Abstract
Cadmium (Cd) and lead (Pb) carry a high heavy-metal-toxic risk for both animals and plants in soil. In this study, iron-based biochar (T-BC) was prepared by co-pyrolysis using wastes of iron tailings and biomass with urea as the functioning agents. Field-emission scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FTIR), and toxicity-characteristic leaching procedure (TCLP) methods were employed to analyze the physicochemical characteristics of T-BC. Additionally, a pot trial was conducted to examine the effects of T-BC on the physiological characteristics of pak choi (Brassica campestris L.), the availability of heavy metals, and enzyme activities in the soils. The results show that toxic metals have been volatilized by the roasting process and immobilized within T-BC via the formation of stable metal-compounds during the co-pyrolysis process, which satisfies the requirements of a soil passivator. Incubation experiments showed that the DTPA-extractable Cd and Pb in contaminated soils decreased with an increasing amendment rate. Moreover, in the pot experiments, by adding 1% (w/w) T-BC into soils, the soils benefited from its large adsorption, complex precipitation, and immobilization capacity. Approximately 36% Cd and 29% Pb concentrations of edible parts in pak choi were reduced. The amendment proved promising for the stabilization of Cd and Pb in contaminated soils, while providing a strategy for solving the residual waste of tailings and biomass.
1. Introduction
Since urbanization and industrialization have spread wildly in China in recent decades, the concentration of toxic heavy metals (Cd and Pb) in soil has increased in various regions [1,2,3]. Heavy metals are usually added to soil through mining and smelting activities [4,5,6], uncontrolled e-waste processing operations, industrial wastewater, and industrials solids [7,8]. Vegetables, with rich vitamins, mineral elements, dietary fiber, and other nutrients, are an indispensable, non-staple, daily food [4,9,10]. However, the quality of vegetables is challenged by the threat of Cd and Pb pollution, which pose a strong threat to people’s health. Therefore, properly addressing soil pollution and the control of vegetable pollution caused by heavy metals in the soil have become very important and concerning social issues.
Currently, in situ immobilization of soil has been developed for the treatment of contaminated soils, a method which is suitable for wide introduction and application [11,12]. This technology is designed to reduce the mobility and biological impact of toxic heavy metals (THMS) in soils through the addition of exogenous soil amendments and a series of physical and chemical reactions affecting the speciation of THMS. Commonly used immobilization amendments of heavy metal contaminated soil mainly include lime substances [13], modified coal fly ash [14], red mud [15,16], clay minerals, biochar [9], phosphates, iron, and iron oxides. In addition, as an important research direction in the field of environmental science, iron-based materials and biochar have great application potential in improving soil remediation. However, major challenges remain unsolved in the field of soil remediation of nano zero-valent iron and synthetic iron oxide. These materials have a relatively high cost and can easily aggregate into large clusters due to their high surface energy and magnetic interaction, which impedes the series of physical and chemical reactions with THMS and reduces their amendment efficiency. Therefore, there is a need for promising strategies to solve the above defects and reduce material costs.
Tailings are one of the largest industrial solid wastes in the world, and the rapid development of the steel industry has produced a large amount of iron tailings. By 2013, 5.00 × 109 t of iron tailings have accumulated in China. At present, most of the iron tailings are treated from two aspects: with the aim of reducing harm and from a resource-based aspect. One focuses on separating heavy metals from iron tailings and reducing risk for he environment; the other on directly utilizing iron tailings as raw material in the production of other materials. Chlorination roasting can effectively separate and remove toxic metal from tailings [17,18]. Hong et al. [17] reported that heavy metals, including Pb, Zn, and Cu, were volatilized at a rate reaching more than 90% through chlorination roasting by NaCl. Moreover, a study by Li et al. [18] found that in roasted iron tailings with CaCl2 as a chlorinating agent, the volatilization rates of the toxic metals Pb, Cd, and Cu were 98%, 97%, and 80%, respectively. This treatment effectively reduced the toxic and hazardous substances in the tailings, with the residual mainly containing iron oxide and silicon dioxide. Thus, it is worth considering how to further utilize the residue after chlorination roasting. Traditionally, iron-based biochar mainly uses chemicals to grow iron-containing mineral phases on the surface of biochar [19,20,21]. Wu et al. [21] utilized rice, straw-derived biochar modified with ferrous chloride (Fe[II]) and (Fe[III]) and found that Fe(II) biochar decreased leaching by 86.4% of P leaching. Yuan et al. [20] reported that biochar was modified by Fe2+/Fe3+ and NaOH, and a C-O-Fe structure was formed on its surface, while the iron components in T-BC, iron oxides (Fe3O4, γ-Fe2O3 and Fe-O-Fe), iron-containing functional groups (-Fe-R-COOH and Fe-R-OH, etc.), and the mineral crystal XiFeYjOk reacted with the cadmium ion in the reaction surface to exchange, form complexes, and precipitate, achieving the purpose of fixing with Cd. In addition, in other studies, Zhou et al. [22] used urea to further functionalize magnetic biochar for the removal of lead. The maximum adsorption capacity of lead on magnetic biochar was 188.18 mg/g. Although a significant amount of research has been on iron-modified biochar, the above-mentioned methods have the disadvantages of many preparation steps and high cost. Based on this, from the perspective of a circular economy and green development, we consider whether waste iron oxides can be embedded in biochar to prepare iron-based biochar by functionalizing with urea and further explore the application of iron-based biochar in soil remediation.
Pak choi (Brassica campestris L.) is an essential edible vegetable widely distributed in China and Southeast Asia [23]. Vegetable safety has become a top priority [9]. In this paper, a “waste-safety” strategy is proposed to stabilize heavy metal in polluted soil and ensure vegetable safety. In this study, T-BC was prepared with iron tailings, fallen leaves, and urea by a simple, one-step, low-temperature, co-pyrolysis method. This study aims to: (1) prepare an iron-based biochar amendment from a residue originating from iron-tailings and biomass by the co-pyrolysis method and investigate the release of toxic-elements from T-BC; (2) evaluate the remediation performance of iron-based biochar in Cd and Pb-contaminated soils using pak choi as model plants.
2. Materials and Methods
2.1. Chemicals and Soil
Iron-tailing residue samples were collected from Guangdong Dabaoshan Mine and then ground by planetary mill (WXQM-8L, Tencan, Changsha, China), and raw samples with a diameter of <0.1 mm were obtained. The main chemical composition of the iron tailings is shown in Table S1. A biomass of mango leaves (ML) was collected from a local orchard, air-dried, and crushed to pieces. After processing with a 10-mesh sieve, powder was collected. Urea and other chemicals in this study were purchased from Sinopharm Chemical Reagent Co., Shanghai, China.
Topsoil (0–20 cm) was collected from farmland near Dabaoshan mine (DS). After they were air dried, the soil samples were sieved (<2 mm and 0.15 mm), homogenized, and preserved prior to analysis. The soil consisted of 53.9% sand, 11.5% silt, and 34.6% clay, indicating a texture of sandy loam. It had an organic-matter content of 18.9 g/kg, a pH of 4.08, a total Cd concentration of 0.57 mg/kg, and a total Pb concentration of 385 mg/kg. The concentrations of total Cd and Pb were above the risk-screening values for the soil contamination of agricultural land (GB 15618-2018). The soil was contaminated with multiple THMs, which might be attributed to mining activities located near the farmland [4]. Table 1 summarizes the soil properties.

Table 1.
Properties of the soil and biochar.
Plant seeds: pak choi (Brassica campestris L.) seeds were obtained from a local seed company in Shaoguan City, Gaungdong Province, China.
2.2. Soil Passivator Preparation
ML were loaded into a ceramic crucible and heated in a muffle furnace. The temperature was raised with an interval of 10 °C·min−1 and kept constant at 600 °C for 2 h with N2 during the pyrolysis process. The resultant biochar was then cooled and labelled MB. The preparation process of the tailings-based biochar (T-BC) was as a tailings-based biochar prepared by the co-pyrolysis of MR and ML in the presence of urea. In brief, the urea and ML with a fixed mass ratio (0, 20, 40, 50, 60, 80, 100% [w/w]) of total mass (10 g) were mixed with MR (10 g) then fully ground and put into a ceramic crucible with a lid. Then the ceramic crucible was loaded into a muffle furnace—the specific pyrolysis process is shown above. The product was coded as T-BC1, T-BC2, T-BC3, T-BC4, T-BC5, T-BC6, and T-BC7, representing the material ratio of urea and biomass at 0, 20, 40, 50, 60, 80, 100% (w/w), respectively. Finally, the products were cooled overnight and homogenized, sieved (100 mesh), and saved prior to analysis.
2.3. Soil Experiments and Pot Experiments
Soil incubation studies were performed with 1000 g Soil. The Soil was amended with 20 g (2% w/w) of MB, T-BC1, T-BC2, T-BC3, T-BC4, T-BC5, T-BC6, and T-BC7, and its moisture content was kept at 70%. A control sample was left without biochar treatment. After 14 days of incubation at 25 °C, the immobilization of metals was used to evaluate the curing and stabilization effect of a series of biochar and collected for chemical analyses.
Plastic pots (16.5 cm outer diameter, 13.5 cm inner diameter, and 11 cm height) were used in the pot experiment. Each pot was filled with 1000 g of soil, and basal fertilizers (NH4NO3, 120 mg N kg−1 soil, K2HPO4, 30 mg P kg−1 soil, and 75.7 mg K kg−1 soil) were added to each pot so that the nutrition supply level could meet the normal growth demands of pak choi. At the same time, four rate of T-BC (0, 0.5, 1, and 2% [w/w]) were applied in soil, i.e., BC0 (0 g/pot), BC0.5 (5 g/pot), BC1 (10 g/pot), and BC2 (20 g/pot), in triplicate. Further, MB doses of 20 g/pot were also used in the soil amendment as a traditional biochar amendment control. Seeds of pak choi were selected and sterilized by 2% H2O2 for 15 min, and ten seeds were planted in each pot in the middle of November 2018. Two weeks after sowing, the seed germination rate was recorded, and then the seedlings were thinned to three per pot and watered every three days by weighing. The crops were harvested at 40 days after seed germination (harvested late in December 2018) on the basis of the growth period for pak choi. Additionally, all the treatment soil samples were collected and then air-dried and stored to measure enzymatic activity and other soil characteristics.
2.4. Analytic Methods
A toxicity characteristic leaching procedure (TCLP) (based on USEPA 1311) was carried out to evaluate the leaching of heavy metals in the T-BC under the co-pyrolysis process. In brief, the soil passivator and buffer solution (HOAc/NaOAc, pH 2.88) were mixed at a solid to liquid ratio of 1:20 in a polyethylene tube. The mixture was shaken at 250 rpm for 18 ± 2 h before filtration. The filtrate was stored at 4 °C and, in time, analyzed. In addition, FTIR spectroscopy (Shimadzu DR-8001) and scanning electron microscopy (SEM) were used for the assessment of surface functional groups and the structural morphology of the T-BC.
Soil properties, such as pH, SOC, CEC, EC, available N (A-N), available P (A-P), available K (A-K), and available K (A-Mg) were determined with the method described by Lu [24]. Soil dissolved organic matter (DOM), as expressed by dissolved organic carbon (DOC), was extracted from the soil samples using the method described by Jiang et al. [25]. The concentrations of HMs in the soil were examined by aqueous extraction using HCl-HNO3-HF-HClO4 digestion. The available silicon content was detected by the molybdenum blue spectrophotometric method [26]. The heavy metal fractions (exchangeable bound, carbonate-bound, Fe-Mn oxide-bound, organic-matter-bound, and residual bound) in the soil were analyzed by a Tessier’s sequential extraction procedure [27]. The available Cd and Pb in the soil particles were extracted by the DTPA extraction method. The colorimetric method was applied to analyze urease (UE), acid phosphatase (ACP), and catalase (CAT) in the soils [28,29,30].
Before the plants were harvested, the plant samples were divided into roots and leaves, and the fresh weight of each part was weighed. The fresh leaves were used to measure the content of chlorophyll a, chlorophyll b, superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) [31,32]. The malondialdehyde (MDA) content of leaves was measured following the method of Heath and Pacher [33]. Further, the harvested plant biomass and plant height was measured, and for total ions and metal determination in the roots and leaves of the plant samples, they were mixed with 10 mL of concentrated HNO3-HClO4 digested. The Cd and Pb content in the solution was recorded prior to analysis. The concentrations of metals in the samples were measured using atomic-absorption spectroscopy (AAS, ice3300 Thermo Scientific, Waltham, MA, USA) and inductively coupled plasma mass spectrometry (ICP-MS, 7900, Agilent, Santa Clara, CA, USA) techniques.
Quality control was done by parallel analysis of a certified reference: a soil certified reference material (GBW07450, National Research Center for Certified Reference Materials, China) and a plant certified reference material (GBW10049, National Research Center for Certified Reference Materials, China). Each treatment in this study was done in triplicate—the samples were processed to ensure the accuracy and precision of the analysis.
2.5. Data Analysis
The biological concentration factor (BCF), enrichment factor (EF), and transfer factor (TF) were used to evaluate the transfer characteristics of Cd and Pb in the soil and pak choi. Moreover, the estimated daily intake of vegetables (EDI, μg/kg·d) of local residents and target hazard quotient method (THQ) were used to assess the potential risks to the human body. The detailed calculated formula is appended in the supplementary information.
The statistical software SPSS 21.0 (IBM Corp, Armonk, NY, USA) was used to conduct the analysis of variance (ANOVA), in which a p value < 0.05 was considered statistically significant. Regression analysis and correlation analysis were conducted to identify the association between the growth indices and soil property. All samples used for analyses were performed in triplicate. Measurement data were shown as mean ± standard deviation.
3. Results and Discussion
3.1. Leaching Toxicity and Soil Amendment
Generally, chlorination roasting can effectively remove residual heavy metals in iron tailings at a lower temperature [34,35]. As shown in Table S2, the leaching concentration of heavy metals Zn, Cu, Cd, and Pb in the roasted iron tailings (MR) was greatly reduced compared with the iron tailings (Table S2). Due to the strong reaction effect between chloride and heavy metals, the Pb and Zn in MR is drastically volatilized in the chlorination roasting process [18,34,35]. Moreover, as shown in Table S2, the leaching concentrations of Pb, Zn, and Cu from T-BC also characterize a trend of reduction as MR addition with the co-pyrolysis method. The Pb, Cu, and Zn immobilization is mainly attributed to the strong adsorption and co-precipitation capacity of BC in T-BC, which is in strong agreement with the results reported by previous studies [20,22,36]. Therefore, treatment by chlorination roasting and co-pyrolysis can reduce heavy metals in waste.
Apart from the soil-characteristics effect influencing the Cd and Pb bioavailability, the direct adsorption and stabilization of Cd and Pb by T-BC was also an important process. As shown in Figure S1a, T-BC and MB application significantly decreased the DTPA extracts Cd and Pb in both soils compared with the control soil samples, which may be attributed to the alkaline substances and iron oxide in BC. Previous studies have found that the pH significantly increased (compared to the control) in acidic soil samples under the treatment of BC [37,38,39]. The increase of organic matter-bound fraction and Fe-Mn oxides-bound fraction of Cd and Pb have been reported to be due to the oxygen-containing functional groups in BC forming complexes with Cd and Pb [37,39]. The data also show that the values of DTPA extracted Cd and Pb was high at T-BC5, T-BC6, and T-BC7 compared with T-BC4. Hence, T-BC4 was finally chosen to be used in further characterization analysis and pot experiments.
3.2. FTIR Analysis and SEM of the Biochar
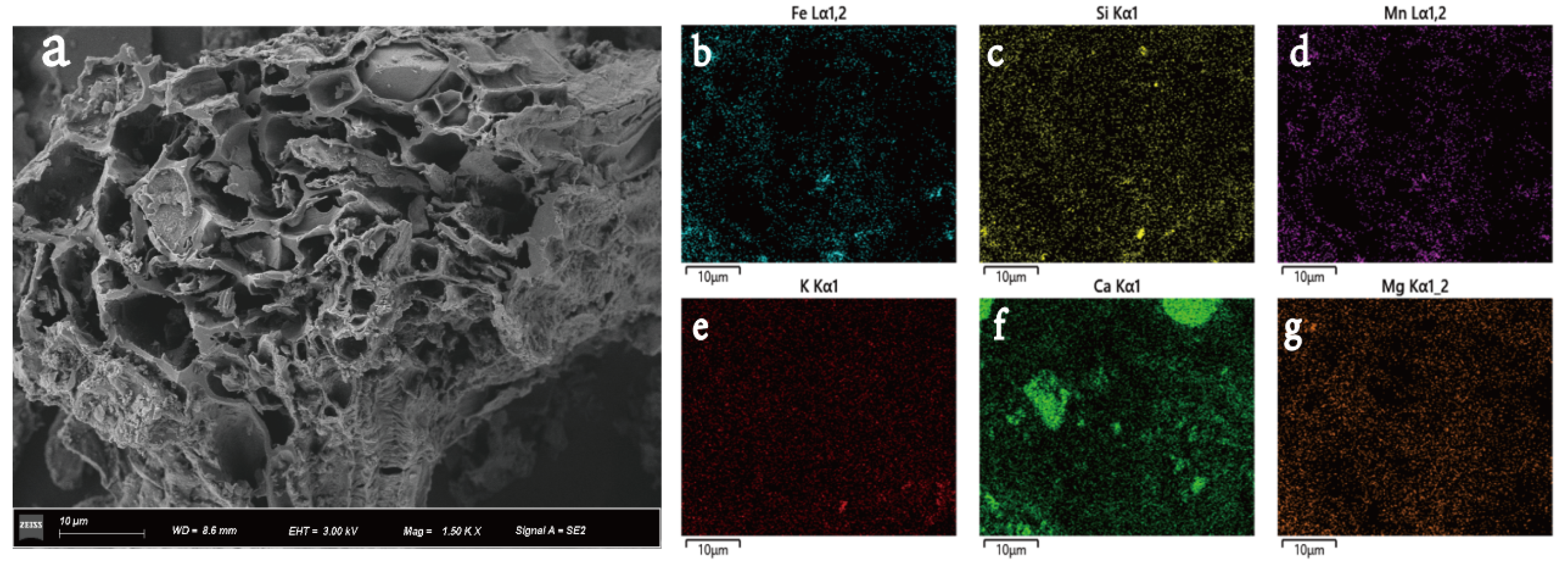
The FTIR spectra of T-BC4 are presented in Figure S2. The bending vibration at 459.88 cm−1 stands for the O-Si-O peak [40]. The peak at 559.98 cm−1 represents the Fe-O. From the T-BC spectra, the peak in the range of 1468–1747 cm−1 is indicative of the C=O, C=C, and C=N groups corresponding to the organic phase of the BC [41]. Moreover, as shown, the T-BC4 samples show strong bands at 580–605 and 971–1079 cm−1 (Figure S2), which can be attributed to the stretching and bending modes of PO43− groups [42]. According to the SEM image, we can see that the abundant pore structures on the surface of the BC with co-pyrolysis treatment with MR is disorderly and bulky (Figure 1a). This indicates that co-pyrolysis plays an active role in the pore formation of biochar, and its pore size is mainly mesoporous and macroporous. These features are also very important for the adsorption of Pb and Cd in soil. Moreover, as found as black, lump particles in the pore structure of the SEM image of T-BC with the co-pyrolysis treatment with MR, some components containing iron oxides are embedded in biochar pores, indicating the successful synthesis of tailings-based biochar. In addition, on the outside of T-BC, a large number of folds and flocculent attachment structures with rough external morphology are found, which can effectively promote the adsorption and stabilization of heavy metals by T-BC (Figure 1a). Figure 1b–g show the chemical elements of T-BC. As illustrated in Figure 1, the major elements of T-BC were contained Fe, Mn, Si, and nutrient elements (K, Ca, and Mg). Apparently, Fe and Mn can be observed in MR, indicating that MR was attached to the BC surface during the co-pyrolysis process.
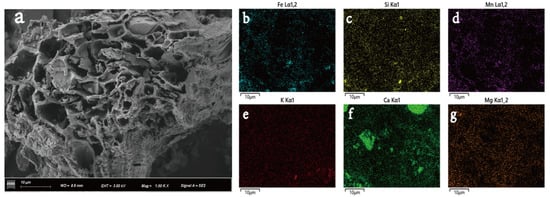
Figure 1.
SEM image (a) and the elements Fe (b), Si (c), Mn (d), K (e), Ca (f), Mg (g) scanning mapping of T-BC.
3.3. Soil Properties
The present study confirms that the addition of T-BC and MB significantly improves and enhances the physicochemical properties and fertility of contaminated soils. The changes in soil physicochemical properties (pH, CEC, SOC, and DOC) varied among biochar types and their added doses (Table 2). The highest increase in soil pH was observed from 4.57 to 5.21 (BC2 treatment) and 5.37 (MB treatment) when T-BC and MB were applied at the 2% rate, respectively. The soil SOC and DOC content of contaminated soil was increased 12% and 20% (SOC), and 69% and 99% (DOC), when amended with 2% T-BC and MB, respectively, relative to the control (Table 2). Similarly, the application of different, plant-derived biochar significantly enhanced physical and chemical properties in previous studies [37,43,44]. Albert et al. [45] reported that mainly due to biochar, pH, CEC, SOC, and DOC were higher than that recorded in the soil. Moreover, the treatment with the application of T-BC and MB significantly increased soil nutrients of available P, Si, K, Mg, and Ca, compared with the control (Table 2). This process appears to also be dependent upon the rich nutrient content of BC [28,46]. Bashir et al. [44] reported similar results when they applied BC with 3% doses, which soil total N, total P and total K were significantly increased compared to the control.

Table 2.
The physical and chemical properties of the different treatments soil.
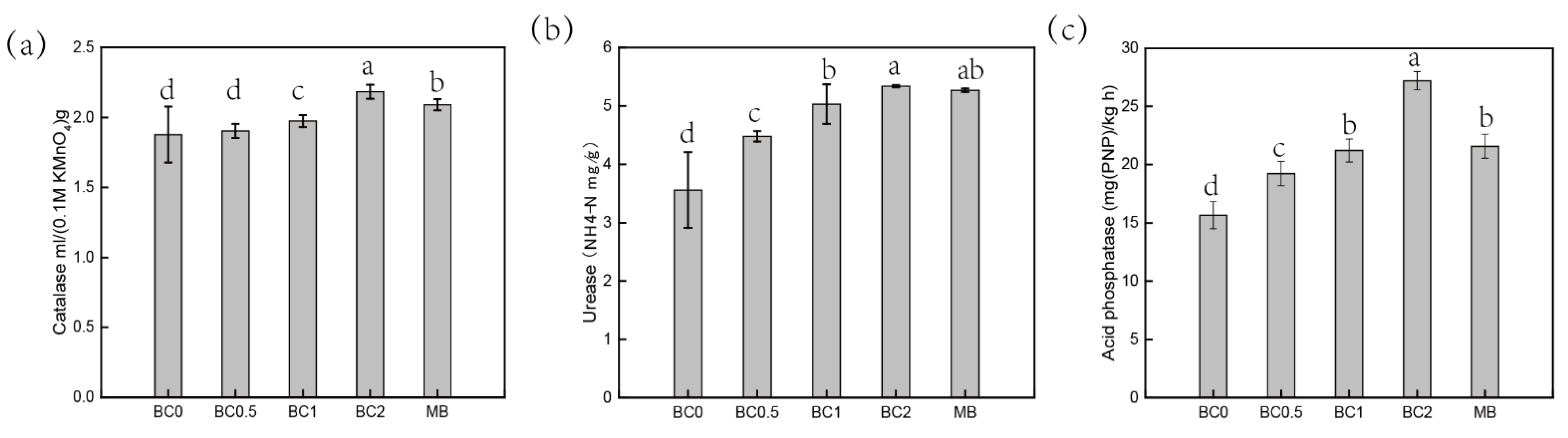
Soil enzymes are sensitive to contamination and therefore can be used to assess soil quality after remediating and restoring the contaminated soils. This is especially useful for evaluating the impact of heavy metal pollution in soil [29]. The BC0 treatment soil showed low catalase and urease activities, indicating that the ability for catalyzing the decomposition of H2O2 and hydrolyze urea was inhibited by heavy-metal pollution (Figure 2a). A significant increase in the activity of CAT was observed in the high-dose BC (BC2 and MB) treatments, as compared to BC0. Soil CAT was significantly enhanced from 1.88 mL/g in the control to 2.18 mL/g in BC2, and to 2.09 mL/g in MB-treated soil (Figure 2a). Increased CAT in amended soil could be ascribed to the decreased toxicity of metal, and applied BC could improve soil physical and chemical properties which leads to changes in the composition and function of soil microbial communities [29,47]. The presence of basic nutrient contents in BC acts as a feed source for microorganisms, which leads to an increase in the degradable composition of T-BC-amended soils. Similarly, a prominent increment in UR occurred due to the significant increase in the soil after BC addition (Figure 2b), which is due to the beneficial effects on the physical and chemical properties in the soil (Table 1). Specific enzyme activity associated with soil N utilization and transformation could be increased after the addition of BC, as reported by Bailey et al. [48] and Dempster et al. [49]. On the other hand, the activity of soil ACP was enhanced in the treatments with an increasing rate of BC application. The ACP increased significantly from 15.67 mg PNP/ kg h in the BC0 treatment to 27.19 mg/ PNP kg h and 24.57 mg PNP/kg h in BC2 and MB, respectively (Figure 2c). Interestingly, soil enzyme activities of ACP in BC2 were lower than in the MB treatment, which is involved in pH after BC is added (Figure 2c). The reduction in ACP could be attributed to the increase in soil pH after BC application with increasing rates as reported by Salam et al. [47]. A previous study by Chen et al. [50] concluded that the incorporation of BC decreased the activity of ACP with an increasing pH, while alkaline phosphatase activity was increased. Previous studies have also reported that exchangeable metals in soils can inhibit enzyme activities, and soil microorganisms played a role in promoting soil enzyme activities.
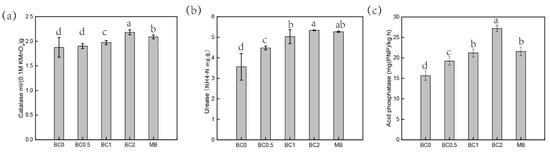
Figure 2.
Influence of the biochar on enzyme activities. CAT (a), Catalase activities; UR (b), Urease activities; ACP (c), Acid phosphatase activities. BC0, BC0.5, BC1, and BC2 mean as soil applied with T-BC of four rate (0, 0.5, 1, and 2% (w/w)); MB means as soil applied with biochar (2% (w/w)) from mango leaves.
3.4. Availability and Fractionation of Cd and Pb in Soil
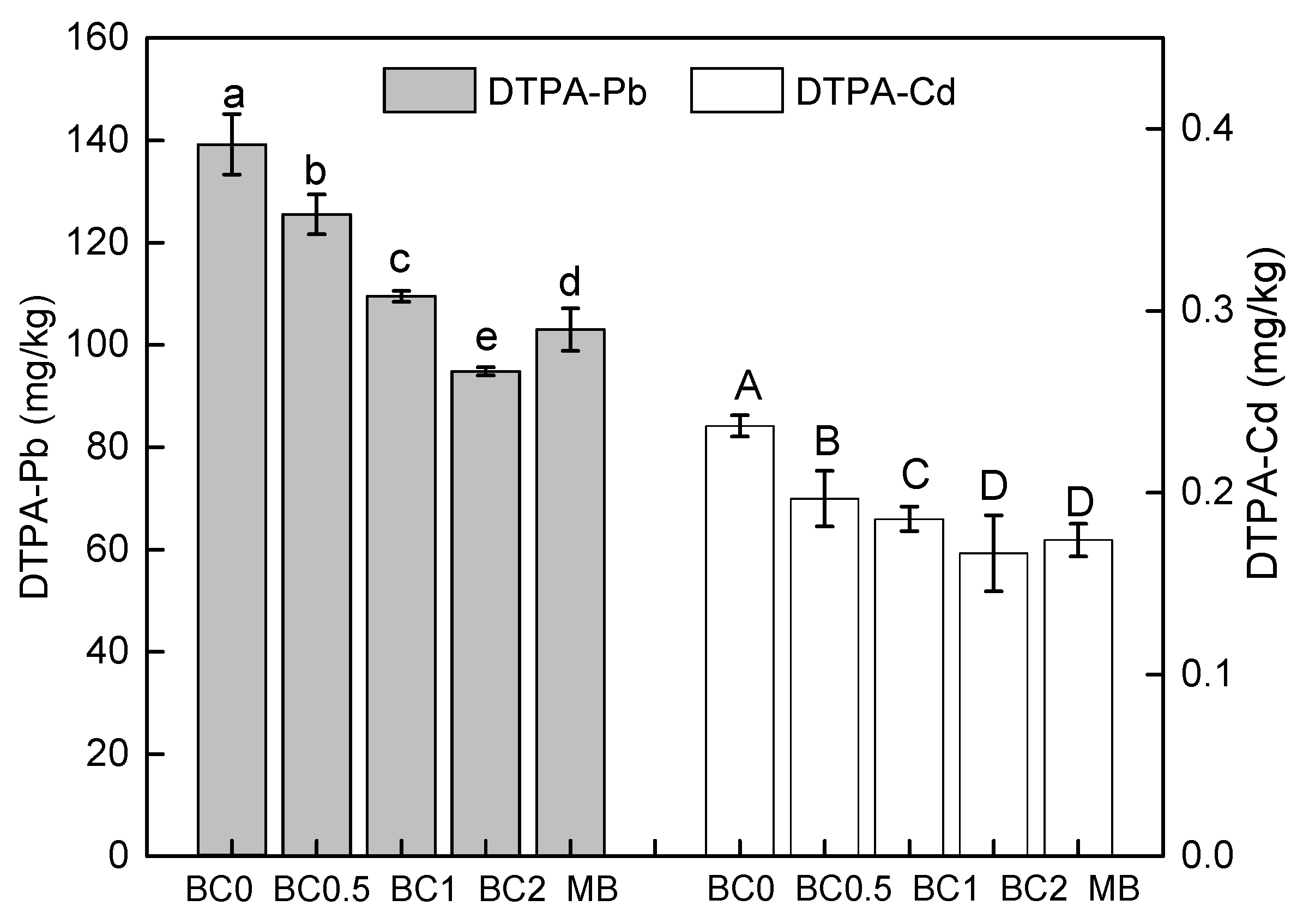
T-BC application can improve soil quality and therefore the reduction in soil Cd and Pb availability to the plant. The concentrations of DTPA-extractable Cd and Pb in the soil after the harvest of the plant is shown in Figure 3. The overall performance of the concentrations of available Pb and Cd change initially decreased and then increased along with the BC application rate. Moreover, with the same dose of MB applied, the ability of T-BC to reduce the content of DTPA-extractable Pb and Cd in soil was stronger than that of BC (Figure 3). Soil DPTA-extractable Cd and Pb was decreased with BC applied by 10%, 21%, 32%, and 26% (Pb), and by 17%, 22%, 30%, and 26% (Cd), respectively, compared with the BC0 (Figure 3). These findings confirm that the concentration of DTPA-extractable Pb and Cd was reduced, and that this resulted from T-BC and MB absorbing the Pb and Cd in the amended soil and thereafter preventing the mobilization of Pb and Cd in the soil. In this study, the increase in soil pH and DOC (Table 2) probably further enhanced the absorption of Pb and Cd because it not only increased the negative charge in the soil components, but it also increased DOC including different types of functional groups such as hydroxyl, carboxyl, and amino groups [36]. A large amount of available Cd and Pb precipitates with organic functional groups, suggesting that the majority of the Cd and Pb is in the form of a Cd or Pb organic complex [51]. Moreover, the higher pH value promoted the exchangeable fraction of Cd and Pb to the residual fraction, resulting in a decrease of the available Cd and Pb content in the soils. Meanwhile, increases in Cd and Pb were bounded to organic matter as soil pH values increased—this has also been observed previously [36]. This may also strongly confirm that T-BC has a stronger ability to stabilize Cd and Pb than MB.
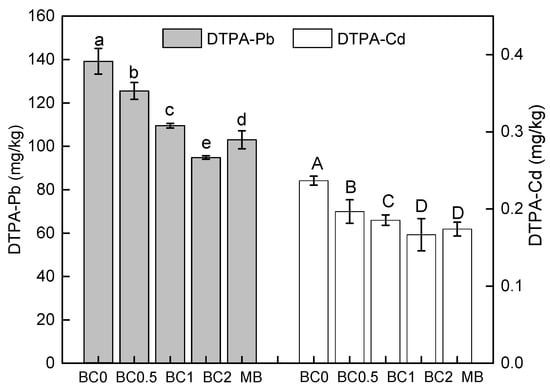
Figure 3.
Soil bioavailable DTPA-Pb and DTPA-Cd. BC0, BC0.5, BC1, and BC2 mean as soil applied with T-BC of four rate (0, 0.5, 1, and 2% (w/w)); MB means as soil applied with biochar (2% (w/w)) from mango leaves.
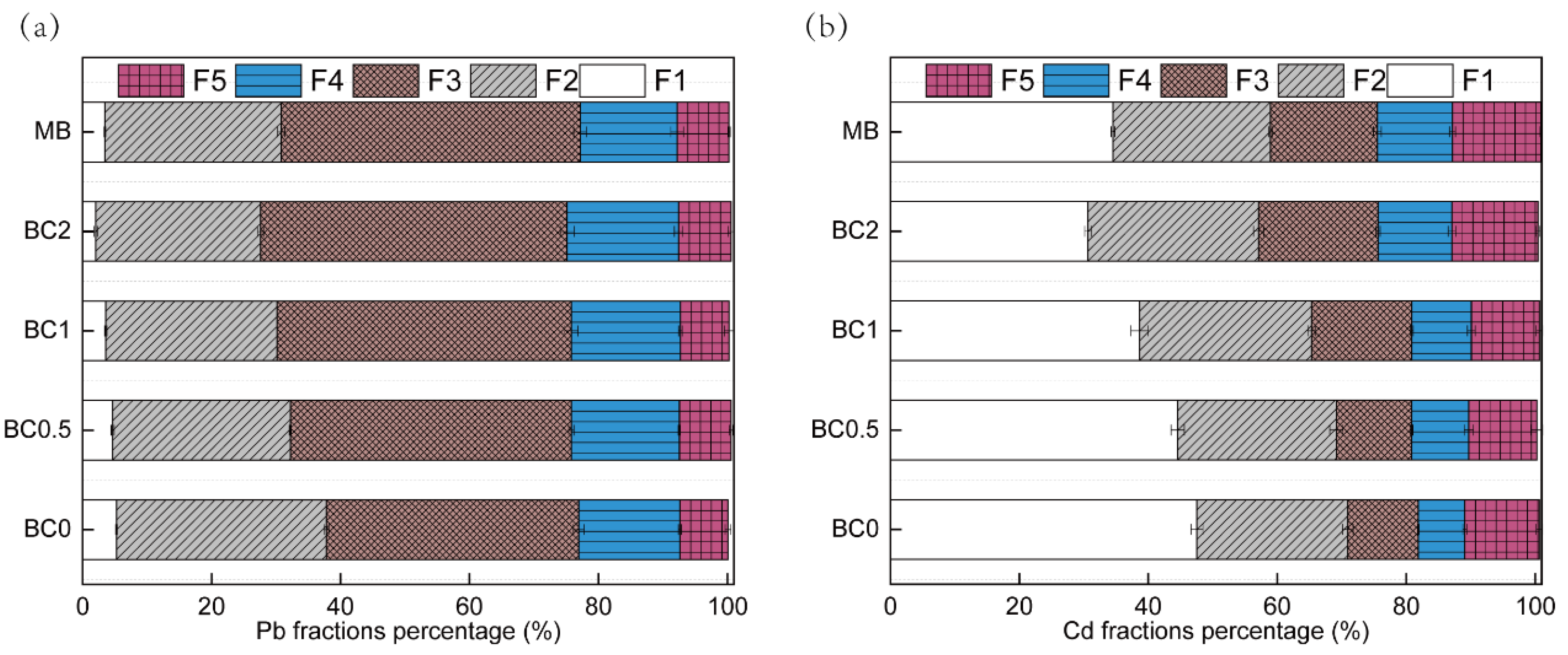
The Pb and Cd in soils was sequentially fractionated, comprising F1, F2, F3, F4, and F5. For the sequential five fractions of Pb and Cd, F1 and F2 are easily absorbed into the plant, and the sum of F1 and F2 can be considered as the bioavailable state. However, F3, F4, and F5 are barely absorbed and utilized by plants. The speciation of Pb in soils is shown in Figure 4a. The sum of the two most labile fractions (F1 and F2) for Pb decreased from 38% to 32, 30, 27, and 31%, and the sum of the two most recalcitrant fractions (F4 and F5) was basically unchanged with 0.5, 1, and 2% T-BC and 2% MB addition, respectively (Figure 4a). One similar study comes from Park et al. [52], for whom the sum of the two fractions, including F1 and F2 for Pb, decreased from 59% to 49, and 31 and 17%, and the sum of the two most recalcitrant fractions (F4 and F5) also increased with three doses of chicken manure-derived biochar addition (1, 5, and 15%), respectively. In previous studies, the possible mechanisms for Pb immobilization by BC are shown to be: (a) the formation of Pb (hydro)oxide, carbonate, or phosphate precipitates [28,52]; (b) electrostatic interactions between metal cations and the activated functional groups by increasing the pH [25,45]; and (c) surface chemisorption between d-electrons of metals and delocalized π-electrons of chars [43]. The applied T-BC have the ability to transform the available forms of Pb into more stable forms. These results are in line with Ahmad et al. [53]. The increase in the reducible forms of Pb might be possibly due to the formation of Fe/Mn oxides and bonds with the mineral phase of BC as reported by Ahmad et al. [53]. As Cd metal properties, Cd content above 90% was distributed principally in F1, F2, F3, and F5 in un-treated soil (Figure 4b). The F1 of Cd resulting from the treatments of BC 0.5, BC1, BC2, and MB were significantly decreased by 3%, 9%, 17%, and 13%, respectively, compared to the control. Meanwhile, the effects of T-BC applications on F1 significantly decreased compared to the pristine biochar treatment (MB). Compared to the control, the sum fraction of F2 and F3, the treatments of BC 0.5, BC1, BC2, and MB significantly increased by 6%, 23%, 30%, and 19%, respectively, and a similar trend was also found in the F4 and F5 fraction (Figure 4b). In contaminated soil, the F1-Cd and Pb are important indicators of the potentially bioavailable elements, and their release and availability into the environment vary with changes in pH and SOC [36]. The immobilization of Cd by BC may have resulted not only from the complex formation with ionized hydroxyl-O-groups, but also from the precipitation of metals with CO3 2– and/or PO4 3− that were rich in the form of F2 [29]. In addition, the application of T-BC enhanced the residual fraction of Cd mainly because of the strong binding of Cd to the inner amendment magnetic particles. Cd was electively immobilized by Fe and Mn oxides as a result of the increased content of Fe and Mn, thus resulting in reducing heavy metal availability [54].
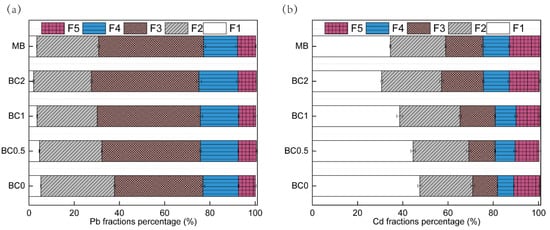
Figure 4.
Percent Pb (a) and Cd (b) species distribution in soil. F1 means exchangeable fractions bound, F2 means carbonate fractions bound, F3 means Fe\Mn oxides fractions bound, F4 means organic fractions bound, and F5 means residual fractions bound. BC0, BC0.5, BC1 and BC2 mean as soil applied with T-BC of four rate (0, 0.5, 1 and 2% (w/w)); MB means as soil applied with 20 g/pot of biochar from mango leaves. BC0, BC0.5, BC1, and BC2 mean as soil applied with T-BC of four rate (0, 0.5, 1, and 2% (w/w)); MB means as soil applied with biochar (2% (w/w)) from mango leaves.
3.5. Physiological Characteristics and Cd, Pb Concentrations in Pak Choi
Quite a few factors may influence crop growth, including soil properties (soil pH, the property of water, nutrient retention, etc.) and toxic elements [3,55,56]. With T-BC/MB addition, the amelioration of metal (-loid) stress and soil environment could lead to an increase in crop yield (Table S4) with the maximum biomass was achieved in BC2 treatments. In addition, the lowest root and shoot biomass was noticed in the control treatment. More specifically, the harvest index of the BC2 and MB treatment of contaminated soil had shoot and root biomasses that were increased by 3% compared to those of the control (Table S4). T-BC has always maintained an advantage over conventional biochar (MB). The T-BC amendment appeared to enhance the yield and harvest index of Chinese cabbage, possibly due to the higher bioavailability of essential nutrients (Table S1) and simultaneous significant immobilization of Cd and Pb (Figure 2 and Figure 3). The obtained results in this study are similar to previous studies of Awasthi et al. [43]. Their results revealed that biochar (4% and 8%) amended with 150 kg/ha N in contaminated soil contributed to the enhancement of nutrient bioavailability for plant uptake, and that this process appears to also be dependent upon soil characteristics.
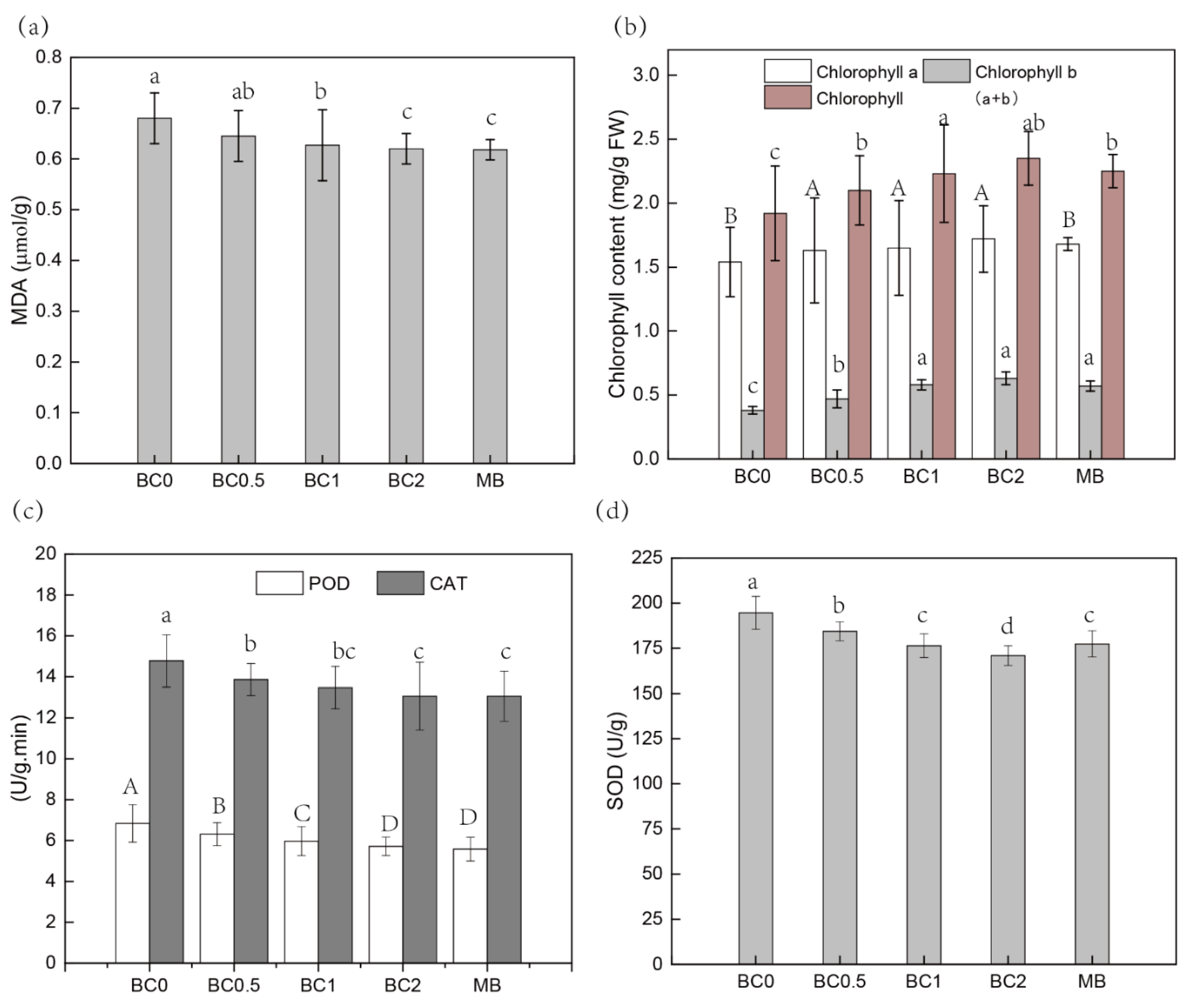
Many studies have confirmed that heavy metals (-loids) can cause oxidative stress by generating reactive oxygen species (ROS) [3,9], hence, resulting in cell membrane disruption (electrolyte leakage), inhibition of nutrient uptake, and enhanced lipid peroxidation (MDA increase) [3,9,23]. The application of biochar and replaced soil could both decrease metal(-loid) content and subsequently the stress on crops through increasing membrane stability [57]. The application of different rates of BC significantly reduced the concentrations of MDA in leaves compared to the plant leaves grown in control soil, and the increasing T-BC amendments positively affected the reduction in oxidative stress (Figure 5a). Similarly, MDA content decreased approximately 9% in leaves in the 2% MB amendments compared to that of the B0 treatment (Figure 5a). At the same time, heavy metals (-loids) can impair photosynthetic activity in plants by causing chloroplast ultrastructure damage [3]. The chlorophyll a, b and chlorophyll a + b content of pak choi leaves significantly increased with T-BC and MB application (Figure 5b). These findings confirm that T-BC application can mitigate the stress effects of Cd and Pb and promote plant growth. Cell peroxidase (POD) activity and catalase (CAT) are mainly used to remove excessive H2O2 in cells by catalytic hydrogen peroxide reaction, so as to maintain plant-cell internal structure stability and improve plant adaptability to stress [9,23,57,58,59]. After the addition of T-BC and MB, POD and CAT enzyme activity of pak choi could be reduced to lower than the control group and the POD and CAT values decreased with the increase in the amount of T-BC and MB additive (Figure 5c). The results show that amendments can effectively reduce POD and CAT content in pak choi, so as to reduce the toxic effect of Cd and Pb stress on pak choi growth. Compared with the control group, CAT content and POD content in pak choi with amendments was significantly reduced after the addition of amendments, which is consistent with the results of Xu et al. [9]. Likewise, the addition of T-BC and MB significantly decreased the activities of SOD in leaves (Figure 5d). In contaminated soil-stressed pak choi, the application of T-BC and MB caused reductions in SOD activities by 5–12% and 9% when increasing the application rate from 0.5% to 2% (T-BC) and 2% (MB), respectively (Figure 5d). These results confirm that T-BC and MB can easily change the form of bioavailable Pb and Cd, and thereby arrive at a more stable state, which might be the reason for the decrease of antioxidant stress to plants and enhanced plant growth.
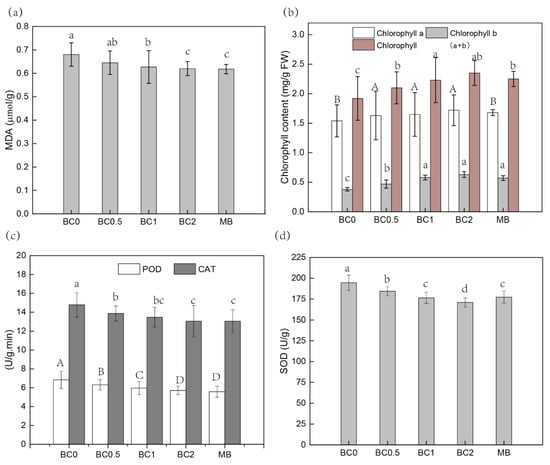
Figure 5.
The physiological characteristics of pak choi. MDA (a); Chlorophyll content (b); POD and CAT activity (c); SOD activity (d). BC0, BC0.5, BC1, and BC2 mean as soil applied with T-BC of four rate (0, 0.5, 1, and 2% (w/w)); MB means as soil applied with biochar (2% (w/w)) from mango leaves.
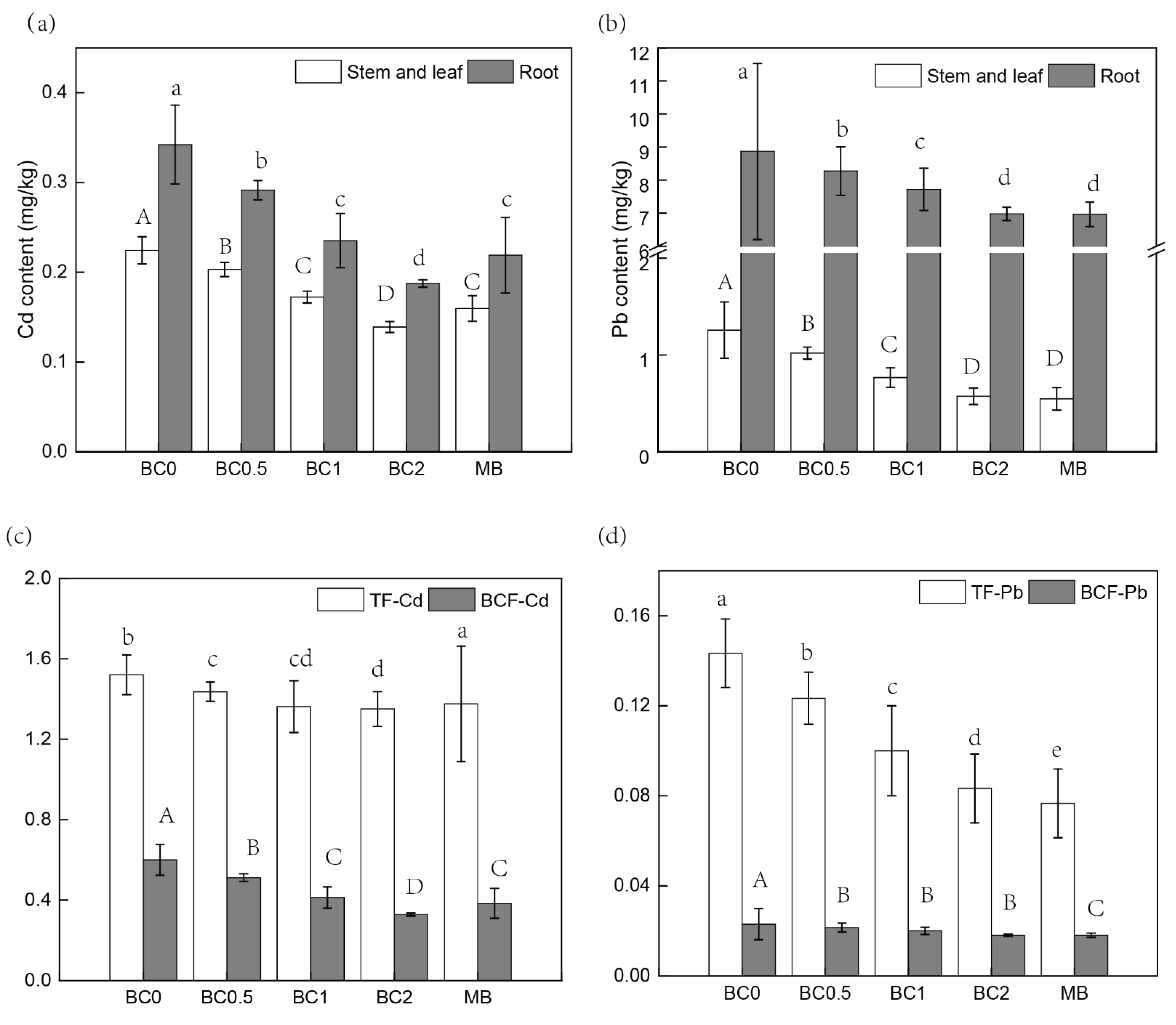
The Cd concentration decreased in roots and shoots (leaves) with the T-BC amendment, and the effect was more pronounced at 2% BC than that of lower BC rates (0.5% and 1%). The application of 0.5%, 1%, and 2% T-BC decreased the Cd concentrations in shoots (leaves) approximately by 9%, 23%, and 36%, as compared to the control, respectively (Figure 6a). In the 2% MB treatment, Cd concentration in shoots (leaves) was 0.16 ± 0.01 mg /kg, which was significantly lower (approximately 27%) than that of the control treatment (0.22 ± 0.01 mg/kg) (Figure 6a). Similarly, Pb concentration was significantly decreased in roots, shoots, and leaves in a dose-additive of T-BC. However, there was a non-significant difference in Pb contents in roots, shoots, and leaves between 2% T-BC and 2% MB (Figure 6b). Clearly, under the T-BC and MB treatments (including the control), the Pb concentrations in the shoots and leaves of pak choi exceeded the standard (0.20 mg/kg) established by the National Food Safety Standard of China (GB2762-2017). Therefore, from the perspective of soil environmental quality and safety and the high yield of Chinese cabbage, higher doses of T-BC or a combination of low-accumulation genotype for the remediation of Cd- and Pb-contaminated soil is recommended. In addition, the accumulation of Cd and Pb in pak choi plants was significantly decreased by BC amendments (Figure 6a,b). The highest Cd and Pb concentrations were found in the roots, followed by shoots in all treatments. The impact of T-BC and MB on the biological concentration factor (BCF) and translocation factor (TF) of Pb and Cd are presented in Figure 6c,d. Soil without T-BC and MB incorporation showed higher Cd and Pb accumulation rates and exhibited higher values of BCF and TF.
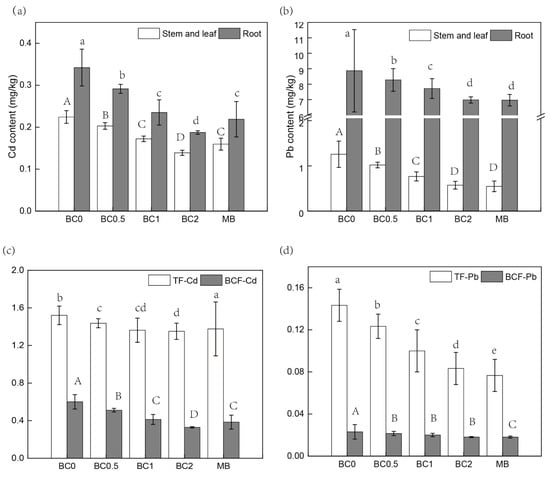
Figure 6.
The Cd (a,c) and Pb (b,d) accumulation in the edible part of pak choi based on fresh weight. BC0, BC0.5, BC1, and BC2 mean as soil applied with T-BC of four rate (0, 0.5, 1, and 2% (w/w)); MB means as soil applied with biochar (2% (w/w)) from mango leaves.
3.6. Relationship among Parameters
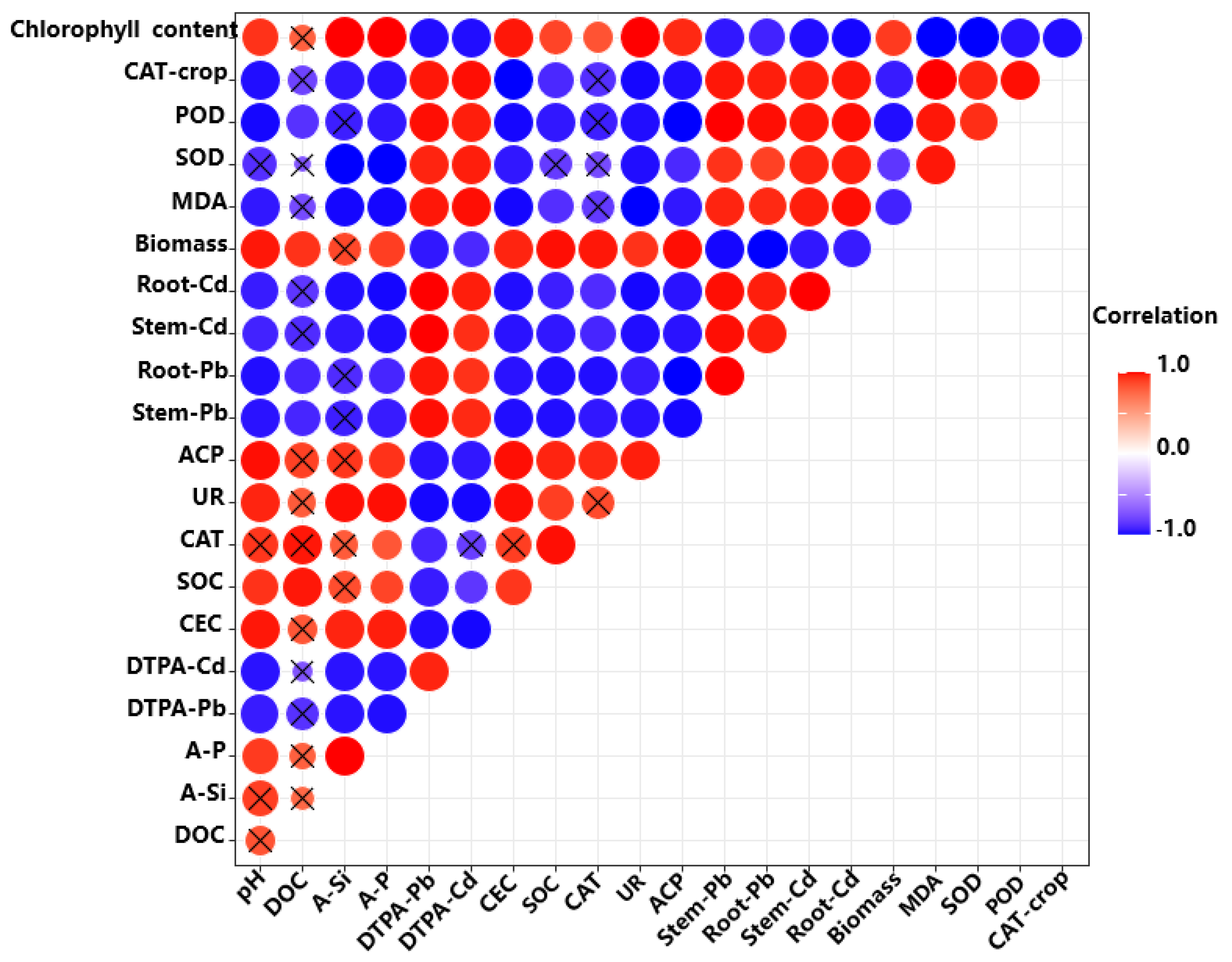
Figure 7 shows the Pearson correlation coefficients of soil properties, soil DTPA-Cd and Pb, pak choi organ Cd and Pb, and physiological characteristics of pak choi. Significant positive correlations were observed in pH, DOC, and A-Si content (p < 0.05). Soil DTPA-extractable Cd and Pb were positively correlated with root and stem Cd and Pb (p < 0.05). Similarly, Cd and Pb concentration in each pak choi root showed a positive correlation with that in stems and leaves. Pak choi biomass and chlorophyll content in leaves were positively correlated with soil pH, SOC, CEC, DOC, and A-Si and A-P content, and negatively correlated with soil DTPA-extractable Cd and Pb and pak choi-organ Cd and Pb (p < 0.05). Moreover, the physiological characteristics of pak choi, including MDA, SOD, and POD, were also significantly and negatively correlated with DTPA-Cd and DTPA-Pb (p < 0.05). This result can also be attributed to the decrease of heavy-metal accumulation in pak choi tissues due to the change in the chemical behavior of the soil with BC application.
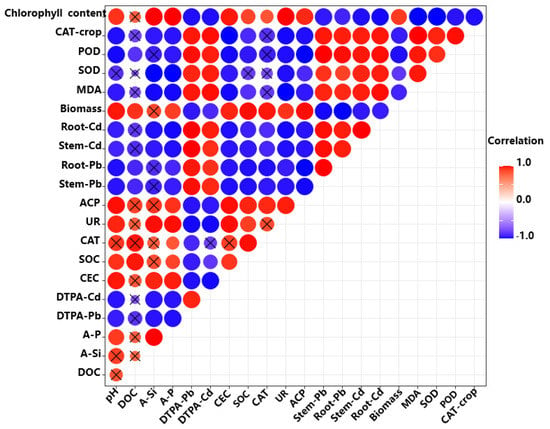
Figure 7.
Pearson correlation coefficients for the relationships among the tested parameters of pak choi and soil (n = 5).
3.7. Health Risk Assessment of Cd and Pb Intake in Pak Choi
The edible parts of pak choi accumulate a large amount of Cd and Pb, which may pose potential risks to human health through the food chain. The estimated daily intake (EDI) of local residents (children and adults) was calculated by a formula. It is shown in Table 3; children ingested (μg·kg−1·d−1) Pb (4.48 and 4.27) and Cd (1.09 and 1.25) daily through vegetable consumption and local adults consume (μg·kg−1·d−1) Pb (3.38 and 3.23) and Cd (0.82 and 0.94) through daily vegetable consumption in the 2% T-BC and MB treatments. The results show that the children’s EDI of Pb and Cd is greater than the adult’s EDI, which means that it will potentially cause greater harm to children. However, the potential health risks of pak choi to the human body with a T-BC and MB treatment in the soil were significantly reduced compared to the control group. In addition, the target risk factor (THQ) has been widely used to assess the health risks of eating contaminated crops [4,10]. Residents (adults and children) eat the THQ value of pak choi THQPb > THQCd. Compared with the control treatment, a 2% T-BC application decreased the THQ value of pak choi Pb by 54% and 54%, and Cd by 39% and 38%, respectively. The application of MB and T-BC shows similar results. Unfortunately, in all the treatments, pak choi still poses a health risk to the human body. This result is similar to those of previous studies—pak choi is a local vegetable that has a serious impact on human health [60].

Table 3.
Estimated daily intake (EDI) and health risk assessment of heavy metals in aboveground part of pak choi.
4. Conclusions
A new “waste-safety” strategy has been demonstrated to simultaneously handle both the iron tailing waste and biomass and soil pollution problems. The mobile metals in iron tailings were volatilized and immobilized by T-BC during the co-pyrolysis process with biomass and urea. While T-BC has been employed to remediate Cd/Pb contaminated soil, protecting photosynthetic systems and increasing nutrient absorption and the growth of plants, at same time it drastically reduces the Pb and Cd accumulation concentrations in the edible parts of pak choi and blocks the diffusion of Cd and Pb into the food chain. Soil remediation with T-BC can increase pH, DOC content, and mineral nutrients, and enhance soil enzyme activity. Overall, this research has strongly demonstrated the possibility and application potential of using waste (iron tailings and biomass) to remediate heavy-metal-contaminated soil. In the next step, more in-depth work is expected to comprehensively evaluate remediation efficiency, cost-effectiveness, long-term performance, and environmental implications.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/pr9111866/s1, Figure S1: The effect of various T-BC on concentrations (mg/kg) of the DTPA-extractable (Cd), (Pb) in soil. Figure S2: The FTIR spectra of T-BC4. Table S1: The composition (%) of tailings by XRF and the heavy metal in tailings and MR. Table S2: Leaching toxicity of the T-BC under different biomass and urea rate. Table S3: Some physical and chemical properties of the different treatments soil.
Author Contributions
Funding acquisition, resources and writing—review and editing, L.L.; data curation, investigation and methodology, writing—review and editing Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Guangdong Natural Science Foundation (2017A030310D05).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Research data can be obtained from the author.
Acknowledgments
The work was supported by Guangdong Natural Science Foundation (2017A030310D05).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lu, A.; Wang, J.; Qin, X.; Wang, K.; Han, P.; Zhang, S. Multivariate and geostatistical analyses of the spatial distribution and origin of heavy metals in the agricultural soils in Shunyi, Beijing, China. Sci. Total Environ. 2012, 425, 66–74. [Google Scholar] [CrossRef]
- Niu, L.; Yang, F.; Xu, C.; Yang, H.; Liu, W. Status of metal accumulation in farmland soils across China: From distribution to risk assessment. Environ. Pollut. 2013, 176, 55–62. [Google Scholar] [CrossRef]
- Jiang, S.; Dai, G.; Zhou, J.; Zhong, J.; Liu, J.; Shu, Y. An assessment of integrated amendments of biochar and soil replacement on the phytotoxicity of metal(loid)s in rotated radish-soya bean-amaranth in a mining acidy soil. Chemosphere 2021, 287, 132082. [Google Scholar] [CrossRef]
- Qin, J.; Niu, A.; Liu, Y.; Lin, C. Arsenic in leafy vegetable plants grown on mine water-contaminated soils: Uptake, human health risk and remedial effects of biochar. J. Hazard. Mater. 2021, 402, 123488. [Google Scholar] [CrossRef]
- Zhong, Q.; Zhou, Y.; Tsang, D.; Liu, J.; Yang, X.; Yin, M.; Wu, S.; Wang, J.; Xiao, T.; Zhang, Z. Cadmium isotopes as tracers in environmental studies: A review. Sci. Total Environ. 2020, 736, 139585. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Yin, M.; Zhang, Q.; Beiyuan, J.; Liu, J.; Yang, X.; Wang, J.; Wang, L.; Jiang, Y.; Xiao, T.; et al. Cadmium isotopic fractionation in lead-zinc smelting process and signatures in fluvial sediments. J. Hazard. Mater. 2021, 411, 125015. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, G.; Murtaza, B.; Niazi, N.K.; Sabir, M. Soil Contaminants: Sources, Effects, and Approaches for Remediation. In Improvement of Crops in the Era of Climatic Changes; Springer: New York, NY, USA, 2014; pp. 171–196. [Google Scholar]
- Venegas, A.; Rigol, A.; Vidal, M. Viability of organic wastes and biochars as amendments for the remediation of heavy metal-contaminated soils. Chemosphere 2015, 119, 190–198. [Google Scholar]
- Xu, C.; Qi, J.; Yang, W.; Chen, Y.; Yang, C.; He, Y.; Wang, J.; Lin, A. Immobilization of heavy metals in vegetable-growing soils using nano zero-valent iron modified attapulgite clay. Sci. Total Environ. 2019, 686, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.; McBride, M.B.; Xia, H.; Li, N.; Li, Z. Health risk from heavy metals via consumption of food crops in the vicinity of Dabaoshan mine, South China. Sci. Total Environ. 2009, 407, 1551–1561. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A comparison of technologies for remediation of heavy metal contaminated soils. J. Geochem. Explor. 2017, 182, 247–268. [Google Scholar] [CrossRef] [Green Version]
- Yao, Z.; Li, J.; Xie, H.; Yu, C. Review on Remediation Technologies of Soil Contaminated by Heavy Metals. Procedia Environ. Sci. 2012, 16, 722–729. [Google Scholar] [CrossRef] [Green Version]
- Duan, M.-M.; Wang, S.; Huang, D.-Y.; Zhu, Q.-H.; Liu, S.-L.; Zhang, Q.; Zhu, H.-H.; Xu, C. Effectiveness of simultaneous applications of lime and zinc/iron foliar sprays to minimize cadmium accumulation in rice. Ecotoxicol. Environ. Saf. 2018, 165, 510–515. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, H.; Hu, X.; Liu, F.; Wang, L.; Zhao, X.; Gao, P.; Ji, P. Optimization of preparation technology for modified coal fly ash and its adsorption properties for Cd2+. J. Hazard. Mater. 2020, 392, 122461. [Google Scholar] [CrossRef]
- Yang, T.; Wang, Y.; Sheng, L.; He, C.; Sun, W.; He, Q. Enhancing Cd(II) sorption by red mud with heat treatment: Performance and mechanisms of sorption. J. Environ. Manag. 2020, 255, 109866. [Google Scholar] [CrossRef]
- Yang, D.; Deng, W.; Tan, A.; Chu, Z.; Wei, W.; Zheng, R.; Shangguan, Y.; Sasaki, A.; Endo, M.; Chen, H. Protonation stabilized high As/F mobility red mud for Pb/As polluted soil remediation. J. Hazard. Mater. 2021, 404, 124143. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.-X.; Ning, X.-A.; Lu, X.-W.; Li, Y.; Lai, X.-J.; Zeng, J.; Chen, C.-H. Effect of chlorine on the volatilization of heavy metals by roasting iron tailings. China Environ. Sci. 2020, 40, 2276–2286. [Google Scholar]
- Li, R.; Cai, H.; Ning, X. Separation of lead, copper, cadmium in iron tailings by CaCl2 chlorination roasting method. Chin. J. Environ. Eng. 2021, 15, 1083–1091. [Google Scholar]
- Sun, T.; Xu, Y.; Sun, Y.; Wang, L.; Liang, X.; Zheng, S. Cd immobilization and soil quality under Fe–modified biochar in weakly alkaline soil. Chemosphere 2021, 280, 130606. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Hong, M.; Li, H.; Ye, Z.; Gong, H.; Zhang, J.; Huang, Q.; Tan, Z. Contributions and mechanisms of components in modified biochar to adsorb cadmium in aqueous solution. Sci. Total Environ. 2020, 733, 139320. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, S.; Wang, J.; Ding, X. Phosphorus retention using iron (II/III) modified biochar in saline-alkaline soils: Adsorption, column and field tests. Environ. Pollut. 2020, 261, 114223. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, Y.; Zhou, J.; Guo, J.; Ren, J.; Zhou, F. Efficient removal of lead from aqueous solution by urea-functionalized magnetic biochar: Preparation, characterization and mechanism study. J. Taiwan Inst. Chem. Eng. 2018, 91, 457–467. [Google Scholar] [CrossRef]
- Kamran, M.; Malik, Z.; Parveen, A.; Zong, Y.; Abbasi, G.H.; Rafiq, M.T.; Shaaban, M.; Mustafa, A.; Bashir, S.; Rafay, M.; et al. Biochar alleviates Cd phytotoxicity by minimizing bioavailability and oxidative stress in pak choi (Brassica chinensis L.) cultivated in Cd-polluted soil. J. Environ. Manag. 2019, 250, 109500. [Google Scholar] [CrossRef]
- Lu, R. Analytical Methods of Soil Agrochemistry; China Agricultural Science and Technology Publishing House: Beijing, China, 1999; pp. 18–99. [Google Scholar]
- Jiang, S.; Liu, J.; Wu, J.; Dai, G.; Wei, D.; Shu, Y. Assessing biochar application to immobilize Cd and Pb in a contaminated soil: A field experiment under a cucumber–sweet potato–rape rotation. Environ. Geochem. Health 2020, 42, 4233–4244. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiao, X.; Zhang, K.; Chen, B. Effects of biochar amendment on the soil silicon cycle in a soil-rice ecosystem. Environ. Pollut. 2019, 248, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Tessier, A.; Campbell, P.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Jin, Y.; Liang, X.; He, M.; Liu, Y.; Tian, G.; Shi, J. Manure biochar influence upon soil properties, phosphorus distribution and phosphatase activities: A microcosm incubation study. Chemosphere 2016, 142, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Fan, Y.; Yang, J.; Xu, L.; Zhou, J.; Zhu, Z. In situ phytoextraction of copper and cadmium and its biological impacts in acidic soil. Chemosphere 2016, 161, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L.; Temple, K.L. Some Variables Affecting the Measurement of “Catalase Activity” in Soil. Soil Sci. Soc. Am. J. 1964, 28, 207–209. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, C.; Zhao, X.; Tan, Q.; Sun, X.; Cao, A.; Cui, M.; Zhang, Y. Molybdenum improves antioxidant and osmotic-adjustment ability against salt stress in Chinese cabbage (Brassica campestris L. ssp. Pekinensis). Plant Soil 2012, 355, 375–383. [Google Scholar] [CrossRef]
- Huang, H.; Rizwan, M.; Li, M.; Song, F.; Zhou, S.; He, X.; Ding, R.; Dai, Z.; Yuan, Y.; Cao, M.; et al. Comparative efficacy of organic and inorganic silicon fertilizers on antioxidant response, Cd/Pb accumulation and health risk assessment in wheat (Triticum aestivum L.). Environ. Pollut. 2019, 255, 113146. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L.J. Photoperoxidation in isolated chloroplasts. i. kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Yu, J.; Sun, L.; Ma, C.; Qiao, Y.; Xiang, J.; Hu, S.; Yao, H. Mechanism on heavy metals vaporization from municipal solid waste fly ash by MgCl2 6H2O. Waste Manag. 2016, 49, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ma, A.; Srinivasakannan, C.; Zhang, L.; Li, S.; Yin, S. Investigation on the recovery of gold and silver from cyanide tailings using chlorination roasting process. J. Alloys Compd. 2018, 763, 241–249. [Google Scholar] [CrossRef]
- Wu, C.; Shi, L.; Xue, S.; Li, W.; Jiang, X.; Rajendran, M.; Qian, Z. Effect of sulfur-iron modified biochar on the available cadmium and bacterial community structure in contaminated soils. Sci. Total Environ. 2019, 647, 1158–1168. [Google Scholar] [CrossRef]
- Sun, T.; Xu, Y.; Sun, Y.; Wang, L.; Liang, X.; Jia, H. Crayfish shell biochar for the mitigation of Pb contaminated water and soil: Characteristics, mechanisms, and applications. Environ. Pollut. 2021, 271, 116308. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, M.; Shi, L.; Wu, C.; Li, W.C.; An, W.; Liu, Z.; Xue, S. Effect of sulfur and sulfur-iron modified biochar on cadmium availability and transfer in the soil–rice system. Chemosphere 2019, 222, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zheng, L.; Wang, S.-L.; Wu, Z.; Wu, W.; Niazi, N.K.; Shaheen, S.M.; Rinklebe, J.; Bolan, N.; Ok, Y.S.; et al. Sorption mechanisms of lead on silicon-rich biochar in aqueous solution: Spectroscopic investigation. Sci. Total Environ. 2019, 672, 572–582. [Google Scholar] [CrossRef]
- Yao, X.; Ji, L.; Guo, J.; Ge, S.; Lu, W.; Cai, L.; Wang, Y.; Song, W.; Zhang, H. Magnetic activated biochar nanocomposites derived from wakame and its application in methylene blue adsorption. Bioresour. Technol. 2020, 302, 122842. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Huang, D.; Liu, X.; Meng, J.; Tang, C.; Xu, J. Remediation of As(III) and Cd(II) co-contamination and its mechanism in aqueous systems by a novel calcium-based magnetic biochar. J. Hazard. Mater. 2018, 348, 10–19. [Google Scholar] [CrossRef]
- Azeem, M.; Ali, A.; Jeyasundar, P.G.S.A.; Li, Y.; Abdelrahman, H.; Latif, A.; Li, R.; Basta, N.; Li, G.; Shaheen, S.M.; et al. Bone-derived biochar improved soil quality and reduced Cd and Zn phytoavailability in a multi-metal contaminated mining soil. Environ. Pollut. 2021, 277, 116800. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Wang, Q.; Chen, H.; Liu, T.; Awasthi, S.K.; Duan, Y.; Varjani, S.; Pandey, A.; Zhang, Z. Role of compost biochar amendment on the (im)mobilization of cadmium and zinc for Chinese cabbage (Brassica rapa L.) from contaminated soil. J. Soils Sediments 2019, 19, 3883–3897. [Google Scholar] [CrossRef]
- Bashir, S.; Hussain, Q.; Shaaban, M.; Hu, H. Efficiency and surface characterization of different plant derived biochar for cadmium (Cd) mobility, bioaccessibility and bioavailability to Chinese cabbage in highly contaminated soil. Chemosphere 2018, 211, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Albert, H.A.; Li, X.; Jeyakumar, P.; Wei, L.; Huang, L.; Huang, Q.; Kamran, M.; Shaheen, S.M.; Hou, D.; Rinklebe, J.; et al. Influence of biochar and soil properties on soil and plant tissue concentrations of Cd and Pb: A meta-analysis. Sci. Total Environ. 2021, 755, 142582. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, X.; Jing, Y.; Li, Q.; Zhang, J.; Huang, Q. Effects of biochar amendment on rapeseed and sweet potato yields and water stable aggregate in upland red soil. Catena 2014, 123, 45–51. [Google Scholar] [CrossRef]
- Salam, A.; Bashir, S.; Khan, I.; Hussain, Q.; Gao, R.; Hu, H. Biochar induced Pb and Cu immobilization, phytoavailability attenuation in Chinese cabbage, and improved biochemical properties in naturally co-contaminated soil. J. Soils Sediments 2019, 19, 2381–2392. [Google Scholar] [CrossRef]
- Bailey, V.L.; Fansler, S.J.; Smith, J.L.; Bolton, H. Reconciling apparent variability in effects of biochar amendment on soil enzyme activities by assay optimization. Soil Biol. Biochem. 2011, 43, 296–301. [Google Scholar] [CrossRef]
- Dempster, D.N.; Gleeson, D.; Solaiman, Z.; Jones, D.L.; Murphy, D. Decreased soil microbial biomass and nitrogen mineralisation with Eucalyptus biochar addition to a coarse textured soil. Plant Soil 2012, 354, 311–324. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Zheng, J.; Zhang, B.; Lu, H.; Chi, Z.; Pan, G.; Li, L.; Zheng, J.; Zhang, X.; et al. Biochar soil amendment increased bacterial but decreased fungal gene abundance with shifts in community structure in a slightly acid rice paddy from Southwest China. Appl. Soil Ecol. 2013, 71, 33–44. [Google Scholar] [CrossRef]
- Guo, X.; Xie, X.; Liu, Y.; Wang, C.; Yang, M.; Huang, Y. Effects of digestate DOM on chemical behavior of soil heavy metals in an abandoned copper mining areas. J. Hazard. Mater. 2020, 393, 122436. [Google Scholar] [CrossRef]
- Park, J.H.; Choppala, G.K.; Bolan, N.S.; Chung, J.W.; Chuasavathi, T. Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant Soil 2011, 348, 439–451. [Google Scholar] [CrossRef]
- Ahmad, M.; Lee, S.S.; Lee, S.E.; Al-Wabel, M.I.; Tsang, D.C.W.; Ok, Y.S. Biochar-induced changes in soil properties affected immobilization/mobilization of metals/metalloids in contaminated soils. J. Soils Sediments 2017, 17, 717–730. [Google Scholar] [CrossRef]
- Fernández-Calviño, D.; Pérez-Armada, L.; Cutillas-Barreiro, L.; Paradelo, R.; Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Álvarez, E.; Arias-Estévez, M. Changes in Cd, Cu, Ni, Pb and Zn Fractionation and Liberation Due to Mussel Shell Amendment on a Mine Soil. Land Degrad. Dev. 2016, 27, 1276–1285. [Google Scholar] [CrossRef]
- Shabbir, A.; Saqib, M.; Murtaza, G.; Abbas, G.; Imran, M.; Rizwan, M.; Naeem, M.A.; Ali, S.; Javeed, H.M.R. Biochar mitigates arsenic-induced human health risks and phytotoxicity in quinoa under saline conditions by modulating ionic and oxidative stress responses. Environ. Pollut. 2021, 287, 117348. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.Z.U.; Waqar, M.; Bashir, S.; Rizwan, M.; Ali, S.; Baroudy, A.A.E.F.E.; Khalid, H.; Ayub, M.A.; Usman, M.; Jahan, S. Effect of biochar and compost on cadmium bioavailability and its uptake by wheat–rice cropping system irrigated with untreated sewage water: A field study. Arab. J. Geosci. 2021, 14, 135. [Google Scholar] [CrossRef]
- Zhao, H.; Huang, X.; Liu, F.; Hu, X.; Zhao, X.; Wang, L.; Gao, P.; Li, J.; Ji, P. Potential of a novel modified gangue amendment to reduce cadmium uptake in lettuce (Lactuca sativa L.). J. Hazard. Mater. 2021, 410, 124543. [Google Scholar] [CrossRef]
- Abbas, T.; Rizwan, M.; Ali, S.; Rehman, M.Z.U.; Qayyum, M.F.; Abbas, F.; Hannan, F.; Rinklebe, J.; Ok, Y.S. Effect of biochar on cadmium bioavailability and uptake in wheat ( Triticum aestivum L.) grown in a soil with aged contamination. Ecotoxicol. Environ. Saf. 2017, 140, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhou, A.-Q.; Cao, H.-B.; Liu, J.-W.; Chen, Y.-J.; Zhang, A.-C. Safety limits of heavy metals in planted soil of Chinese cabbage based on health risk assessment. J. Agro-Environ. Sci. 2020, 39, 1213–1220. [Google Scholar]
- Cao, C.; Zhang, S.; Zhang, P. Heavy metal contamination in soil–vegetable systems and its health risks in an area irrigated with acid mine drainage in Dabaoshan, Guangdong, China. J. Agro-Environ. Sci. 2020, 39, 1–16. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).