A Study on the Hydrolysis of Urea Contained in Wastewater and Continuous Recovery of Ammonia by an Enzymatic Membrane Reactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Batch Hydrolysis
2.2. Materials Used
2.3. Enzyme Hydrolysis Reaction
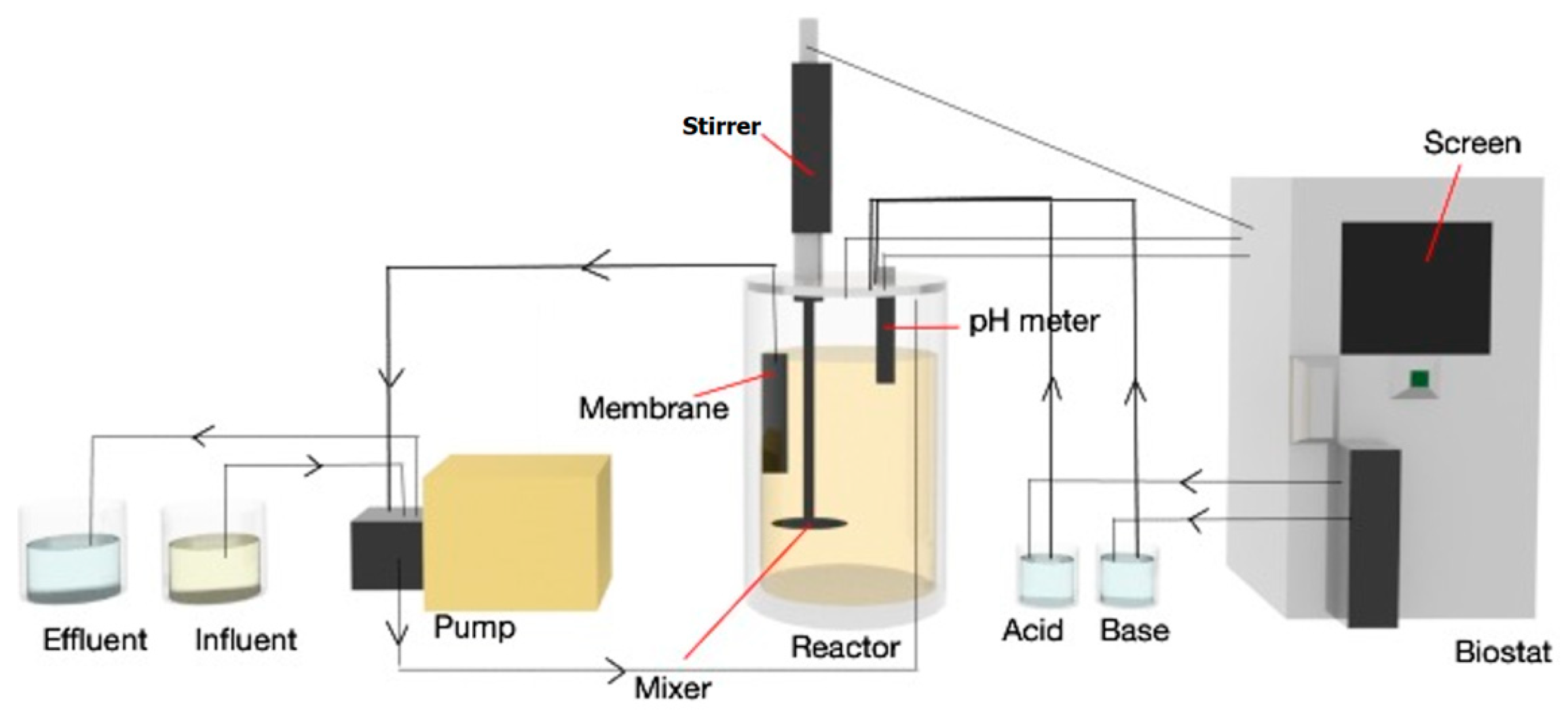
2.4. Continuous Enzymatic Hydrolysis of Urea
2.5. Data Analysis and Experimental Reproducibility
2.6. SEM and EDAX Technique
3. Results and Discussions
3.1. Effect of pH on Enzyme (Urease) Activity
3.2. Effect of Enzyme Concentration on Batch Hydrolysis of Urea
3.3. Effect of Feed Concentration on Urease Activity
3.4. Effect of Ammonium Sulphate (NH4)2SO4 Addition on Urease Activity
3.5. Effect of Feed Concentration pH on Urease Activity
3.6. Effect of Ionic Strength on Urease Activity
3.7. Analysis of Membrane Surface and Fouling
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Urbańczyk, E.; Sowa, M.; Simka, W. Urea removal from aqueous solutions—A review. J. Appl. Electrochem. 2016, 46, 1011–1029. [Google Scholar] [CrossRef] [Green Version]
- Lilong, Y.; Weiguang, L.; Yuan, R.; Yu, L.; Ying, Z. Study on the way of urea removal by BAF. J. Chem. Soc. Pak. 2013, 35, 1081–1087. [Google Scholar]
- Glibert, P.M.; Harrison, J.; Heil, C.; Seitzinger, S. Escalating Worldwide use of Urea—A Global Change Contributing to Coastal Eutrophication. Biogeochemistry 2006, 77, 441–463. [Google Scholar] [CrossRef]
- Qin, Y.; Cabral, J.M. Review Properties and Applications of Urease. Biocatal. Biotransform. 2002, 20, 1–14. [Google Scholar] [CrossRef]
- Hasanoğlu, A.; Romero, J.; Pérez, B.; Plaza, A. Ammonia removal from wastewater streams through membrane contactors: Experimental and theoretical analysis of operation parameters and configuration. Chem. Eng. J. 2010, 160, 530–537. [Google Scholar] [CrossRef]
- Vecino, X.; Reig, M.; Bhushan, B.; Gibert, O.; Valderrama, C.; Cortina, J.L. Liquid fertilizer production by ammonia recovery from treated ammonia-rich regenerated streams using liquid-liquid membrane contactors. Chem. Eng. J. 2019, 360, 890–899. [Google Scholar] [CrossRef]
- Kyriakou, V.; Garagounis, I.; Vourros, A.; Vasileiou, E.; Stoukides, M. An Electrochemical Haber-Bosch Process. Joule 2020, 4, 142–158. [Google Scholar] [CrossRef]
- Ghimire, U.; Jang, M.; Jung, S.P.; Park, D.; Park, S.J.; Yu, H.; Oh, S.-E. Electrochemical Removal of Ammonium Nitrogen and COD of Domestic Wastewater using Platinum Coated Titanium as an Anode Electrode. Energies 2019, 12, 883. [Google Scholar] [CrossRef] [Green Version]
- Simka, W.; Piotrowski, J.; Robak, A.; Nawrat, G. Electrochemical treatment of aqueous solutions containing urea. J. Appl. Electrochem. 2009, 39, 1137–1143. [Google Scholar] [CrossRef]
- Guo, J.; Lee, J.-G.; Tan, T.; Yeo, J.; Wong, P.W.; Ghaffour, N.; An, A.K. Enhanced ammonia recovery from wastewater by Nafion membrane with highly porous honeycomb nanostructure and its mechanism in membrane distillation. J. Membr. Sci. 2019, 590, 117265. [Google Scholar] [CrossRef]
- Zarebska, A.; Nieto, D.R.; Christensen, K.; Søtoft, L.F.; Norddahl, B. Ammonium Fertilizers Production from Manure: A Critical Review. Crit. Rev. Environ. Sci. Technol. 2014, 45, 1469–1521. [Google Scholar] [CrossRef]
- Grimrath, A.; Berends, P.; Rabe, S.; Berger, R.G.; Linke, D. Koji fermentation based on extracellular peptidases of Flammulinavelutipes. Eur. Food Res. Technol. 2011, 232, 415–424. [Google Scholar] [CrossRef]
- American Public Health Association. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association—American Water Works Association and Water Environmental Federation: Washington, DC, USA, 1998. [Google Scholar]
- Qin, Y.; Cabral, J.M.S. Kinetic studies of the urease-catalyzed hydrolysis of urea in a buffer-free system. Appl. Biochem. Biotechnol. 1994, 49, 217–240. [Google Scholar] [CrossRef]
- Muñoz-Aguado, M.; Wiley, D.; Fane, A. Enzymatic and detergent cleaning of a polysulfone ultrafiltration membrane fouled with BSA and whey. J. Membr. Sci. 1996, 117, 175–187. [Google Scholar] [CrossRef]
- Shi, X.; Tal, G.; Hankins, N.P.; Gitis, V. Fouling and cleaning of ultrafiltration membranes: A review. J. Water Process. Eng. 2014, 1, 121–138. [Google Scholar] [CrossRef]
- Bremner, J.M.; Chai, H.S. Effect of phosphoroamides on ammonia volatilization in soil treated with urea. Biol. SMD Fertil. Soil. 1989, 8, 227–230. [Google Scholar]
- Tong, D.; Xu, R. Effects of urea and (NH4)2SO4 on nitrification and acidification of Ultisols from Southern China. J. Environ. Sci. 2012, 24, 682–689. [Google Scholar] [CrossRef]
- Thenabadu, M.W.; Dharmakeerthi, R.S. Review urease activity in soils: A review. Nutr. Plant Sri Lanka 1996, 24, 159–195. [Google Scholar]
- Tikhonenko, S.A.; Saburova, E.A.; Durdenko, E.N.; Sukhorukov, B.I. Enzyme-polyelectrolyte complex: Salt effects on the reaction of urease with polyallylamine. Russ. J. Phys. Chem. A 2009, 83, 1781–1788. [Google Scholar] [CrossRef]
- Engelhardt, S.; Vogel, J.; Duirk, S.E.; Moore, F.B.; Barton, H.A. Assessment of urea hydrolysis as a pretreatment strategy to improve total nitrogen rejection from urine using aquaporin-based membranes in forward osmosis. J. Water Process. Eng. 2020, 34, 101135. [Google Scholar] [CrossRef]
- Nicolau, E.; Fonseca, J.J.; Rodriguez-Martinez, J.A.; Richardson, T.-M.J.; Flynn, M.; Griebenow, K.; Cabrera, C.R. Evaluation of a Urea Bioelectrochemical System for Wastewater Treatment Processes. ACS Sustain. Chem. Eng. 2014, 2, 749–754. [Google Scholar] [CrossRef]
- Nelson, D.L.; Cox, M.M. Lehninger’s Principles of Biochemistry, 4th ed.; W.H. Freeman and Company: New York, NY, USA, 2004. [Google Scholar]
- Revilla, M.; Alexander, J.; Glibert, P.M. Oceanography: Methods Urea analysis in coastal waters: Comparison of enzymatic and direct methods. Limnol. Oceanogr. Methods 2005, 3, 290–299. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Nidetzky, B. The Microenvironment in Immobilized Enzymes: Methods of Characterization and Its Role in Determining Enzyme Performance. Molecules 2019, 24, 3460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosco, F.; Capolongo, A.; Ruggeri, B. Effect of Temperature, pH, Ionic Strength, and Sodium Nitrate on Activity of LiPs: Implications for Bioremediation. Bioremediat. J. 2002, 6, 65–76. [Google Scholar] [CrossRef]






| pH | Kinetic Parameters () | Lineweaver–Burk | Langmuir |
|---|---|---|---|
| No control pH | mg/L | 223.65 | 354.15 |
| mg/L·min | 13.83 | 15.48 | |
| determining coefficient, R2 | 0.9446 | 0.9973 | |
| pH 4 | mg/L | 334.73 | 724.69 |
| mg/L·min | 15.77 | 20.2 | |
| determining coefficient, R2 | 0.9601 | 0.9824 | |
| pH 6 | mg/L | 353.35 | 675.26 |
| mg/L·min | 12.37 | 15.8 | |
| Determining coefficient, R2 | 0.8066 | 0.9854 | |
| pH 7 | mg/L | 418.47 | 525.23 |
| mg/L·min | 15.1 | 16 | |
| Determining coefficient, R2 | 0.9724 | 0.9873 | |
| pH 8 | mg/L | 630.25 | 1042 |
| mg/L·min | 15.43 | 16.81 | |
| Determining coefficient, R2 | 0.9084 | 0.9078 |
| Spot 1 | Element | Conc. (%mass) | Net Int. Error | Spot 2 | Element | Conc. (%mass) | Net Int. Error |
|---|---|---|---|---|---|---|---|
| C | 58.37 | 0.01 | C | 18.57 | 0.01 | ||
| O | 26.93 | 0.01 | O | 42.16 | 0.01 | ||
| F | 3.12 | 0.06 | F | 8.48 | 0.01 | ||
| Na | 3 | 0.04 | Na | 2.78 | 0.02 | ||
| Ca | 4.76 | 0.05 | Mg | 1.48 | 0.03 | ||
| Fe | 1.61 | 0.32 | Al | 0.78 | 0.03 | ||
| Ni | 2.21 | 0.21 | Ca | 25.48 | 0.01 | ||
| Total | 100 | - | Fe | 0.13 | 0.57 | ||
| Ni | 0.12 | 0.56 | |||||
| Total | 100 | - |
| Spot 3 | Element | Conc. (%mass) | Net Int. Error | Spot 4 | Element | Conc. (%mass) | Net Int. Error |
|---|---|---|---|---|---|---|---|
| C | 13.8 | 0.01 | C | 10.96 | 0.01 | ||
| O | 40.32 | 0.01 | O | 46.67 | 0.01 | ||
| F | 7.98 | 0.02 | Al | 0.79 | 0.02 | ||
| Na | 2.9 | 0.03 | Ca | 41.58 | 0 | ||
| Al | 1.18 | 0.02 | |||||
| Ca | 33.52 | 0.01 | |||||
| Fe | 0.18 | 0.58 | |||||
| Ni | 0.13 | 0.58 | |||||
| Total | 100 | Total | 100 |
| Spot 3 | Element | Conc. (%mass) | Net Int. Error |
|---|---|---|---|
| C | 40.32 | 0.01 | |
| O | 6.55 | 0.03 | |
| F | 48.17 | 0.01 | |
| Al | 1.06 | 0.03 | |
| Ca | 3.67 | 0.06 | |
| Fe | 0.18 | 0.59 | |
| Ni | 0.15 | 0.57 | |
| Total | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yahya, M.N.; Gökçekuş, H.; Orhon, D.; Keskinler, B.; Karagunduz, A.; Omwene, P.I. A Study on the Hydrolysis of Urea Contained in Wastewater and Continuous Recovery of Ammonia by an Enzymatic Membrane Reactor. Processes 2021, 9, 1703. https://doi.org/10.3390/pr9101703
Yahya MN, Gökçekuş H, Orhon D, Keskinler B, Karagunduz A, Omwene PI. A Study on the Hydrolysis of Urea Contained in Wastewater and Continuous Recovery of Ammonia by an Enzymatic Membrane Reactor. Processes. 2021; 9(10):1703. https://doi.org/10.3390/pr9101703
Chicago/Turabian StyleYahya, Mukhtar Nuhu, Hüseyin Gökçekuş, Derin Orhon, Bülent Keskinler, Ahmet Karagunduz, and Philip Isaac Omwene. 2021. "A Study on the Hydrolysis of Urea Contained in Wastewater and Continuous Recovery of Ammonia by an Enzymatic Membrane Reactor" Processes 9, no. 10: 1703. https://doi.org/10.3390/pr9101703
APA StyleYahya, M. N., Gökçekuş, H., Orhon, D., Keskinler, B., Karagunduz, A., & Omwene, P. I. (2021). A Study on the Hydrolysis of Urea Contained in Wastewater and Continuous Recovery of Ammonia by an Enzymatic Membrane Reactor. Processes, 9(10), 1703. https://doi.org/10.3390/pr9101703






