A New Clark-Type Layered Double Hydroxides-Enzyme Biosensor for H2O2 Determination in Highly Diluted Real Matrices: Milk and Cosmetics
Abstract
:1. Introduction
2. Materials and Methods
2.1. LDH Preparation
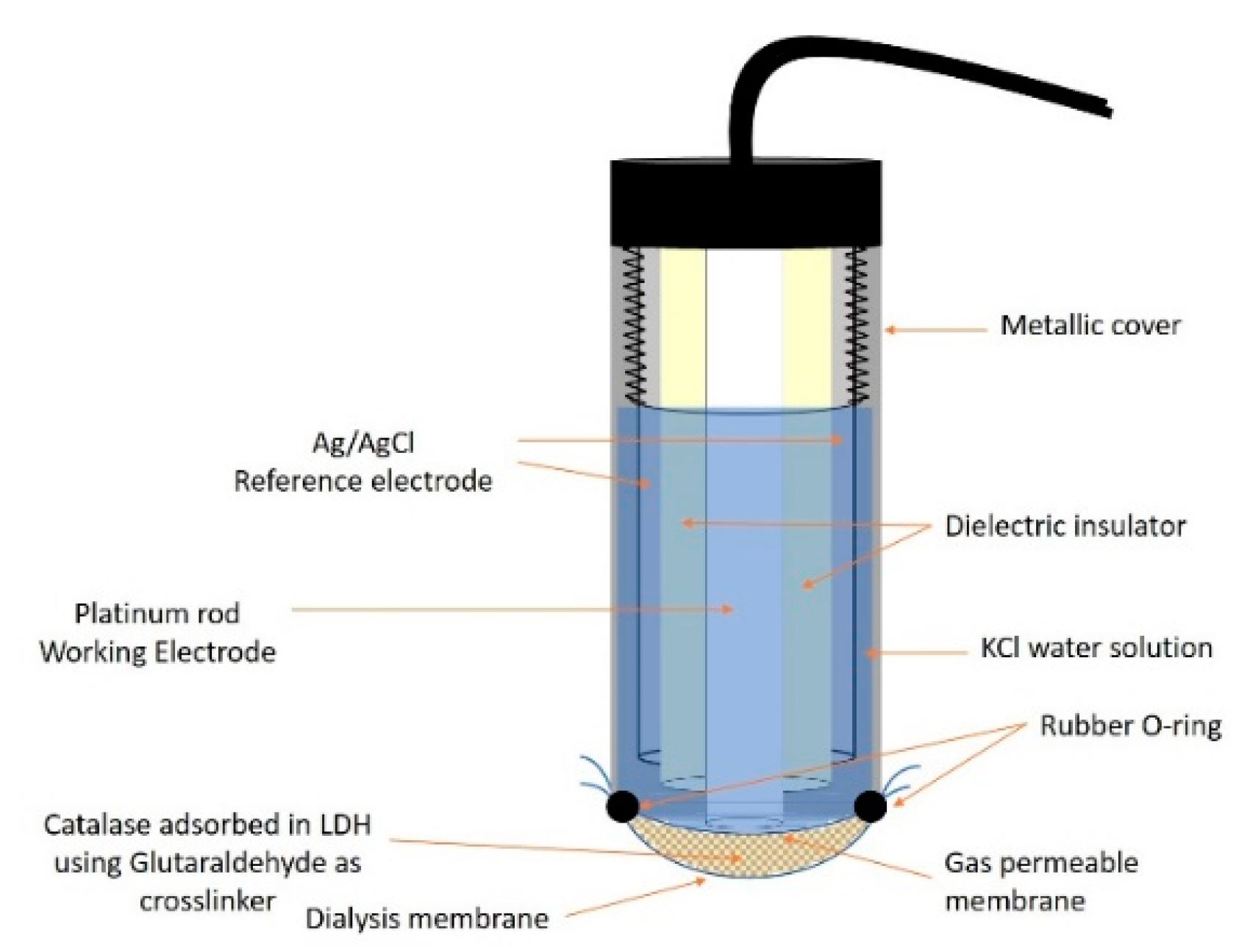
2.2. Enzyme Biosensor Preparation and Measurements
2.3. Apparatus and Survey
3. Results
3.1. Characterization of the LDH-Enzyme Compound
3.2. Characterization of the Biosensor
3.3. Interference Overcoming
3.4. Measurement in Real Matrices
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forano, C.; Costantino, U.; Prévot, V.; Gueho, C.T. Layered Double Hydroxides (LDH). In Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 745–782. ISBN 978-0-08-099364-5. [Google Scholar]
- Laipan, M.; Yu, J.; Zhu, R.; Zhu, J.; Smith, A.T.; He, H.; O’Hare, D.; Sun, L. Functionalized Layered Double Hydroxides for Innovative Applications. Mater. Horiz. 2019, 7, 715–745. [Google Scholar] [CrossRef]
- de Roy, A.; Forano, C.; Malki, K.E.; Besse, J.-P. Anionic Clays: Trends in Pillaring Chemistry; Springer: Boston, MA, USA, 1992. [Google Scholar]
- Zhang, F.; Zhang, C.; Song, L.; Zeng, R.; Liu, Z.; Cui, H. Corrosion of In-Situ Grown MgAl-LDH Coating on Aluminum Alloy. Trans. Nonferrous Met. Soc. China 2015, 25, 3498–3504. [Google Scholar] [CrossRef]
- Bi, X.; Zhang, H.; Dou, L. Layered Double Hydroxide-Based Nanocarriers for Drug Delivery. Pharmaceutics 2014, 6, 298–332. [Google Scholar] [CrossRef] [PubMed]
- Kuthati, Y.; Kankala, R.K.; Lee, C.-H. Layered Double Hydroxide Nanoparticles for Biomedical Applications: Current Status and Recent Prospects. Appl. Clay Sci. 2015, 112–113, 100–116. [Google Scholar] [CrossRef]
- Han, J.; Meng, X.; Lu, L.; Wang, Z.L.; Sun, C. Triboelectric Nanogenerators Powered Electrodepositing Tri-Functional Electrocatalysts for Water Splitting and Rechargeable Zinc-Air Battery: A Case of Pt Nanoclusters on NiFe-LDH Nanosheets. Nano Energy 2020, 72, 104669. [Google Scholar] [CrossRef]
- Ma, L.; Wang, Q.; Islam, S.M.; Liu, Y.; Ma, S.; Kanatzidis, M.G. Highly Selective and Efficient Removal of Heavy Metals by Layered Double Hydroxide Intercalated with the MoS42− Ion. J. Am. Chem. Soc. 2016, 138, 2858–2866. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; He, J.; Wei, M.; Evans, D.G.; Duan, X. Uptake of Chloride Ion from Aqueous Solution by Calcined Layered Double Hydroxides: Equilibrium and Kinetic Studies. Water Res. 2006, 40, 735–743. [Google Scholar] [CrossRef]
- Shan, D.; Cosnier, S.; Mousty, C. Layered Double Hydroxides: An Attractive Material for Electrochemical Biosensor Design. Anal. Chem. 2003, 75, 3872–3879. [Google Scholar] [CrossRef]
- Shan, D.; Mousty, C.; Cosnier, S. Subnanomolar Cyanide Detection at Polyphenol Oxidase/Clay Biosensors. Anal. Chem. 2003, 76, 178–183. [Google Scholar] [CrossRef]
- Mousty, C.; Vieille, L.; Cosnier, S. Laccase Immobilization in Redox Active Layered Double Hydroxides: A Reagentless Amperometric Biosensor. Biosens. Bioelectron. 2007, 22, 1733–1738. [Google Scholar] [CrossRef]
- Shan, D.; Cosnier, S.; Mousty, C. HRP/[Zn–Cr–ABTS] Redox Clay-Based Biosensor: Design and Optimization for Cyanide Detection. Biosens. Bioelectron. 2004, 20, 390–396. [Google Scholar] [CrossRef]
- Chen, H.; Mousty, C.; Cosnier, S.; Silveira, C.; Moura, J.J.G.; Almeida, M.G. Highly Sensitive Nitrite Biosensor Based on the Electrical Wiring of Nitrite Reductase by [ZnCr-AQS] LDH. Electrochem. Commun. 2007, 9, 2240–2245. [Google Scholar] [CrossRef]
- Chen, H.; Mousty, C.; Chen, L.; Cosnier, S. A New Approach for Nitrite Determination Based on a HRP/Catalase Biosensor. Mater. Sci. Eng. C 2008, 28, 726–730. [Google Scholar] [CrossRef]
- Shan, D.; Wang, Y.; Zhu, M.; Xue, H.; Cosnier, S.; Wang, C. Development of a High Analytical Performance-Xanthine Biosensor Based on Layered Double Hydroxides Modified-Electrode and Investigation of the Inhibitory Effect by Allopurinol. Biosens. Bioelectron. 2009, 24, 1171–1176. [Google Scholar] [CrossRef]
- Ding, S.-N.; Shan, D.; Xue, H.-G.; Zhu, D.-B.; Cosnier, S. Glucose Oxidase Immobilized in Alginate/Layered Double Hydroxides Hybrid Membrane and Its Biosensing Application. Anal. Sci. 2009, 25, 1421–1425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shan, D.; Yao, W.; Xue, H. Amperometric Detection of Glucose with Glucose Oxidase Immobilized in Layered Double Hydroxides. Electroanalysis 2006, 18, 1485–1491. [Google Scholar] [CrossRef]
- Fernández, L.; Ledezma, I.; Borrás, C.; Martínez, L.A.; Carrero, H. Horseradish Peroxidase Modified Electrode Based on a Film of Co–Al Layered Double Hydroxide Modified with Sodium Dodecylbenzenesulfonate for Determination of 2-Chlorophenol. Sens. Actuators B Chem. 2013, 182, 625–632. [Google Scholar] [CrossRef]
- Zhai, C.; Guo, Y.; Sun, X.; Zheng, Y.; Wang, X. An Acetylcholinesterase Biosensor Based on Graphene–Gold Nanocomposite and Calcined Layered Double Hydroxide. Enzym. Microb. Technol. 2014, 58–59, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Z.; Rui, Y.; Li, M. Horseradish Peroxidase Immobilization on Carbon Nanodots/CoFe Layered Double Hydroxides: Direct Electrochemistry and Hydrogen Peroxide Sensing. Biosens. Bioelectron. 2015, 64, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Xu, S.; Zeng, H.-Y.; Cao, X.; Dan Pan, A.; Xiao, G.-F.; Ding, P.-X. Hydrogen Peroxide Biosensor Based on Chitosan/2D Layered Double Hydroxide Composite for the Determination of H2O2. Bioelectrochemistry 2018, 123, 94–102. [Google Scholar] [CrossRef]
- Vajedi, F.S.; Dehghani, H. A High-Sensitive Electrochemical DNA Biosensor Based on a Novel ZnAl/Layered Double Hydroxide Modified Cobalt Ferrite-Graphene Oxide Nanocomposite Electrophoretically Deposited onto FTO Substrate for Electroanalytical Studies of Etoposide. Talanta 2020, 208, 120444. [Google Scholar] [CrossRef]
- Li, J. A Novel Electrochemical Biosensor Based on Layered Hydroxide Nanosheets/DNA Composite for the Determination of Phenformin Hydrochloride. Int. J. Electrochem. Sci. 2021, 210237. [Google Scholar] [CrossRef]
- Farhat, H.; Célier, J.; Forano, C.; Mousty, C. Evaluation of Hierarchical Glucose Oxidase/Co3Mn-CO3 LDH Modified Electrodes for Glucose Detection. Electrochim. Acta 2021, 376, 138050. [Google Scholar] [CrossRef]
- Campanella, L.; Lelo, D.; Martini, E.; Tomassetti, M. Organophosphorus and Carbamate Pesticide Analysis Using an Inhibition Tyrosinase Organic Phase Enzyme Sensor; Comparison by Butyrylcholinesterase+choline Oxidase Opee and Application to Natural Waters. Anal. Chim. Acta 2007, 587, 22–32. [Google Scholar] [CrossRef]
- Tomassetti, M.; Martini, E.; Campanella, L.; Favero, G.; Carlucci, L.; Mazzei, F. Comparison of Three Immunosensor Methods (Surface Plasmon Resonance, Screen-Printed and Classical Amperometric Immunosensors) for Immunoglobulin G Determination in Human Serum and Animal or Powdered Milks. J. Pharm. Biomed. Anal. 2013, 73, 90–98. [Google Scholar] [CrossRef]
- Tomassetti, M.; Angeloni, R.; Martini, E.; Castrucci, M.; Campanella, L. Enzymatic DMFC Device Used for Direct Analysis of Chloramphenicol and a Comparison with the Competitive Immunosensor Method. Sens. Actuators B Chem. 2018, 255, 1545–1552. [Google Scholar] [CrossRef]
- Tomassetti, M.; Pezzilli, R.; Prestopino, G.; Natale, C.D.; Medaglia, P.G. Fabrication and Characterization of a Layered Double Hydroxide Based Catalase Biosensor and a Catalytic Sensor for Hydrogen Peroxide Determination. Microchem. J. 2021, 170, 106700. [Google Scholar] [CrossRef]
- Boclair, J.W.; Braterman, P.S. Layered Double Hydroxide Stability. 1. Relative Stabilities of Layered Double Hydroxides and Their Simple Counterparts. Chem. Mater. 1999, 11, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Lopez, T.; Bosch, P.; Ramos, E.; Gomez, R.; Novaro, O.; Acosta, D.; Figueras, F. Synthesis and Characterization of Sol−Gel Hydrotalcites. Structure and Texture †. Langmuir 1996, 12, 189–192. [Google Scholar] [CrossRef]
- Laipan, M.; Xiang, L.; Yu, J.; Martin, B.R.; Zhu, R.; Zhu, J.; He, H.; Clearfield, A.; Sun, L. Layered Intercalation Compounds: Mechanisms, New Methodologies, and Advanced Applications. Prog. Mater. Sci. 2020, 109, 100631. [Google Scholar] [CrossRef]
- Xu, Z.P.; Stevenson, G.; Lu, C.-Q.; Lu, G.Q. (Max). Dispersion and Size Control of Layered Double Hydroxide Nanoparticles in Aqueous Solutions. J. Phys. Chem. B 2006, 110, 16923–16929. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, F.; Evans, D.G.; Duan, X. Layered Double Hydroxide Films: Synthesis, Properties and Applications. Chem. Commun. 2010, 46, 5197. [Google Scholar] [CrossRef] [PubMed]
- Scarpellini, D.; Leonardi, C.; Mattoccia, A.; Di Giamberardino, L.; Medaglia, P.G.; Mantini, G.; Gatta, F.; Giovine, E.; Foglietti, V.; Falconi, C.; et al. Solution-Grown Zn/Al Layered Double Hydroxide Nanoplatelets onto Al Thin Films: Fine Control of Position and Lateral Thickness. J. Nanomater. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Richetta, M.; Digiamberardino, L.; Mattoccia, A.; Medaglia, P.G.; Montanari, R.; Pizzoferrato, R.; Scarpellini, D.; Varone, A.; Kaciulis, S.; Mezzi, A.; et al. Surface Spectroscopy and Structural Analysis of Nanostructured Multifunctional (Zn, Al) Layered Double Hydroxides: XPS and UPS Investigation of Nanostructured Multifunctional LDH. Surf. Interface Anal. 2016, 48, 514–518. [Google Scholar] [CrossRef] [Green Version]
- Zeng, R.-C.; Li, X.-T.; Liu, Z.-G.; Zhang, F.; Li, S.-Q.; Cui, H.-Z. Corrosion Resistance of Zn–Al Layered Double Hydroxide/Poly(Lactic Acid) Composite Coating on Magnesium Alloy AZ31. Front. Mater. Sci. 2015, 9, 355–365. [Google Scholar] [CrossRef]
- Chai, H.; Xu, X.; Lin, Y.; Evans, D.G.; Li, D. Synthesis and UV Absorption Properties of 2,3-Dihydroxynaphthalene-6-Sulfonate Anion-Intercalated Zn–Al Layered Double Hydroxides. Polym. Degrad. Stab. 2009, 94, 744–749. [Google Scholar] [CrossRef]
- Feng, Y.; Li, D.; Wang, Y.; Evans, D.G.; Duan, X. Synthesis and Characterization of a UV Absorbent-Intercalated Zn–Al Layered Double Hydroxide. Polym. Degrad. Stab. 2006, 91, 789–794. [Google Scholar] [CrossRef]
- Arizaga, G.G.C.; Gardolinski, J.E.F.D.C.; Schreiner, W.H.; Wypych, F. Intercalation of an Oxalatooxoniobate Complex into Layered Double Hydroxide and Layered Zinc Hydroxide Nitrate. J. Colloid Interface Sci. 2009, 330, 352–358. [Google Scholar] [CrossRef]
- Sel, E.; Ulu, A.; Ateş, B.; Köytepe, S. Comparative Study of Catalase Immobilization via Adsorption on P(MMA-Co-PEG500MA) Structures as an Effective Polymer Support. Polym. Bull. 2021, 78, 2663–2684. [Google Scholar] [CrossRef]
- El-Shishtawy, R.M.; Ahmed, N.S.E.; Almulaiky, Y.Q. Immobilization of Catalase on Chitosan/ZnO and Chitosan/ZnO/Fe2O3 Nanocomposites: A Comparative Study. Catalysts 2021, 11, 820. [Google Scholar] [CrossRef]
- Barreca, D.; Neri, G.; Scala, A.; Fazio, E.; Gentile, D.; Rescifina, A.; Piperno, A. Covalently Immobilized Catalase on Functionalized Graphene: Effect on the Activity, Immobilization Efficiency, and Tetramer Stability. Biomater. Sci. 2018, 6, 3231–3240. [Google Scholar] [CrossRef] [PubMed]
- Prestopino, G.; Arrabito, G.; Generosi, A.; Mattoccia, A.; Paci, B.; Perez, G.; Verona-Rinati, G.; Medaglia, P.G. Emerging Switchable Ultraviolet Photoluminescence in Dehydrated Zn/Al Layered Double Hydroxide Nanoplatelets. Sci. Rep. 2019, 9, 11498. [Google Scholar] [CrossRef]
- Geraud, E.; Prevot, V.; Forano, C.; Mousty, C. Spongy Gel-like Layered Double Hydroxide–Alkaline Phosphatase Nanohybrid as a Biosensing Material. Chem. Commun. 2008, 1554–1556. [Google Scholar] [CrossRef]
- Nascimento, C.F.; Santos, P.M.; Pereira-Filho, E.R.; Rocha, F.R.P. Recent Advances on Determination of Milk Adulterants. Food Chem. 2017, 221, 1232–1244. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.S.; Merkuleva, A.D.; Andreev, S.V.; Sakharov, K.A. Method for Determination of Hydrogen Peroxide in Adulterated Milk Using High Performance Liquid Chromatography. Food Chem. 2019, 283, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Moody, G.J.; Thomas, J.D.R. Selective Ion-Sensitive Electrodes. Sel. Annu. Rev. Anal. 1973, 3, 59. [Google Scholar] [CrossRef]
- Mascini, M. Uses of Known Addition, Gran’s Plots and the Related Methods with Ion-Selective Electrodes. In Ion-Selective Electrode Reviews; Elsevier: Amsterdam, The Netherlands, 1981; Volume 2, pp. 17–71. ISBN 978-0-08-028434-7. [Google Scholar]
- Campanella, L.; Capesciotti, G.; Russo, M.; Tomassetti, M. Study of the Catalytic Mechanism of the Enzyme Catalase on Organic Hydroperoxides in Non-Polar Organic Solvent. Curr. Enzym. Inhib. 2008, 4, 86–92. [Google Scholar] [CrossRef]






| Linear Regression (y = μA; x = mM) | Linearity Range (mM) | R2 | LOD (mM) | RSD% | Response Time (s) | |
|---|---|---|---|---|---|---|
| Present Work | y = (0.1704 ± 0.0044)∙x + (0.2599 ± 0.0460) | 0.021–17.5 | 0.9954 | 0.021 | 0.36 | 65 |
| Ref. [29] | y = (10.09 ± 0.29)∙x + (115.1 ± 29.1) | 0.25–158 | 0.9976 | 0.2 | 0.5 | 8.5 |
| Percent Interferences of Oxidant or Antioxidant Compounds to H2O2 Analysis, Using LDH-Clark Enzymatic Catalase Biosensor | Percent Interferences of Oxidant or Antioxidant Compounds to H2O2 Analysis, Using LDH-Glassy Carbon Catalase Biosensor | |
|---|---|---|
| % response increase, to H2O2 response, in presence of potassium permanganate aqueous solution. | 0% | 260% |
| % response increase, to H2O2 response, in presence of sodium nitrate aqueous solution. | 0% | 3% |
| % response decrease, to H2O2 response, in presence of Fe3+ aqueous solution. | 0% | 32.3% (transient peak 124%) |
| % response decrease, to H2O2 response, in presence of sodium ascorbate aqueous solution. | 0% | 5.6% |
| % response decrease, to H2O2 response, in presence of sodium nitrite aqueous solution. | 0% | 1% |
| Sample | Found Concentration in Cosmetic Sample (g L−1) | H2O2 Additions (g L−1) | Found + Added Nominal Value (g L−1) | Experimental Value (g L−1) | Δ (%) (RSD% = 0.7) | Percent Recovery (RSD% = 0.7) |
|---|---|---|---|---|---|---|
| LB1 | 0.78 | 1.30 | 2.08 | 2.24 | 7.69 | 107.69 |
| LB1 | 0.78 | 3.80 | 4.58 | 4.78 | 4.37 | 104.37 |
| LB2 | 1.30 | 1.80 | 3.10 | 3.32 | 7.10 | 107.10 |
| LB2 | 1.30 | 3.80 | 5.10 | 5.14 | 0.78 | 100.78 |
| Found H2O2 Concentration in Milk Sample (mg L−1) | Additions of H2O2 (mg L−1) | Found + Added Nominal Value (mg L−1) | Experimental Value (mg L−1) | Δ (%) (RSD% = 1.5) | Percent Recovery (RSD% = 1.5) |
|---|---|---|---|---|---|
| 2.2 | 200 | 202.2 | 218.0 | 7.8 | 107.8 |
| 2.2 | 500 | 502.2 | 462.1 | −8.0 | 92.0 |
| 2.2 | 700 | 702.2 | 726.5 | 3.5 | 103.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomassetti, M.; Pezzilli, R.; Prestopino, G.; Di Biagio, F.; Di Natale, C.; Medaglia, P.G. A New Clark-Type Layered Double Hydroxides-Enzyme Biosensor for H2O2 Determination in Highly Diluted Real Matrices: Milk and Cosmetics. Processes 2021, 9, 1878. https://doi.org/10.3390/pr9111878
Tomassetti M, Pezzilli R, Prestopino G, Di Biagio F, Di Natale C, Medaglia PG. A New Clark-Type Layered Double Hydroxides-Enzyme Biosensor for H2O2 Determination in Highly Diluted Real Matrices: Milk and Cosmetics. Processes. 2021; 9(11):1878. https://doi.org/10.3390/pr9111878
Chicago/Turabian StyleTomassetti, Mauro, Riccardo Pezzilli, Giuseppe Prestopino, Francesco Di Biagio, Corrado Di Natale, and Pier Gianni Medaglia. 2021. "A New Clark-Type Layered Double Hydroxides-Enzyme Biosensor for H2O2 Determination in Highly Diluted Real Matrices: Milk and Cosmetics" Processes 9, no. 11: 1878. https://doi.org/10.3390/pr9111878
APA StyleTomassetti, M., Pezzilli, R., Prestopino, G., Di Biagio, F., Di Natale, C., & Medaglia, P. G. (2021). A New Clark-Type Layered Double Hydroxides-Enzyme Biosensor for H2O2 Determination in Highly Diluted Real Matrices: Milk and Cosmetics. Processes, 9(11), 1878. https://doi.org/10.3390/pr9111878







