Combining Kinetic and Constraint-Based Modelling to Better Understand Metabolism Dynamics
Abstract
1. Introduction
2. Kinetic Models and Constraint-Based Approaches: Two Complementary Views of Metabolic Dynamics
2.1. Using Kinetic Models to Analyse the Dynamics of Metabolism
- () is the vector of the concentration of extracellular metabolites i.e. metabolites being outside the cells, directly in the reactor,
- () is the vector of the concentration of intracellular metabolites i.e. metabolites being inside the cells, expressed as a quantity of matter per cells (per for example),
- () represents the cell population,
- () is the volume of the reactor.
- batch reactor with
- fedbatch reactor (continuously fed) with and
- chemostat with .
2.2. Using Constraint-Based Approaches to Describe Metabolic Configurations
- ,
- e is nondecomposable, i.e. there is no nonzero vector such that where .
- Minimal Constraint Flux Modes (MCFMs) by Morterol et al. [25] to take into account Boolean constraints on the reactions,
- Elementary Flux Vectors (EFV) by Klamt et al. [26] to take into account linear constraint on the reaction rates’ values,
- Elementary Growth Modes (EGM) by Müller [27] to bring ODEs and CBM closer by taking into account the dilution term in the definition of CBM.
2.3. Comparison of the Two Approaches
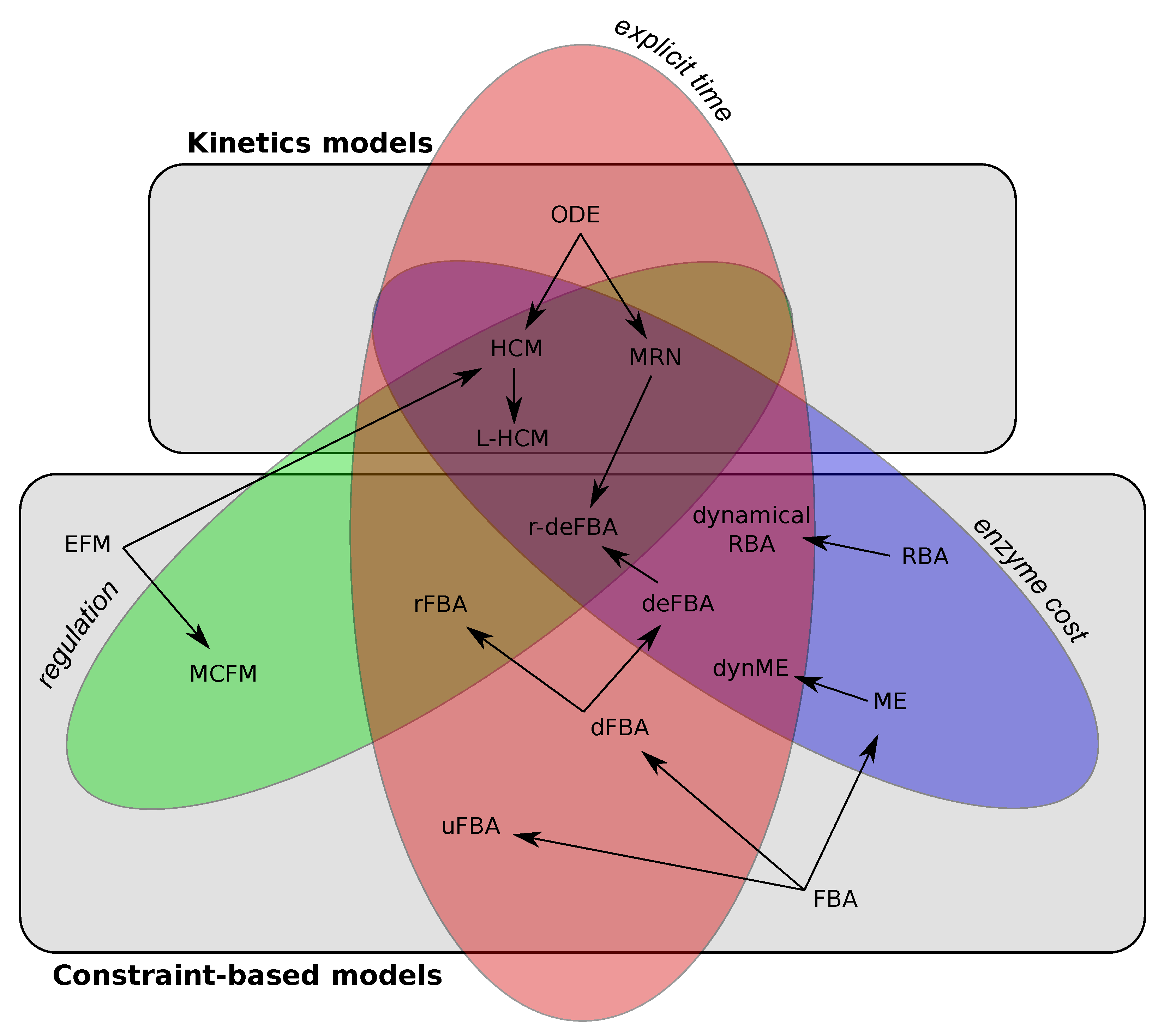
3. Review of Different Frameworks to Study the Dynamics of Metabolism
3.1. Incorporation of Time into FBA
3.1.1. Dynamic Flux Balance Analysis (dFBA)
Static Optimisation Approach (SOA)
Dynamic Optimisation Approach (DOA)
Direct Approach (DA)
3.1.2. Unsteady-State FBA (uFBA)
3.2. Integration of Gene Regulations into CBM
3.2.1. Regulatory Flux Balance Analysis (rFBA)
3.2.2. Genetic Regulation and EFMs: SMT/ASP
3.3. Integration of Enzyme Cost Production Recovers Complex Dynamical Behaviours
3.3.1. Resource Balance Analysis (RBA)
3.3.2. Dynamic Enzyme-Cost FBA (deFBA)
3.4. Hybrid Models Extending Dynamical Systems by Incorporating Regulations and Enzyme Cost Production
3.4.1. Hybrid Cybernetic Models
3.4.2. Hybrid Automata
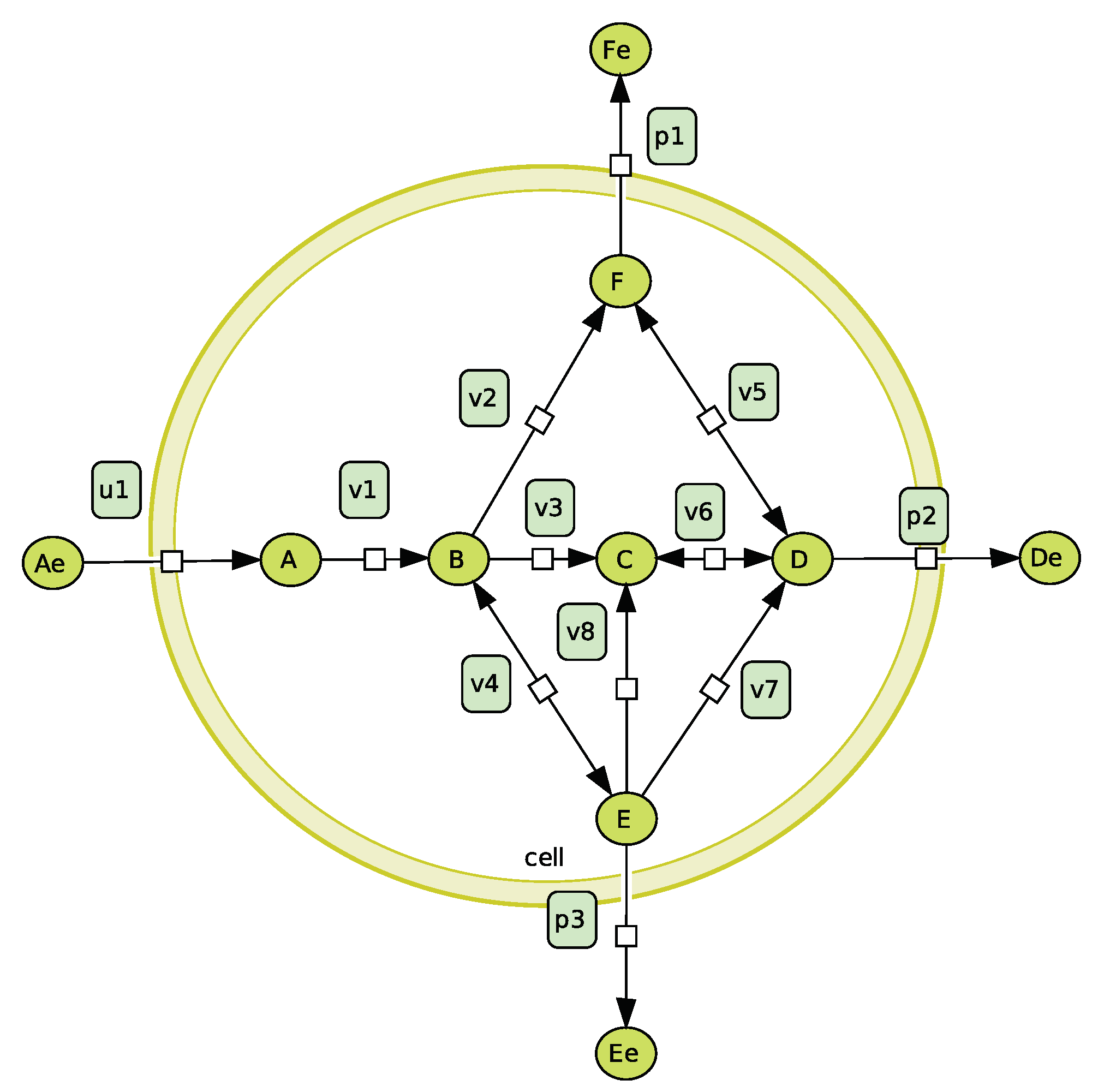
4. Combination of a Kinetic Model and Constraint-Based Approaches
| Name | |||||||||
| Value | 0.5 | 10 | 1 | 5 | 100 | 3.4 | 0.1 | 0.1 | 0.5 |
| Name | |||||||||
| Value | 7 | 0.1 | 4 | 10 | 1 | 10 | 0.1 |
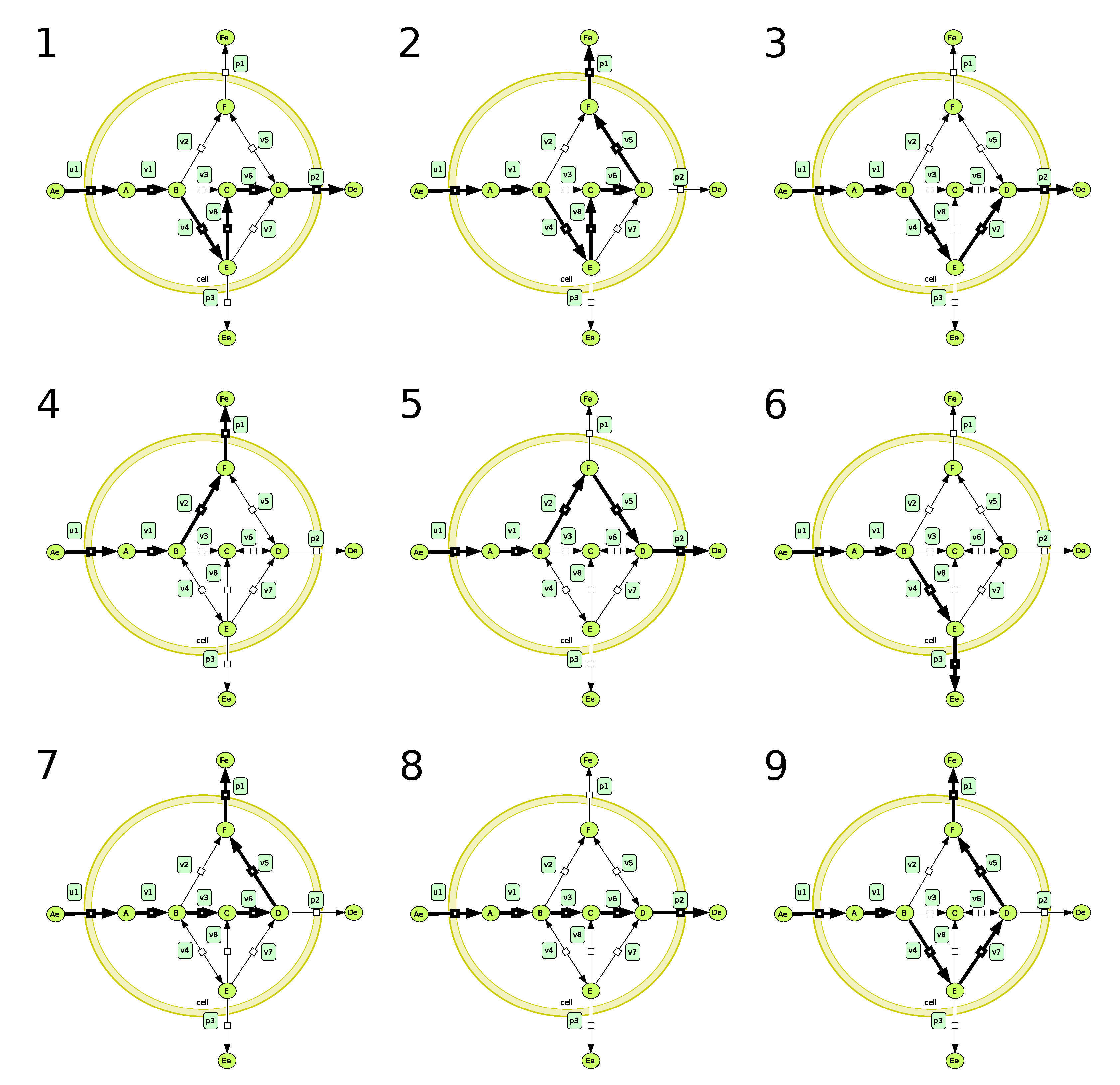
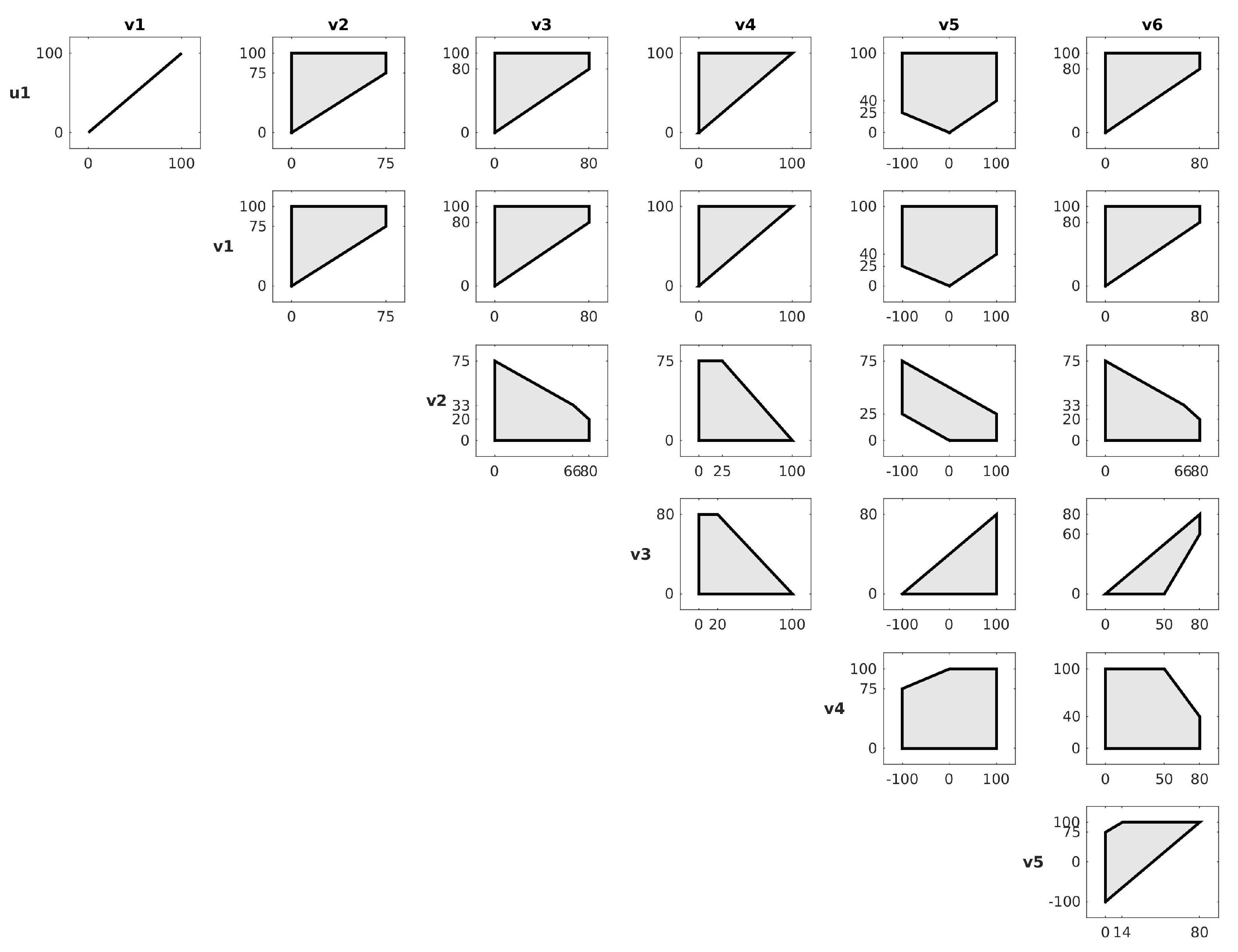
4.1. Using the Differential System to Reduce the Solution Space
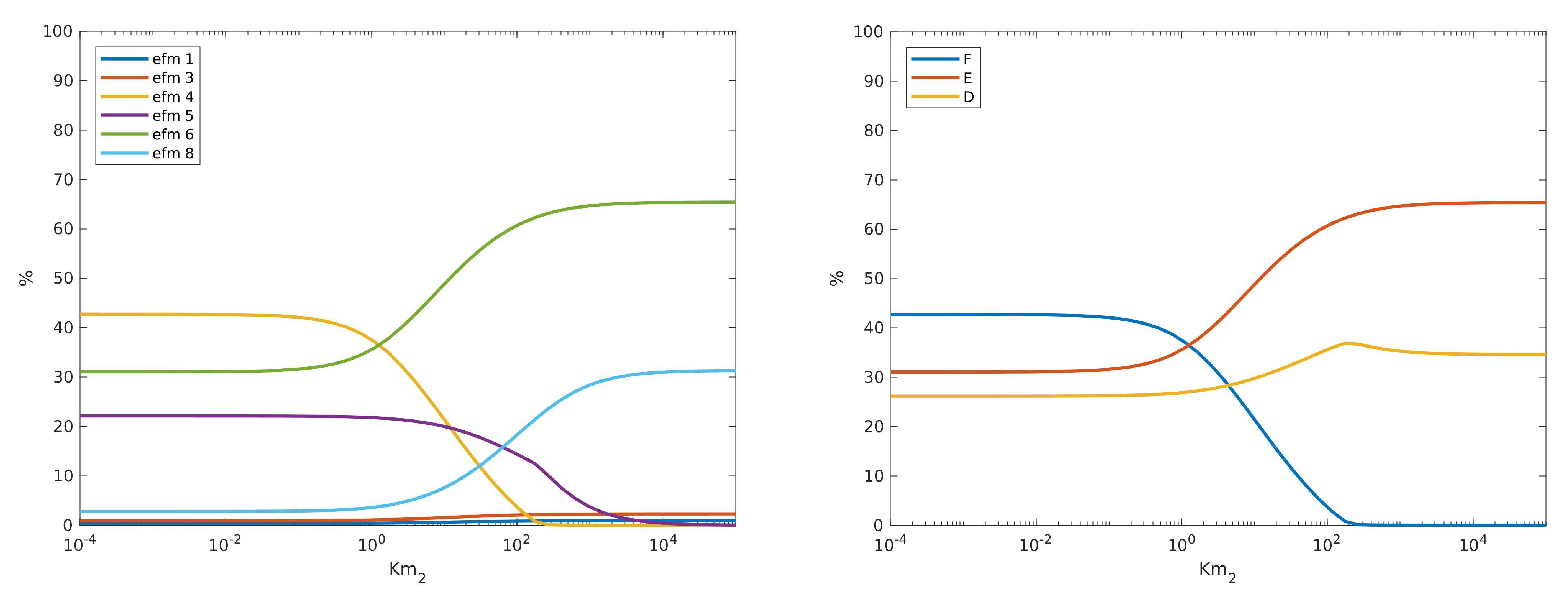
4.2. Using EFMs to Help Analyse the Differential System
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CBM | Constraint-Based Modelling |
| EFM | Elementary Flux Modes |
| FBA | Flux Balance Analysis |
| ODE | Ordinary Differential Equations |
| LP | Linear Programming |
References
- da Veiga Moreira, J.; Hamraz, M.; Abolhassani, M.; Schwartz, L.; Jolicoeur, M.; Peres, S. Metabolic Therapies Inhibit Tumor Growth in Vivo and in Silico. Sci. Rep. 2019, 9, 3153. [Google Scholar] [CrossRef] [PubMed]
- Robitaille, J.; Chen, J.; Jolicoeur, M. A Single Dynamic Metabolic Model Can Describe mAb Producing CHO Cell Batch and Fed-Batch Cultures on Different Culture Media. PLoS ONE 2015, 10, e0136815. [Google Scholar] [CrossRef] [PubMed]
- Ciesielski, A.; Grzywacz, R. Process Maps with Metabolic Constraints for Bioethanol Production by Continuous Fermentation. Chem. Eng. Sci. 2021, 229, 116134. [Google Scholar] [CrossRef]
- Park, H.; Patel, A.; Hunt, K.A.; Henson, M.A.; Carlson, R.P. Artificial Consortium Demonstrates Emergent Properties of Enhanced Cellulosic-Sugar Degradation and Biofuel Synthesis. NPJ Biofilms Microbiomes 2020, 6, 59. [Google Scholar] [CrossRef]
- Orth, J.D.; Conrad, T.M.; Na, J.; Lerman, J.A.; Nam, H.; Feist, A.M.; Palsson, B.O. A Comprehensive Genome-Scale Reconstruction of Escherichia Coli Metabolism–2011. Mol. Syst. Biol. 2014, 7, 535. [Google Scholar] [CrossRef]
- Brunk, E.; Sahoo, S.; Zielinski, D.C.; Altunkaya, A.; Dräger, A.; Mih, N.; Gatto, F.; Nilsson, A.; Preciat Gonzalez, G.A.; Aurich, M.K.; et al. Recon3D Enables a Three-Dimensional View of Gene Variation in Human Metabolism. Nat. Biotechnol. 2018, 36, 272–281. [Google Scholar] [CrossRef]
- da Veiga Moreira, J.; Peres, S.; Steyaert, J.M.; Bigan, E.; Paulevé, L.; Nogueira, M.L.; Schwartz, L. Cell Cycle Progression Is Regulated by Intertwined Redox Oscillators. Theor. Biol. Med. Model. 2015, 12, 10. [Google Scholar] [CrossRef]
- Moulin, C.; Tournier, L.; Peres, S. Using a Hybrid Approach to Model Central Carbon Metabolism Across the Cell Cycle. In Hybrid Systems Biology; Češka, M., Paoletti, N., Eds.; Springer International Publishing: Cham, Switzerland, 2019; Volume 11705, pp. 132–146. [Google Scholar] [CrossRef]
- Monod, J.; Wyman, J.; Changeux, J.P. On the Nature of Allosteric Transitions: A Plausible Model. J. Mol. Biol. 1965, 12, 88–118. [Google Scholar] [CrossRef]
- Gillespie, D.T. Exact Stochastic Simulation of Coupled Chemical Reactions. J. Phys. Chem. 1977, 81, 2340–2361. [Google Scholar] [CrossRef]
- Amar, P.; Bernot, G.; Norris, V. HSIM: A Simulation Programme to Study Large Assemblies of Proteins. J. Biol. Phys. Chem. 2004, 4, 79–84. [Google Scholar] [CrossRef][Green Version]
- Klann, M.; Ganguly, A.; Koeppl, H. Hybrid Spatial Gillespie and Particle Tracking Simulation. Bioinformatics 2012, 28, i549–i555. [Google Scholar] [CrossRef]
- Baldan, P.; Cocco, N.; Marin, A.; Simeoni, M. Petri Nets for Modelling Metabolic Pathways: A Survey. Nat. Comput. 2010, 9, 955–989. [Google Scholar] [CrossRef]
- Ao, P. Metabolic Network Modelling: Including Stochastic Effects. Comput. Chem. Eng. 2005, 29, 2297–2303. [Google Scholar] [CrossRef][Green Version]
- Bettenbrock, K.; Fischer, S.; Kremling, A.; Jahreis, K.; Sauter, T.; Gilles, E.D. A Quantitative Approach to Catabolite Repression in Escherichia coli. J. Biol. Chem. 2006, 281, 2578–2584. [Google Scholar] [CrossRef] [PubMed]
- Chassagnole, C.; Noisommit-Rizzi, N.; Schmid, J.W.; Mauch, K.; Reuss, M. Dynamic modeling of the central carbon metabolism ofEscherichia coli. Biotechnol. Bioeng. 2002, 79, 53–73. [Google Scholar] [CrossRef]
- Kim, O.D.; Rocha, M.; Maia, P. A Review of Dynamic Modeling Approaches and Their Application in Computational Strain Optimization for Metabolic Engineering. Front. Microbiol. 2018, 9, 1690. [Google Scholar] [CrossRef]
- Steuer, R.; Gross, T.; Selbig, J.; Blasius, B. Structural Kinetic Modeling of Metabolic Networks. Proc. Natl. Acad. Sci. USA 2006, 103, 11868–11873. [Google Scholar] [CrossRef]
- Grimbs, S.; Selbig, J.; Bulik, S.; Holzhütter, H.G.; Steuer, R. The Stability and Robustness of Metabolic States: Identifying Stabilizing Sites in Metabolic Networks. Mol. Syst. Biol. 2007, 3, 146. [Google Scholar] [CrossRef] [PubMed]
- Carbonaro, N.J.; Thorpe, I.F. Using Structural Kinetic Modeling To Identify Key Determinants of Stability in Reaction Networks. J. Phys. Chem. A 2017, 121, 4982–4992. [Google Scholar] [CrossRef] [PubMed]
- Girbig, D.; Grimbs, S.; Selbig, J. Systematic Analysis of Stability Patterns in Plant Primary Metabolism. PLoS ONE 2012, 7, e34686. [Google Scholar]
- Schuster, S.; Hilgetag, C. On Elementary Flux Modes in Biochemical Reaction Systems at Steady State. J. Biol. Syst. 1994, 2, 165–182. [Google Scholar] [CrossRef]
- Hunt, K.A.; Folsom, J.P.; Taffs, R.L.; Carlson, R.P. Complete Enumeration of Elementary Flux Modes through Scalable Demand-Based Subnetwork Definition. Bioinformatics 2014, 30, 1569–1578. [Google Scholar] [CrossRef]
- Acuña, V.; Chierichetti, F.; Lacroix, V.; Marchetti-Spaccamela, A.; Sagot, M.F.; Stougie, L. Modes and Cuts in Metabolic Networks: Complexity and Algorithms. Biosystems 2009, 95, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Morterol, M.; Dague, P.; Peres, S.; Simon, L. Minimality of Metabolic Flux Modes under Boolean Regulation Constraints. In Proceedings of the 12th International Workshop on Constraint-Based Methods for Bioinformatics (WCB’16), Toulouse, France, 5 September 2016; pp. 65–81. [Google Scholar]
- Klamt, S.; Regensburger, G.; Gerstl, M.P.; Jungreuthmayer, C.; Schuster, S.; Mahadevan, R.; Zanghellini, J.; Müller, S. From Elementary Flux Modes to Elementary Flux Vectors: Metabolic Pathway Analysis with Arbitrary Linear Flux Constraints. PLoS Comput. Biol. 2017, 13, e1005409. [Google Scholar] [CrossRef] [PubMed]
- Müller, S. Elementary Growth Modes/Vectors and Minimal Autocatalytic Sets for Kinetic/Constraint-Based Models of Cellular Growth. bioRxiv 2021. [Google Scholar] [CrossRef]
- Varma, A.; Palsson, B. Metabolic Flux Balancing: Basic concepts, scientific and practical use. Nat. Biotechnol. 1994, 12, 994–998. [Google Scholar] [CrossRef]
- Mahadevan, R.; Schilling, C. The effects of alternate optimal solutions in constraint-based genome-scale metabolic models. Metab. Eng. 2003, 5, 264–276. [Google Scholar] [CrossRef]
- Feist, A.M.; Palsson, B.O. The Biomass Objective Function. Curr. Opin. Microbiol. 2010, 13, 344–349. [Google Scholar] [CrossRef]
- García Sánchez, C.E.; Torres Sáez, R.G. Comparison and Analysis of Objective Functions in Flux Balance Analysis. Biotechnol. Prog. 2014, 30, 985–991. [Google Scholar] [CrossRef]
- Montezano, D.; Meek, L.; Gupta, R.; Bermudez, L.E.; Bermudez, J.C.M. Flux Balance Analysis with Objective Function Defined by Proteomics Data—Metabolism of Mycobacterium Tuberculosis Exposed to Mefloquine. PLoS ONE 2015, 10, e0134014. [Google Scholar] [CrossRef]
- Peres, S.; Jolicoeur, M.; Moulin, C.; Dague, P.; Schuster, S. How Important Is Thermodynamics for Identifying Elementary Flux Modes? PLoS ONE 2017, 12, e0171440. [Google Scholar] [CrossRef] [PubMed]
- Segel, I.H. Enzyme Kinetics: Behavior and Analysis of Rapid Equilibrium and Steady State Enzyme Systems; Wiley: New York, NY, USA, 1993. [Google Scholar]
- Smallbone, K.; Mendes, P. Large-Scale Metabolic Models: From Reconstruction to Differential Equations. Ind. Biotechnol. 2013, 9, 179–184. [Google Scholar] [CrossRef]
- Khodayari, A.; Zomorrodi, A.R.; Liao, J.C.; Maranas, C.D. A kinetic model of Escherichia coli core metabolism satisfying multiple sets of mutant flux data. Metab. Eng. 2014, 25, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Khodayari, A.; Maranas, C.D. A genome-scale Escherichia coli kinetic metabolic model k-ecoli457 satisfying flux data for multiple mutant strains. Nat. Commun. 2016, 7, 13806. [Google Scholar] [CrossRef] [PubMed]
- Varma, A.; Palsson, B.O. Stoichiometric Flux Balance Models Quantitatively Predict Growth and Metabolic By-Product Secretion in Wild-Type Escherichia Coli W3110. Appl. Environ. Microbiol. 1994, 60, 3724–3731. [Google Scholar] [CrossRef]
- Mahadevan, R.; Edwards, J.; Doyle, F. Dynamic Flux Balance Analysis of Diauxic Growth in Escherichia coli. Biophys. J. 2002, 83, 1331–1340. [Google Scholar] [CrossRef]
- Höffner, K.; Harwood, S.M.; Barton, P.I. A Reliable Simulator for Dynamic Flux Balance Analysis. Biotechnol. Bioeng. 2013, 110, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Hjersted, J.L.; Henson, M.A. Optimization of Fed-Batch Saccharomyces Cerevisiae Fermentation Using Dynamic Flux Balance Models. Biotechnol. Prog. 2008, 22, 1239–1248. [Google Scholar] [CrossRef]
- da Veiga Moreira, J.; Jolicoeur, M.; Schwartz, L.; Peres, S. Fine-tuning mitochondrial activity in Yarrowia lipolytica for citrate overproduction. Sci. Rep. 2021, 11, 878. [Google Scholar] [CrossRef]
- Kuriya, Y.; Araki, M. Dynamic Flux Balance Analysis to Evaluate the Strain Production Performance on Shikimic Acid Production in Escherichia coli. Metabolites 2020, 10, 198. [Google Scholar] [CrossRef]
- Hanly, T.J.; Henson, M.A. Dynamic flux balance modeling of microbial co-cultures for efficient batch fermentation of glucose and xylose mixtures. Biotechnol. Bioeng. 2011, 108, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Noack, S.; Wiechert, W.; von Lieres, E. Dynamic Flux Balance Analysis with Nonlinear Objective Function. J. Math. Biol. 2017, 75, 1487–1515. [Google Scholar] [CrossRef]
- Dromms, R.A.; Lee, J.Y.; Styczynski, M.P. LK-DFBA: A Linear Programming-Based Modeling Strategy for Capturing Dynamics and Metabolite-Dependent Regulation in Metabolism. BMC Bioinform. 2020, 21, 93. [Google Scholar] [CrossRef] [PubMed]
- Gomez, J.A.; Höffner, K.; Barton, P.I. DFBAlab: A fast and reliable MATLAB code for dynamic flux balance analysis. BMC Bioinform. 2014, 15, 409. [Google Scholar] [CrossRef] [PubMed]
- Scott, F.; Wilson, P.; Conejeros, R.; Vassiliadis, V.S. Simulation and Optimization of Dynamic Flux Balance Analysis Models Using an Interior Point Method Reformulation. Comput. Chem. Eng. 2018, 119, 152–170. [Google Scholar] [CrossRef]
- Bordbar, A.; Yurkovich, J.T.; Paglia, G.; Rolfsson, O.; Sigurjónsson, O.E.; Palsson, B.O. Elucidating dynamic metabolic physiology through network integration of quantitative time-course metabolomics. Sci. Rep. 2017, 7, 46249. [Google Scholar] [CrossRef]
- Covert, M.W.; Schilling, C.H.; Palsson, B. Regulation of Gene Expression in Flux Balance Models of Metabolism. J. Theor. Biol. 2001, 213, 73–88. [Google Scholar] [CrossRef]
- Covert, M.W.; Knight, E.M.; Reed, J.L.; Herrgard, M.J.; Palsson, B.Ø. Integrating High-Throughput and Computational Data Elucidates Bacterial Networks. Nature 2004, 429, 92–96. [Google Scholar] [CrossRef]
- Covert, M.W.; Xiao, N.; Chen, T.J.; Karr, J.R. Integrating Metabolic, Transcriptional Regulatory and Signal Transduction Models in Escherichia coli. Bioinformatics 2008, 24, 2044–2050. [Google Scholar] [CrossRef]
- Min Lee, J.; Gianchandani, E.P.; Eddy, J.A.; Papin, J.A. Dynamic Analysis of Integrated Signaling, Metabolic, and Regulatory Networks. PLoS Comput. Biol. 2008, 4, e1000086. [Google Scholar] [CrossRef]
- Thuillier, K.; Baroukh, C.; Bockmayr, A.; Cottret, L.; Paulevé, L.; Siegel, A. Learning Boolean Controls in Regulated Metabolic Networks: A Case-Study. In Proceedings of the 19th International Conference on Computational Methods in Systems Biology, Bordeaux, France, 22 September 2021. [Google Scholar]
- Peres, S.; Morterol, M.; Simon, L. SAT-Based Metabolics Pathways Analysis without Compilation. In Lecture Note in Bioinformatics; Mendes, J.D.P., Smallbone, K., Eds.; Springer International Publishing: Berlin, Germany, 2014; Volume 8859, pp. 20–31. [Google Scholar]
- Mahout, M.; Carlson, R.P.; Peres, S. Answer Set Programming for Computing Constraints-Based Elementary Flux Modes: Application to Escherichia coli Core Metabolism. Processes 2020, 8, 1649. [Google Scholar] [CrossRef]
- Janhunen, T.; Kaminski, R.; Ostrowski, M.; Schaub, T.; Schellhorn, S.; Wanko, P. Clingo Goes Linear Constraints over Reals and Integers. arXiv 2017, arXiv:1707.04053. [Google Scholar] [CrossRef]
- Goelzer, A.; Fromion, V.; Scorletti, G. Cell design in bacteria as a convex optimization problem. In Proceedings of the 48th IEEE Conference on Decision and Control, Shanghai, China, 15–18 December 2009; pp. 4517–4522. [Google Scholar]
- Goelzer, A.; Fromion, V.; Scorletti, G. Cell design in bacteria as a convex optimization problem. Automatica 2011, 47, 1210–1218. [Google Scholar] [CrossRef]
- Goelzer, A.; Muntel, J.; Chubukov, V.; Jules, M.; Prestel, E.; Nolker, R.; Mariadassou, M.; Aymerich, S.; Hecker, M.; Noirot, P.; et al. Quantitative prediction of genome-wide resource allocation in bacteria. Metab. Eng. 2015, 32, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Bulović, A.; Fischer, S.; Dinh, M.; Golib, F.; Liebermeister, W.; Poirier, C.; Tournier, L.; Klipp, E.; Fromion, V.; Goelzer, A. Automated Generation of Bacterial Resource Allocation Models. Metab. Eng. 2019, 55, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Tournier, L.; Goelzer, A.; Fromion, V. Optimal Resource Allocation Enables Mathematical Exploration of Microbial Metabolic Configurations. J. Math. Biol. 2017, 75, 1349–1380. [Google Scholar] [CrossRef] [PubMed]
- Jeanne, G.; Goelzer, A.; Tebbani, S.; Dumur, D.; Fromion, V. Dynamical resource allocation models for bioreactor optimization. IFAC-PapersOnLine 2018, 51, 20–23. [Google Scholar] [CrossRef]
- Jeanne, G.; Goelzer, A.; Tebbani, S.; Dumur, D.; Fromion, V. Towards a Realistic and Integrated Strain Design in Batch Bioreactor. In Proceedings of the 2018 IEEE Conference on Decision and Control (CDC), Miami, FL, USA, 17–19 December 2018; pp. 2698–2703. [Google Scholar] [CrossRef]
- Lerman, J.A.; Hyduke, D.R.; Latif, H.; Portnoy, V.A.; Lewis, N.E.; Orth, J.D.; Schrimpe-Rutledge, A.C.; Smith, R.D.; Adkins, J.N.; Zengler, K.; et al. In silico method for modelling metabolism and gene product expression at genome scale. Nat. Commun. 2012, 3, 929. [Google Scholar] [CrossRef]
- Liu, J.K.; O’Brien, E.J.; Lerman, J.A.; Zengler, K.; Palsson, B.O.; Feist, A.M. Reconstruction and Modeling Protein Translocation and Compartmentalization in Escherichia coli at the Genome-Scale. BMC Syst. Biol. 2014, 8, 110. [Google Scholar] [CrossRef]
- Lloyd, C.J.; Ebrahim, A.; Yang, L.; King, Z.A.; Catoiu, E.; O’Brien, E.J.; Liu, J.K.; Palsson, B.O. COBRAme: A Computational Framework for Genome-Scale Models of Metabolism and Gene Expression. PLoS Comput. Biol. 2018, 14, e1006302. [Google Scholar] [CrossRef]
- Yang, L.; Ebrahim, A.; Lloyd, C.J.; Saunders, M.A.; Palsson, B.O. DynamicME: Dynamic simulation and refinement of integrated models of metabolism and protein expression. BMC Syst. Biol. 2019, 13, 2. [Google Scholar] [CrossRef]
- Waldherr, S.; Oyarzun, D.A.; Bockmayr, A. Dynamic optimization of metabolic networks coupled with gene expression. J. Theor. Biol. 2015, 365, 469–485. [Google Scholar] [CrossRef]
- Ramkrishna, D. A Cybernetic Perspective of Microbial Growth. In Foundations of Biochemical Engineering; American Chemical Society: Washington, DC, USA, 1983; Chapter 7; pp. 161–178. [Google Scholar] [CrossRef]
- Young, J.D.; Henne, K.L.; Morgan, J.A.; Konopka, A.E.; Ramkrishna, D. Integrating cybernetic modeling with pathway analysis provides a dynamic, systems-level description of metabolic control. Biotechnol. Bioeng. 2008, 100, 18. [Google Scholar] [CrossRef]
- Kim, J.I.; Varner, J.D.; Ramkrishna, D. A hybrid model of anaerobic E. coli GJT001: Combination of elementary flux modes and cybernetic variables. Biotechnol. Prog. 2008, 24, 993–1006. [Google Scholar] [CrossRef]
- Song, H.S.; Ramkrishna, D. When is the Quasi-Steady-State Approximation Admissible in Metabolic Modeling? When Admissible, What Models are Desirable? Ind. Eng. Chem. Res. 2009, 48, 7976–7985. [Google Scholar] [CrossRef]
- Song, H.S.; Ramkrishna, D. Prediction of metabolic function from limited data: Lumped hybrid cybernetic modeling (L-HCM). Biotechnol. Bioeng. 2010, 106, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Song, H.S.; Ramkrishna, D.; Pinchuk, G.E.; Beliaev, A.S.; Konopka, A.E.; Fredrickson, J.K. Dynamic modeling of aerobic growth of Shewanella oneidensis. Predicting triauxic growth, flux distributions, and energy requirement for growth. Metab. Eng. 2013, 15, 25–33. [Google Scholar] [CrossRef]
- Tourigny, D.S. Dynamic metabolic resource allocation based on the maximum entropy principle. J. Math. Biol. 2020, 80, 2395–2430. [Google Scholar] [CrossRef]
- Lunze, J.; Lamnabhi-Lagarrigue, F. (Eds.) Handbook of Hybrid Systems Control: Theory, Tools, Applications; Cambridge University Press: Cambridge, MA, USA, 2009. [Google Scholar] [CrossRef]
- Liu, L.; Bockmayr, A. Formalizing Metabolic-Regulatory Networks by Hybrid Automata. Acta Biotheor. 2020, 68, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Bockmayr, A. Regulatory Dynamic Enzyme-Cost Flux Balance Analysis: A Unifying Framework for Constraint-Based Modeling. J. Theor. Biol. 2020, 501, 110317. [Google Scholar] [CrossRef] [PubMed]
- Machado, D.; Costa, R.S.; Ferreira, E.C.; Rocha, I.; Tidor, B. Exploring the Gap between Dynamic and Constraint-Based Models of Metabolism. Metab. Eng. 2012, 14, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.M.; Kanehisa, M. A Quadratic Programming Approach for Decomposing Steady-State Metabolic Flux Distributions onto Elementary Modes. Bioinformatics 2005, 21, ii204–ii205. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.M.; Kanehisa, M. Quantitative Elementary Mode Analysis of Metabolic Pathways: The Example of Yeast Glycolysis. BMC Bioinform. 2006, 7, 186. [Google Scholar] [CrossRef]
- Nookaew, I.; Meechai, A.; Thammarongtham, C.; Laoteng, K.; Ruanglek, V.; Cheevadhanarak, S.; Nielsen, J.; Bhumiratana, S. Identification of Flux Regulation Coefficients from Elementary Flux Modes: A Systems Biology Tool for Analysis of Metabolic Networks. Biotechnol. Bioeng. 2007, 97, 1535–1549. [Google Scholar] [CrossRef]
- Trinh, C.T.; Wlaschin, A.; Srienc, F. Elementary Mode Analysis: A Useful Metabolic Pathway Analysis Tool for Characterizing Cellular Metabolism. Appl. Microbiol. Biotechnol. 2009, 81, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Poolman, M.G.; Venkatesh, K.V.; Pidcock, M.K.; Fell, D.A. A Method for the Determination of Flux in Elementary Modes, and Its Application to Lactobacillus Rhamnosus. Biotechnol. Bioeng. 2004, 88, 13. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.H.J.; Ji, P. Decomposing Flux Distributions into Elementary Flux Modes in Genome-Scale Metabolic Networks. Bioinformatics 2011, 27, 2256–2262. [Google Scholar] [CrossRef] [PubMed]
- Folch-Fortuny, A.; Marques, R.; Isidro, I.A.; Oliveira, R.; Ferrer, A. Principal Elementary Mode Analysis (PEMA). Mol. Biosyst. 2016, 12, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Folch-Fortuny, A.; Teusink, B.; Hoefsloot, H.C.; Smilde, A.K.; Ferrer, A. Dynamic Elementary Mode Modelling of Non-Steady State Flux Data. BMC Syst. Biol. 2018, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, S.; Blomberg, P.; Castillo, S.; Rousu, J. Principal Metabolic Flux Mode Analysis. Bioinformatics 2018, 34, 2409–2417. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moulin, C.; Tournier, L.; Peres, S. Combining Kinetic and Constraint-Based Modelling to Better Understand Metabolism Dynamics. Processes 2021, 9, 1701. https://doi.org/10.3390/pr9101701
Moulin C, Tournier L, Peres S. Combining Kinetic and Constraint-Based Modelling to Better Understand Metabolism Dynamics. Processes. 2021; 9(10):1701. https://doi.org/10.3390/pr9101701
Chicago/Turabian StyleMoulin, Cecile, Laurent Tournier, and Sabine Peres. 2021. "Combining Kinetic and Constraint-Based Modelling to Better Understand Metabolism Dynamics" Processes 9, no. 10: 1701. https://doi.org/10.3390/pr9101701
APA StyleMoulin, C., Tournier, L., & Peres, S. (2021). Combining Kinetic and Constraint-Based Modelling to Better Understand Metabolism Dynamics. Processes, 9(10), 1701. https://doi.org/10.3390/pr9101701





