Abstract
The electrocoagulation (EC) process has been widely studied in recent years to remove a wide range of contaminants present in different types of water: fluorides, arsenic, heavy metals, organic matter, colorants, oils, and recently, pharmaceutical compounds. However, most of the studies have been aimed at understanding the process factors that have the most significant effect on efficiency, and these studies have been mainly on a batch process. Therefore, this review is focused on elucidating the current state of development of this process and the challenges it involves transferring to continuous processes and the recent exploration of its potential use in the removal of pharmaceutical contaminants and its implementation with other technologies.
1. Introduction
Various pollutants in water for human use and consumption, municipal wastewater, and industrial effluents represent a public health problem and a threat to ecosystems. Contaminants such as fluoride, arsenic, heavy metals, dyes, fats and oils, and pharmaceuticals, to name a few, come from various sources, and their removal represents a challenge due to the characteristics of each type of water. Even so, the EC process has shown high versatility since it allows one to efficiently remove these contaminants [1,2,3,4,5,6,7] in underground, surface, and residual water.
Until now, extensive studies have been carried out on the parameters that have a more significant effect on the batch-scale EC process. For example, the initial concentration of the pollutant, current density, pH, applied voltage or current, treatment time, temperature, the distance between electrodes, electrode arrangement, stirring speed, and support electrolyte are the parameters that have been most evaluated [8,9,10,11,12,13]. However, evaluating the removal of pollutants in a continuous process, determining which aspects significantly influence removal efficiencies, and keeping the operating system efficient are some of the aspects to be addressed urgently to implement this technology. In addition, being able to scale up to larger dimensions than those used in the laboratory, to couple this process to other removal processes, purification plants, or wastewater treatment, has to be considered.
In the last decade, in addition to efforts to understand the removal mechanisms and the most critical operational parameters, some research has focused on bringing the process to continuous flow. For this, some authors have chosen to evaluate the process in batch and subsequently in continuous [14,15,16,17,18,19,20]. From the exploration of the batch process, the authors obtain the optimal parameters of the process. Then, the experiments in the continuous process are carried out, generally in the same reactor.
Designing an EC reactor through modeling tools is crucial since it affects performance and is decisive when scaling [21]. Having so many parameters to consider, some authors have focused on the cell geometry and the effect that it has on the flow regime [22,23,24]. Other authors have focused on the design of the electrodes to minimize energy consumption [25] or through an analysis of the current and potential distribution [26], as well as the design of processes where sedimentation and sludge separation units are considered [27,28,29]. The flow rate and residence time have been the most evaluated operational parameters inherent to a continuous process.
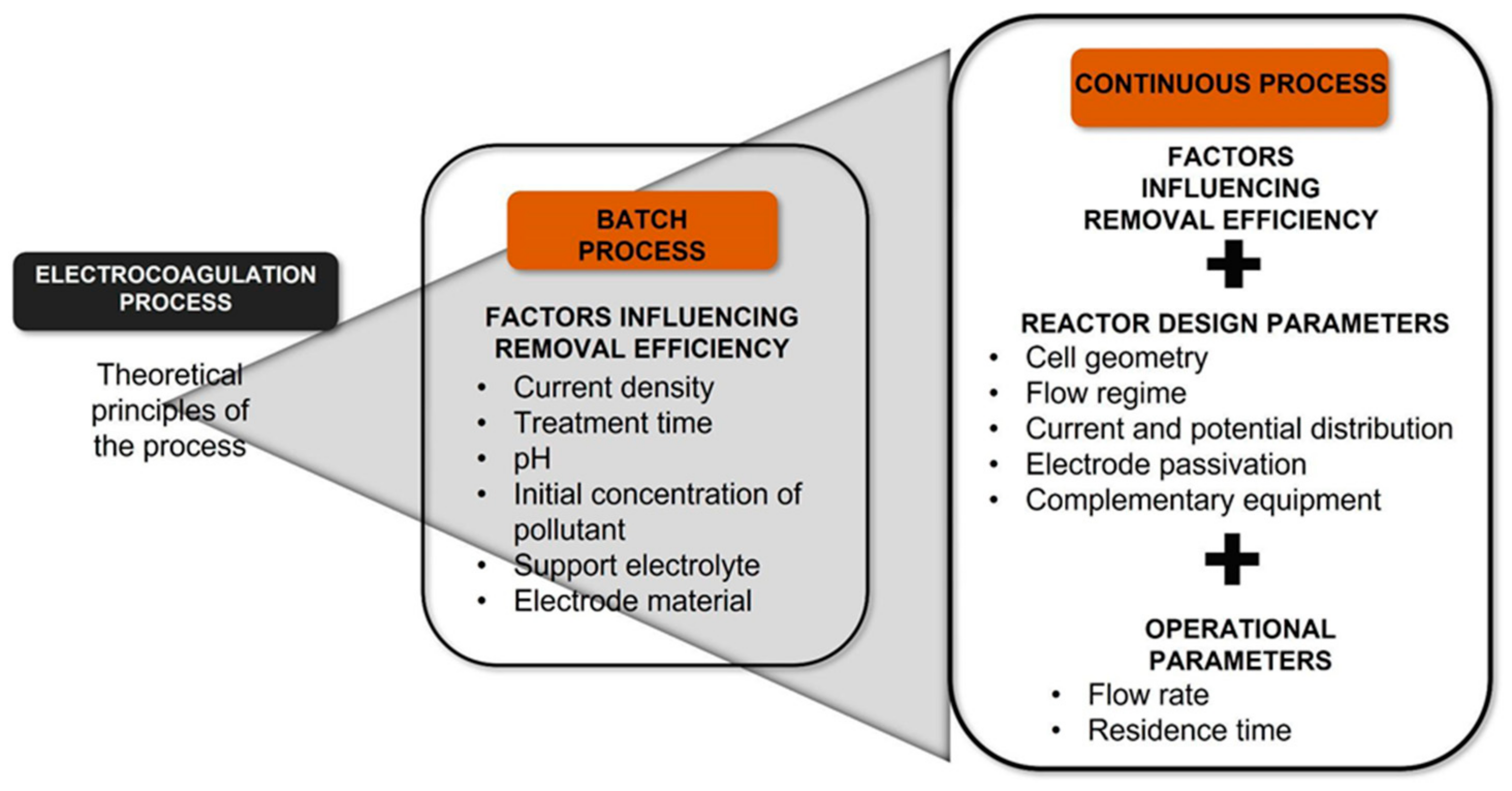
There is no universal design; instead, reactor designs have been developed for each pollutant evaluated by various authors. However, although the geometric and electrode configurations are different, it can be said that all the continuous processes designs come from a deep understanding of the EC process’s theoretical principles evaluated on a batch scale (Figure 1). Furthermore, in recent years, the new research trend has been directed to incorporate this process with other water treatment technologies to increase efficiencies or attack different pollutants, organic and inorganic. Due to the EC’s success, its application has recently been explored in pharmaceuticals removal, detected in surface and groundwater [30]. This review aims to present the current panorama of the development of the EC process concerning its implementation in a continuous flow, which brings this technology closer to the application on a larger scale and its coupling with other processes. This fact makes its application more feasible to remove pharmaceutical contaminants present in the water.
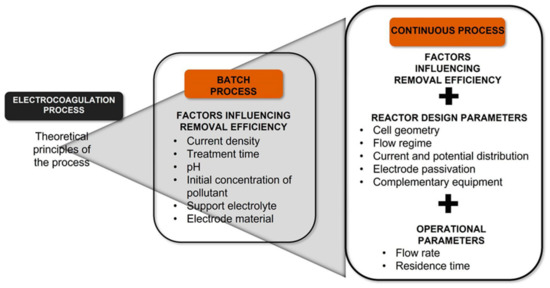
Figure 1.
Steps of design continuous EC process.
2. Theoretical Principles of the EC Process
The EC process consists of the electrochemical generation of species within the solution by applying an electrical current through the sacrificial electrodes. The produced species destabilize the colloidal particles through the neutralization of charges, which produces the formation of larger flocs, in which the contaminants are trapped. These flocs can be separated from the solution by floating or sedimentation [31,32].
The generation of metal ions takes place at the anode. At the same time, hydrogen gas is produced at the cathode, which can also help flocculate particles float out of the water. Therefore, this process is sometimes also called electro flocculation. Aluminum and iron electrodes effectively remove pollutants under favorable operating conditions [11]. The mechanisms of the reactions that occur can vary because it is a very dynamic process, and it changes as it progresses or when the operating parameters are changed; it is also variable for each type of pollutant. However, it can be generalized regarding the following reactions that take place at the anode and the cathode [33]:
Anode:
Cathode:
Two mechanisms for the production of iron hydroxides have been proposed for the iron electrode, Fe(OH)n, where n = 2 or 3 [11]. In the first mechanism, occurs (Equations (5)–(8)) while the second mechanism results in (Equations (9)–(12)).
Anode:
Cathode:
General reaction:
Anode:
Cathode:
General reaction:
On the other hand, with the use of aluminum electrodes, the following reactions are carried out [34]:
Anode:
Cathode:
After the generation of the ions and , reactions occur that form various monomeric and polymeric species that have different charges [35] which eventually transform into the insoluble hydroxide forming a kind of “sweep flocs” that have large surface areas where adsorption takes place [34]. These flocs can be present in the solution depending on the pH and the concentration of the coagulating metal.
An increase in the concentration of and favor the equilibrium and the formation of this insoluble amorphous species that remove pollutants through co-precipitation [35]. Species is formed through a series of reactions that take place as the process progresses (Equations (15)–(17)) [36], while as the process progresses, the pH increases and the insoluble species begin to appear (Equation (18)).
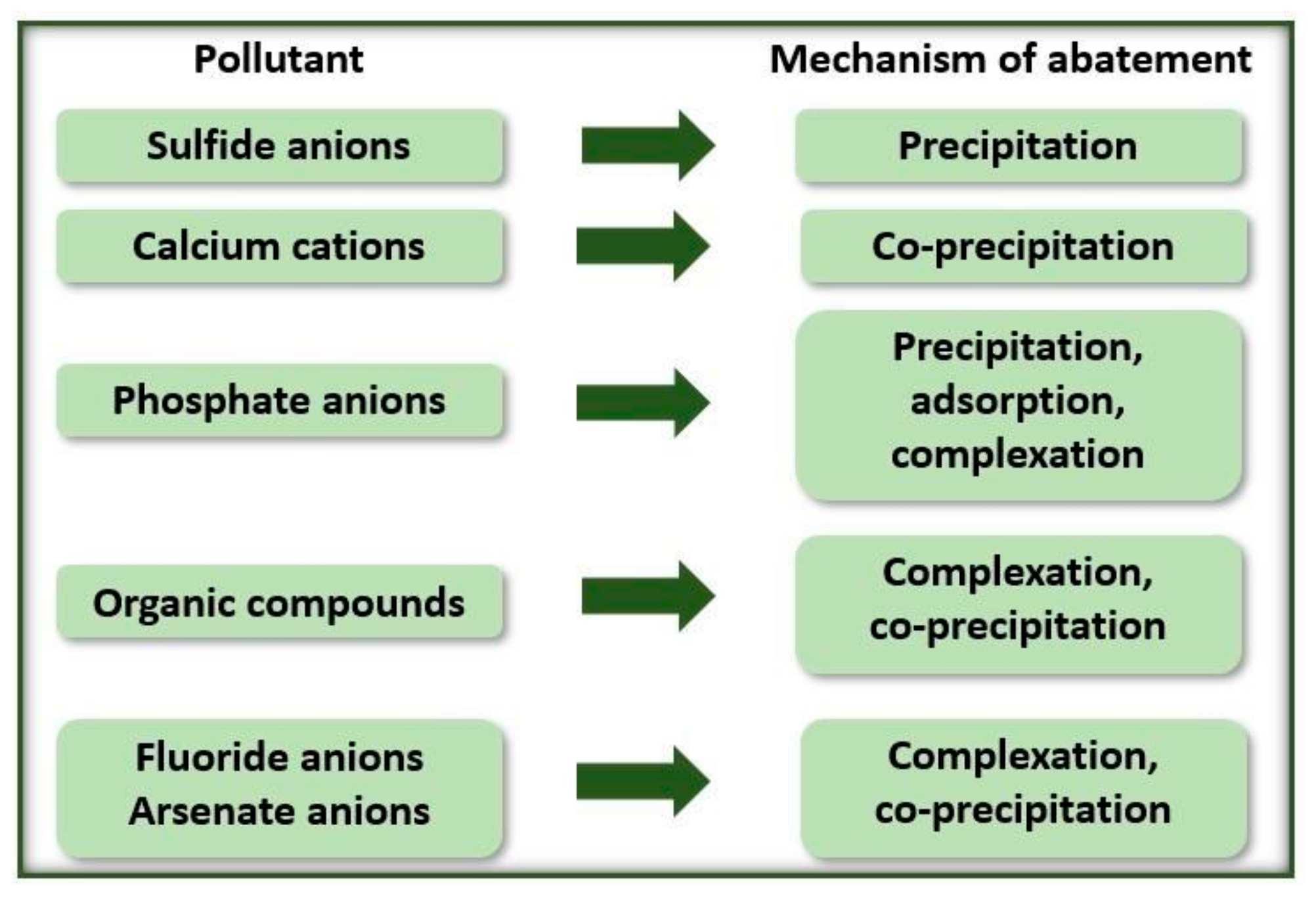
According to Hakizimana et al. [31], there are different equilibrium reactions of the metal hydroxides formed with the soluble contaminants in the solution. Due to this, removal occurs by different mechanisms (Figure 2).
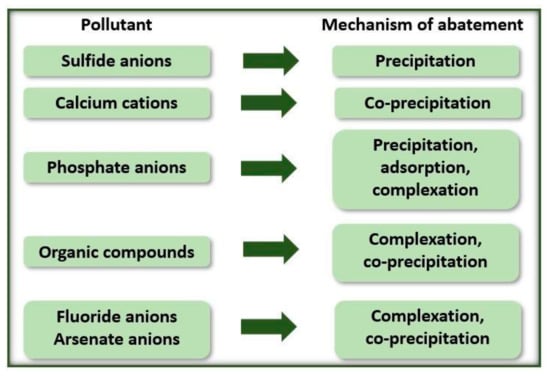
Figure 2.
EC process removal mechanisms according to the type of contaminant.
Main Operational Parameters
Recent advances in EC processes indicate that the removal rate depends on specific operational parameters such as the initial concentration, current density, pH, material, number, arrangement of the electrodes and the distance between them, the conductivity of the water to be treated, as well as the treatment time [9,10,12,33].
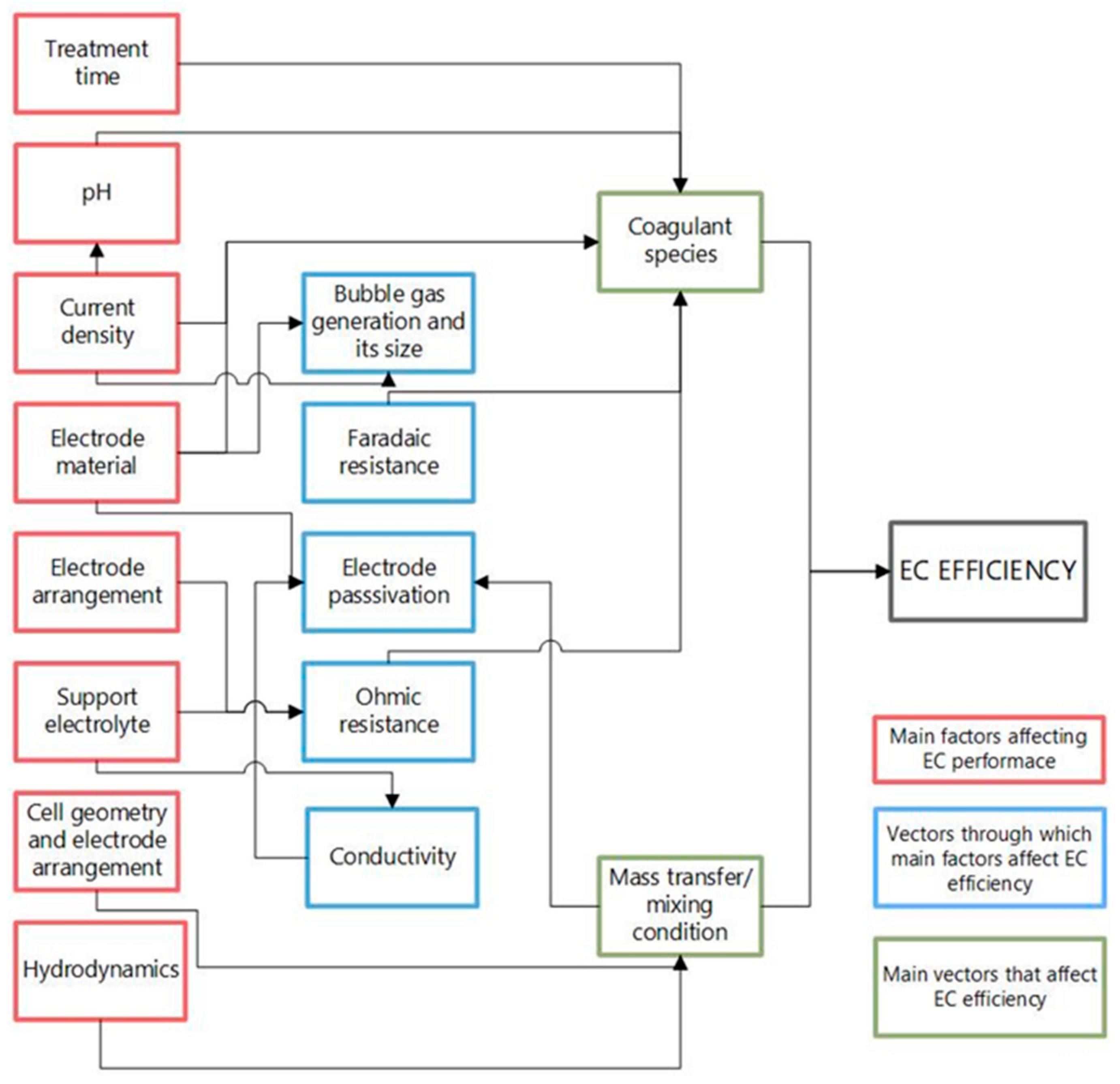
Figure 3 outlines how the main parameters affect the efficiency of the EC process. These factors have been widely discussed and evaluated with various pollutants, and further reference can be found elsewhere [21,31,33,37,38]. In general, all effects contribute to two phenomena determining the efficiency: the coagulating species formed and the mass transfer/mixing conditions. In addition, operating parameters such as current density, pH, electrode material, supporting electrolyte, conductivity, electrode arrangement, and treatment time are factors that govern the coagulant species generation through other variables which affect the efficiency of the process, such as the generation and size of gas bubbles, Faradaic resistance, due to the oxidation-reduction reactions occurring in the cell, the ratio between the potential difference and the current applied to the system (ohmic resistance), as well as the passivation of the electrodes.
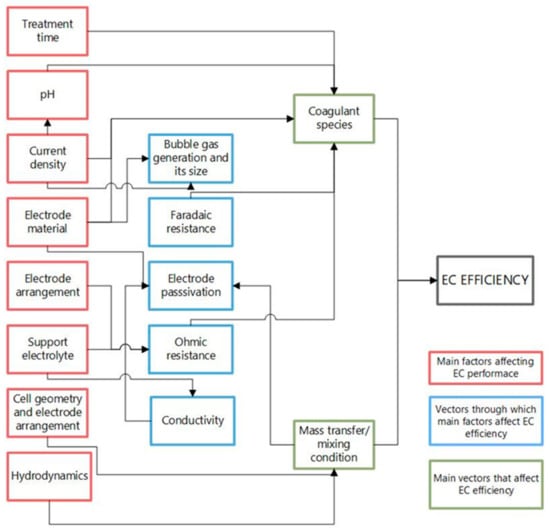
Figure 3.
Main factors affecting the EC process (Modified Hakizimana et al. [31]).
The mass transfer/mixing condition is another factor affecting the efficiency of the process. Two main effects that are parameters of the physical design of the reactor come together: the cell geometry and electrode arrangement, and the hydrodynamics. In addition, the current density, an operational parameter, also influences the mass transfer and mixing conditions through the generation of the bubbles. Therefore, it can be deduced that the EC process removal efficiency depends on the optimal reactor physical design and the operating parameters.
3. Aspects to Be Considered in the Design of EC Reactors
Reactor design is the focal point of an electrochemical water treatment process; the performance of the reactor has a direct effect on the process operation, and the cost since it affects many of the other units of the process, such as settlers and filters [39]. Therefore, it is necessary to analyze this aspect if it is intended to develop systems that operate in continuous flow with careful attention to develop an appropriate geometrical design, to select the electrode material and the complementary equipment such as pumps and settling tanks, among others. It is especially relevant to minimize the potential drop between the electrodes to improve conversion efficiency and energy requirements in the process. It should also be taken into account that the effluent and various solid species affect the hydrodynamic conditions of the reactor during electrolytic processes [21].
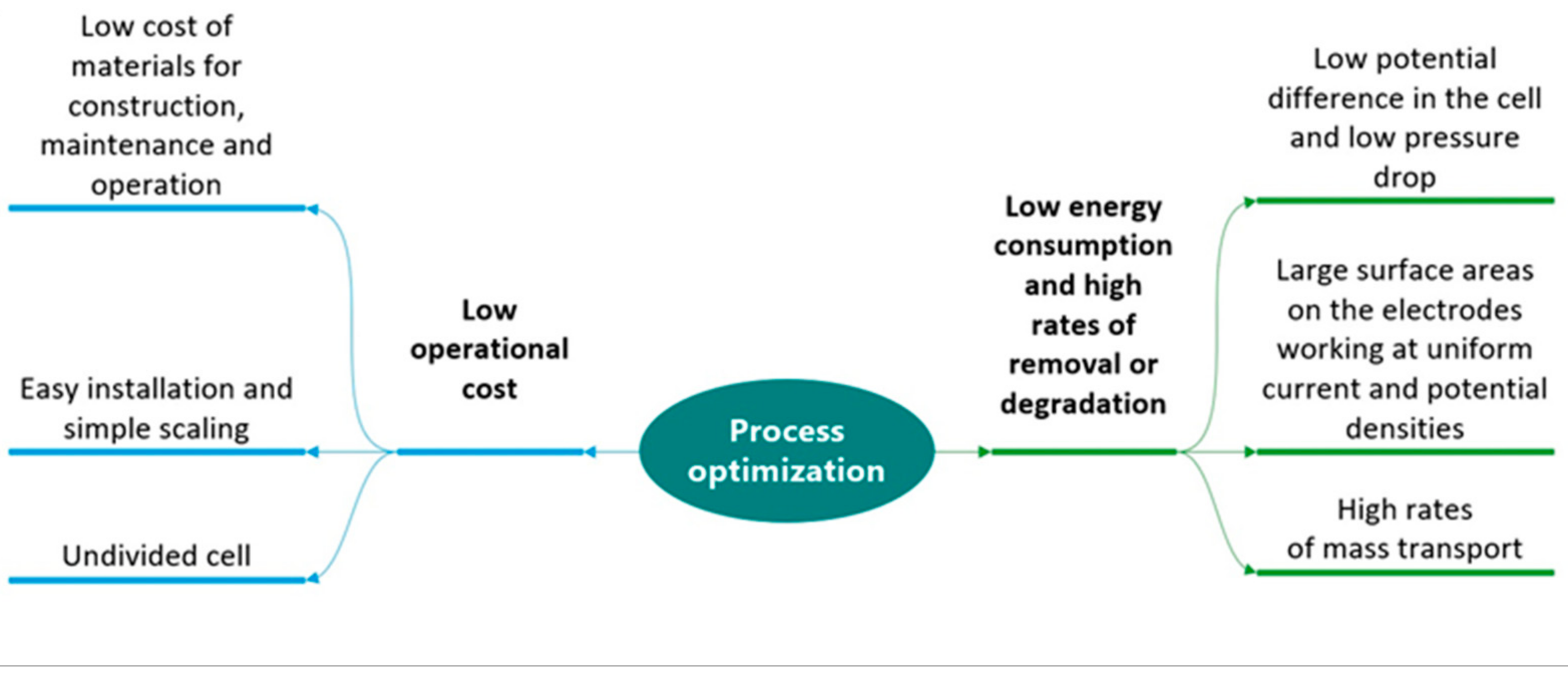
In order to optimize the process in terms of energy consumption and increase the oxidation rate, it is desirable to have a suitable reactor design based on the characteristics listed in Figure 4 according to Nava and Ponce de Leon [40], which are associated with selecting a suitable geometry and the arrangement of the electrodes.
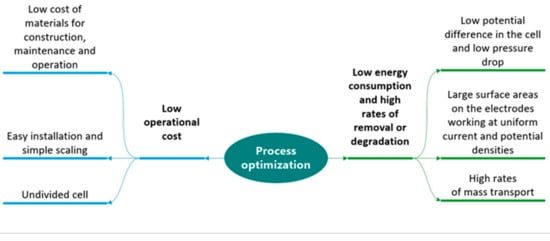
Figure 4.
Characteristics of a reactor for optimization of the process in terms of operating costs, energy consumption and removal rate.
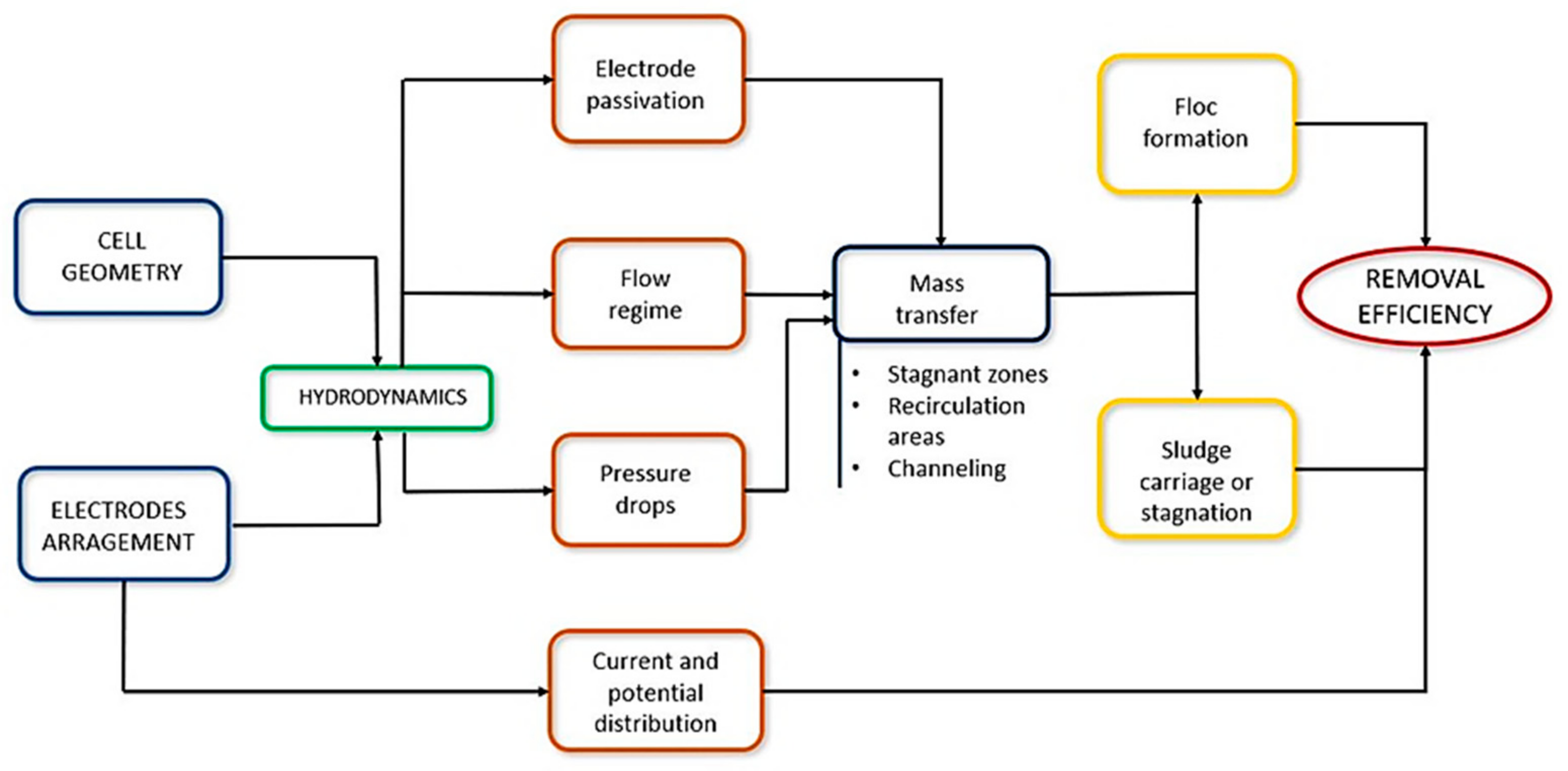
In addition, these aspects influence the system’s hydrodynamics and impact other phenomena and conditions that occur inside the reactor, such as the passivation of the electrodes, the flow regime, and the pressure drop. For example, the mass transfer depends on the previous phenomena and determines the formation of flocs and the carry or stagnation of sludge. On the other hand, the arrangement of the electrodes determines the current and potential distribution in the cell. Thus, the conjunction of design factors, phenomena, and conditions mentioned above influences the efficiency of the process (Figure 5).
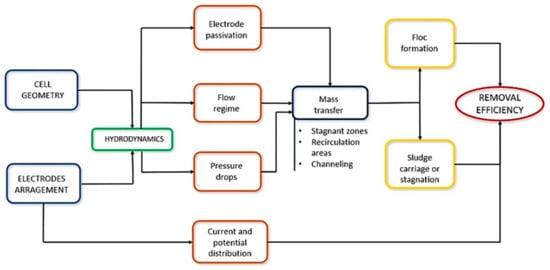
Figure 5.
Conditions that influence the efficiency of an EC process for water treatment.
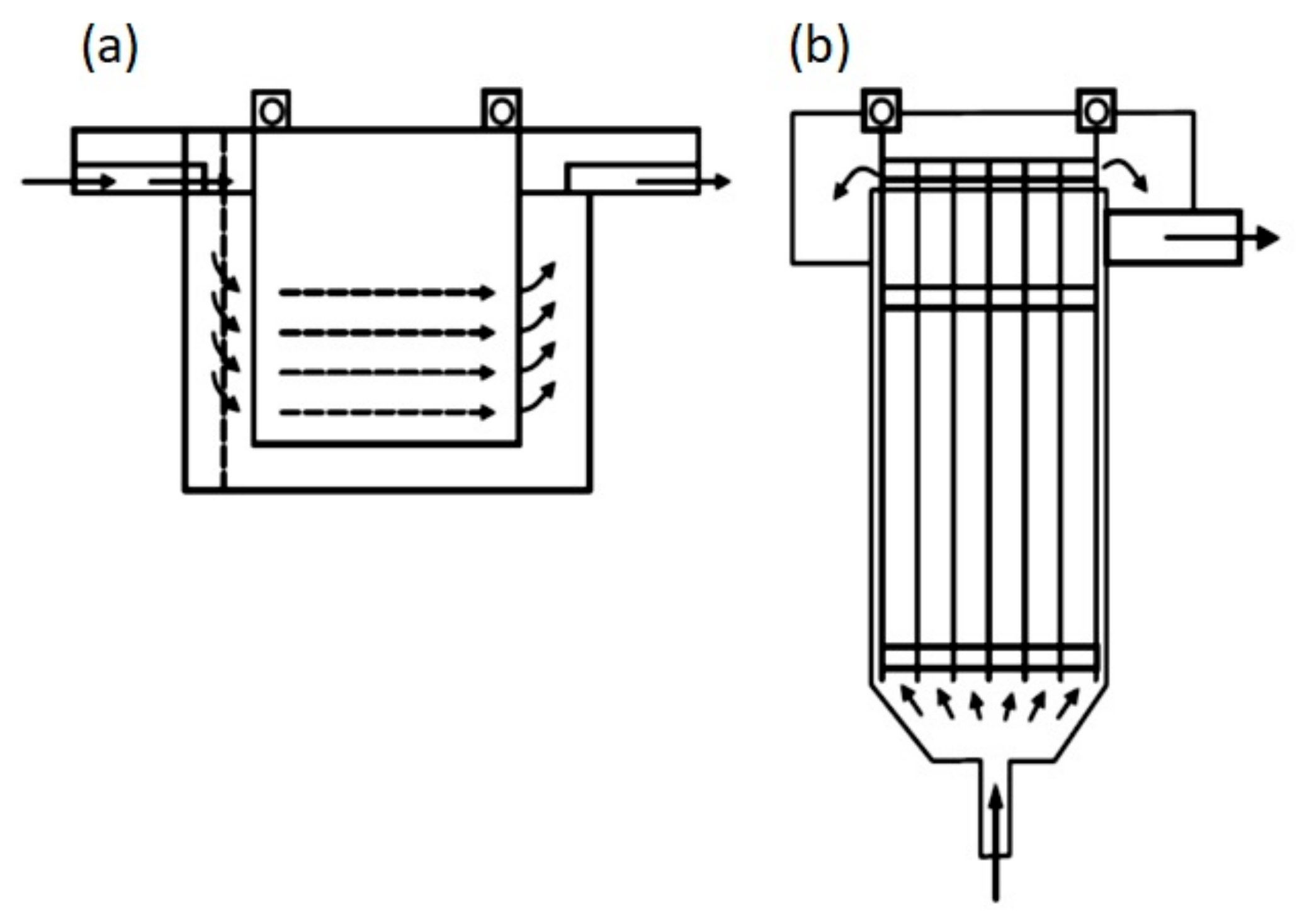
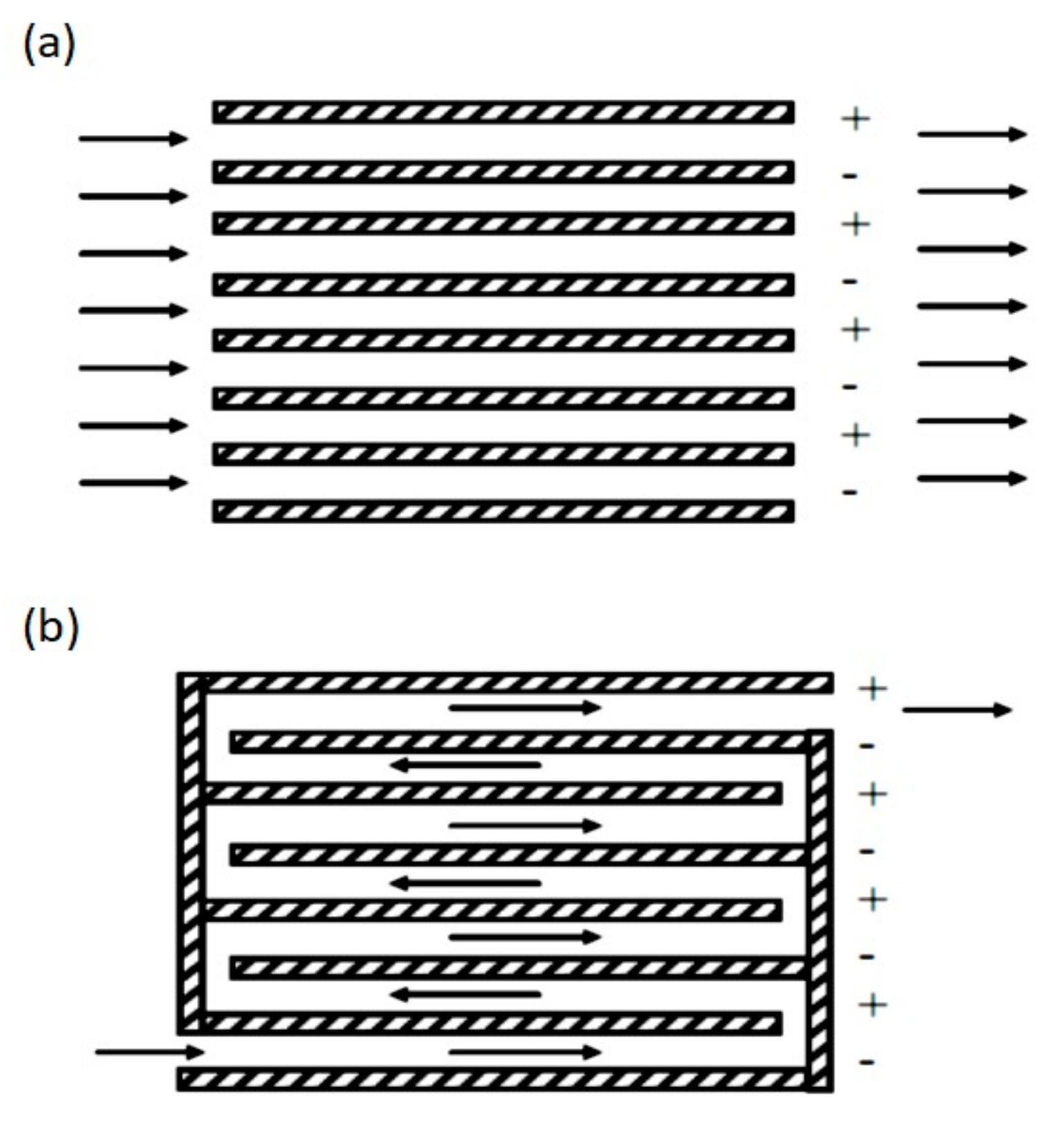
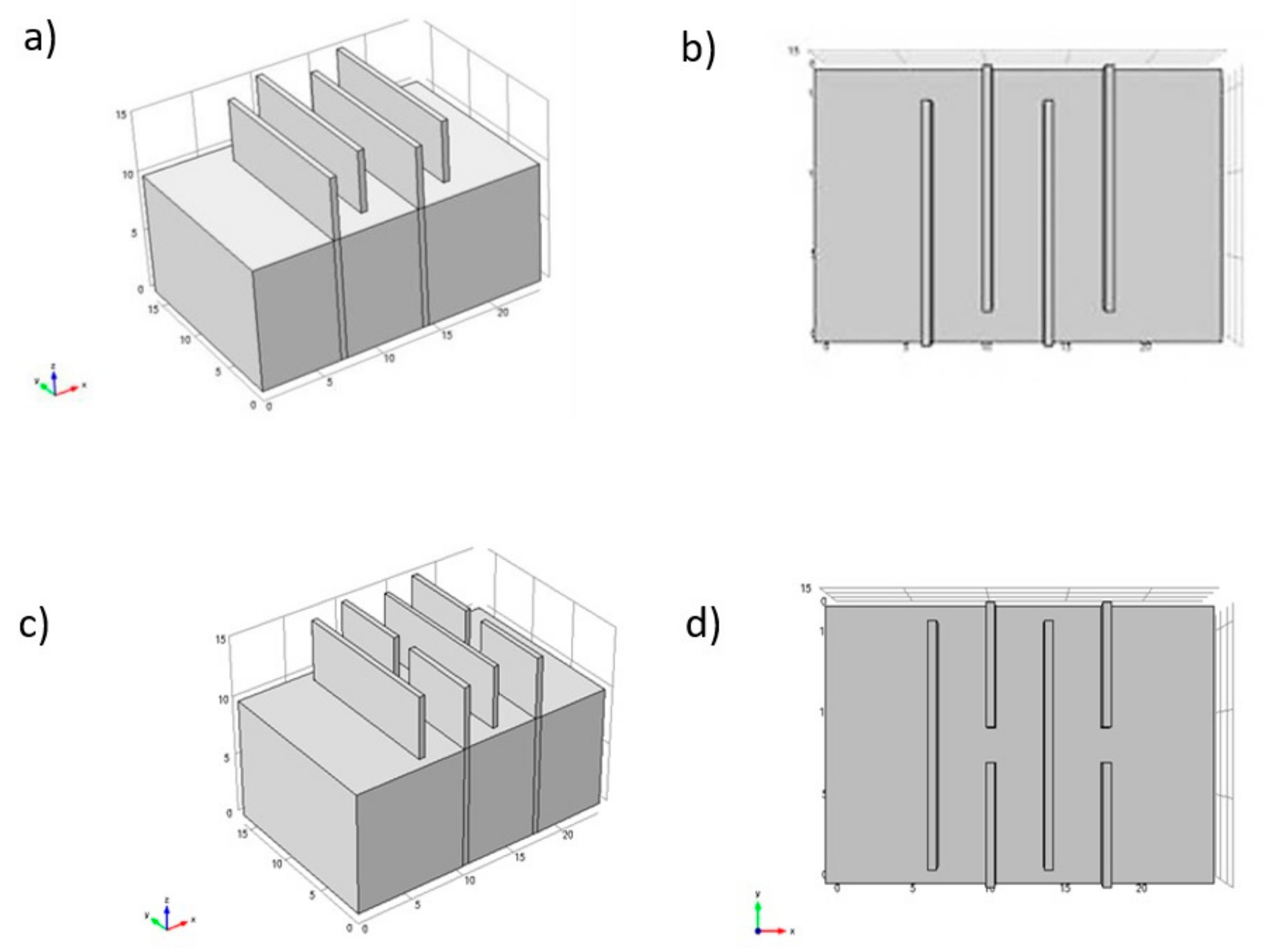
The reactor efficiency is affected by the cell geometry and by the configurations of the electrodes and mixing implements [41]. For example, depending on the orientation of the plates that work as electrodes, the flow in the reactors can be horizontal or vertical (Figure 6), and the flow through the electrodes can be divided into multiple channels or a single channel (Figure 7).
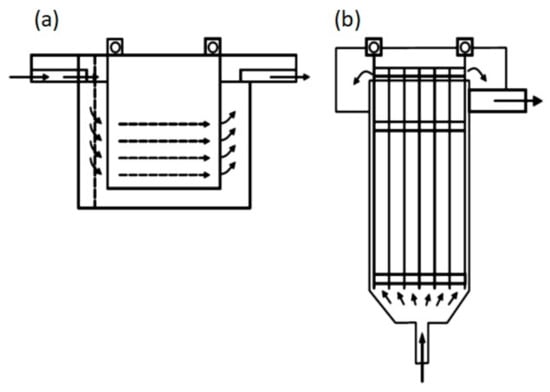
Figure 6.
Types of reactors: (a) Horizontal flow; (b) Vertical flow [42].
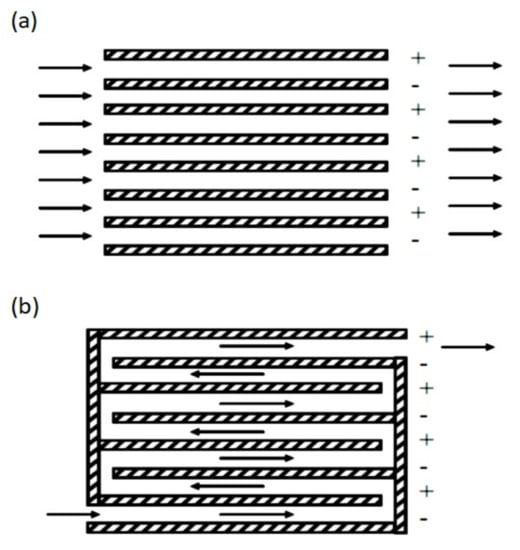
Figure 7.
Flow types according to the arrangement of the electrodes: (a) Multiple channels; (b) single channel [42].
Multiple channels offer more accessible accommodation but offer a small flow rate, and some drawbacks such as electrode passivation are impossible to address [42]. On the other hand, a simple channel offers a higher flow rate. Some factors that are affected by the cell geometry and configuration are the system hydrodynamics, the mass transfer, the current and potential distribution, and the passivation of the electrode. The cell design must ensure that the current and potential distribution is uniform to optimize energy consumption, oxidation rate, and selectivity [40]. Generally, configurations in which the electrodes are parallel arranged offer a uniform distribution of current and potential. In addition, it is easier to control the distance between the electrodes and has a high mass transfer.
The characterization of hydrodynamics allows knowing variables such as fluid velocity profiles. Those profiles influence the formation of flocs and the transport of the formed sludge. The production of sludge within the EC process must be considered when designing a continuous flow reactor. Stagnation of sludge can become a problem, which can be largely avoided or remedied by increasing the volumetric flow [26]. By determining the flow patterns in the fluid, the residence time distribution can be determined, which influences the efficiency of the process because the fluid elements have different residence times [40]. These fluctuations generate a difference in the contact of the coagulant or oxidizing species with the pollutants; they can even promote or inhibit the formation of flocs. In addition, the distribution of residence time allows to know the existence of stagnation zones or dead zones in the reactor that results in the fluid taking a longer time than expected to leave the reactor [24].
Electrode passivation is another problem that can be prevented or corrected by controlling the hydrodynamics of the process. In reactors that do not have a mixing element, or in those in which the electrodes are static, it is common to form an oxide layer on the surface of the electrodes, resulting in their passivation and, along with it, a decrease of mass transfer and process efficiency, and increase the electrical energy consumption [41]. It is also essential to know and determine the magnitude of the pressure drops within the reactor to determine the energy requirements for pumping to transport the electrolyte through the cell [40]. Pressure drops are highly dependent on hydrodynamics generated from cell design and electrode placement.
The EC technique presents scaling problems mainly because the designs used have been mainly empirical, based on operational parameters that determine the removal kinetics of a specific contaminant, for example, temperature, current density, treatment time, electrode material, among others. Analysis of the potential and current distribution must be considered for a viable reactor design. The selectivity of the EC process is determined by a uniform potential distribution within the reactor since this parameter determines the removal rate of the contaminant, and improving the use of cell energy may be possible through a current and potential distribution analysis [43].
4. Types of Continuous Flow EC Reactors
Even though the EC technique for the removal of various pollutants has been studied a lot in recent decades, until now, there is no established reactor design since it is configured for each process and each specific pollutant; therefore, it is difficult to compare their performance [44]. This section is intended to describe the types of continuous flow reactors that have been reported. These are filter press type reactors, mainly, horizontal flow reactors and vertical flow reactors. More details of the various continuous processes discussed in this section can be found in Table S1 in Supplementary Materials.
4.1. Filter Press Reactors
The reactor in filter press configuration has been widely used and therefore studied. This type of reactor consists of a filter press mechanism in which the coagulants are produced through metal plates that function as electrodes, which are supported by frames. Subsequently, the treated water goes to a jar test where agitation is carried out to promote the flocs growth and remove the contaminants. Finally, the precipitation and clarification stages are carried out [45]. The flow pattern is apparently plug flow; however, this largely depends on the design of the collectors at the inlet and outlet of the reactor. The design of the collectors or distributors causes that throughout the reaction area, the speed of the fluid is not homogeneous, since in the case that the distributors are asymmetric, the elements of the fluid move at a higher speed in the shorter channels than in long canals. These velocity gradients within the reactor produce recirculation and stagnation zones, which affect the mass transfer [22,23].
Several designs of filter press reactors have been developed based on removal efficiency results and the hydrodynamic behavior of the fluid. Some reactors of this type contain membranes, and it is a divided cell. For example, Hernández et al. [46] conducted a laboratory-scale continuous flow EC reactor design for arsenic (V) removal. The reactor consisted of 6 channels (8.1 cm in length), four cathodes, and three anodes, whose separation was 0.6 cm. This design has been the basis for the studies carried out by Flores et al. [45], who also evaluated an EC process to remove arsenic. The arrangement of this reactor, of the serpentine type, favors turbulence and mass transfer of the coagulant produced towards the solution. After the EC process, the treated water goes to a jar test where it is mixed for 15 min at 30 rpm, to later go to a sedimentation stage lasting 1 h. The process completely removed the arsenic at a current density of 5 mA/cm2 and a mean linear flow of 0.91 cm/s. In a later study, Guzmán et al. [47] evaluated the same system for the simultaneous removal of fluoride and arsenic, present in groundwater from the Bajío region in Guanajuato, Mexico with initial fluoride concentrations of 2.5 mg/L and 43 μg/L of arsenic. After passing through the reactor, the solution was stirred at 45 rpm in the jar tests for 15 min, and then a precipitation step was carried out for 3 h. The best results were obtained at a current density of 4 mA/cm2 and a mean linear flow of 1.82 cm/s. Under these conditions, the final fluoride concentration was <0.5 mg/L, while arsenic was wholly removed.
The removal results and operating parameters of this reactor in these three studies are presented in Table 1. By using the same reactor, a comparison can be made between these three processes. When there is a higher pollutant (arsenic) concentration, a higher current density is necessary to achieve similar removal results. However, there is an essential variation in the mean linear flows, which may be due to the type of water; in the process with the highest flow, arsenic was removed from synthetic water; in contrast, the other two processes were evaluated in groundwater. The removal of the same pollutant in the same reactor, but contained in different water matrices, allows us to visualize that, although a systematic reactor design for the EC process is developed, the performance with each specific pollutant should be evaluated, considering the type of water in which it is contained.

Table 1.
EC processes comparison carried out in the same filter-press reactor.
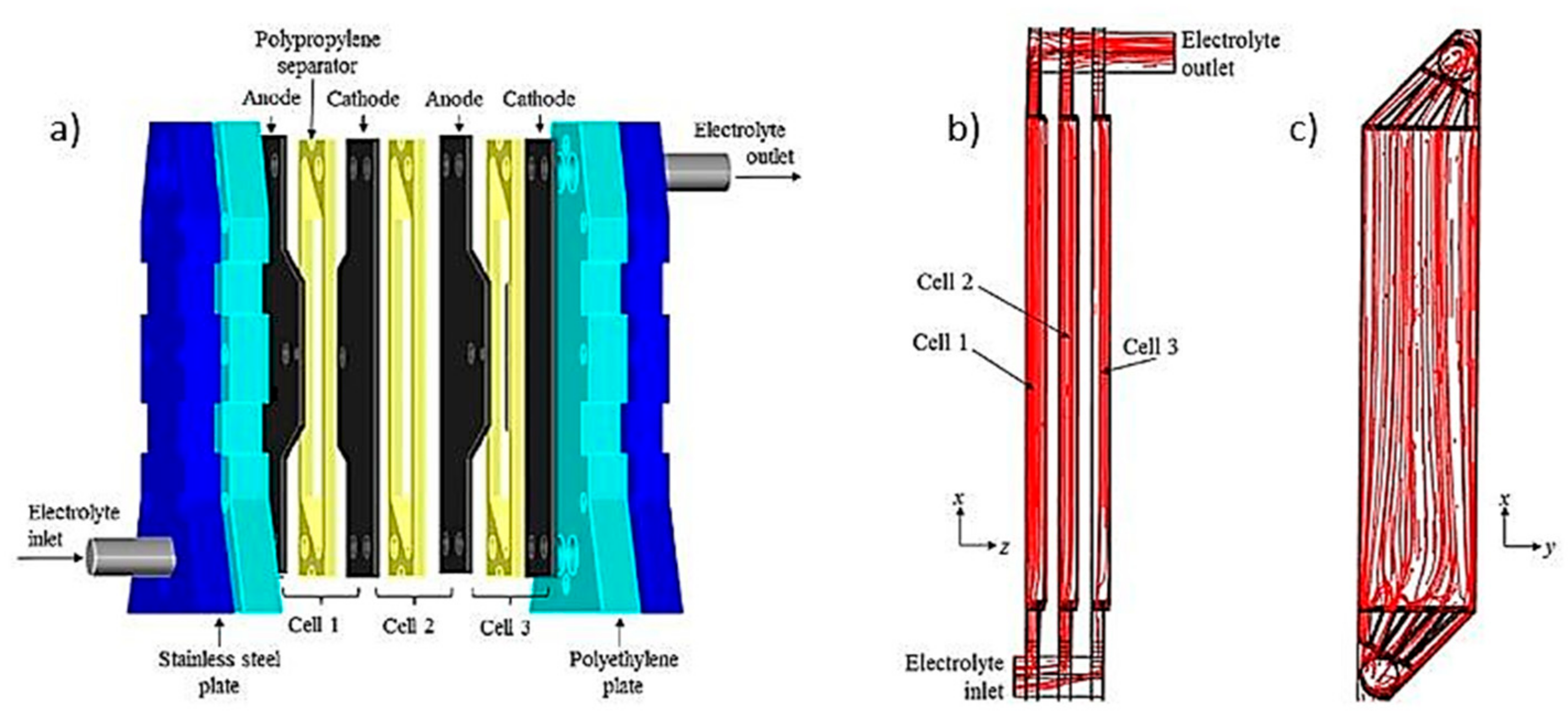
The FM01-LC filter press reactor is a laboratory-scale electrochemical cell [48] and has been widely used for advanced electrochemical oxidation processes [40]. In this type of reactor, the electrolyte is distributed within the reactor channel through internal collectors incorporated in the cell spacers (Figure 8). This type of reactor has also been used for EC processes to remove fluoride and arsenic simultaneously with aluminum electrodes.
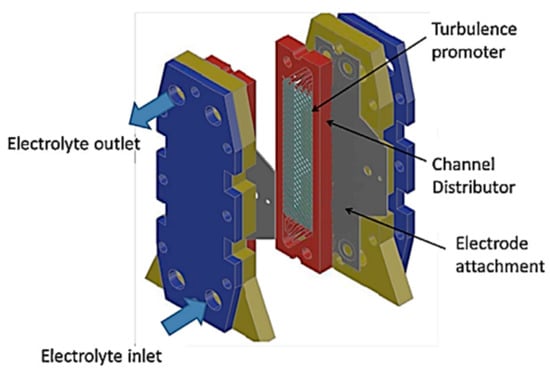
Figure 8.
The FM01-LC electrochemical reactor [48].
Sandoval et al. [49] used an electrochemical filter press type FM01-LC reactor with three undivided cells with four aluminum electrodes separated 0.60 cm through polypropylene flow distributors (Figure 9a) to simultaneously remove fluoride and arsenic. The hydrodynamic behavior of this reactor had been previously analyzed by Sandoval et al. [50]. By computational fluid dynamics (CFD), it was determined that the flow pattern in this reactor was closer to continuous mixing flow. The flow pattern of a single cell FM01-LC reactor has been reported as quasi-plug flow. According to the authors, the flow pattern is affected by the pipe that communicates the outputs of the three cells, which acts as a mixer. The flow inlet is at the bottom while the outlet is at the top, so the electrolyte flows up through each of the three channels (Figure 9b,c). The flow distributors used as separators allow a turbulent flow regime, which improves the mass transport of the coagulating species from the anode to the solution, also avoiding passivation. In this process, after the coagulants production, the solution passes to another flocculation stage, with agitation for 15 min (45 rpm) to promote the flocs’ growth and favor the contaminants’ adsorption.
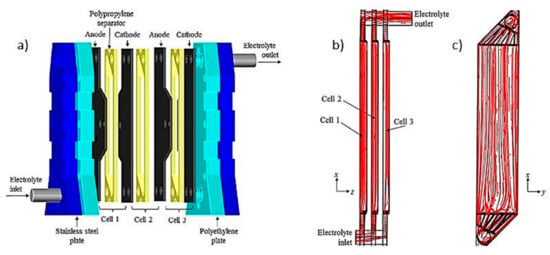
Figure 9.
(a) Reactor FM01-LC with three cells; Streamlines (b) Lateral view; (c) back view (Adapted from Sandoval et al. [50]).
Vázquez et al. [51] also evaluated the hydrodynamic behavior of the flow in a FM01-LC reactor using CFD. The authors conclude that the main reasons why there is no fully developed flow in this reactor are the asymmetric design of the flow distributor at the inlet and the fact that the distributor is inverted at the outlet. To correct this problem, Cruz-Díaz et al. [23] developed a new geometry in the inlet and outlet distributors of a FM01-LC reactor (Figure 10a,b). In this study, the authors compared the flow distribution using CFD between the typical FM01-LC reactor and their modified one. As a result, the new reactor configuration has a more homogeneous velocity field in the reaction zone. Immediately after the flow inlet, an inverted V-shaped obstacle is encountered, which helps the mixing at the inlet distribute the same amount of mass in the ducts formed in the first line of obstacles next, which are intended for the fluid to move with approximately the same speed. Subsequently, the second line of obstacles promotes the fluid entering the electroactive zone with the same speed. At the same height of the second line of obstacles, there is a reduction in both walls of the reactor to avoid the channeling of the flow near the walls in the typical FM01-LC reactor. On the other hand, the geometry of the outlet manifolds helps to concentrate the flow to have a uniform velocity in the outlet tube. With the new geometry developed by these authors, the velocity of the fluid element is the same at the beginning of the active zone, and when entering the outlet ducts (Figure 10c), high and low-speed zones are minimized in the electroactive area, and it is possible to have a fully developed flow.
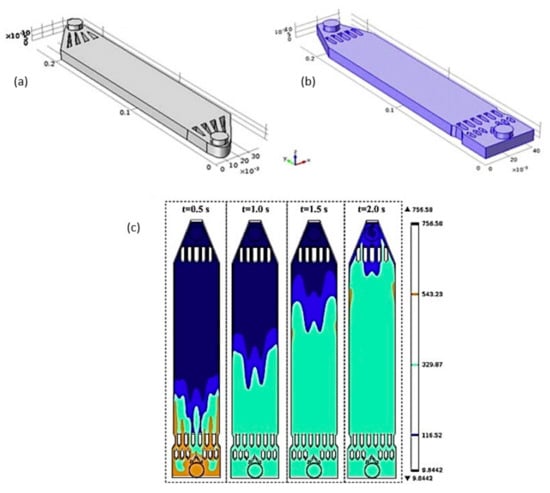
Figure 10.
Inlet and outlet geometry: (a) common FM01-LC; (b) new design; (c) mass transfer distribution of tracer (adapted from Cruz-Díaz et al. [23]).
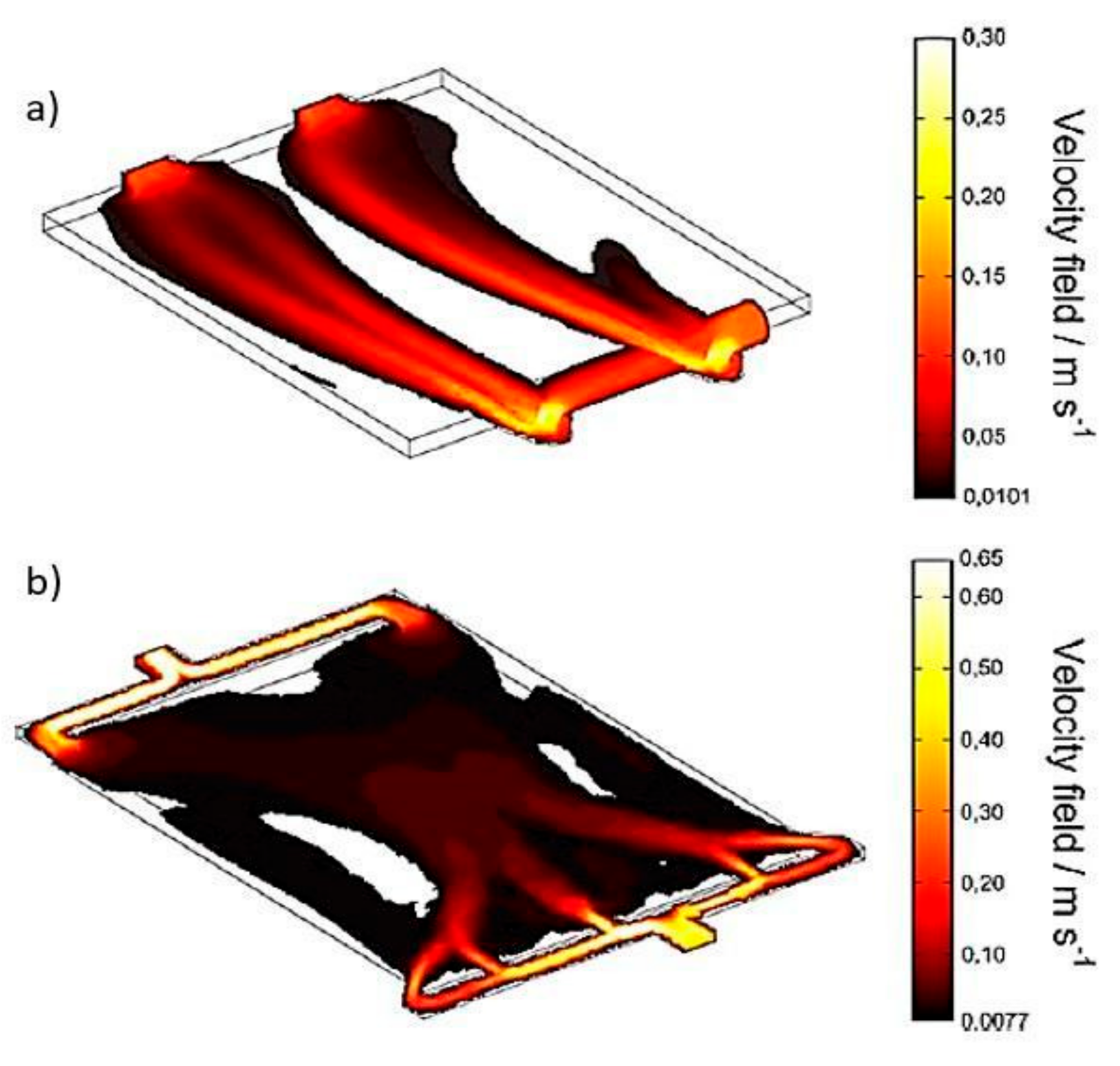
Frías-Ferrer et al. [22] also adapted the inlets and outlets distributors of a filter press reactor used for electrodialysis, water treatment, and organic synthesis. Through CFD and experimental validation, it was determined that a new configuration of the inlet and outlet distributors promotes optimal mixing conditions due to a more uniform distribution (Figure 11) and avoids harmful processes such as corrosion of the electrodes and the formation of unwanted products.
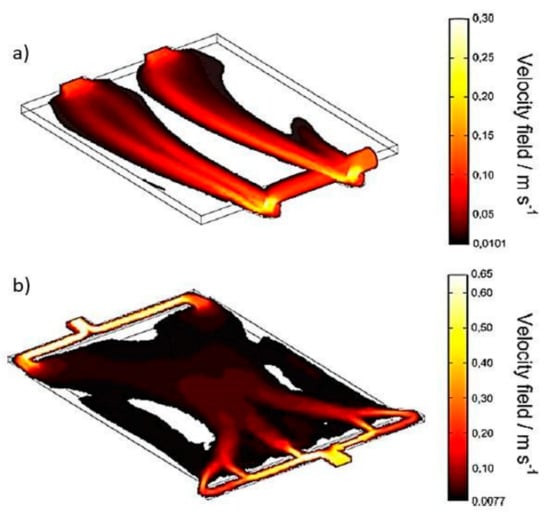
Figure 11.
Velocity field in reactors: (a) established design; (b) new design. Empty zones designate stagnant zones [22].
4.2. Rectangular Horizontal Flow Reactors
This type of design is generally an adaptation of the batch reactor, the water to be treated is introduced with a pump, achievieng the continuos flow. This reactor can overflow into which the treated water comes out, and in some cases, a settler or a system can be added to separate the sludge from the water. The reactor can be configured in a single channel; the electrode plates act as flow deflectors. There are also suspended plates in different configurations and designs or rod electrodes.
Kim et al. [52] developed an EC process to remove various dyes in a single channel continuous rectangular reactor. The authors point out that due to the arrangement of the zigzag electrodes and the fact that the spacing between them was minimal, it could be considered that there is no mixing of the flow in the longitudinal direction. Therefore, it is considered a plug flow tubular reactor (PFTR). The process allows one to obtain high efficiencies for the dye removal in synthetic water. However, a decrease in efficiency was observed compared to synthetic solutions when dyeing wastewater is treated since it was only possible to remove 41.4% of color and 51.4% of chemical oxygen demand (COD) under optimal operating conditions.
In another study to remove another dye (Orange II), Mollah et al. [53] evaluated the EC process in a single channel continuous flow apparatus distributed by Kaselco EC Treatment Systems, Texas, USA. Under optimal conditions, this process allowed the removal of 98.5% of the dye in synthetic water. Hence, the authors concluded that the apparatus designed by the company could be a viable option to remove textile dyes.
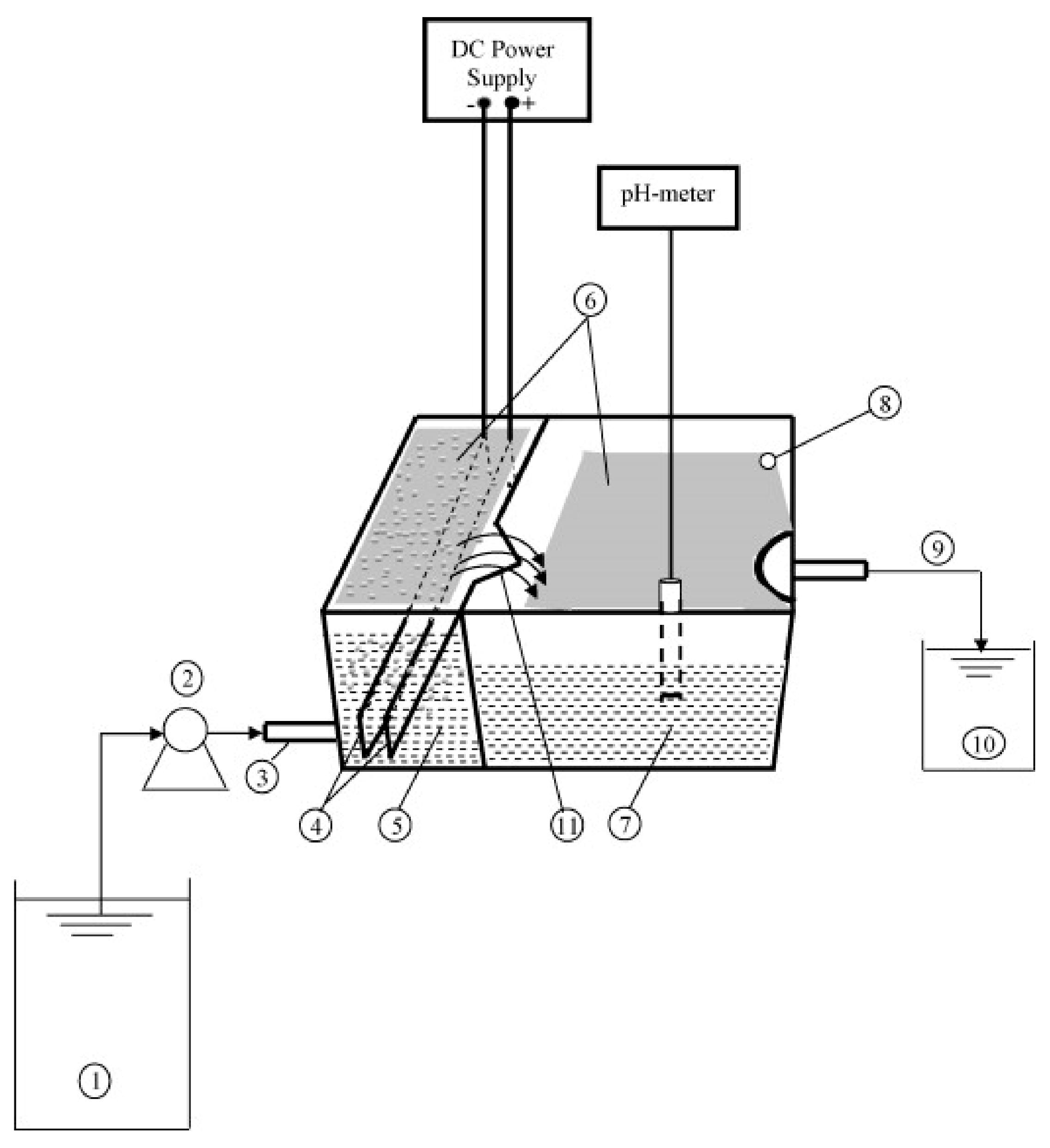
Merzouk et al. [1] evaluated the performance of a rectangular horizontal flow reactor in which the EC and electroflotation processes are carried out. The cell was divided into two compartments; in the first one, the EC process is carried out. The treated water passes to the second compartment by overflowing, where the sludge is separated from the water by flotation or sedimentation (Figure 12). In this process, the optimal parameters to remove red dye and COD, efficiencies greater than 85% and 80%, respectively, were obtained.
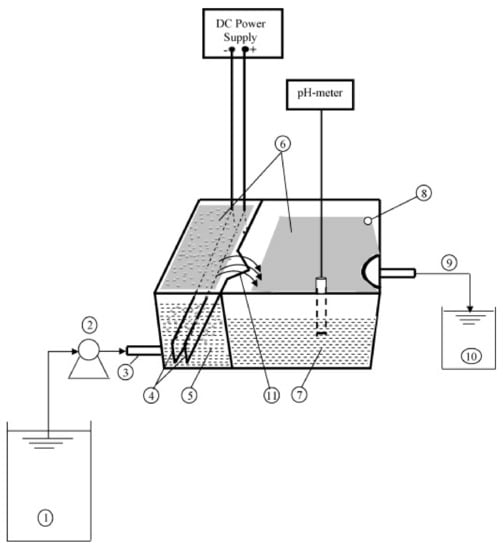
Figure 12.
Experimental setup of continuous EC cell: (1) wastewater tank; (2) peristaltic pump; (3) inlet of the first compartment; (4) electrodes; (5) first compartment; (6) sludge; (7) second compartment; (8) sludge exit (overflow); (9) treated effluent outlet; (10) treated water tank; (11) outfall [1].
Kobya et al. [14] conducted a study to treat zinc phosphate coating wastewater from an automotive assembly plant. The authors first evaluated the batch process to determine the optimal operational parameters. Subsequently, they carried out the process in a continuous flow, varying the flow from 50 to 400 mL/min, and the electrode material, iron, and aluminum. The aluminum electrodes showed to be more efficient in removing zinc and phosphate, obtaining removal efficiencies for both pollutants greater than 95% in all the evaluated flows. In comparison, the iron electrodes allowed efficiencies in the range of 85.5% to 99.6%. In addition, the increase in flow was shown to be the factor that most influenced the efficiency decrease.
Vázquez et al. [43] performed an analysis of primary potential and current distributions using CFD, considering these parameters as critical factors in the energy-efficient design of an EC reactor. The reactor consisted of a single channel rectangular cell with eleven aluminum electrodes, six cathodes and five anodes in parallel connection, with a continuous flow of 1.6 L/min. The process was performed in wastewater from a paper mill to remove turbidity, evaluating COD and biochemical oxygen demand (BOD) values. Optimization of the process was carried out through uniform distribution of potential and current. However, the authors conclude that a more uniform current distribution does not significantly affect the final COD and BOD concentrations. Nevertheless, the proper current distribution, achieved when both edges of the anodes are insulated, is a determining factor in energy consumption. This, in turn, promotes a higher rate of aluminum ion production, which increases the removal of colloidal matter.
Vázquez et al. [26] also conducted a CFD study to determine the hydrodynamics and the current and potential distribution in a multichannel cell used for an EC process. The reactor consisted of an open cell with thirteen aluminum electrodes, six anodes and seven cathodes. An outstanding aspect in the design of this reactor was the modification in the aluminum plates used as cathodes by making holes in them to minimize the production of hydrogen bubbles and thus reduce the potential increase in the cell. Therefore, the authors sought to optimize the process in terms of energy consumption by analyzing the distribution of current and potential and by modifying the design of the electrodes. As a result, the authors conclude from their analysis that it is better not to isolate the electrodes in this reactor design because this reduces the formation of aluminum flocs. Under optimal conditions, this process efficiently removed turbidity (97% removal) Recently McBeath et al. [54] also carried out a CFD modeling of the hydrodynamics and current distribution in a horizontal continuous flow EC reactor. This study observed that the areas of low current density have a higher flow velocity. Therefore, the distance between electrodes was increased to avoid this phenomenon, and a more uniform current distribution was observed, although a higher ohmic resistance was also generated.
Song et al. [24] studied the hydrodynamics and species generation process in a rectangular reactor with four iron electrodes, acting as flow deflectors. These authors tested two configurations in the arrangement of the electrodes to determine which was the optimal one. In this type of reactor, the configuration of Figure 13a,b is generally used. In this study, the authors conclude that the traditional configuration is better than the new proposed configuration (Figure 13c,d) because the original configuration offers more significant mass transfer and more uniform mixing. They evaluated four flow rates (0.035, 0.043, 0.055, 0.077 L/min) to determine this parameter’s effect on the removal efficiency of arsenic and antimony. The best efficiencies were obtained at a flow of 0.043 L/min, 99.73% for arsenic, and 94.87% for antimony. An efficiency decrease was observed at higher flow values. The authors conclude that even though the increase in flow favors mass transfer by reducing stagnation zones, the removal efficiency of pollutants decreases because the formation of flocs is not favored under these conditions. In this study, a CFD theoretical evaluation and experimental of the current and potential distribution was also carried out to observe the effect of insulating the edges of the anodes. The results showed that the highest efficiencies are obtained when both edges are insulated due to a more uniform current distribution. They consider that this parameter is essential in designing an EC reactor, as Vázquez et al. [43].
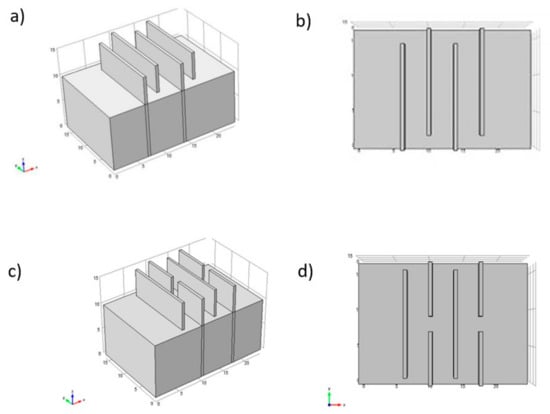
Figure 13.
Diagram of three-dimensional configuration and cross-sections: (a) typical configuration; (b) X-Y cross section; (c) new configuration; (d) X-Y cross section (adapted from Song et al. [24]).
Mohora et al. [29] evaluated an EC-flocculation (ECF) system to remove arsenic in groundwater. The reactor used was a continuous horizontal flow. The reactor geometry, within which the coagulation, flocculation, flotation, and sedimentation units were found together, caused the processes to occur in parallel. The pollutants were evacuated by flotation and constantly removed into a tank. The polarity of the electrodes was changed every 30 and 15 min. In this reactor, no additional complementary equipment such as the sedimentation tank is considered, nor the separation of the sludge from the treated water; samples were taken from the reactor through a sampling point at the determined residence time of the treatment. With this process, arsenic was removed with 93% efficiency from an initial concentration of 51 µg/L. In another study, Mohora et al. [55] evaluated the removal of arsenic in the same reactor using iron electrodes treating a volume of groundwater of 7.9 L. The initial concentration of arsenic was 38.15 µg/L, and they were able to remove 96%. The same reactor was used in both processes, but the electrode material was different. However, although the removal efficiencies are similar, the operating conditions were different because, in the first study, a current density of 8.86 mA/cm2 and a flow of 7 L/h were applied. In contrast, in the second study, these values were 1.98 A/m2 and 12 L/h.
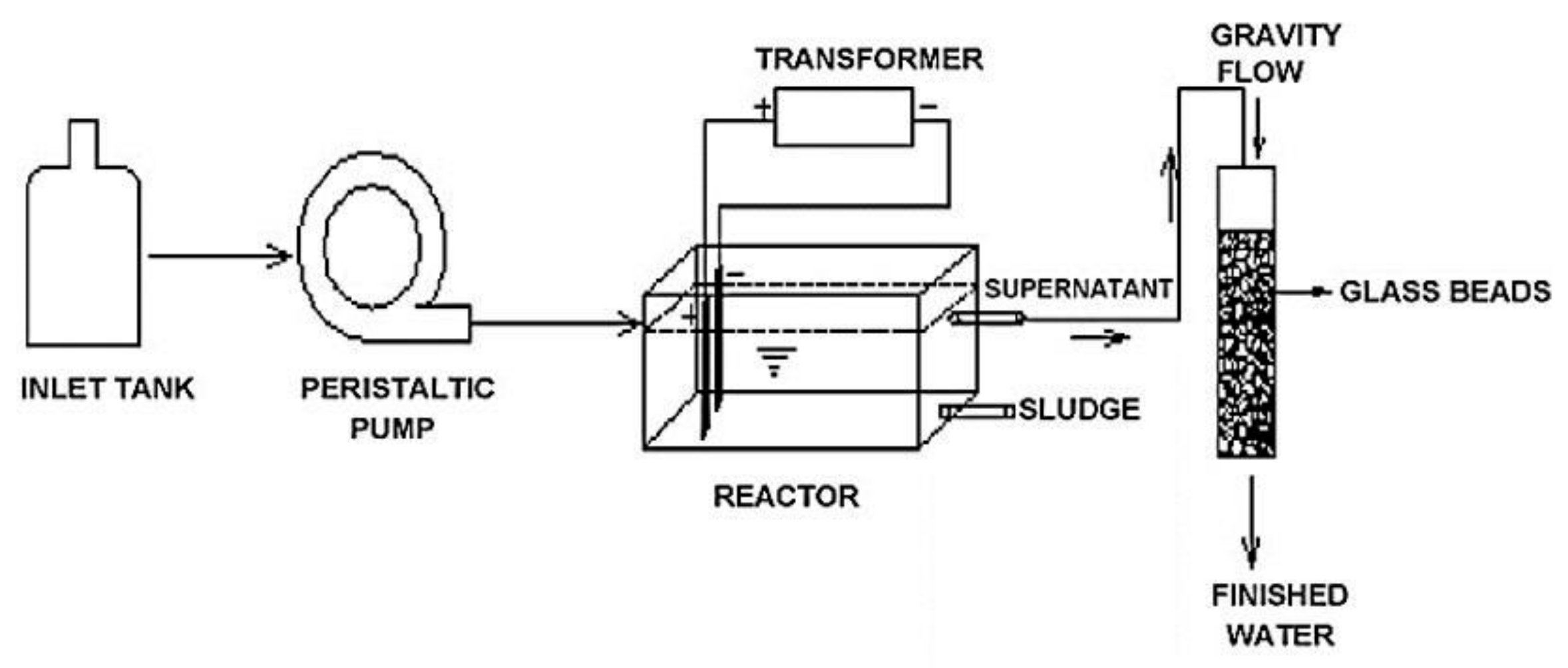
Kumar and Goel [28] designed and built an EC reactor manufactured in Perspex with 36 cm × 12 cm × 11.5 cm dimensions. A flow of 2 L/h was supplied with an estimated retention time of 2 h. Two steel electrodes with 14.5 cm × 2.5 cm dimensions and 0.1 cm of thickness were used, which were immersed (9 cm), and the distance between them was 7 cm. First, the coagulation, flocculation, sedimentation, and flotation stages occur inside the reactor. Then, the sludge was separated through the lower part of the reactor; and the treated water was passed to a 20 mm filtration column, in which the flow was by gravity, filled with 6 mm glass beads, whose purpose was to remove suspended solids and turbidity (Figure 14). In this study, an analysis using a tracer to determine if the flow in the reactor approached a plug flow reactor (PFR) or continuous stirred tank reactor (CSTR) and obtained the real residence time was also carried out. The results showed that this design is closer to a CSTR and that the residence time was 3.86 h versus the design time of 2 h.
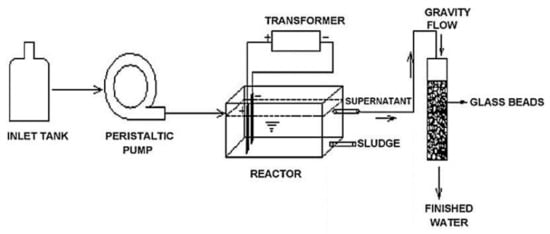
Figure 14.
Experimental set-up diagram [28].
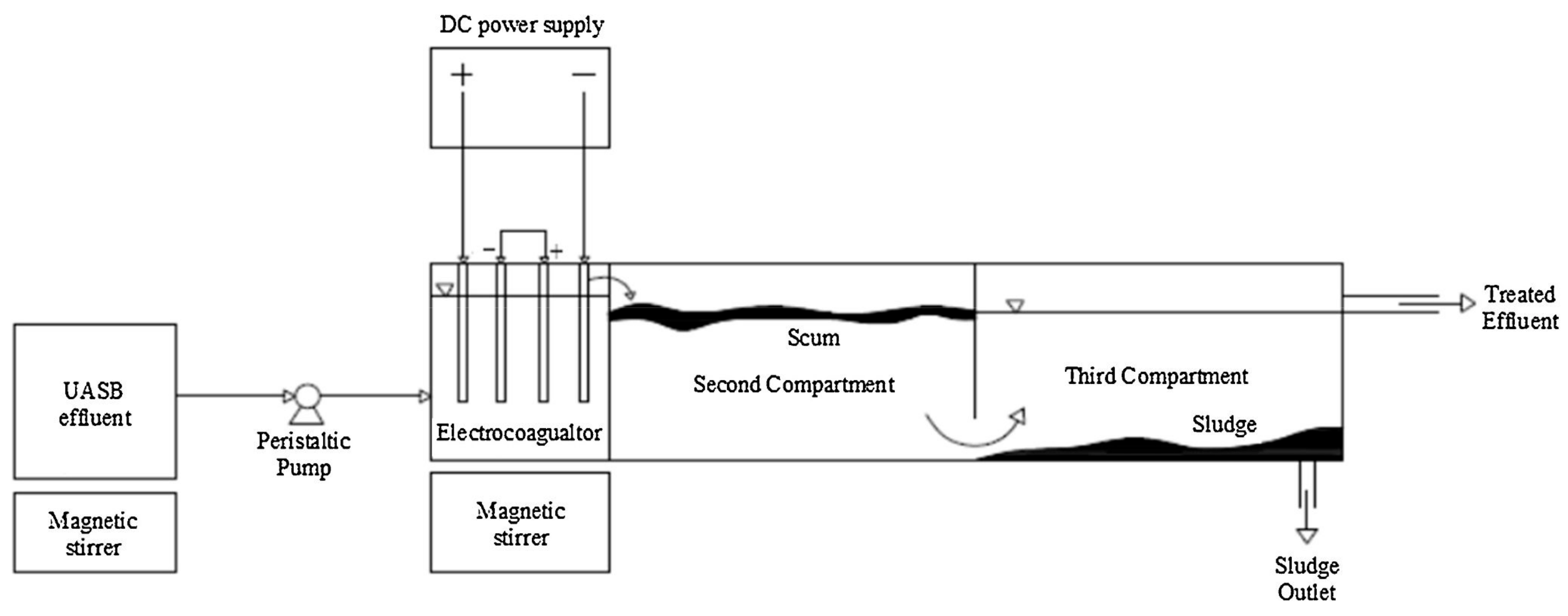
Another reactor design that considers the separation of sludge and effluent is the one developed by Makwana and Ahammed [56]. It consisted of a reactor with a capacity of 8.3 L and was divided into three compartments (Figure 15); in the first one, with a capacity of 1.5 L, the EC process was carried out. Later, the water passed to the second compartment in which the foam was removed, and finally, in the third compartment, it settled and removed the sludge from the effluent. The mentioned unit was used as a post-treatment of anaerobically treated wastewater. COD removal was evaluated by varying the current density and residence time parameters, achieving removal of 67.15% of COD. As an extra, the authors mention that they obtained 99.91% removal of total coliforms and 99.86% of fecal coliforms, so this process as post-treatment in wastewater treatment plants (WWTP) could be auspicious.
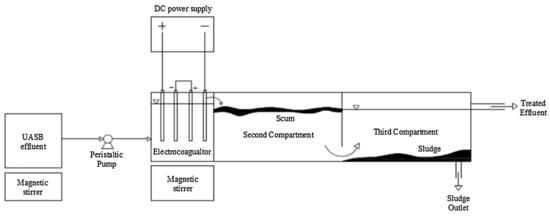
Figure 15.
Experimental set-up [56].
Karamati-Niaragh et al. [57] used a horizontal flow reactor to remove nitrate. A section for the settler after the EC process is also considered in this design. The authors evaluate the efficiency of the process in terms of removal and cost, considering mainly the effect of the application of alternating current and direct current. The results showed that the use of alternating current has an effect mainly in reducing the consumption of the electrodes. As a result, the process allows obtaining removal efficiencies greater than 60% at the cost of USD $54/(kg nitrate removal) applying direct current and USD $29/(kg nitrate removal) for alternating current.
Abbasi et al. [58] evaluated a continuous process for the treatment of licorice processing wastewater through a reactor that consisted of the EC cell and a sedimentation/flotation cell where the sludge was removed. In this reactor, four iron electrodes in vertically arranged plates were used, connected to direct current, while two pairs of four plates were placed without connection between the vertical plates for a bipolar connection. An external stirrer carried out stirring. The process allowed removal with efficiencies of 90.1% for color, 89.4 for COD, 82% for turbidity, and 73.3 for alkalinity. Ezechi et al. [59] evaluated the process in a rectangular reactor in which four pairs of electrodes are suspended, and stirring is provided using a magnetic stirrer. In this study, iron and aluminum were evaluated as the electrode material to remove boron from produced water, which comes from the gas and oil extraction process. Aluminum as electrode material turned out to be more efficient to remove boron, achieving a removal percentage of 84% in a residence time of 45 min. The authors point out that reducing the residence time results in a removal decrease; because a longer residence time implies a longer contact time between the electroactive species and boron, and therefore, a more significant flocs formation.
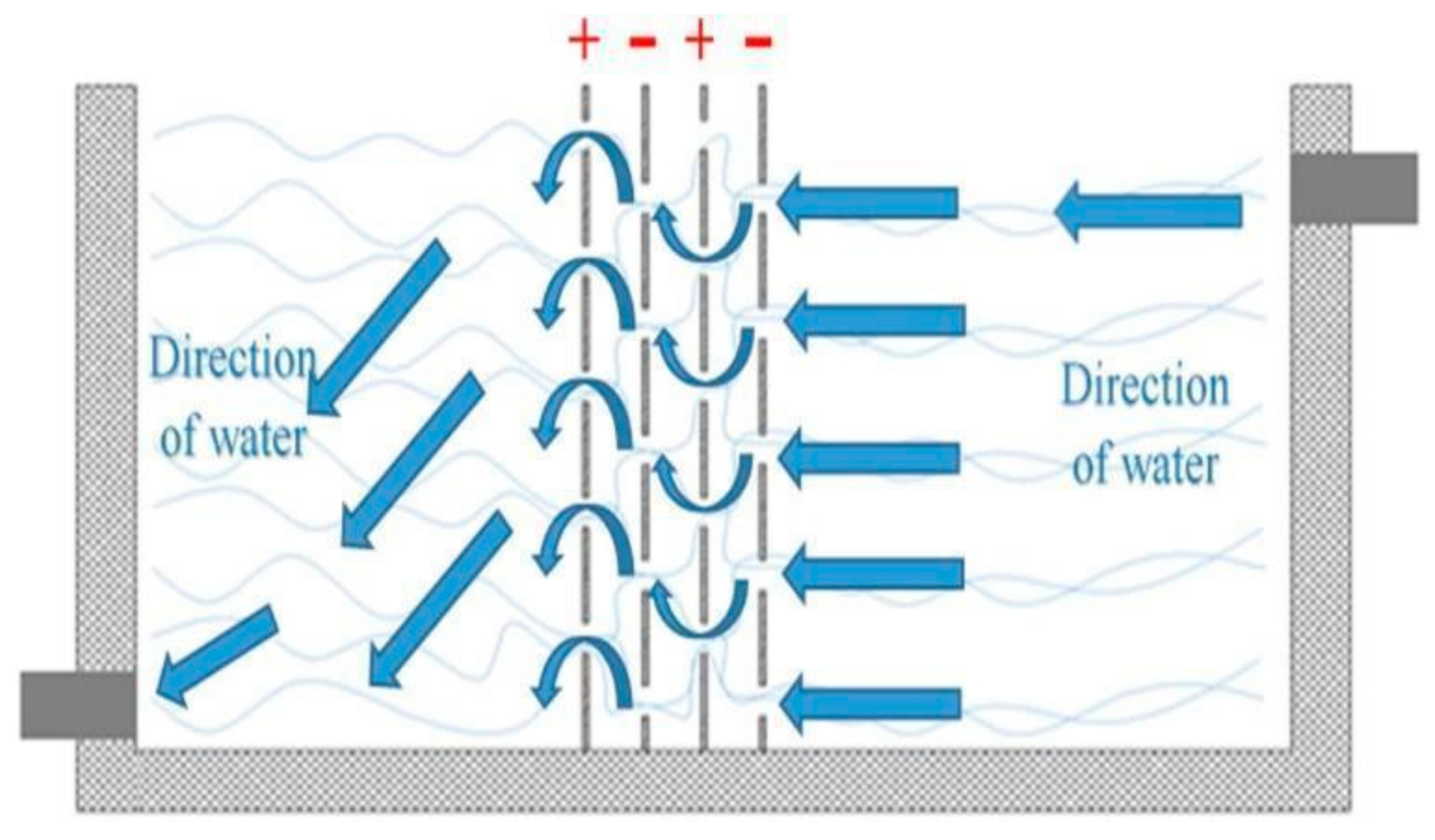
In most rectangular continuous reactors, the mixing occurs by the flow and the electrodes’ geometry arrangement. Some authors have chosen to test novel configurations on the plates used as electrodes to promote mass transfer. Such is the case of Abdulhadi et al. [25], who used plates with 35 circular perforations to promote flow mixing without the need for an external mixing element. The different distribution of the perforations in anodes and cathodes promotes even better mixing (Figure 16), which reduces energy consumption. The process was able to remove 99.9% of the iron in 50 min of treatment.
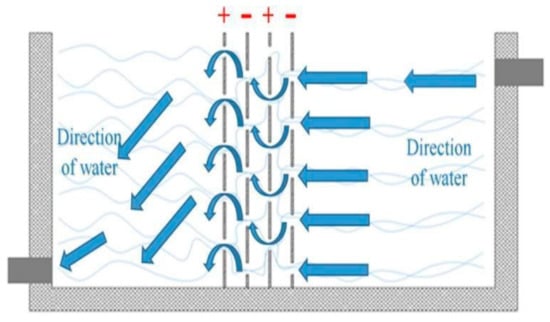
Figure 16.
Electrode design and electrode arrangement to promote mixing [25].
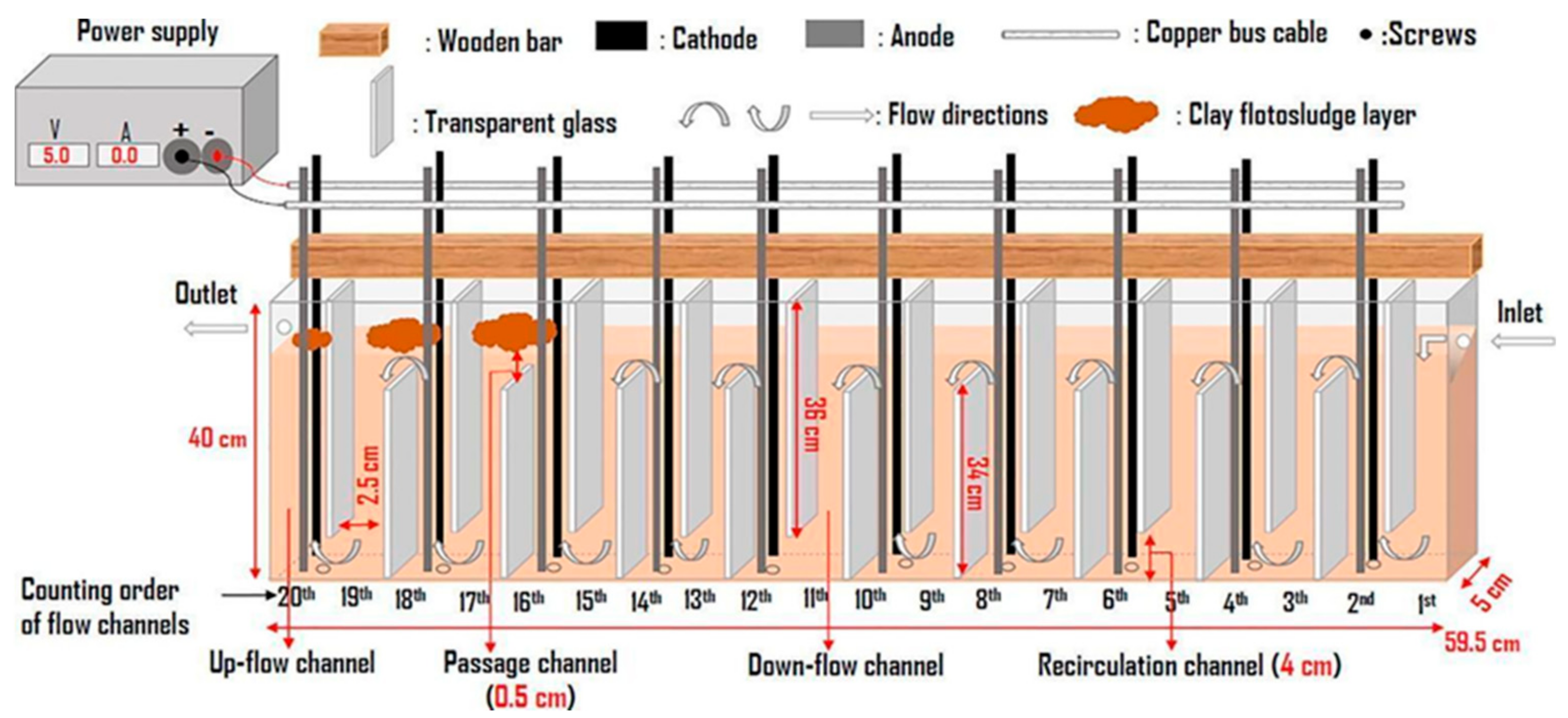
In addition to the designs in the plates that promote mixing, other elements have been added in the reactor which acts as flow deflectors and promote more effective hydrodynamics inside the reactor, reducing dead zones or stagnation. Ntambwe Kambuyi et al. [60] present in their recent research a novel system of a single channel continuous flow reactor in which the baffles are glass plates. The reactor consists of 20 channels which, according to the arrangement of the plates, can be upward or downward flow, with a pair of aluminum electrodes in the upward flow channels; in total, there are ten pairs of electrodes (Figure 17). The authors evaluate with this design the convenience of using many electrodes or not, reducing the pairs of active electrodes until the optimal configuration is found. Since the electrodes do not act as baffles, the hydrodynamic advantages that glass baffles offer by inactivating the single or multi-channel electrode pair are not sacrificed. The authors conclude that the optimal configuration is four pairs of electrodes working in the first eight channels of the reactor, while the last channels act as mixers and promoters of floc formation. This is because when there are more electrodes in the cell, the electro-dissolved aluminum from the last channels will not have sufficient residence time. Therefore, its contribution will be minimal for removal efficiency.
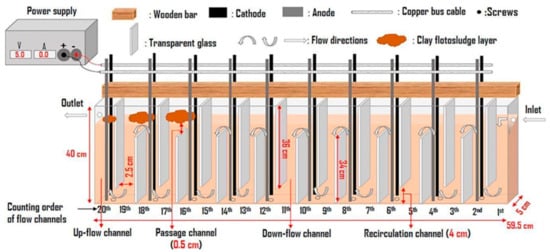
Figure 17.
Configuration of a recent continuous flow reactor design [60].
The application of electric current, necessary for the EC process, is the aspect that most influences the cost of the process and has been one of the limitations to apply this technology. Aside from optimizing the process in terms of operational parameters, cell and electrode designs, some researchers have also explored the possibility of supplying the electricity needed for the process through renewable energies such as solar. For example, Nguyen et al. [19] evaluated the possibility of using solar energy for the process in a continuous flow rectangular reactor using a 12 V solar panel, in addition to comparing the efficiency using a direct current source. With promising results for its application in rural areas, this system treated 12 L/h with solar energy achieving an arsenic removal >91%. Furthermore, it was estimated that the anode could be replaced after 500 days if the system was operated for 4 h per day.
Hendaoui et al. [61] developed a continuous EC process to treat effluent from the textile industry, including a smectite-rich clay adsorption unit. The process consists of two rectangular reactors, the first for the EC process and the second for the adsorption process. The authors point out that the process can remove color, COD, and total suspended solids (TSS) with efficiencies of 96.87%, 89.77%, and 84.46%, respectively, with the addition of a clay suspension in a flow of 100 mL/min. Another relevant result of this process combination is the adsorption process’s contribution in removing the measured parameters. Compared to the efficiencies of the EC process alone, clay adsorption promotes color removal from 87.3% to 96.58%, for COD from 81.4% to 89.62%, and for TSS from 73.75% to 84.5%.
Kuokkanen et al. [20] used a reactor based on the patent FI127889B of Rajaniemi et al. [62]. The process is evaluated to remove turbidity, COD, Cr, Zn, and Al from wastewater from a metallurgical industry, obtaining removal efficiencies of 91%, >91%, 95%, 93%, and 85%, respectively. In addition, the authors carried out filtration tests to determine the effect that the filter size has on the metal concentration in the treated water from the dissolution of the electrodes; the results showed that the filter size has a significant influence on the residual concentrations of the anode material, in this case, iron or aluminum. Although residual aluminum is present in those samples filtered at sizes of 10 μm and 5 μm, the filter sizes that most remove aluminum are those with a size of 0.45 μm to 3 μm. This is an essential contribution to the study of the feasibility of the EC process since the quality of the treated water can vary according to the degree of filtration that occurs at the end of the process. However, the authors of this study emphasize that this type of analysis should be done in other EC processes to treat another type of water because the characteristics of the study water may influence its results.
Wu et al. [63] developed a process to remove methyl orange in a continuous flow reactor in which the electrodes are iron plates bent in a zigzag. In this research, the effect of the flow rate, the spacing between the electrodes, and the bending angle in the plates used as electrodes was studied on the removal efficiency. The flow rate study showed that both high and low flow rates do not promote high color removal efficiencies; in a range of 15 to 105 L/h, a flow of 60 L/h was optimal. According to the results, the distance between electrodes was established at a value of 2.5 cm, while the bending angle on the plate was 75°, thereby obtaining a color removal efficiency of 99.23%. After obtaining these parameters, the authors evaluate the effect of the initial concentration, the current density, and the treatment time, the first of the variables being the one that has a more significant influence on the discoloration, in addition to indicating that the interactions between the factors mentioned above have no significant effect. From these operational parameters, an optimization of the process is carried out through the statistical model. A removal of 90.53% was estimated for an initial concentration of 134.03 mg/L of dye. Experimental validation of this model was carried out, obtaining an average removal percentage of 92.35%. In addition, the process performance with flat plates was compared under the same conditions, and the efficiency was 58.9%. The authors of this study emphasize that it is necessary to carry out a statistical study for each reactor and, in this way, obtain the critical variables of each process.
4.3. Vertical Flow Reactors
This type of reactor has also been studied in various designs and has shown good efficiencies to remove various pollutants. For example, Parga et al. [64] developed a continuous EC system to remove arsenic. This design has a porous tube through which contaminated water passes. The air is injected into the tube before passing through the vertical electrodes found in the cell, with seven carbon steel electrodes used as anode and cathode. The vertical geometry of the electrodes through which the flow received passes the use of oxygen and hydrogen gas generated in the hydrolysis of the water will facilitate the removal of the ferric and ferrous species in which the pollutants are removed. The electrode spacing was 6 mm, and their dimensions were 10.0 cm × 15.4 cm, while the cell volume was 1.2 L. These same authors subsequently applied the process on a larger scale (pilot plant) for the treatment of well water with 0.040 mg/L of arsenic at a flow of 30 L/min. The system was coupled to pumps and separation tanks using a filter press to collect residual sludge after the process. The arsenic removal achieved with this system was around 99%. Parga et al. [65] evaluated the same system for arsenic and chromium removal; air injection improved the efficiencies from 95.54% to 99.77% for arsenic removal, while for chromium, the increase was from 97.3% to 99.9%.
Emamjomeh and Sivakumar [27] designed and built a continuous system for EC. The cell consisted of a rectangular Perspex acrylic container. In addition, sedimentation and flotation tanks were configured, with a volume of 49 L. This is a complete design since it considers the separation of the sludge from the treated water through the sedimentation of the sludge particles by gravity. In this way, it is possible to have a container for the sludge and another for the treated water. This study evaluated the effect of flow rate in the range of 150 to 400 mL/min on removal efficiency. It was observed that as the flow increases, the removal efficiency decreases for the same current density because as the flow increases, the retention time decreases.
Moussavi et al. [15] evaluated an EC process to treat petroleum-contaminated groundwater. These authors evaluated the batch process’s and once the optimal parameters were obtained, these were applied to evaluate the performance of the continuous process. As a result, an increase in removal was obtained from 67.2% to 93.4% of the total petroleum hydrocarbons, increasing the residence time from 10 to 60 min. Thus, the authors conclude that the performance of a continuous process depends on the residence time.
Lu et al. [66] evaluated a monopolar continuous design for vertical flow, consisting of a single compartment reactor with two aluminum electrodes arranged in parallel (Figure 18). The flow considered in this system was laminar, and the purpose of the study was to determine a mathematical model for mass transfer and the generation of ionic species. Furthermore, this model was experimentally validated. This system had already been used in the study to remove nickel ions from water by Lu et al. [67]. These authors made an analysis based on the mass transfer through the channel and proposed a new optimization parameter for the design of continuous flow reactors based on a molar ratio of the adsorbed pollutant ions and the aluminum ions produced by the anode (M/Al ratio). In addition, the molar ratio in the sludge produced was analyzed. This study determined that current density and residence time influence the molar ratio; as current density and residence time increase, the M/Al molar ratio decreases. This reactor was also used to remove fluoride [68] and compare the batch’s performance and continuous process. The results showed that the performance of the continuous process in this reactor design is more efficient to remove fluoride than the batch process for the range of current densities evaluated from 2.5 to 12.5 A/m2. This was because in the batch process of this reactor, there is no mixing, and therefore, there is not enough floc distribution. On the other hand, when evaluating the effect of residence time in both processes, it was concluded that, as the residence time increases, the continuous process was less efficient than the batch process since back mixing occurs due to the turbulence generated by the different phases present in the process, and therefore there is a gradient in the residence time, which does not exist in the batch process. However, the continuous process allowed the removal of 92.74% of fluorides in a residence time of 20 min.
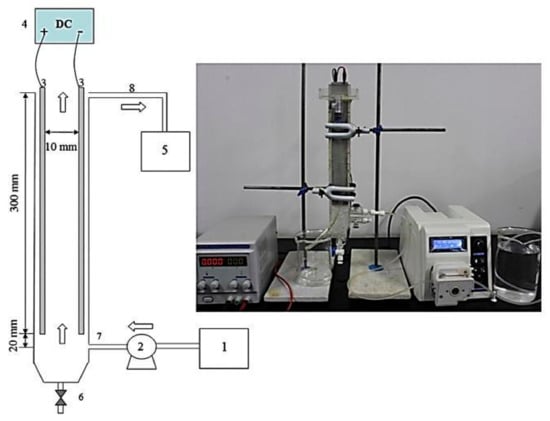
Figure 18.
Schematic diagram and experimental set-up of EC process: (1) Raw water tank; (2) Peristaltic pump; (3) Aluminum electrodes; (4) DC power supply; (5) Outflow tank; (6) Sludge disposal; (7) Influent; (8) Effluent [67].
Gaalova et al. [69] designed a continuous EC treatment system to remove Fe3+ ions. The system operated at a flow of 40 L/h with a residence time of 46 s. The cathode used was stainless steel, while aluminum and mild steel were used as the anode. After the EC treatment, a stirring of 10 to 25 rpm was carried out to promote the entry of oxygen and the formation of the flocs, thus allowing sedimentation for a time of 60 min.
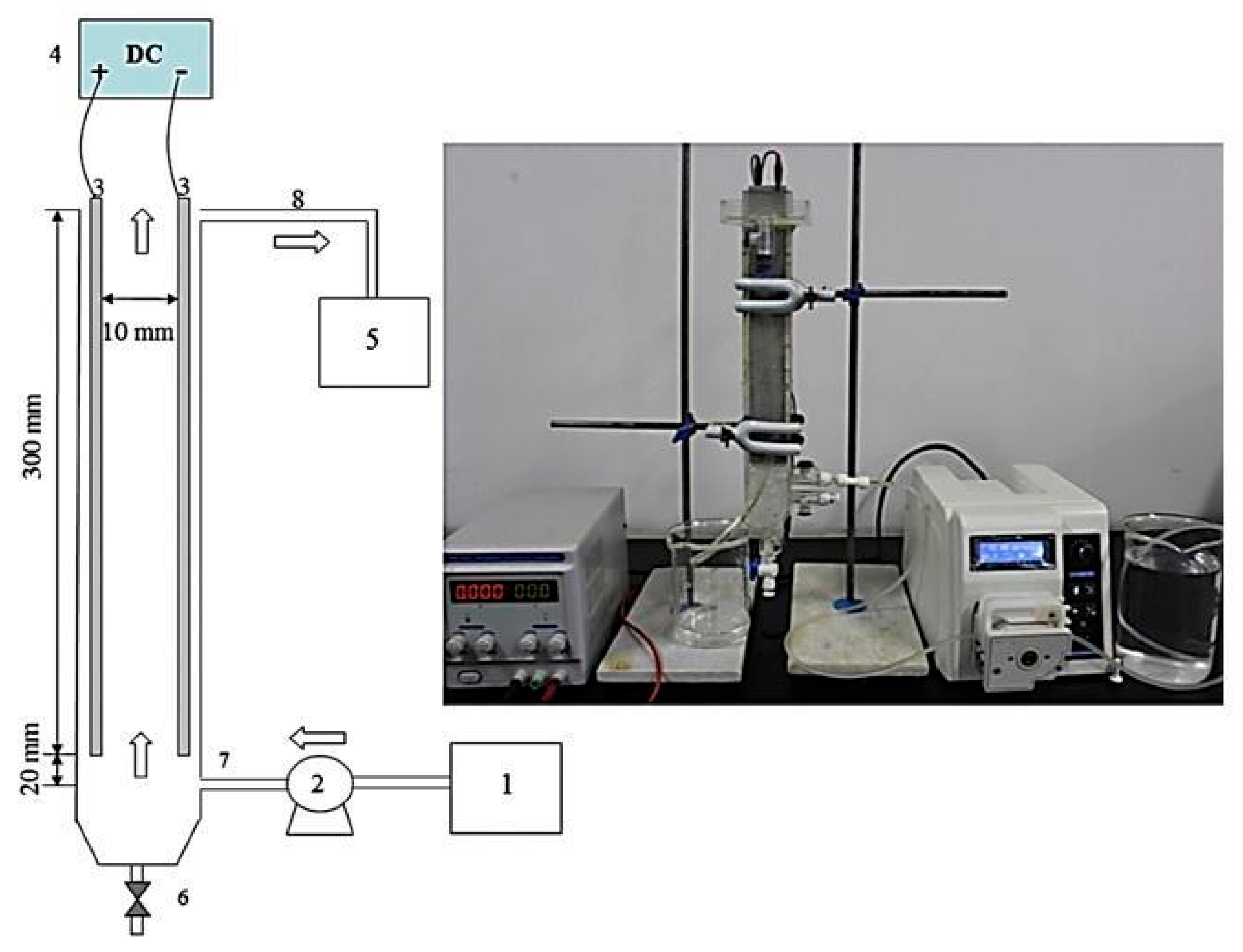
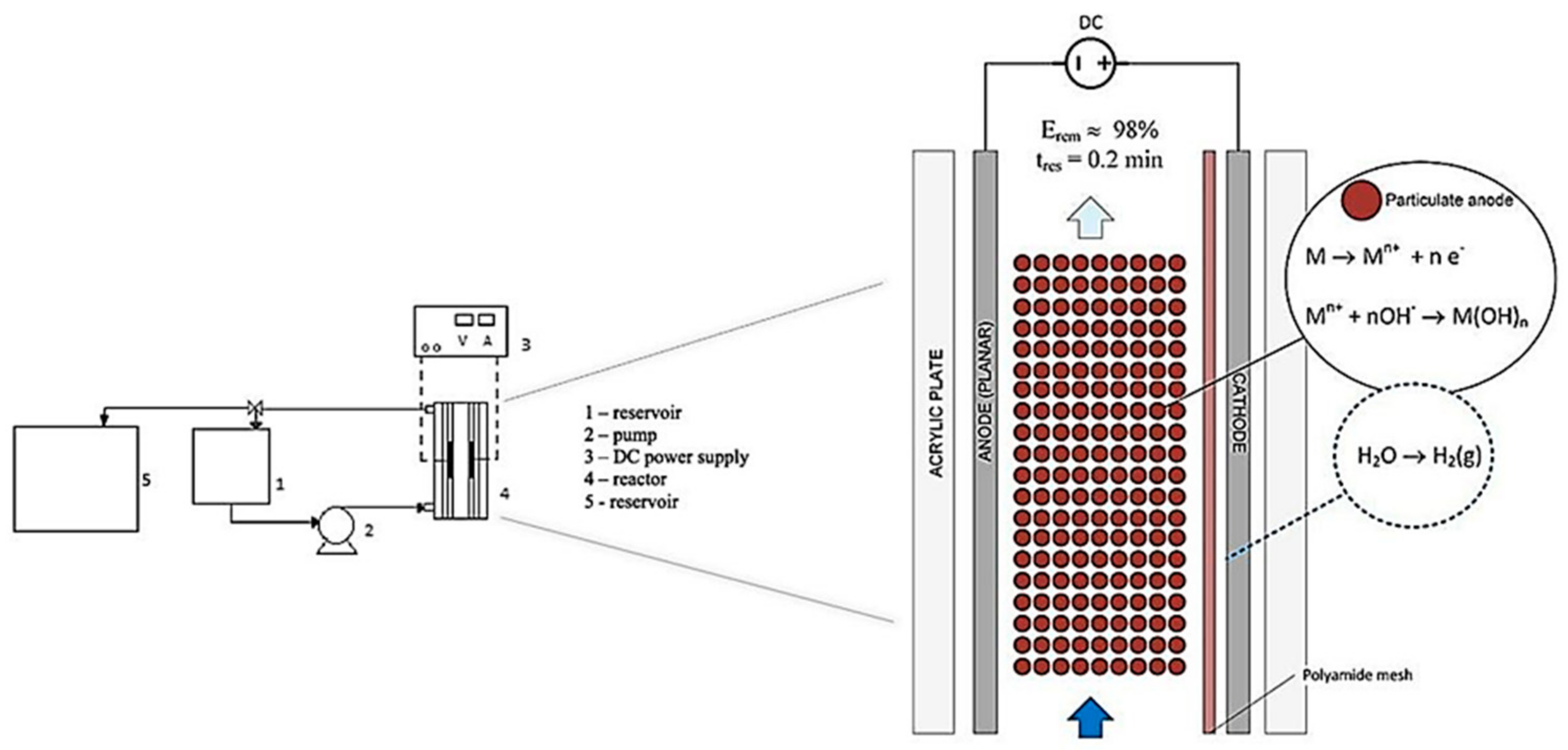
Rodrigues et al. [18] used a new vertical flow EC reactor design to remove color, using a fixed bed anode made of metal spheres with a diameter of 2.4 mm (Figure 19). This reactor was evaluated for the batch process to determine the electrode material that provided better efficiencies (aluminum or steel particles). This batch study determined that the best material was iron; therefore, these particles were used for the continuous process achieving 98% of color removal in optimal conditions. Furthermore, the authors compare the same reactor with a flat anode, and the result was a higher performance using the particulate anode in most of the operating conditions. In addition, a comparison of the results of this research against other processes evaluated with plate electrodes concludes that the study reactor being equally efficient in removal percentages, but in a shorter treatment time, only 0.2 min. Therefore, this reactor type can be a viable alternative for continuous EC processes.
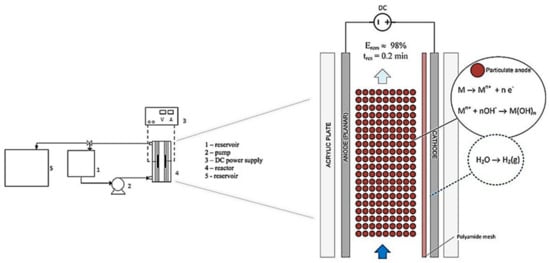
Figure 19.
Schematic representation of the experimental system (adapted from Rodrigues et al. [18]).
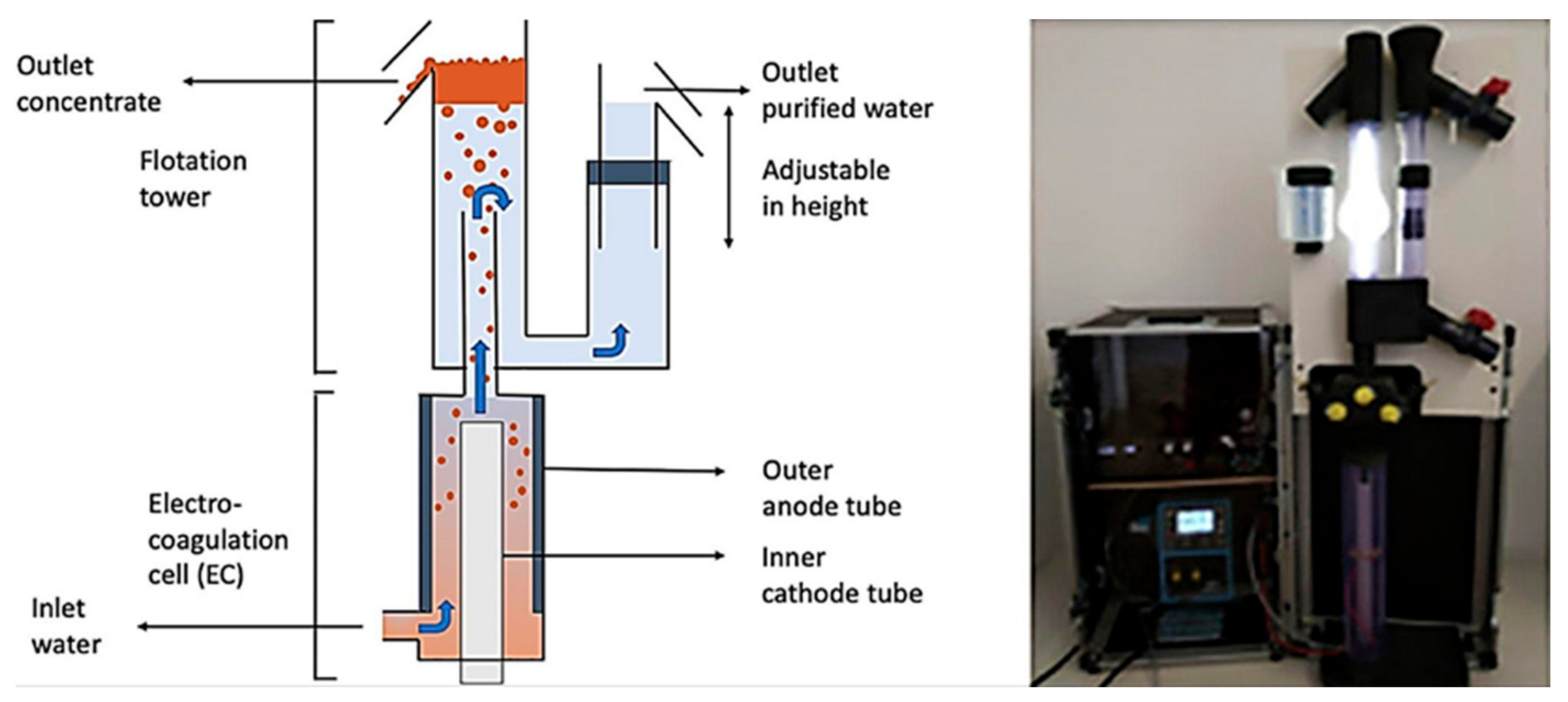
Another novel vertical flow design developed is recently used for algae harvesting by Parmentier et al. [70]. This consists of a commercial flotation–electrocoagulation tubular reactor distributed by Noah Water Solutions. The EC device is like a tower, where the EC process is carried out in the bottom, and the flotation/flocculation at the top with the solids and water separation. This commercial design includes a DC converter and a pump to feed water to the system (Figure 20). In this study, the removal of 88% of Chlorella vulgaris microalgae was achieved.
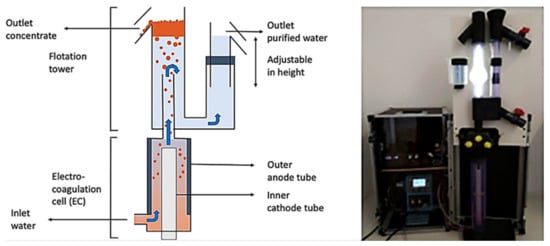
Figure 20.
Schematic diagram and setup of the electrocoagulation-flotation tubular reactor (adapted from Parmentier et al. [70]).
5. Application of the EC Process in the Removal of Pharmaceuticals
In recent years, the presence of pharmaceutical compounds has been detected in surface waters and groundwater. These compounds were designed to act and be effective at small doses. Therefore, it is possible that, although the doses found in aquatic bodies are low, they may have an effect and be pharmacologically active in other microorganisms for which they were not originally designed [71]. Therefore, its presence in water poses a potential risk to aquatic organisms and humans. The primary source of pharmaceutical compounds to surface water bodies is wastewater treatment plants. Some compounds can be degraded to a certain degree during biological treatment [72]. However, there is the possibility that such degradation results in a metabolite that can become more toxic than the parent compound [73]. Sanitary landfills are also an important source of emerging pollutants since they generate leachates that, if not adequately contained, can infiltrate into groundwater or drain into a surface water source [74]. In some cases, the source of these substances is directly poor disposal of pharmaceutical products, which are dumped directly into the drainage system [75]. The use of wastewater for irrigation of various plantations results in the release of pharmaceuticals to the soil. Gibson et al. [76] conducted a study in the irrigation district of Valle de Tula in Mexico, in which untreated wastewater was used. This study determined that some acidic pharmaceuticals and endocrine disruptors have poor mobility through the soil horizons; however, carbamazepine was present in the most superficial and deep horizons, so it can be considered a persistent pharmaceutical compound with a high potential to contaminate groundwater. In their study, Lesser et al. [77] also found carbamazepine and sulfamethoxazole in groundwater from the Mezquital Valley in Mexico.
In the case of veterinary products, these are of special consideration since, in most cases, they do not pass through the drainage system. They therefore do not have a biological treatment in the treatment plants that degrades them in some measure [75]. These substances are released directly into the environment and can reach bodies of water through runoff. Once pharmaceuticals have reached a surface water body, such as a river or lake, they can reach groundwater in lower concentrations. On the other hand, aquifer recharge is also a potential source of emerging pollutants in groundwater [78].
Although its presence in water has not yet been regulated, concern about the possible impact on human health and the environment has led to the search for a solution to the problem posed by pharmaceutical compounds. In this context, recent studies for the removal of pharmaceuticals have shown that the EC process is efficient and can become a viable alternative to remove these types of pollutants (Table 2).

Table 2.
Electrocoagulation processes for the removal of pharmaceuticals.
Arsand et al. [79] applied the EC process to remove dexamethasone from hospital wastewater. In this process, up to 38.1% of the drug was removed. It was observed that dexamethasone removal begins up to 15 min of treatment due to competition with other organic pollutants present in wastewater; therefore, the authors recommend this process as a pretreatment for advanced oxidation processes. The removal mechanism is by trapping colloidal particles by sweep flocs.
Ouaissa et al. [80] removed 96.5% of tetracycline in two minutes of treatment from a synthetic solution with an initial concentration of 10 mg/L. However, they emphasize that these results must be corroborated in a real effluent. Nariyan et al. [7] evaluated the process of removing oxytetracycline hydrochloride, comparing the performance of the anode material, achieving removal efficiencies of 93.2% and 87.75% for iron and aluminum, respectively. According to the authors, this process is feasible to remove oxytetracycline in the range of 50 to 200 mg/L. Finally, Baran et al. [81] evaluated the removal efficiency of four antibiotics: ampicillin, doxycycline, sulfathiazole, and Tylosin. Of all of them, only doxycycline, belonging to the tetracycline antibiotics subgroup, was removed completely, while the only removal of 3.6%, 3.3%, and 3.1% was achieved for ampicillin, sulfathiazole, and Tylosin, respectively. These three studies demonstrate that the tetracycline group of antibiotics is susceptible to removal by the EC process.
Barışçı and Turkay [82] in their study obtained removal of 86.6% of ciprofloxacin in 10 min of treatment, from an initial concentration of 5 mg/L and an applied current density of 4.325 mA/cm2. Another study to remove this drug was carried out by Yoosefian et al. [83]; the process evaluated by these authors was able to remove practically all the ciprofloxacin from a concentration of 60 mg/L in 20 min applying a current density of 15 mA/cm2. Both processes had the iron anode as material. The differences between these studies are mainly due to the initial concentration and the applied current density. In general, it is necessary to apply a higher current density at higher concentrations to achieve high efficiencies. Ahmadzadeh et al. [84] also evaluated the removal of ciprofloxacin through the EC process, although the anode material was aluminum in this process. The study was carried out in synthetic water with a content of 32.5 mg/L of ciprofloxacin, current density 12.5 mA/cm2, and a time of 20 min, reaching a removal of 88.57%. Under the same operating conditions, a complete removal is achieved in hospital wastewater with an initial concentration of 154 µg/L of ciprofloxacin.
Ensano et al. [85] evaluated the feasibility of removing diclofenac, carbamazepine, and amoxicillin from municipal wastewater obtained from the WWTP primary clarifier. Removal efficiencies increase as treatment time increases while increasing current density from 0.3 to 0.5 mA/cm2 shows an increase in efficiency; however, at higher current densities, the efficiency decreases due to passivation at the electrode. The authors conclude that amoxicillin and diclofenac are removed by charge neutralization and electro-flotation, while carbamazepine is adsorbed on coagulants.
Oulebsir et al. [86] compared the EC process and nanofiltration (NF) process on the efficiency of each process to remove amoxicillin; They also evaluated a combined system of both processes to verify the contribution that the EC process can have on the NF process. In both processes, pH was a determining factor in removal efficiency. As a result, the EC process removed 52.7% of amoxicillin at pH 3, while in the NF process, an efficiency of 99% was obtained at pH 10. On the other hand, in the combined process, EC followed by NF, similar efficiencies were observed regardless of pH, since the efficiencies were 98.2% and 97.5% at pH 2.5 and 10, respectively. In this study, the use of Ca(NO3)2 as electrolyte in the EC process is noteworthy, which was transformed into Ca(OH)2 deposits in the cathode in which some of the amoxicillin was trapped. According to the authors, calcium was involved in the products generated.
Negarestani et al. [87] remove acetaminophen and ibuprofen with efficiencies of 33.15% and 59.32%, respectively. Applying a double-stage EC process, it was possible to increase these efficiencies to 48% and 78%. The authors suggest that ibuprofen removal may be due to charge neutralization, whereas both could precipitate on the coagulant surface, and sweep coagulation could occur.
Padmaja et al. [88] carried out a comparative study between the efficiency of chemical coagulation and EC to treat water from a pharmaceutical effluent, the leading indicators being the removal of COD and total dissolved solids (TDS). Chemical coagulation was able to remove 93.7% of COD using Alum and 90.0% of COD with FeCl3 and 14.5% of TDS; on the other hand, the EC process removed 92.3% and 91.5% of COD and TDS, respectively. In conclusion, the EC process did not require additional chemicals. Furthermore, it produced less sludge and clearer water than the chemical coagulation process, so it is considered a process with greater efficiency and applicability.
Govindan et al. [89] obtained the removal of >90% of TOC by EC process in three compounds: acetaminophen, antipyrine, and atenolol. According to the analysis carried out, the possible degradation routes of the pollutants can be degraded by direct anodic oxidation and other redox reactions. It was observed that the degradation of atenolol and acetaminophen results in less dangerous molecules. Due to their low hydrophobicity, the compounds could be removed by neutralization of charges.
Kumari and Kumar [90] removed 60% of acetaminophen from river water in 120 min of treatment. In this study, the effect of the electrode material (iron, stainless steel, and aluminum) was evaluated, being the aluminum electrode with which the highest efficiencies were achieved. According to the sludge analysis, the removal mechanism was by trapping and adsorption on the Al(OH)3 flocs. According to the results obtained, the authors point out that this process is feasible to remove concentrations of up to 2 mg/L efficiently.
Lu et al. [91] evaluated the process to remove tetracycline-Cu (TC-Cu) complexes, which are formed when the drug and metal meet in water; these complexes are very stable and are considered more difficult to remove than other contaminants. The study evaluates the removal of TC, TOC, and Cu2+, and the efficiencies obtained were 100%, 80.2%, and 88.1%, respectively. TC and Cu2+ are removed by adsorption, and the latter could also be removed due to an electroreduction and flotation process. The electrode material was iron, coinciding with the use of this material with the study carried out by Nariyan et al. [7] to obtain 93.2% removal of oxytetracycline.
Oliveira et al. [92] showed in their study that the anode material dramatically influences the removal efficiency. When evaluating the process to remove trimethoprim and amoxicillin separately and in a mixture; it was observed that trimethoprim is better removed when the anode is stainless steel, while amoxicillin and the mixture are removed in major degree with aluminum anode.
6. The EC Process Application in Hybrid Processes
Because of how versatile the EC process can be, in terms of the removal of pollutants of diverse nature, in the last decade there has been interest from various groups of researchers around the world to determine the contribution that this process can have by coupling it with other pollutant removal technologies in various types of water.
The EC process has been coupled with technologies such as electrooxidation [93,94,95,96], ozonation [97,98,99], electro-Fenton [97,100,101,102], adsorption [103,104,105], advanced oxidation processes [97,106], biofiltration [107], ultrafiltration [108], reverse osmosis [106], sonication [109], ultrasonication [110,111], electrocatalysis [112], photocatalysis [113], oxidative media filtration [114], catalytic wet air oxidation [105], E-peroxone process [115], gravity membrane bioreactor [116,117] and microflotation [118], to name a few. In this context, the EC process has increased the efficiencies, either pre-treatment or post-treatment, of other processes (Table 3). Contaminants such as chromium complexes (Cr-EDTA), which are difficult to remove by chemical coagulation, have been efficiently removed (> 99%) by electrooxidation (EO) followed by an EC process [93]. These two processes were also evaluated by García-García et al. [94] but in the reverse order, that is, EC process followed by EO process, to remove COD, total organic carbon (TOC), color and turbidity of industrial wastewater, achieving efficiencies of 99.7%, 70.26%, 100% and 95%, respectively. The hybrid EC-EO process has proven to be efficient in removing nitrates. In the EC process, it is possible to remove nitrate. However, at the end of this, ammonium ions are presented as by-products, which are removed by the EO process [96].

Table 3.
Hybrid processes and efficiency improvement with the electrocoagulation process as pre-treatment or post-treatment.
Zazou et al. [97] evaluated the effect of each of three advanced oxidation processes (electro-Fenton (EF), anodic oxidation (AO), and peroxy-coagulation (PC)) after the EC process to determine which is the most viable for treating wastewater from the textile industry. The efficiencies of any combination of processes were more significant than the EC process alone; however, the performance of the EC-EF process was the most efficient, removing 97% of TOC under the same operating conditions in model water. This process was applied in the treatment of textile wastewater, removing 98.1% of TOC and 100% of turbidity.
Different pollutants may be present simultaneously in the water, and some of them may not be susceptible to removal through the EC process; this is the importance of evaluating hybrid processes that allow the removal of two or more pollutants of different nature present in the water. McBeath et al. [114] evaluate in their study the effect of the EC process combined with an oxidative media filtration for the removal of arsenic and manganese. The results showed that arsenic is efficiently removed by the EC process (80% removal), which did not occur with manganese (only 10% removal). However, a significant reduction of manganese (56%) was observed in the filtration process, while the arsenic was removed almost entirely.
The combination of adsorption (AD) and EC has been evaluated to remove TOC, turbidity, color, metals, and organic compounds. Bulca et al. [105] used as adsorbent rice husk-based activated carbon (RHAC) after the EC process. In the latter, a 42% removal of TOC was achieved, and by coupling both systems, it was possible to remove 91% of TOC. The authors compare this combination of processes and the EC process followed by catalytic wet air oxidation (CWAO). The EC-CWAO combination had a lower efficiency with removal of only 62% of TOC. However, the study concludes that this last hybrid system may be a promising alternative considering that organic pollutants are degraded by CWAO and not only transferred from one phase to another, as in adsorption. Another EC-AD system evaluated by Nigri et al. [103] to remove organic compounds, calcium, and strontium from a water effluent from the oil industry showed that when these processes are combined, metals are removed by the EC process (88% calcium removal and 72% strontium removal), while organic compounds are removed by adsorption (52% removal of organic matter). Because industrial effluents can contain organic and inorganic pollutants, this hybrid system can be an option for their treatment. Hussin et al. [104] also evaluated the EC-AD combination. However, in this system, both processes were carried out in the EC reactor, adding the adsorbent to it. As a result, 99.88 % of Pb(II) removal was obtained with the hybrid system, while the EC process and adsorption separately only had efficiencies of 78.5% and 79.3%, respectively.
On the other hand, the sonication (S) process in conjunction with the EC process has shown itself to be efficient in removing arsenic. However, the cost increases due to the energy requirement of both processes [111]. As an advantage of this combination, the S process improves the EC process’s efficiency by cleaning the electrodes’ surface, which allows a uniform generation of coagulants [109]. The combination of the EC process with this type of technology to inactivate Escherichia coli has recently been explored; Hashim et al. [110] developed a hybrid process applying an ultrasonic field to an EC reactor through which it was possible to inactivate all the E. coli. On the other hand, Fan et al. [113] obtained similar results, 99.92% inactivation, through an EC process combined with electrocatalysis. These results show that the EC process coupled with various treatment technologies is a promising alternative to solve the presence of various pollutants. However, it is necessary to balance the convenience of these coupled systems in terms of efficiency and the increase in the cost of the final treatment, either due to energy requirements or consumables.
7. Known Aspects of the EC Process and Perspectives
The intense research work on the EC process has resulted in significant contributions to the knowledge and understanding of this process. It has also highlighted its versatility to treat almost any type of water and its efficiency in removing many pollutants. As a result of decades of research, the works of various authors have been pooled and discussed in a considerable amount of reviews [21,31,32,33,34,37,44,119,120,121,122], the last two years being those in which more works of this type have been published [38,123,124,125,126,127,128,129,130,131,132,133,134,135].
In retrospect, the theoretical aspects of the process, the pollutants removed, the critical operating parameters, the characterization of sludge, and the optimization by various techniques have been the objects of study most addressed. Recently, emphasis has been placed on energy consumption and the viability of using renewable energies [21,33,119,129], and the application of the process in the removal of organic and pharmaceutical pollutants [38,134]. In addition, suggested as the next level of development and application of this technology has been the evaluation in continuous flow reactors and treatment of natural water instead of synthetic water, as well as the optimization of the process through a suitable design of the reactor assisted by CFD [21,31,34,37,119,131]. The latest research trends indicate that it is necessary to address the growth and structure of flocs as part of the reactor design [132], and implement strategies to avoid passivation, and therefore understand this phenomenon [127]. Hybrid and combined processes have a strong trend in recent years [135]. As discussed above, combining the EC process with other technologies has proven to be feasible and efficient. However, there is still no escalation of these processes [129]. On the other hand, it has also been recommended to carry out studies on strategies to reduce the consumption of the electrodes [135] such as developing new electrode designs [38,128] and explore the use of another type of electrode material that is more ecological [129] such as Mg [133].
The EC process for the recovery of metals and elements of interest has recently been studied. Zhang et al. [136] reported having recovered 95% of lithium ions from a synthetic solution with the use of aluminum electrodes; the parameters evaluated were practically the same that have been evaluated for the elimination of contaminants such as current density, treatment time, initial pH and spacing between the electrodes. Despite obtaining promising results in the recovery of lithium, it is not established how the separation of the metal from the precipitates, identified as lithium aluminum chloride hydroxide hydrate (LiCl·2Al(OH)3·xH2O) and crystal structures of LiCl·2Al(OH)3, could be carried out. In addition, the authors make a comparison of the costs of the EC process versus chemical precipitation with aluminate, the latter being higher in cost (0.108 USD/g Li) versus 0.023 USD/g Li of the EC process. Other electrochemical technologies such as electrodialysis, capacitive deionization, and electrochemically switchable ion exchange have been used to recover lithium. However, most studies have been carried out only at the laboratory level [137]. Due to the importance of this metal for its application in batteries, the EC process can be outlined as a viable method for its recovery, especially if it is possible to develop efficient systems in continuous flow. Another metal that has been the subject of study for its recovery through the EC process is copper. In their recent study, Mehdipoor and Moosavirad [138] obtained a copper recovery more significant than 90%, so it is concluded that this process could be a viable option for use in mineral processing plants. On the other hand, Li et al. [139] have evaluated the removal and recovery of uranium from wastewater from the uranium extraction process in a mine using a chelation process through chelating ligands and EC with iron and aluminum, and magnesium anodes. The use of the iron anode showed the highest uranium removal capacity (99.16%). From the sludge produced, the flocs were eluted, digested in acid, and later, the uranium amide product was obtained by alkaline precipitation. The authors detail that they obtain a uranium recovery of 89.71%, so they suggest that this technique can help to remove and recycle uranium from this type of wastewater.
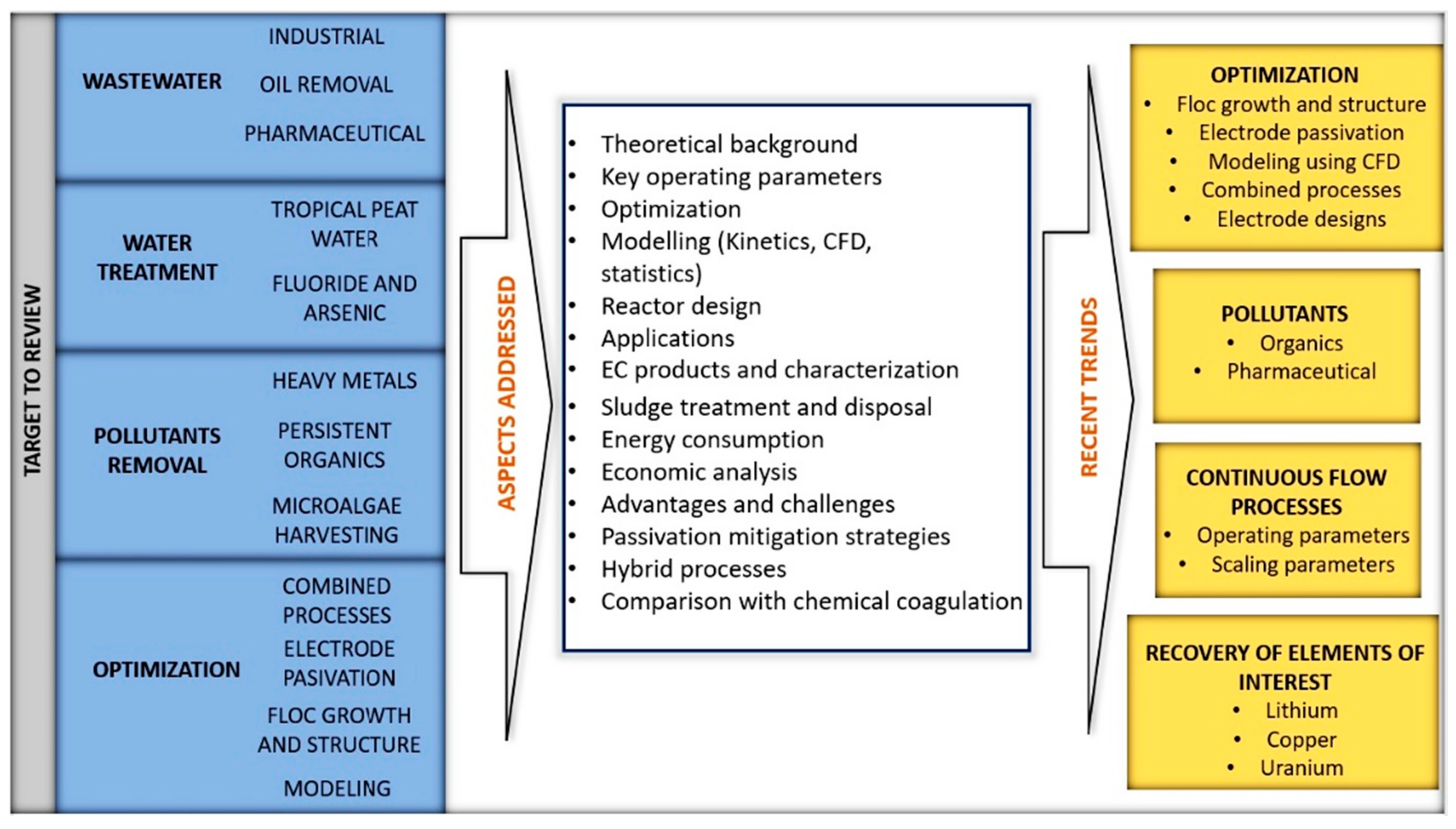
Figure 21 summarizes the objectives and aspects addressed so far and the most recent research trends, according to what has been reviewed in the available literature. It is essential to mention that new information appears periodically due to the interest in the EC process. Therefore, it is necessary to update the reviews on the most critical topics.
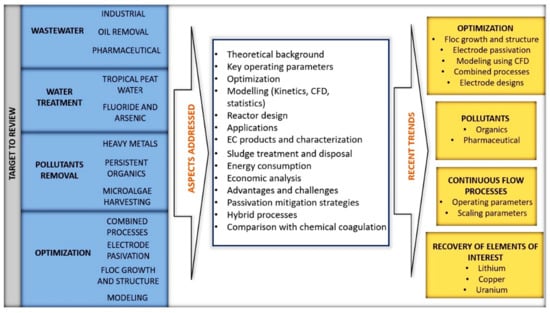
Figure 21.
Targets, aspects addressed and recent trends of the EC process.
8. Conclusions
The EC process is a treatment technology that has proven to be very versatile since it allows the removal of a wide variety of contaminants, including pharmaceuticals. The studies carried out have made it possible to know that the removal mechanisms of these compounds can be direct anodic degradation, charge neutralization, and adsorption in coagulants. Furthermore, this process has been shown to contribute to efficiency when coupling it with other treatment technologies. Nevertheless, it is necessary to consider the cost-benefit of using hybrid technologies.
The need to give treatment and remove contaminants of different nature present in the water makes it necessary for this process to be evaluated on a larger scale and in continuous flow. Most of the continuous processes evaluated have been on a small scale. However, they have made it possible to visualize that the factors that have the most effect in a continuous process are the residence time and the flow pattern in the reactor, which is strongly influenced by the shape and configuration of the electrodes. Due to this, a reactor must be designed based on the analysis of these aspects. To achieve more efficient processes in removal and energy consumption, computational modeling using CFD is a tool that has proven to be very useful since efficiency is largely determined by hydrodynamics. For more robust models that allow describing the complex phenomena in the process, it is necessary to consider multiphase flows and the generation of species preferably.
Additionally, the water matrix in which the contaminants are contained is of great importance and influences the performance of the process. For this reason, it is necessary to obtain the operating parameters for each type of water to be treated in the process, even when a systematic and established design for the EC reactor is achieved. In addition, it is necessary to consider a complete treatment system with designs that include complimentary equipment such as settlers and filtration methods for a continuous flow process to be viable and applicable. Finally, the recovery of metals and elements of interest is more reason to develop more efficient continuous flow systems.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/pr9101831/s1. Table S1: Continuous flow electrocoagulation processes.
Author Contributions
Conceptualization, M.L.-G. and L.R.-C.; writing—original draft preparation, M.L.-G., M.A.F.-H., and L.R.-C.; writing—review and editing, M.L.-G., M.A.F.-H., and L.R.-C.; visualization, M.L.-G. and L.R.-C.; supervision, L.R.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Council of Science and Technology (CONACYT) with the scholarship grant number 727401.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not available.
Acknowledgments
Thanks to M.S.A. Luis A. Torres-Castañón of CIMAV-DGO, for his valuable technical support and collaboration. Thanks to Consejo de Ciencia y Tecnología del Estado de Durango (COCYTED).
Conflicts of Interest
The authors declare that they have no conflict of interest. The National Council of Science and Technology, which provides the scholarships grants, was not involved at any stage of the study.
References
- Merzouk, B.; Gourich, B.; Sekki, A.; Madani, K.; Vial, C.; Barkaoui, M. Studies on the decolorization of textile dye wastewater by continuous electrocoagulation process. Chem. Eng. J. 2009, 149, 207–214. [Google Scholar] [CrossRef]
- Cataldo Hernández, M.; Barletta, L.; Dogliotti, M.B.; Russo, N.; Fino, D.; Spinelli, P. Heavy metal removal by means of electrocoagulation using aluminum electrodes for drinking water purification. J. Appl. Electrochem. 2012, 42, 809–817. [Google Scholar] [CrossRef]
- Kim, K.; Baek, K.; Ji, S.; Cheong, Y.; Yim, G.; Jang, A. Study on electrocoagulation parameters (current density, pH, and electrode distance) for removal of fluoride from groundwater. Environ. Earth Sci. 2016, 75, 1–8. [Google Scholar] [CrossRef]
- Song, P.; Yang, Z.; Xu, H.; Huang, J.; Yang, X.; Wang, L. Investigation of Influencing Factors and Mechanism of Antimony and Arsenic Removal by Electrocoagulation Using Fe–Al Electrodes. Ind. Eng. Chem. Res. 2014, 53, 12911–12919. [Google Scholar] [CrossRef]
- Genc, A.; Bakirci, B. Treatment of emulsified oils by electrocoagulation: Pulsed voltage applications. Water Sci. Technol. 2015, 71, 1196–1202. [Google Scholar] [CrossRef] [PubMed]
- Khemila, B.; Merzouk, B.; Chouder, A.; Zidelkhir, R.; Leclerc, J.P.; Lapicque, F. Removal of a textile dye using photovoltaic electrocoagulation. Sustain. Chem. Pharm. 2018, 7, 27–35. [Google Scholar] [CrossRef]
- Nariyan, E.; Aghababaei, A.; Sillanpää, M. Removal of pharmaceutical from water with an electrocoagulation process; effect of various parameters and studies of isotherm and kinetic. Sep. Purif. Technol. 2017, 188, 266–281. [Google Scholar] [CrossRef]
- García-Lara, A.M.; Montero-Ocampo, C. Improvement of arsenic electro-removal from underground water by lowering the interference of other ions. Water. Air. Soil Pollut. 2010, 205, 237–244. [Google Scholar] [CrossRef]
- Kobya, M.; Ulu, F.; Gebologlu, U.; Demirbas, E.; Oncel, M.S. Treatment of potable water containing low concentration of arsenic with electrocoagulation: Different connection modes and Fe-Al electrodes. Sep. Purif. Technol. 2011, 77, 283–293. [Google Scholar] [CrossRef]
- Ali, I.; Khan, T.A.; Asim, M. Removal of arsenate from groundwater by electrocoagulation method. Environ. Sci. Pollut. Res. 2012, 19, 1668–1676. [Google Scholar] [CrossRef]
- Can, B.Z.; Boncukcuoglu, R.; Yilmaz, A.E.; Fil, B.A. Effect of some operational parameters on the Arsenic removal by electrocoagulation using iron electrodes. J. Environ. Health Sci. Eng. 2014, 12, 95. [Google Scholar] [CrossRef] [Green Version]
- Banerji, T.; Chaudhari, S. Arsenic removal from drinking water by electrocoagulation using iron electrodes- an understanding of the process parameters. J. Environ. Chem. Eng. 2016, 4, 3990–4000. [Google Scholar] [CrossRef]
- Thakur, L.S.; Mondal, P. Simultaneous arsenic and fluoride removal from synthetic and real groundwater by electrocoagulation process: Parametric and cost evaluation. J. Environ. Manag. 2017, 190, 102–112. [Google Scholar] [CrossRef]
- Kobya, M.; Demirbas, E.; Dedeli, A.; Sensoy, M.T. Treatment of rinse water from zinc phosphate coating by batch and continuous electrocoagulation processes. J. Hazard. Mater. 2010, 173, 326–334. [Google Scholar] [CrossRef]
- Moussavi, G.; Khosravi, R.; Farzadkia, M. Removal of petroleum hydrocarbons from contaminated groundwater using an electrocoagulation process: Batch and continuous experiments. Desalination 2011, 278, 288–294. [Google Scholar] [CrossRef]
- Kobya, M.; Akyol, A.; Demirbas, E.; Oncel, M.S. Removal of arsenic from drinking water by batch and continuous electrocoagulation processes using hybrid Al-Fe plate electrodes. Environ. Prog. Sustain. Energy 2014, 33, 131–140. [Google Scholar] [CrossRef]
- Thakur, L.S.; Goyal, H.; Mondal, P. Journal of Environmental Chemical Engineering Simultaneous removal of arsenic and fluoride from synthetic solution through continuous electrocoagulation: Operating cost and sludge utilization. J. Environ. Chem. Eng. 2019, 7, 102829. [Google Scholar] [CrossRef]
- Rodrigues, A.R.; Seki, C.C.; Ramalho, L.S.; Argondizo, A.; Silva, A.P. Separation and Puri fi cation Technology Electrocoagulation in a fixed bed reactor—Color removal in batch and continuous mode. Sep. Purif. Technol. 2020, 253, 117481. [Google Scholar] [CrossRef]
- Nguyen, T.T.Q.; Loganathan, P.; Dinh, B.K.; Nguyen, T.V.; Vigneswaran, S.; Ngo, H.H. Removing arsenate from water using batch and continuous-flow electrocoagulation with diverse power sources. J. Water Process Eng. 2021, 41, 102028. [Google Scholar] [CrossRef]
- Kuokkanen, V.; Kuokkanen, M.; Hynynen, I.; Kuokkanen, T. Electrocoagulation treatment of metallurgical industry wastewater—A laboratory scale batch and pilot scale continuous study. Hydrometallurgy 2021, 202, 105596. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Eiband, M.M.S.G.; de Melo, J.V.; Martínez-Huitle, C.A. Electrocoagulation and advanced electrocoagulation processes: A general review about the fundamentals, emerging applications and its association with other technologies. J. Electroanal. Chem. 2017, 801, 267–299. [Google Scholar] [CrossRef] [Green Version]
- Frías-Ferrer, Á.; Tudela, I.; Louisnard, O.; Sáez, V.; Esclapez, M.D.; Díez-García, M.I.; Bonete, P.; González-García, J. Optimized design of an electrochemical filter-press reactor using CFD methods. Chem. Eng. J. 2011, 169, 270–281. [Google Scholar] [CrossRef]
- Cruz-Díaz, M.R.; Rivero, E.P.; Almazán-Ruiz, F.J.; Torres-Mendoza, Á.; González, I. Design of a new FM01-LC reactor in parallel plate configuration using numerical simulation and experimental validation with residence time distribution (RTD). Chem. Eng. Process. Process Intensif. 2014, 85, 145–154. [Google Scholar] [CrossRef]
- Song, P.; Song, Q.; Yang, Z.; Zeng, G.; Xu, H.; Li, X.; Xiong, W. Numerical simulation and exploration of electrocoagulation process for arsenic and antimony removal: Electric field, flow field, and mass transfer studies. J. Environ. Manag. 2018, 228, 336–345. [Google Scholar] [CrossRef]
- Abdulhadi, B.; Kot, P.; Hashim, K.; Shaw, A.; Muradov, M.; Al-Khaddar, R. Continuous-flow electrocoagulation (EC) process for iron removal from water: Experimental, statistical and economic study. Sci. Total Environ. 2021, 760, 143417. [Google Scholar] [CrossRef]
- Vázquez, A.; Nava, J.L.; Cruz, R.; Lázaro, I.; Rodríguez, I. The importance of current distribution and cell hydrodynamic analysis for the design of electrocoagulation reactors. J. Chem. Technol. Biotechnol. 2014, 89, 220–229. [Google Scholar] [CrossRef]
- Emamjomeh, M.M.; Sivakumar, M. Fluoride removal by a continuous flow electrocoagulation reactor. J. Environ. Manag. 2009, 90, 1204–1212. [Google Scholar] [CrossRef]
- Kumar, N.S.; Goel, S. Factors influencing arsenic and nitrate removal from drinking water in a continuous flow electrocoagulation (EC) process. J. Hazard. Mater. 2010, 173, 528–533. [Google Scholar] [CrossRef]
- Mohora, E.; Rončević, S.; Agbaba, J.; Tubić, A.; Mitić, M.; Klašnja, M.; Dalmacija, B. Removal of arsenic from groundwater rich in natural organic matter (NOM) by continuous electrocoagulation/flocculation (ECF). Sep. Purif. Technol. 2014, 136, 150–156. [Google Scholar] [CrossRef]
- Yang, Y.; Ok, Y.S.; Kim, K.H.; Kwon, E.E.; Tsang, Y.F. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review. Sci. Total Environ. 2017, 596–597, 303–320. [Google Scholar] [CrossRef] [PubMed]
- Hakizimana, J.N.; Gourich, B.; Chafi, M.; Stiriba, Y.; Vial, C.; Drogui, P.; Naja, J. Electrocoagulation process in water treatment: A review of electrocoagulation modeling approaches. Desalination 2017, 404, 1–21. [Google Scholar] [CrossRef]
- Emamjomeh, M.M.; Sivakumar, M. Review of pollutants removed by electrocoagulation and electrocoagulation/flotation processes. J. Environ. Manag. 2009, 90, 1663–1679. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.M.D. Electrocoagulation (EC) technology for wastewater treatment and pollutants removal. Sustain. Water Resour. Manag. 2019, 5, 359–380. [Google Scholar] [CrossRef]
- Bazrafshan, E.; Mohammadi, L.; Ansari-Moghaddam, A.; Mahvi, A.H. Heavy metals removal from aqueous environments by electrocoagulation process—A systematic review. J. Environ. Health Sci. Eng. 2015, 13, 74. [Google Scholar] [CrossRef] [Green Version]
- Kenova, T.A.; Vasil’eva, I.S.; Kornienko, V.L. Removal of heavy metal ions from aqueous solutions by electrocoagulation using Al and Fe anodes. Russ. J. Appl. Chem. 2015, 88, 693–698. [Google Scholar] [CrossRef]
- Bektaş, N.; Öncel, S.; Akbulut, H.Y.; Dimoglo, A. Removal of boron by electrocoagulation. Environ. Chem. Lett. 2004, 2, 51–54. [Google Scholar] [CrossRef]
- Moussa, D.T.; El-Naas, M.H.; Nasser, M.; Al-Marri, M.J. A comprehensive review of electrocoagulation for water treatment: Potentials and challenges. J. Environ. Manag. 2017, 186, 24–41. [Google Scholar] [CrossRef]
- Zaied, B.K.; Rashid, M.; Nasrullah, M.; Zularisam, A.W.; Pant, D.; Singh, L. A comprehensive review on contaminants removal from pharmaceutical wastewater by electrocoagulation process. Sci. Total Environ. 2020, 726, 138095. [Google Scholar] [CrossRef]
- Mann, U. Principles of Chemical Reactor Analysis and Design, 2nd ed.; Mann, U., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; ISBN 9780471261803. [Google Scholar]
- Nava, J.L.; Ponce de León, C. Reactor Design for Advanced Oxidation Processes. In Electro-Fenton Process: New Trends and Scale-Up; Zhou, M., Oturan, M.A., Sirés, I., Eds.; Springer International Publishing: Singapore, 2018; pp. 263–286. [Google Scholar]
- Martinez-Delgadillo, S.; Mollinedo-Ponce, H.; Mendoza-Escamilla, V.; Gutiérrez-Torres, C.; Jiménez-Bernal, J.; Barrera-Diaz, C. Performance evaluation of an electrochemical reactor used to reduce Cr(VI) from aqueous media applying CFD simulations. J. Clean. Prod. 2012, 34, 120–124. [Google Scholar] [CrossRef]
- Chen, G. Electrochemical technologies in wastewater treatment. Sep. Purif. Technol. 2004, 38, 11–41. [Google Scholar] [CrossRef]
- Vázquez, A.; Rodríguez, I.; Lázaro, I. Primary potential and current density distribution analysis: A first approach for designing electrocoagulation reactors. Chem. Eng. J. 2012, 179, 253–261. [Google Scholar] [CrossRef]
- Sahu, O.; Mazumdar, B.; Chaudhari, P.K. Treatment of wastewater by electrocoagulation: A review. Environ. Sci. Pollut. Res. 2014, 21, 2397–2413. [Google Scholar] [CrossRef]
- Flores, O.J.; Nava, J.L.; Carreño, G.; Elorza, E.; Martínez, F. Arsenic removal from groundwater by electrocoagulation in a pre-pilot-scale continuous filter press reactor. Chem. Eng. Sci. 2013, 97, 1–6. [Google Scholar] [CrossRef]
- Hernández, J.R.; Nava, J.L.; Carreño, G.; Martínez, F. Removal of As (V) by electro-coagulation process. In Proceedings of the 13th International Conference on Water-Rock Interaction, Guanajuato, Mexico, 16–20 August 2010; Birkle, P., Torres-Alvarado, I.S., Eds.; CRC Press Taylor & Francis Group: London, UK, 2010; pp. 16–20. [Google Scholar]
- Guzmán, A.; Nava, J.L.; Coreño, O.; Rodríguez, I.; Gutiérrez, S. Arsenic and fluoride removal from groundwater by electrocoagulation using a continuous filter-press reactor. Chemosphere 2016, 144, 2113–2120. [Google Scholar] [CrossRef]
- Rivera, F.F.; Ponce De León, C.; Nava, J.L.; Walsh, F.C. The filter-press FM01-LC laboratory flow reactor and its applications. Electrochim. Acta 2015, 163, 338–354. [Google Scholar] [CrossRef] [Green Version]
- Sandoval, M.A.; Fuentes, R.; Nava, J.L.; Coreño, O.; Li, Y.; Hernández, J.H. Simultaneous removal of fluoride and arsenic from groundwater by electrocoagulation using a filter-press flow reactor with a three-cell stack. Sep. Purif. Technol. 2019, 208, 208–216. [Google Scholar] [CrossRef]
- Sandoval, M.A.; Fuentes, R.; Walsh, F.C.; Nava, J.L.; Ponce de León, C. Computational fluid dynamics simulations of single-phase flow in a filter-press flow reactor having a stack of three cells. Electrochim. Acta 2016, 216, 490–498. [Google Scholar] [CrossRef] [Green Version]
- Vázquez, L.; Alvarez-Gallegos, A.; Sierra, F.Z.; Ponce de León, C.; Walsh, F.C. Simulation of velocity profiles in a laboratory electrolyser using computational fluid dynamics. Electrochim. Acta 2010, 55, 3437–3445. [Google Scholar] [CrossRef]
- Kim, T.-H.; Park, C.; Shin, E.-B.; Kim, S. Decolorization of disperse and reactive dyes by continuous electrocoagulation process. Desalination 2002, 150, 165–175. [Google Scholar] [CrossRef]
- Mollah, M.Y.A.; Pathak, S.R.; Patil, P.K.; Vayuvegula, M.; Agrawal, T.S.; Gomes, J.A.G.; Kesmez, M.; Cocke, D.L. Treatment of orange II azo-dye by electrocoagulation (EC) technique in a continuous flow cell using sacrificial iron electrodes. J. Hazard. Mater. 2004, 109, 165–171. [Google Scholar] [CrossRef] [PubMed]
- McBeath, S.T.; Nouri-Khorasani, A.; Mohseni, M.; Wilkinson, D.P. In-situ determination of current density distribution and fluid modeling of an electrocoagulation process and its effects on natural organic matter removal for drinking water treatment. Water Res. 2020, 171, 115404. [Google Scholar] [CrossRef] [PubMed]
- Mohora, E.; Rončević, S.; Agbaba, J.; Zrnić, K.; Tubić, A.; Dalmacija, B. Arsenic removal from groundwater by horizontal-flow continuous electrocoagulation (EC) as a standalone process. J. Environ. Chem. Eng. 2018, 6, 512–519. [Google Scholar] [CrossRef]
- Makwana, A.R.; Ahammed, M.M. Continuous electrocoagulation process for the post-treatment of anaerobically treated municipal wastewater. Process Saf. Environ. Prot. 2016, 102, 724–733. [Google Scholar] [CrossRef]
- Karamati-Niaragh, E.; Alavi Moghaddam, M.R.; Emamjomeh, M.M.; Nazlabadi, E. Evaluation of direct and alternating current on nitrate removal using a continuous electrocoagulation process: Economical and environmental approaches through RSM. J. Environ. Manag. 2019, 230, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, S.; Mirghorayshi, M.; Zinadini, S.; Zinatizadeh, A.A. A novel single continuous electrocoagulation process for treatment of licorice processing wastewater: Optimization of operating factors using RSM. Process Saf. Environ. Prot. 2020, 134, 323–332. [Google Scholar] [CrossRef]
- Ezechi, E.H.; Isa, M.H.; Muda, K.; Kutty, S.R.M. A comparative evaluation of two electrode systems on continuous electrocoagulation of boron from produced water and mass transfer resistance. J. Water Process Eng. 2020, 34, 101133. [Google Scholar] [CrossRef]
- Ntambwe Kambuyi, T.; Zmirli, Z.; Bejjany, B.; Mellouk, H.; Digua, K.; Lekhlif, B.; Dani, A. Performance analysis of a continuous-flow single-channel reactor for surface water treatment using electrocoagulation process. Chem. Eng. J. 2022, 428, 131261. [Google Scholar] [CrossRef]
- Hendaoui, K.; Trabelsi-Ayadi, M.; Ayari, F. Optimization of continuous electrocoagulation-adsorption combined process for the treatment of a textile effluent. Chinese J. Chem. Eng. 2021, in press. [Google Scholar] [CrossRef]
- Rajaniemi, K.; Metsavainio, V.P.; Kuokkanen, V. Apparatus and Method for Treatment of Various Types of Water and Wastewater Based on Electrocoagulation Field. Finnish Patent and Registration Office, Helsinki FI 127889B, 2019. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2017178707 (accessed on 11 October 2021).
- Wu, Z.; Dong, J.; Yao, Y.; Yang, Y.; Wei, F. Continuous flowing electrocoagulation reactor for efficient removal of azo dyes: Kinetic and isotherm studies of adsorption. Environ. Technol. Innov. 2021, 22, 101448. [Google Scholar] [CrossRef]
- Parga, J.R.; Cocke, D.L.; Valenzuela, J.L.; Gomes, J.A.; Kesmez, M.; Irwin, G.; Moreno, H.; Weir, M. Arsenic removal via electrocoagulation from heavy metal contaminated groundwater in la Comarca Lagunera México. J. Hazard. Mater. 2005, 124, 247–254. [Google Scholar] [CrossRef]
- Parga, J.R.; Cocke, D.L.; Valverde, V.; Gomes, J.A.G.; Kesmez, M.; Moreno, H.; Weir, M.; Mencer, D. Characterization of electrocoagulation for removal of chromium and arsenic. Chem. Eng. Technol. 2005, 28, 605–612. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Z.; Ma, X.; Tang, Q.; Li, Y. Modeling of the electrocoagulation process: A study on the mass transfer of electrolysis and hydrolysis products. Chem. Eng. Sci. 2017, 165, 165–176. [Google Scholar] [CrossRef]
- Lu, J.; Li, Y.; Yin, M.; Ma, X.; Lin, S. Removing heavy metal ions with continuous aluminum electrocoagulation: A study on back mixing and utilization rate of electro-generated Al ions. Chem. Eng. J. 2015, 267, 86–92. [Google Scholar] [CrossRef]
- Lu, J.; Tang, Q.; Wang, Z.R.; Xu, C.; Lin, S.L. A study on continuous and batch electrocoagulation process for fluoride removal. Desalin. Water Treat. 2016, 57, 28417–28425. [Google Scholar] [CrossRef]
- Gaalova, J.; Krystynik, P.; Dytrych, P.; Kluson, P. Elimination of dissolved Fe3+ ions from water by electrocoagulation. J. Sol-Gel Sci. Technol. 2018, 88, 49–56. [Google Scholar] [CrossRef]
- Parmentier, D.; Manhaeghe, D.; Baccini, L.; Van Meirhaeghe, R.; Rousseau, D.P.L.; Van Hulle, S. A new reactor design for harvesting algae through electrocoagulation-flotation in a continuous mode. Algal Res. 2020, 47, 101828. [Google Scholar] [CrossRef]
- Ebele, A.J.; Abou-Elwafa Abdallah, M.; Harrad, S. Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerg. Contam. 2017, 3, 1–16. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ferro-García, M.Á.; Prados-Joya, G.; Ocampo-Pérez, R. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, A.; Mazumder, P.; Tyagi, V.K.; Tushara Chaminda, G.G.; An, A.K.; Kumar, M. Occurrence and fate of emerging contaminants in water environment: A review. Groundw. Sustain. Dev. 2018, 6, 169–180. [Google Scholar] [CrossRef]
- Li, W.C. Occurrence, sources, and fate of pharmaceuticals in aquatic environment and soil. Environ. Pollut. 2014, 187, 193–201. [Google Scholar] [CrossRef]
- Klatte, S.; Schaefer, H.C.; Hempel, M. Pharmaceuticals in the environment—A short review on options to minimize the exposure of humans, animals and ecosystems. Sustain. Chem. Pharm. 2017, 5, 61–66. [Google Scholar] [CrossRef]
- Gibson, R.; Durán-Álvarez, J.C.; Estrada, K.L.; Chávez, A.; Jiménez Cisneros, B. Accumulation and leaching potential of some pharmaceuticals and potential endocrine disruptors in soils irrigated with wastewater in the Tula Valley, Mexico. Chemosphere 2010, 81, 1437–1445. [Google Scholar] [CrossRef] [PubMed]
- Lesser, L.E.; Mora, A.; Moreau, C.; Mahlknecht, J.; Hernández-Antonio, A.; Ramírez, A.I.; Barrios-Piña, H. Survey of 218 organic contaminants in groundwater derived from the world’s largest untreated wastewater irrigation system: Mezquital Valley, Mexico. Chemosphere 2018, 198, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Lapworth, D.J.; Baran, N.; Stuart, M.E.; Ward, R.S. Emerging organic contaminants in groundwater: A review of sources, fate and occurrence. Environ. Pollut. 2012, 163, 287–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arsand, D.R.; Kümmerer, K.; Martins, A.F. Removal of dexamethasone from aqueous solution and hospital wastewater by electrocoagulation. Sci. Total Environ. 2013, 443, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Ouaissa, Y.A.; Chabani, M.; Amrane, A.; Bensmaili, A. Removal of tetracycline by electrocoagulation: Kinetic and isotherm modeling through adsorption. J. Environ. Chem. Eng. 2014, 2, 177–184. [Google Scholar] [CrossRef] [Green Version]
- Baran, W.; Adamek, E.; Jajko, M.; Sobczak, A. Removal of veterinary antibiotics from wastewater by electrocoagulation. Chemosphere 2018, 194, 381–389. [Google Scholar] [CrossRef]
- Barışçı, S.; Turkay, O. Optimization and modelling using the response surface methodology (RSM) for ciprofloxacin removal by electrocoagulation. Water Sci. Technol. 2016, 73, 1673–1679. [Google Scholar] [CrossRef]
- Yoosefian, M.; Ahmadzadeh, S.; Aghasi, M.; Dolatabadi, M. Optimization of electrocoagulation process for efficient removal of ciprofloxacin antibiotic using iron electrode; kinetic and isotherm studies of adsorption. J. Mol. Liq. 2017, 225, 544–553. [Google Scholar] [CrossRef]
- Ahmadzadeh, S.; Asadipour, A.; Pournamdari, M.; Behnam, B.; Rahimi, H.R.; Dolatabadi, M. Removal of ciprofloxacin from hospital wastewater using electrocoagulation technique by aluminum electrode: Optimization and modelling through response surface methodology. Process Saf. Environ. Prot. 2017, 109, 538–547. [Google Scholar] [CrossRef]
- Ensano, B.M.B.; Borea, L.; Naddeo, V.; Belgiorno, V.; de Luna, M.D.G.; Balakrishnan, M.; Ballesteros, F.C. Applicability of the electrocoagulation process in treating real municipal wastewater containing pharmaceutical active compounds. J. Hazard. Mater. 2019, 361, 367–373. [Google Scholar] [CrossRef]
- Oulebsir, A.; Chaabane, T.; Tounsi, H.; Omine, K.; Sivasankar, V.; Flilissa, A.; Darchen, A. Treatment of artificial pharmaceutical wastewater containing amoxicillin by a sequential electrocoagulation with calcium salt followed by nanofiltration. J. Environ. Chem. Eng. 2020, 8, 104597. [Google Scholar] [CrossRef]
- Negarestani, M.; Motamedi, M.; Kashtiaray, A.; Khadir, A.; Sillanpää, M. Simultaneous removal of acetaminophen and ibuprofen from underground water by an electrocoagulation unit: Operational parameters and kinetics. Groundw. Sustain. Dev. 2020, 11, 100474. [Google Scholar] [CrossRef]
- Padmaja, K.; Cherukuri, J.; Anji Reddy, M. A comparative study of the efficiency of chemical coagulation and electrocoagulation methods in the treatment of pharmaceutical effluent. J. Water Process Eng. 2020, 34, 101153. [Google Scholar] [CrossRef]
- Govindan, K.; Angelin, A.; Kalpana, M.; Rangarajan, M.; Shankar, P.; Jang, A. Electrocoagulants Characteristics and Application of Electrocoagulation for Micropollutant Removal and Transformation Mechanism. ACS Appl. Mater. Interfaces 2020, 12, 1775–1788. [Google Scholar] [CrossRef]
- Kumari, S.; Kumar, R.N. River water treatment using electrocoagulation for removal of acetaminophen and natural organic matter. Chemosphere 2021, 273, 128571. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, W.; Zhang, X.; Si, G.; Zhang, P.; Li, B.; Su, R.; Gao, X. Efficient removal of Tetracycline-Cu complexes from water by electrocoagulation technology. J. Clean. Prod. 2021, 289, 125729. [Google Scholar] [CrossRef]
- Oliveira, J.T.; de Sousa, M.C.; Martins, I.A.; de Sena, L.M.G.; Nogueira, T.R.; Vidal, C.B.; Neto, E.F.A.; Romero, F.B.; Campos, O.S.; do Nascimento, R.F. Electrocoagulation/oxidation/flotation by direct pulsed current applied to the removal of antibiotics from Brazilian WWTP effluents. Electrochim. Acta 2021, 388, 138499. [Google Scholar] [CrossRef]
- Durante, C.; Cuscov, M.; Isse, A.A.; Sandonà, G.; Gennaro, A. Advanced oxidation processes coupled with electrocoagulation for the exhaustive abatement of Cr-EDTA. Water Res. 2011, 45, 2122–2130. [Google Scholar] [CrossRef] [PubMed]
- García-García, A.; Martínez-Miranda, V.; Martínez-Cienfuegos, I.G.; Almazán-Sánchez, P.T.; Castañeda-Juárez, M.; Linares-Hernández, I. Industrial wastewater treatment by electrocoagulation-electrooxidation processes powered by solar cells. Fuel 2015, 149, 46–54. [Google Scholar] [CrossRef]
- Ryan, D.R.; Maher, E.K.; Heffron, J.; Mayer, B.K.; McNamara, P.J. Electrocoagulation-electrooxidation for mitigating trace organic compounds in model drinking water sources. Chemosphere 2021, 273, 129377. [Google Scholar] [CrossRef] [PubMed]
- Benekos, A.K.; Tziora, F.E.; Tekerlekopoulou, A.G.; Pavlou, S.; Qun, Y.; Katsaounis, A.; Vayenas, D.V. Nitrate removal from groundwater using a batch and continuous flow hybrid Fe-electrocoagulation and electrooxidation system. J. Environ. Manag. 2021, 297, 113387. [Google Scholar] [CrossRef] [PubMed]
- Zazou, H.; Afanga, H.; Akhouairi, S.; Ouchtak, H.; Addi, A.A.; Akbour, R.A.; Assabbane, A.; Douch, J.; Elmchaouri, A.; Duplay, J.; et al. Treatment of textile industry wastewater by electrocoagulation coupled with electrochemical advanced oxidation process. J. Water Process Eng. 2019, 28, 214–221. [Google Scholar] [CrossRef]
- Ahangarnokolaei, M.A.; Ayati, B.; Ganjidoust, H. Simultaneous and sequential combination of electrocoagulation and ozonation by Al and Fe electrodes for DirectBlue71 treatment in a new reactor: Synergistic effect and kinetics study. Chemosphere 2021, 285, 131424. [Google Scholar] [CrossRef] [PubMed]
- Das, P.P.; Anweshan, A.; Mondal, P.; Sinha, A.; Biswas, P.; Sarkar, S.; Purkait, M.K. Integrated ozonation assisted electrocoagulation process for the removal of cyanide from steel industry wastewater. Chemosphere 2021, 263, 128370. [Google Scholar] [CrossRef]
- Ding, J.; Jiang, M.; Zhao, G.; Wei, L.; Wang, S.; Zhao, Q. Treatment of leachate concentrate by electrocoagulation coupled with electro-Fenton-like process: Efficacy and mechanism. Sep. Purif. Technol. 2021, 255, 117668. [Google Scholar] [CrossRef]
- Dai, C.; Shi, S.; Chen, D.; Liu, J.; Huang, L.; Zhang, J.; Feng, Y. Study on the mechanism of tetracycline removal in electrocoagulation coupled with electro-fenton reaction system with Fe anode and carbon nanotube cathode. Chem. Eng. J. 2022, 428, 131045. [Google Scholar] [CrossRef]
- Xiao, Z.; Cui, T.; Wang, Z.; Dang, Y.; Zheng, M.; Lin, Y.; Song, Z.; Wang, Y.; Liu, C.; Xu, B.; et al. Energy-efficient removal of carbamazepine in solution by electrocoagulation-electrofenton using a novel P-rGO cathode. J. Environ. Sci. 2022, 115, 88–102. [Google Scholar] [CrossRef]
- Nigri, E.M.; Santos, A.L.A.; Rocha, S.D.F. Removal of organic compounds, calcium and strontium from petroleum industry effluent by simultaneous electrocoagulation and adsorption. J. Water Process Eng. 2020, 37, 101442. [Google Scholar] [CrossRef]
- Hussin, F.; Aroua, M.K.; Szlachtac, M. Combined solar electrocoagulation and adsorption processes for Pb(II) removal from aqueous solution. Chem. Eng. Process.-Process Intensif. 2019, 143, 107619. [Google Scholar] [CrossRef]
- Bulca, Ö.; Palas, B.; Atalay, S.; Ersöz, G. Performance investigation of the hybrid methods of adsorption or catalytic wet air oxidation subsequent to electrocoagulation in treatment of real textile wastewater and kinetic modelling. J. Water Process Eng. 2021, 40, 101821. [Google Scholar] [CrossRef]
- Azerrad, S.P.; Isaacs, M.; Dosoretz, C.G. Integrated treatment of reverse osmosis brines coupling electrocoagulation with advanced oxidation processes. Chem. Eng. J. 2019, 356, 771–780. [Google Scholar] [CrossRef]
- Dia, O.; Drogui, P.; Buelna, G.; Dubé, R. Hybrid process, electrocoagulation-biofiltration for landfill leachate treatment. Waste Manag. 2018, 75, 391–399. [Google Scholar] [CrossRef] [Green Version]
- Sardari, K.; Askegaard, J.; Chiao, Y.H.; Darvishmanesh, S.; Kamaz, M.; Wickramasinghe, S.R. Electrocoagulation followed by ultrafiltration for treating poultry processing wastewater. J. Environ. Chem. Eng. 2018, 6, 4937–4944. [Google Scholar] [CrossRef]
- Prajapati, A.K. Sono-assisted electrocoagulation treatment of rice grain based distillery biodigester effluent: Performance and cost analysis. Process Saf. Environ. Prot. 2021, 150, 314–322. [Google Scholar] [CrossRef]
- Hashim, K.S.; Ali, S.S.M.; AlRifaie, J.K.; Kot, P.; Shaw, A.; Al Khaddar, R.; Idowu, I.; Gkantou, M. Escherichia coli inactivation using a hybrid ultrasonic–electrocoagulation reactor. Chemosphere 2020, 247, 125868. [Google Scholar] [CrossRef] [PubMed]
- Oza, H.; Anantha Singh, T.S.; Sasikumar Jampa, S. Removal of arsenic from aqueous solution using combined ultrasonic and electrocoagulation process. Mater. Today Proc. 2021, 47, 728–732. [Google Scholar] [CrossRef]
- Fan, T.; Deng, W.; Feng, X.; Pan, F.; Li, Y. An integrated electrocoagulation—Electrocatalysis water treatment process using stainless steel cathodes coated with ultrathin TiO2 nanofilms. Chemosphere 2020, 254, 126776. [Google Scholar] [CrossRef]
- Shams, M.; Balouchi, H.; Alidadi, H.; Asadi, F.; Goharshadi, E.K.; Rezania, S.; Rtimi, S.; Anastopoulos, I.; Bonyadi, Z.; Mehranzamir, K.; et al. Coupling electrocoagulation and solar photocatalysis for electro- and photo-catalytic removal of carmoisine by Ag/graphitic carbon nitride: Optimization by process modeling and kinetic studies. J. Mol. Liq. 2021, 340, 116917. [Google Scholar] [CrossRef]
- McBeath, S.T.; Hajimalayeri, A.; Jasim, S.Y.; Mohseni, M. Coupled electrocoagulation and oxidative media filtration for the removal of manganese and arsenic from a raw ground water supply. J. Water Process Eng. 2021, 40, 101983. [Google Scholar] [CrossRef]
- Wang, Y.; Kong, F.; Yang, D.; Liu, Q.; Lin, X.; Chen, J.; Zhang, Y. The synergistic effect of electrocoagulation coupled with E-peroxone process for shale gas fracturing flowback water treatment. Chemosphere 2021, 262, 127968. [Google Scholar] [CrossRef]
- Du, X.; Zhao, W.; Wang, Z.; Ma, R.; Luo, Y.; Wang, Z.; Sun, Q.; Liang, H. Rural drinking water treatment system combining solar-powered electrocoagulation and a gravity-driven ceramic membrane bioreactor. Sep. Purif. Technol. 2021, 276, 119383. [Google Scholar] [CrossRef]
- Xu, J.; Du, X.; Zhao, W.; Wang, Z.; Lu, X.; Zhu, L.; Wang, Z.; Liang, H. Roofing rainwater cleaner production using pilot-scale electrocoagulation coupled with a gravity-driven membrane bioreactor (EC-GDMBR): Water treatment and energy efficiency. J. Clean. Prod. 2021, 314, 128055. [Google Scholar] [CrossRef]
- Abdulrazzaq, N.N.; Al-Sabbagh, B.H.; Shanshool, H.A. Coupling of electrocoagulation and microflotation for the removal of textile dyes from aqueous solutions. J. Water Process Eng. 2021, 40, 101906. [Google Scholar] [CrossRef]
- Song, P.; Yang, Z.; Zeng, G.; Yang, X.; Xu, H.; Wang, L.; Xu, R.; Xiong, W.; Ahmad, K. Electrocoagulation treatment of arsenic in wastewaters: A comprehensive review. Chem. Eng. J. 2017, 317, 707–725. [Google Scholar] [CrossRef]
- Butler, E.; Hung, Y.T.; Yeh, R.Y.L.; Al Ahmad, M.S. Electrocoagulation in wastewater treatment. Water 2011, 3, 495–525. [Google Scholar] [CrossRef]
- Kabdaşlı, I.; Arslan-Alaton, I.; Ölmez-Hancı, T.; Tünay, O. Electrocoagulation applications for industrial wastewaters: A critical review. Environ. Technol. Rev. 2012, 1, 2–45. [Google Scholar] [CrossRef]
- An, C.; Huang, G.; Yao, Y.; Zhao, S. Emerging usage of electrocoagulation technology for oil removal from wastewater: A review. Sci. Total Environ. 2017, 579, 537–556. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, M.A.; Fuentes, R.; Thiam, A.; Salazar, R. Arsenic and fluoride removal by electrocoagulation process: A general review. Sci. Total Environ. 2021, 753, 142108. [Google Scholar] [CrossRef] [PubMed]
- Rahman, N.A.; Jol, C.J.; Linus, A.A.; Ismail, V. Emerging Application of Electrocoagulation for Tropical Peat Water Treatment: A Review. Chem. Eng. Process.-Process Intensif. 2021, 165, 108449. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, P.; Li, J. Electrocoagulation technology for water purification: An update review on reactor design and some newly concerned pollutants removal. J. Environ. Manag. 2021, 296, 113259. [Google Scholar] [CrossRef]
- Castañeda, L.F.; Rodríguez, J.F.; Nava, J.L. Electrocoagulation as an affordable technology for decontamination of drinking water containing fluoride: A critical review. Chem. Eng. J. 2021, 413, 127529. [Google Scholar] [CrossRef]
- Ingelsson, M.; Yasri, N.; Roberts, E.P.L. Electrode passivation, faradaic efficiency, and performance enhancement strategies in electrocoagulation—A review. Water Res. 2020, 187, 116433. [Google Scholar] [CrossRef]
- Shahedi, A.; Darban, A.K.; Taghipour, F.; Jamshidi-Zanjani, A. A review on industrial wastewater treatment via electrocoagulation processes. Curr. Opin. Electrochem. 2020, 22, 154–169. [Google Scholar] [CrossRef]
- Al-Qodah, Z.; Tawalbeh, M.; Al-Shannag, M.; Al-Anber, Z.; Bani-Melhem, K. Combined electrocoagulation processes as a novel approach for enhanced pollutants removal: A state-of-the-art review. Sci. Total Environ. 2020, 744, 140806. [Google Scholar] [CrossRef] [PubMed]
- Tahreen, A.; Jami, M.S.; Ali, F. Role of electrocoagulation in wastewater treatment: A developmental review. J. Water Process Eng. 2020, 37, 101440. [Google Scholar] [CrossRef]
- Kobya, M.; Soltani, R.D.C.; Omwene, P.I.; Khataee, A. A review on decontamination of arsenic-contained water by electrocoagulation: Reactor configurations and operating cost along with removal mechanisms. Environ. Technol. Innov. 2020, 17, 100519. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Jiang, W.M.; Wu, M.R.; Li, Z.H. Comprehensive review of floc growth and structure using electrocoagulation: Characterization, measurement, and influencing factors. Chem. Eng. J. 2021, 417, 129310. [Google Scholar] [CrossRef]
- Visigalli, S.; Barberis, M.G.; Turolla, A.; Canziani, R.; Berden Zrimec, M.; Reinhardt, R.; Ficara, E. Electrocoagulation–flotation (ECF) for microalgae harvesting—A review. Sep. Purif. Technol. 2021, 271, 118684. [Google Scholar] [CrossRef]
- Titchou, F.E.; Zazou, H.; Afanga, H.; El Gaayda, J.; Akbour, R.A.; Hamdani, M. Removal of Persistent Organic Pollutants (POPs) from water and wastewater by adsorption and electrocoagulation process. Groundw. Sustain. Dev. 2021, 13, 134425. [Google Scholar] [CrossRef]
- Almukdad, A.; Hafiz, M.A.; Yasir, A.T.; Alfahel, R.; Hawari, A.H. Unlocking the application potential of electrocoagulation process through hybrid processes. J. Water Process Eng. 2021, 40, 101956. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, R.; Sun, W.; Wang, L.; Tang, H. Li extraction from model brine via electrocoagulation: Processing, kinetics, and mechanism. Sep. Purif. Technol. 2020, 250, 117234. [Google Scholar] [CrossRef]
- Zavahir, S.; Elmakki, T.; Gulied, M.; Ahmad, Z.; Al-Sulaiti, L.; Shon, H.K.; Chen, Y.; Park, H.; Batchelor, B.; Han, D.S. A review on lithium recovery using electrochemical capturing systems. Desalination 2021, 500, 114883. [Google Scholar] [CrossRef]
- Mehdipoor, M.A.; Moosavirad, S.M. Effect of Holed Ferrum electrodes (HFE) on the efficiency of the electrocoagulation process for copper recovery and optimization of parameters, using RSM. Hydrometallurgy 2020, 194, 105313. [Google Scholar] [CrossRef]
- Li, P.; Chen, P.; Wang, G.; Wang, L.; Wang, X.; Li, Y.; Zhang, W.; Jiang, H.; Chen, H. Uranium elimination and recovery from wastewater with ligand chelation-enhanced electrocoagulation. Chem. Eng. J. 2020, 393, 124819. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).