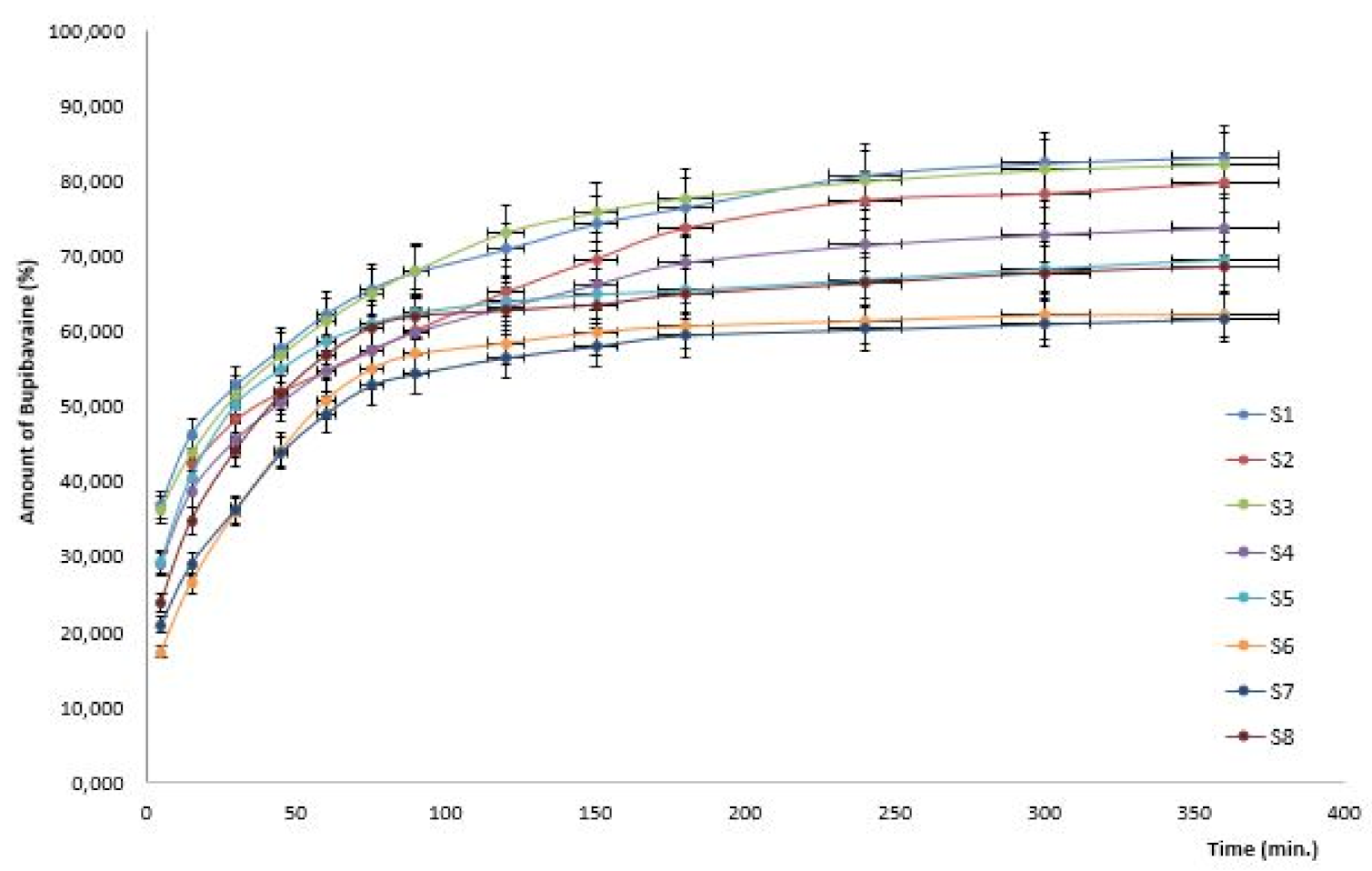
| System | X1 Chitosan (%) | X2 HPMC (%) | X3 Chitosan/ HPMC Ratio | Amount of Bupivacaine 120 min (%) | Amount of Bupivacaine 240 min (%) | Amount of Bupivacaine 360 min (%) |
|---|---|---|---|---|---|---|
| 1 | 0.5 | 2 | 1 | 70.89 ± 4.68 | 80.78 ± 4.97 | 83.19 ± 5.62 |
| 2 | 1.0 | 2 | 1 | 65.26 ± 3.93 | 77.38 ± 3.71 | 79.71 ± 4.56 |
| 3 | 0.5 | 5 | 1s | 74.78 ± 4.86 | 79.99 ± 4.59 | 82.29 ± 5.49 |
| 4 | 0.5 | 2 | 2 | 63.23 ± 3.12 | 71.47 ± 4.39 | 73.83 ± 4.35 |
| 5 | 1.0 | 5 | 1 | 63.94 ± 2.75 | 66.89 ± 3.64 | 69.55 ± 3.77 |
| 6 | 0.5 | 5 | 2 | 58.45 ± 1.87 | 61.41 ± 2.79 | 62.18 ± 4.56 |
| 7 | 1.0 | 2 | 2 | 56.55 ± 1.66 | 60.30 ± 2.58 | 61.77 ± 2.94 |
| 8 | 1.0 | 5 | 2 | 62.75 ± 3.32 | 66.32 ± 3.73 | 68.52 ± 3.23 |
| System | Korsmeyer-Peppas Model | Model Higuchi | ||
|---|---|---|---|---|
| K | n | R2 | R2 | |
| 1 | 0.265 | 0.205 | 0.998 | 0.129 |
| 2 | 0.239 | 0.210 | 0.990 | 0.473 |
| 3 | 0.249 | 0.219 | 0.992 | 0.264 |
| 4 | 0.202 | 0.237 | 0.995 | 0.340 |
| 5 | 0.196 | 0.265 | 0.991 | 0.574 |
| 6 | 0.086 | 0.426 | 0.997 | 0.972 |
| 7 | 0.118 | 0.342 | 0.994 | 0.924 |
| 8 | 0.140 | 0.338 | 0.997 | 0.873 |
| Formulation Variables | Response Parameters (Optimized) | ||||||
|---|---|---|---|---|---|---|---|
| System | X1 Chitosan (%) | X2 HPMC (%) | X3 Chitosan/ HPMC (p/p) | Gelling Capacity | Y1 Contact Angle CA (35 °C) (°) | Y2 Viscosity (35 °C) (cP) | Y3 Hydrochloride Bupivacaine (%) |
| 1 | 0.50 | 2 | 1 | ++ | 39.77 ± 3.97 | 13.72 ± 0.83 | 83.19 ± 5.62 |
| 2 | 1.00 | 2 | 1 | +++ | 46.39 ± 3.46 | 47.60 ± 2.74 | 79.71 ± 4.56 |
| 3 | 0.50 | 5 | 1 | ++ | 44.19 ± 0.58 | 19.52 ± 1.15 | 82.29 ± 5.49 |
| 4 | 0.50 | 2 | 2 | ++ | 46.11 ± 1.56 | 14.28 ± 0.48 | 73.83 ± 4.35 |
| 5 | 1.00 | 5 | 1 | +++ | 49.39 ± 2.02 | 70.36 ± 2.99 | 69.55 ± 3.77 |
| 6 | 0.50 | 5 | 2 | ++ | 50.41 ± 3.87 | 14.44 ± 0.14 | 62.18 ± 4.56 |
| 7 | 1.00 | 2 | 2 | +++ | 46.18 ± 3.77 | 62.32 ± 3.85 | 61.77 ± 2.94 |
| 8 | 1.00 | 5 | 2 | +++ | 51.42 ± 2.98 | 119.87 ± 4.02 | 68.52 ± 3.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popa, L.; Ghica, M.V.; Popescu, R.; Irimia, T.; Dinu-Pîrvu, C.-E. Development and Optimization of Chitosan-Hydroxypropyl Methylcellulose In Situ Gelling Systems for Ophthalmic Delivery of Bupivacaine Hydrochloride. Processes 2021, 9, 1694. https://doi.org/10.3390/pr9101694
Popa L, Ghica MV, Popescu R, Irimia T, Dinu-Pîrvu C-E. Development and Optimization of Chitosan-Hydroxypropyl Methylcellulose In Situ Gelling Systems for Ophthalmic Delivery of Bupivacaine Hydrochloride. Processes. 2021; 9(10):1694. https://doi.org/10.3390/pr9101694
Chicago/Turabian StylePopa, Lăcrămioara, Mihaela Violeta Ghica, Roxana Popescu, Teodora Irimia, and Cristina-Elena Dinu-Pîrvu. 2021. "Development and Optimization of Chitosan-Hydroxypropyl Methylcellulose In Situ Gelling Systems for Ophthalmic Delivery of Bupivacaine Hydrochloride" Processes 9, no. 10: 1694. https://doi.org/10.3390/pr9101694
APA StylePopa, L., Ghica, M. V., Popescu, R., Irimia, T., & Dinu-Pîrvu, C.-E. (2021). Development and Optimization of Chitosan-Hydroxypropyl Methylcellulose In Situ Gelling Systems for Ophthalmic Delivery of Bupivacaine Hydrochloride. Processes, 9(10), 1694. https://doi.org/10.3390/pr9101694









