New Approach Study on Dry Coal Cleaning System with Two-Stage Corona Electrostatic Processes for High-Sulfur Low-Grade Fine Coals
Abstract
:1. Introduction
2. Test Samples and Methods
2.1. Testing Devices
2.2. High-Sulfur Raw Coal
2.3. Experimental Methods
3. Results and Discussion
3.1. Factors Influencing Separation Efficiency
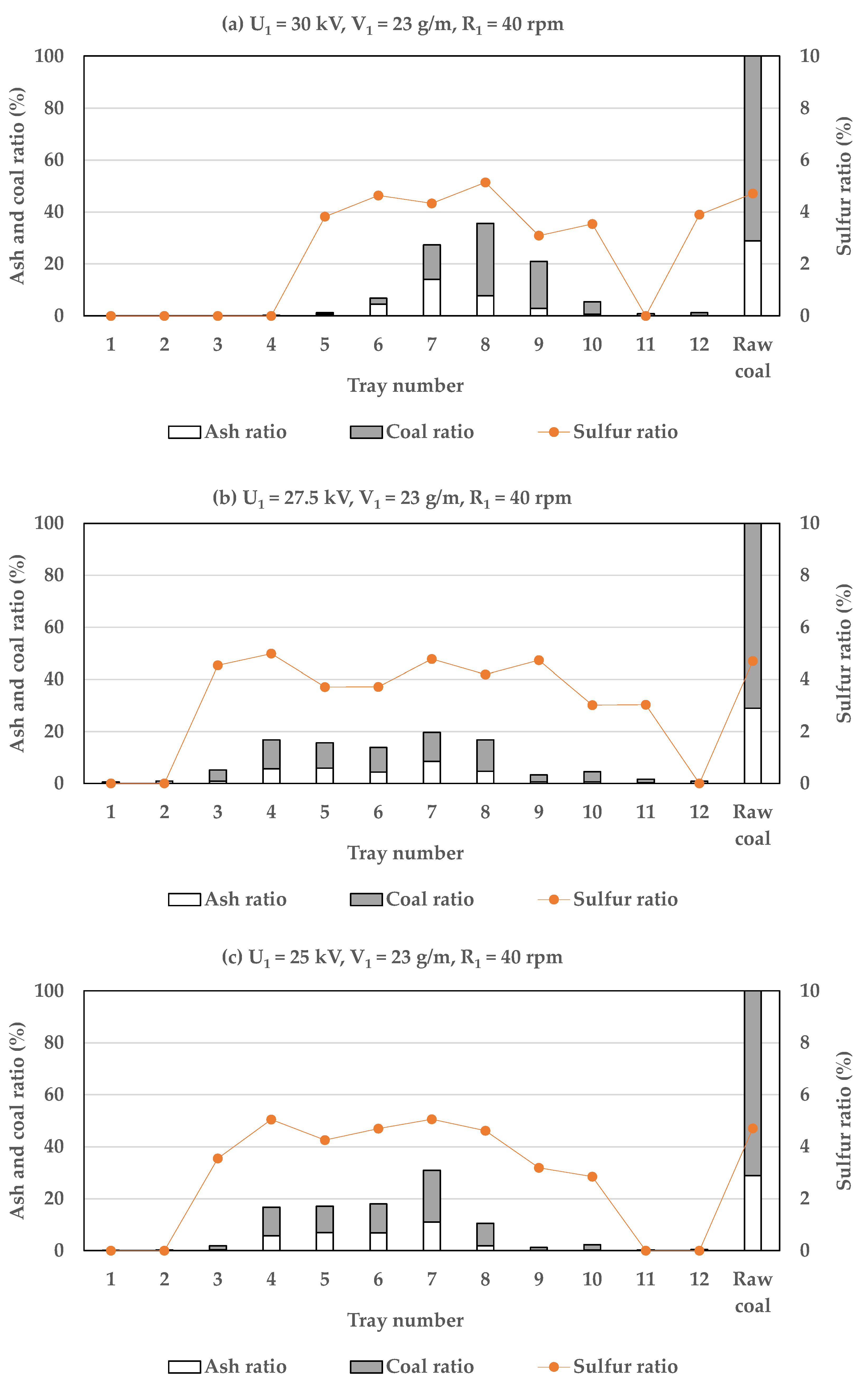
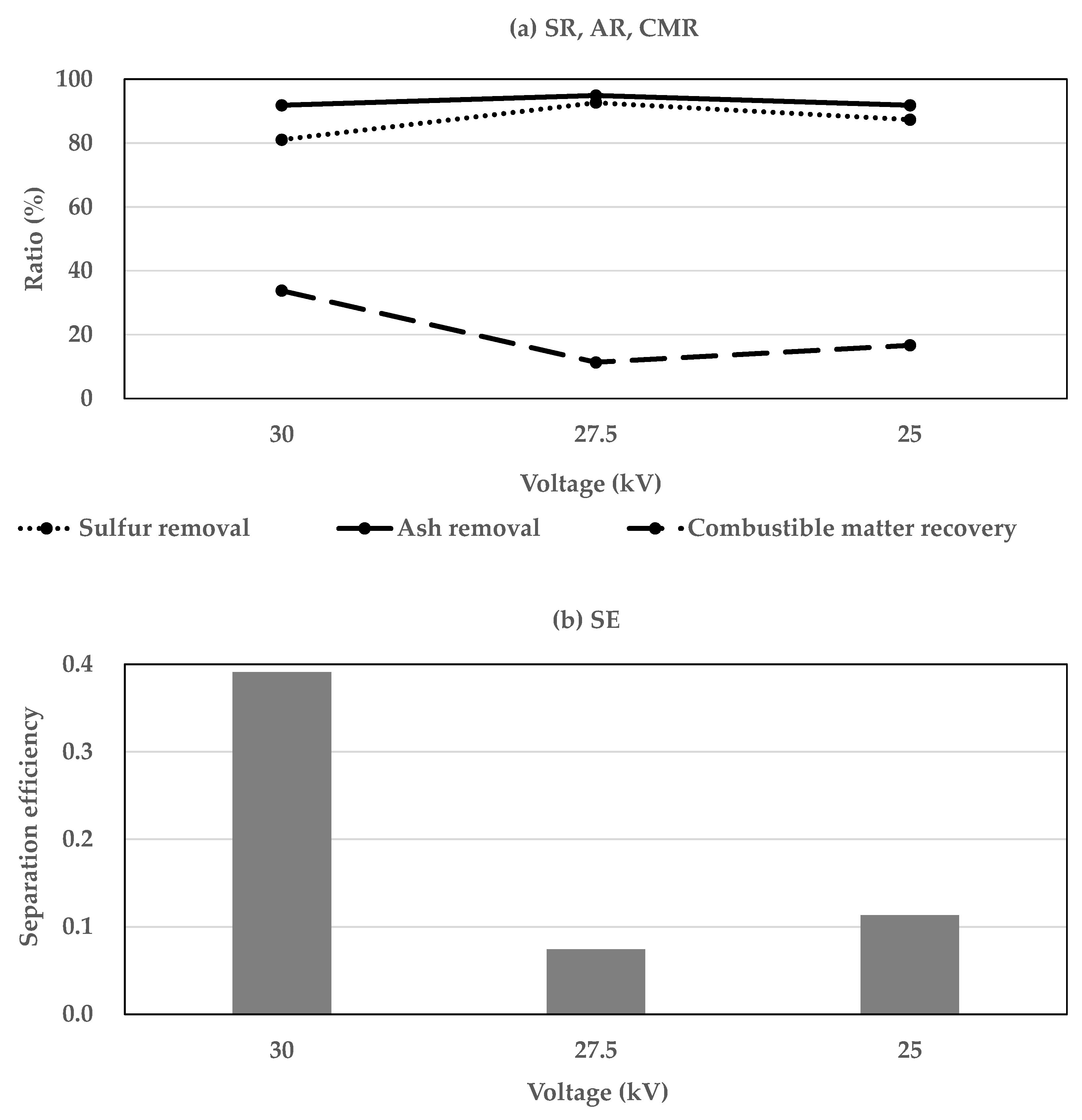
3.1.1. The Influence of Electrode Voltage
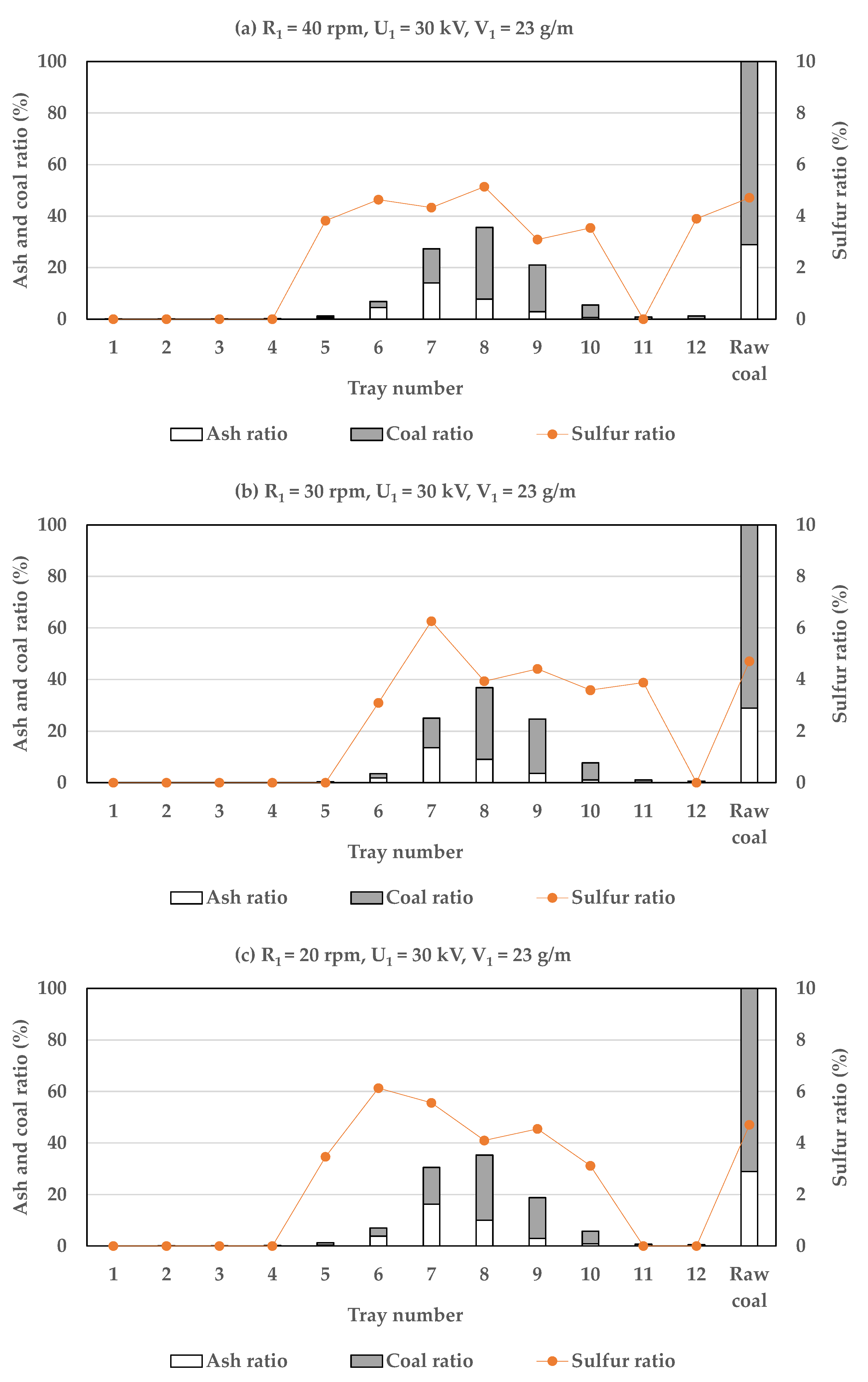
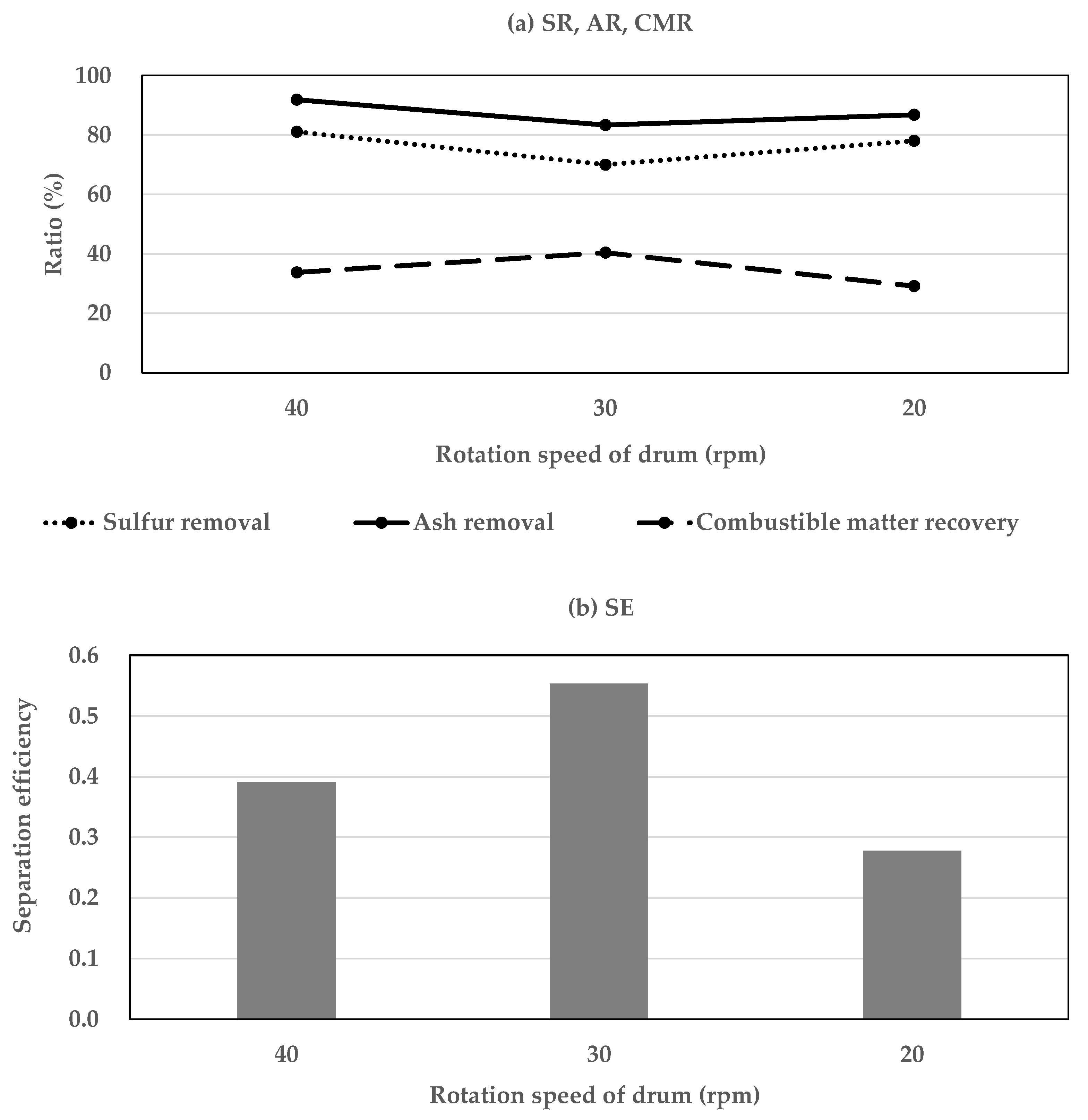
3.1.2. The Influence of Drum Rotational Speed
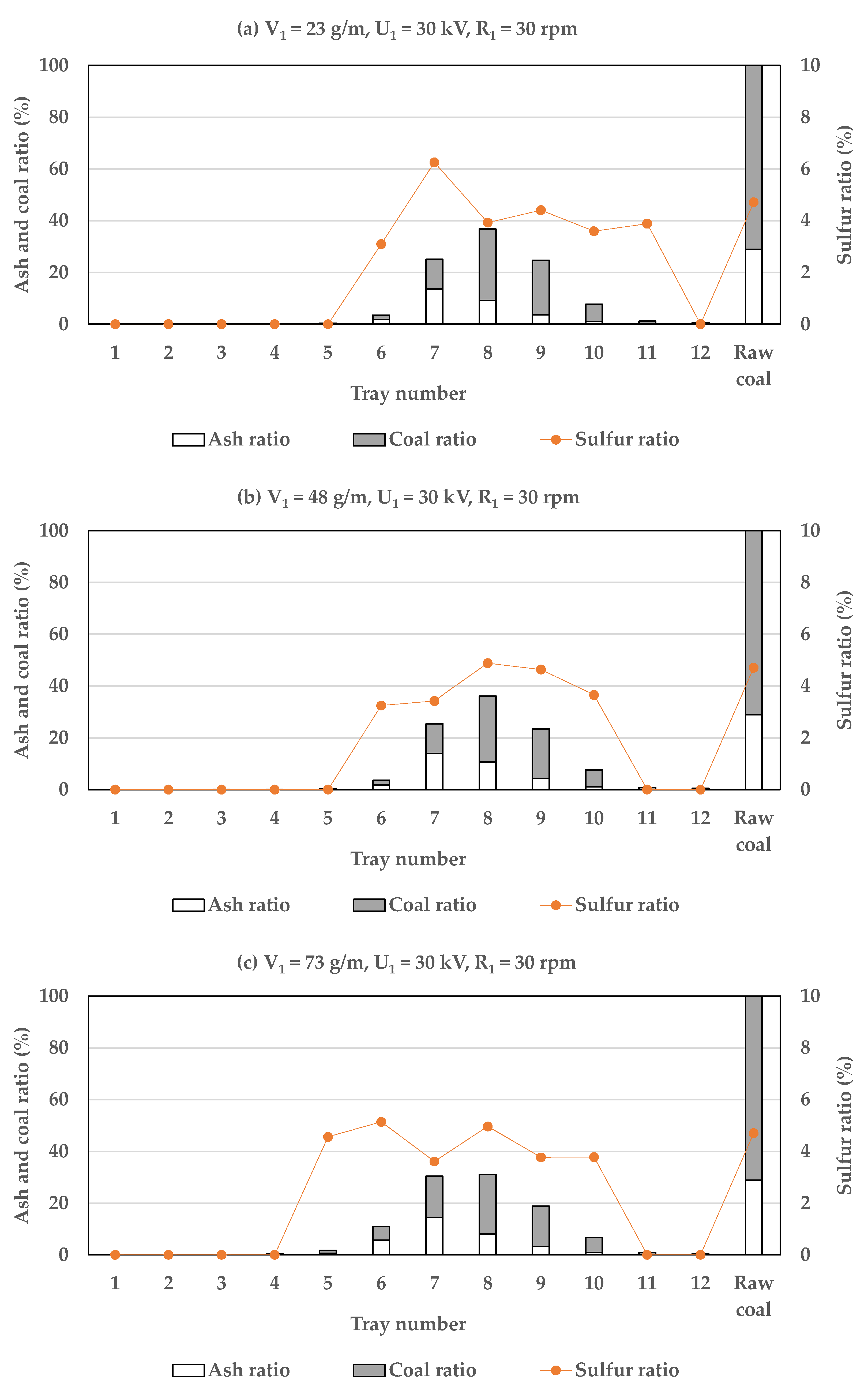
3.1.3. The Influence of Conveyor Speed
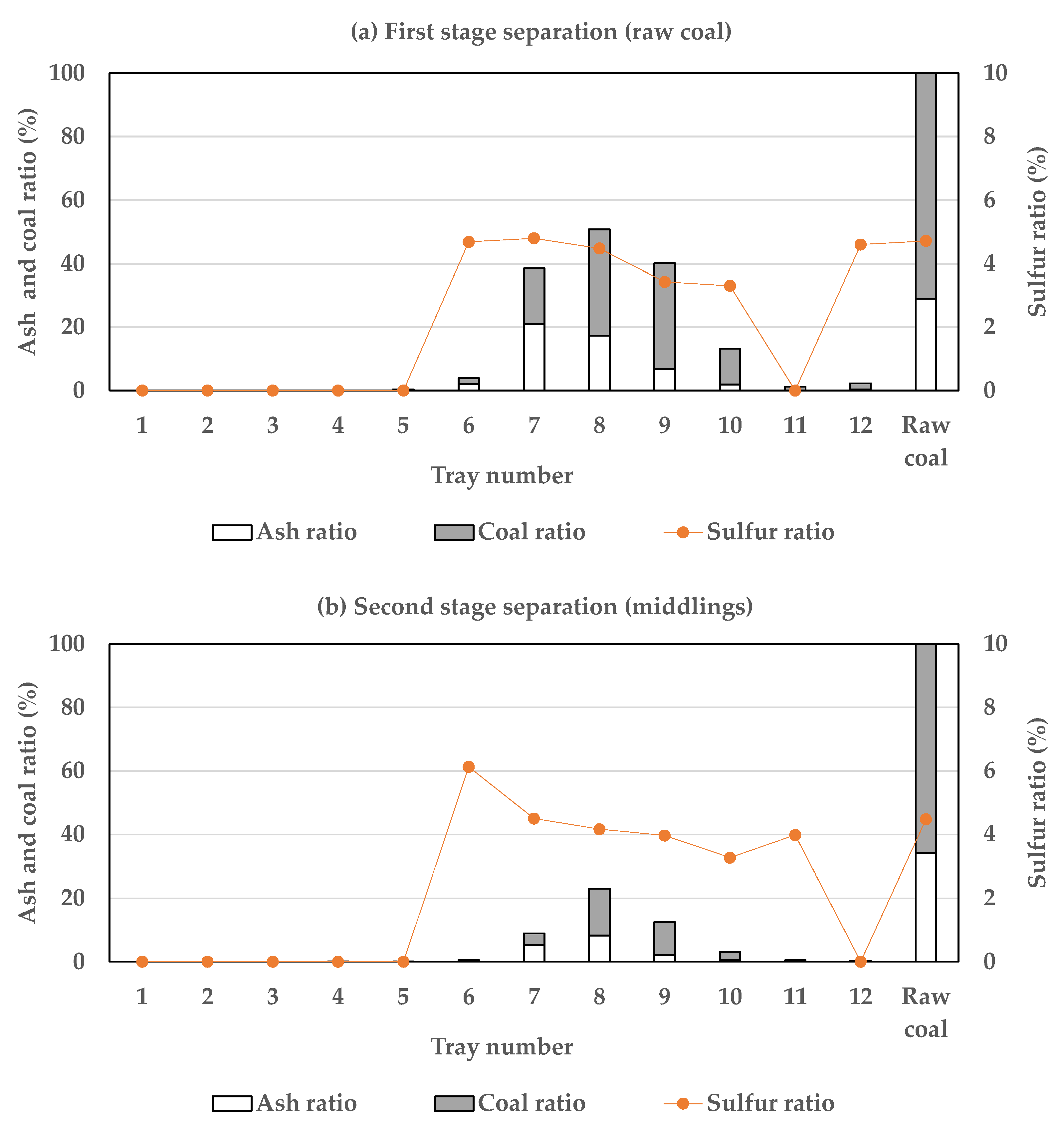
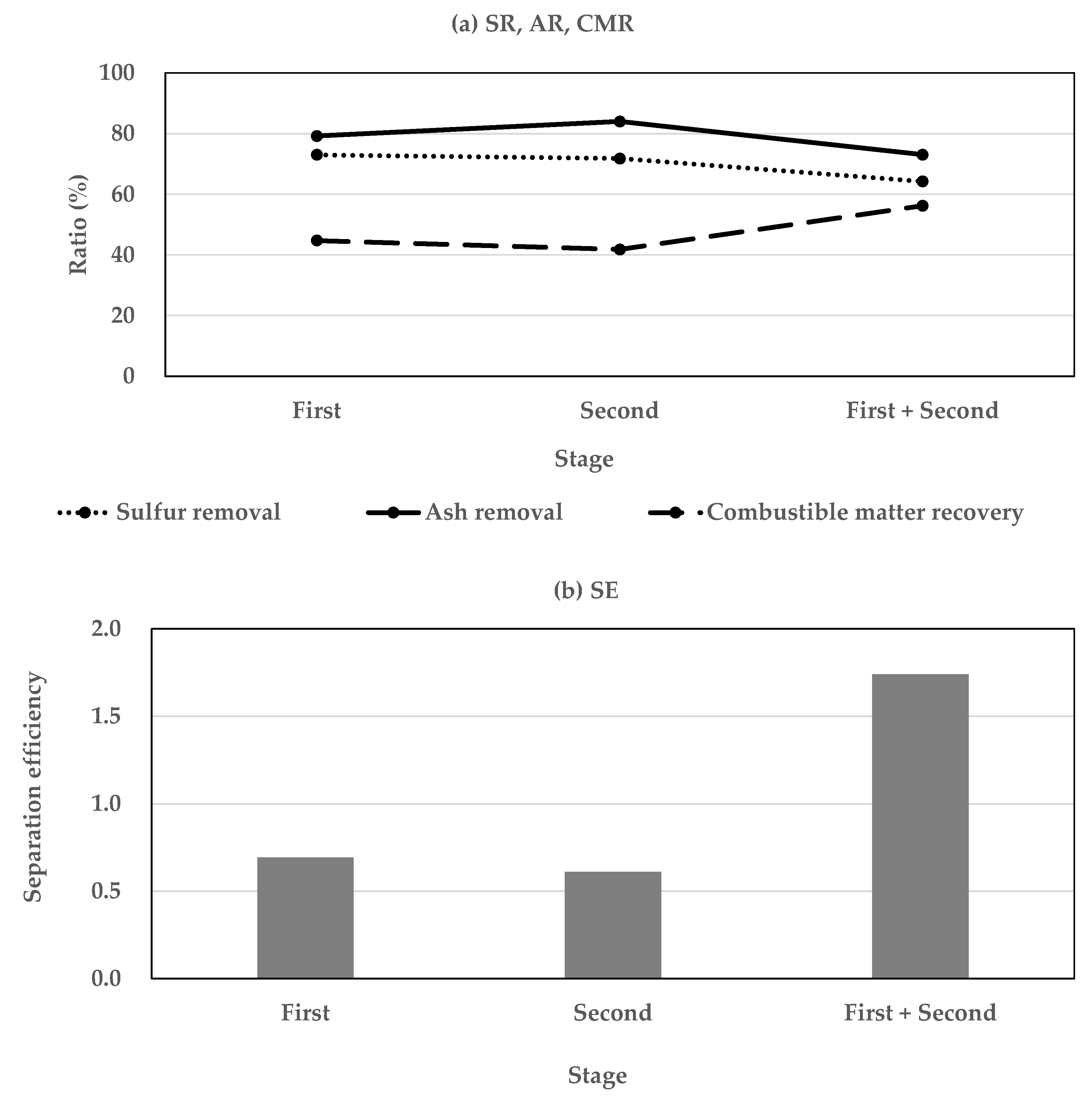
3.2. Efficiency of Two-Stage Coal Separation
3.3. Comparative Analysis of the Composition and Properties of the High-Sulfur Raw Coal and Clean Coal
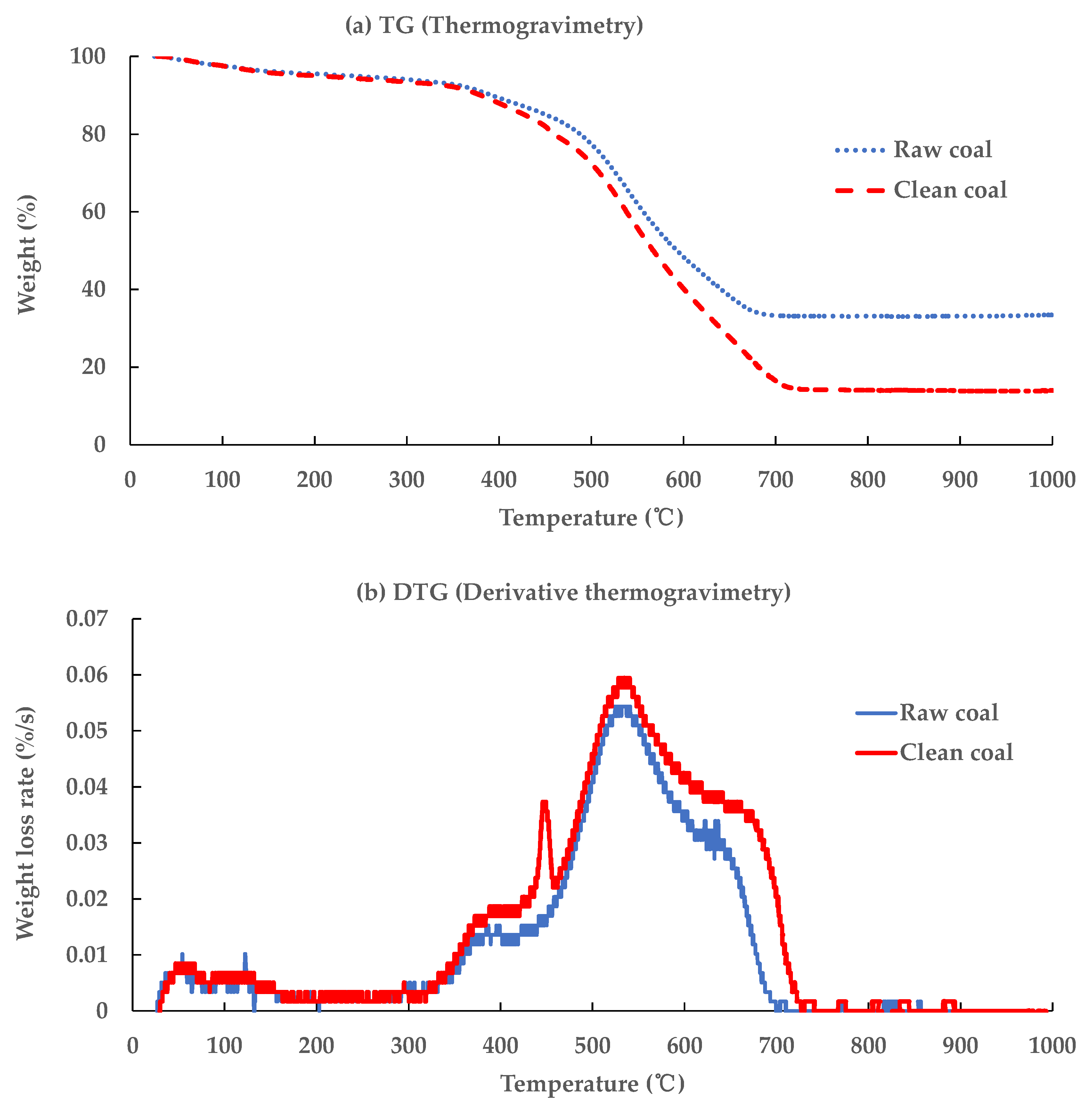
3.3.1. Combustion Behavior
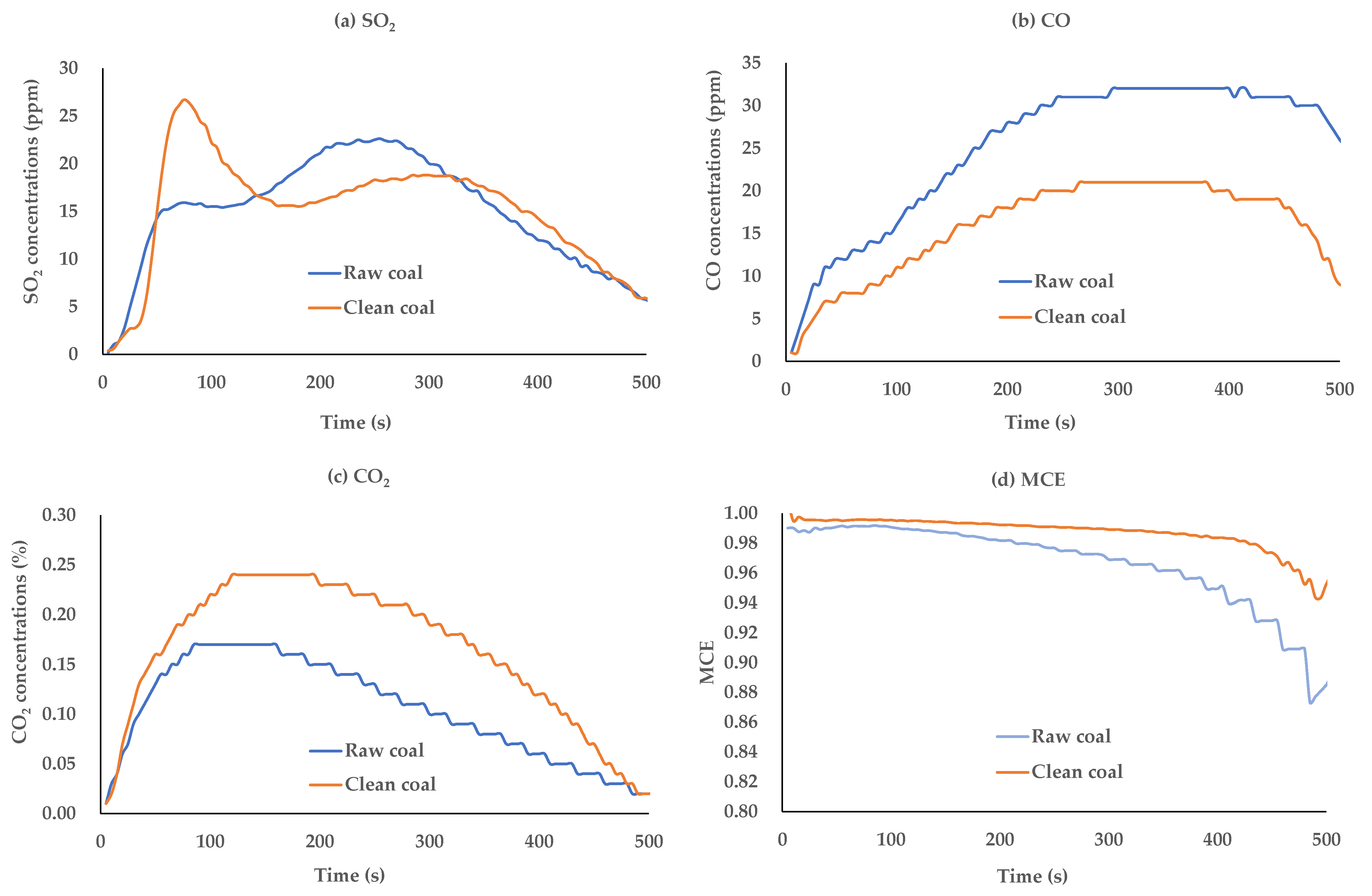
3.3.2. Experimental Investigation of Air Pollutant Emissions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| SR | Sulfur removal |
| AR | Ash removal |
| CMR | Combustible matter recover |
| SE | Separation efficiency |
| Wtfeed | Mass of raw coal supply |
| Wtclean | Mass of recovery of clean coal |
| Sulfurfeed ratio | Mass of sulfur rate of raw coal |
| Sulfurclean ratio | Mass of sulfur rate of clean coal |
| U | Electrode voltages |
| S | Distance between the electrode and the drum |
| θ | Angle between the line from the electrode to the center of the drum circle and the vertical line of the drum |
| T | Room temperature |
| RH | Relative humidity |
| R | Drum rotational speed |
| V | Conveyor speed |
| rpm | Revolution(s) Per Minute |
| g/m | Grams Per Minute |
| M | Moisture |
| V.M | Volatile matter |
| F.C | Fixed carbon |
| Ad | Ash content |
| Sd | Sulfur content |
| COx | Oxocarbon |
| SOx | Sulfur oxides |
| TG | Thermogravimetry |
| DTG | Derivative thermogravimetry |
| L/min | Liters Per Minute |
References
- Petroleum, B. BP Statistical Review of World Energy Report; BP: London, UK, 2019. [Google Scholar]
- Wang, D.; Ding, R.; Gong, Y.; Wang, R.; Wang, J.; Huang, X. Feasibility of the Northern Sea Route for oil shipping from the economic and environmental perspective and its influence on China’s oil imports. Mar. Policy 2020, 118, 104006. [Google Scholar] [CrossRef]
- Zeng, S.; Gu, J.; Yang, S.; Zhou, H.; Qian, Y. Comparison of techno-economic performance and environmental impacts between shale gas and coal-based synthetic natural gas (SNG) in China. J. Clean. Prod. 2019, 215, 544–556. [Google Scholar] [CrossRef]
- Hache, E. Do renewable energies improve energy security in the long run? Int. Econ. 2018, 156, 127–135. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Hu, S. History and future of the coal and coal chemical industry in China. Resour. Conserv. Recycl. 2017, 124, 13–24. [Google Scholar] [CrossRef]
- Meshram, P.; Purohit, B.K.; Sinha, M.K.; Sahu, S.K.; Pandey, B.D. Demineralization of low grade coal—A review. Renew. Sustain. Energy Rev. 2015, 41, 745–761. [Google Scholar] [CrossRef]
- Xie, X.; Liu, X.; Wang, H.; Wang, Z. Effects of aerosols on radiative forcing and climate over East Asia with different SO2 emissions. Atmosphere 2016, 7, 99. [Google Scholar] [CrossRef] [Green Version]
- Kaufmann, R.K.; Kauppi, H.; Mann, M.L.; Stock, J.H. Reconciling anthropogenic climate change with observed temperature 1998–2008. Proc. Natl. Acad. Sci. USA 2011, 108, 11790–11793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, H.; Chen, S.; Dong, X.; Dong, K. Has China’s coal consumption actually reached its peak? National and regional analysis considering cross-sectional dependence and heterogeneity. Energy Econ. 2019, 84, 104509. [Google Scholar] [CrossRef]
- Qian, Y.; Scherer, L.; Tukker, A.; Behrens, P. China’s potential SO2 emissions from coal by 2050. Energy Policy 2020, 147, 111856. [Google Scholar] [CrossRef]
- Su, S.; Li, B.; Cui, S.; Tao, S. Sulfur dioxide emissions from combustion in China: From 1990 to 2007. Environ. Sci. Technol. 2011, 45, 8403–8410. [Google Scholar] [CrossRef] [PubMed]
- Shammas, N.K.; Wang, L.K.; Wang, M.H.S. Sources, chemistry and control of acid rain in the environ-ment. In Handbook of Environment and Waste Management: Acid Rain and Greenhouse Gas Pollution Control; World Scientific Publishing Co.: Singapore, 2020; pp. 1–26. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, C.; Yuan, C.; Xu, H.; Ma, L.; Fang, Q.; Chen, G. Investigation on the influence of sulfur and chlorine on the initial deposition/fouling char-acteristics of a high-alkali coal. Fuel Process. Technol. 2020, 198, 106234. [Google Scholar] [CrossRef]
- Breeze, P. Combustion plant emissions: Sulfur dioxide, nitrogen oxides, and acid rain. In Electricity Generation and the Environment; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Chen, Q.; Sun, H.; Wang, J.; Shan, M.; Yang, X.; Deng, M.; Zhang, L. Long-life type—The dominant fraction of EPFRs in combustion sources and ambient fine particles in Xi’an. Atmos. Environ. 2019, 219, 117059. [Google Scholar] [CrossRef]
- Yuegang, T.; Xin, H.; Aiguo, C.; Wei-wei, L.; Xiu-jie, D.; Qiang, W.; Long, L. Occurrence and sedimentary control of sulfur in coals of China. J. China Coal Soc. 2015, 40, 1976–1987. [Google Scholar] [CrossRef]
- Cai, S.; Zhang, S.; Wei, Y.; Sher, F.; Wen, L.; Xu, J.; Dang, J.; Hu, L. A novel method for removing organic sulfur from high-sulfur coal: Migration of organic sulfur during microwave treatment with NaOH-H2O2. Fuel 2021, 289, 119800. [Google Scholar] [CrossRef]
- Wang, F.; Liu, B.; Zhang, B. Embodied environmental damage in interregional trade: A MRIO-based assessment within China. J. Clean. Prod. 2017, 140, 1236–1246. [Google Scholar] [CrossRef] [Green Version]
- Cheng, G.; Zhang, C. Desulfurization and Denitrification Technologies of Coal-fired Flue Gas. Pol. J. Environ. Stud. 2018, 27, 481–489. [Google Scholar] [CrossRef]
- Tripathy, S.K.; Ramamurthy, Y.; Kumar, C.R. Modeling of high-tension roll separator for separation of titanium bearing minerals. Powder Technol. 2010, 201, 181–186. [Google Scholar] [CrossRef]
- Higashiyama, Y.; Asano, K. Recent progress in electrostatic separation technology. Part. Sci. Technol. 1998, 16, 77–90. [Google Scholar] [CrossRef]
- Iuga, A.; Dǎscǎlescu, L.; Morar, R.; Csorvassy, I.; Neamiu, V. Corona-electrostatic separators for recovery of waste non-ferrous metals. J. Electrost. 1989, 23, 235–243. [Google Scholar] [CrossRef]
- Venter, J.A.; Vermaak, M.K.G.; Bruwer, J.G. Influence of surface effects on the electrostatic separation of zircon and rutile. J. S. Afr. Inst. Min. Metall. 2008, 108, 55–60. [Google Scholar]
- Veit, H.M.; Diehl, T.R.; Salami, A.P.; Rodrigues, J.D.S.; Bernardes, A.M.; Tenório, J.A.S. Utilization of magnetic and elec-tro-static separation in the recycling of printed circuit boards scrap. Waste Manag. 2005, 25, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Tilmatine, A.; Medles, K.; Younes, M.; Bendaoud, A.; Dascalescu, L. Roll-type versus free-fall electrostatic separation of tribocharged plastic particles. IEEE Trans. Ind. Appl. 2010, 46, 1564–1569. [Google Scholar] [CrossRef]
- Zhang, X. Study on Desulfurization and Ash Reduction of fine Coal. D. Xuzhou China Univ. Min. Technol. 1994. [Google Scholar]
- Liu, C.; Wang, Q. Study on Electrostatic Preparation High-Ash Coal from China Using Roll-Type Electrostatic Separator and the Combustion Characteristics of the Cleaned Coal. Processes 2021, 9, 1139. [Google Scholar] [CrossRef]
- Wang, Q.; Sarkar, J.k. Investigations of the pyrolysis behaviors of coconut shell and husk waste biomasses. Int. J. Energy Prod. Manag. 2018, 3, 34–43. [Google Scholar] [CrossRef]
- Lazaroiu, G.; Pop, E.; Negreanu, G.; Pisa, I.; Mihaescu, L.; Bondrea, A.; Berbece, V. Biomass combustion with hydrogen injection for energy applications. Energy 2017, 127, 351–357. [Google Scholar] [CrossRef]

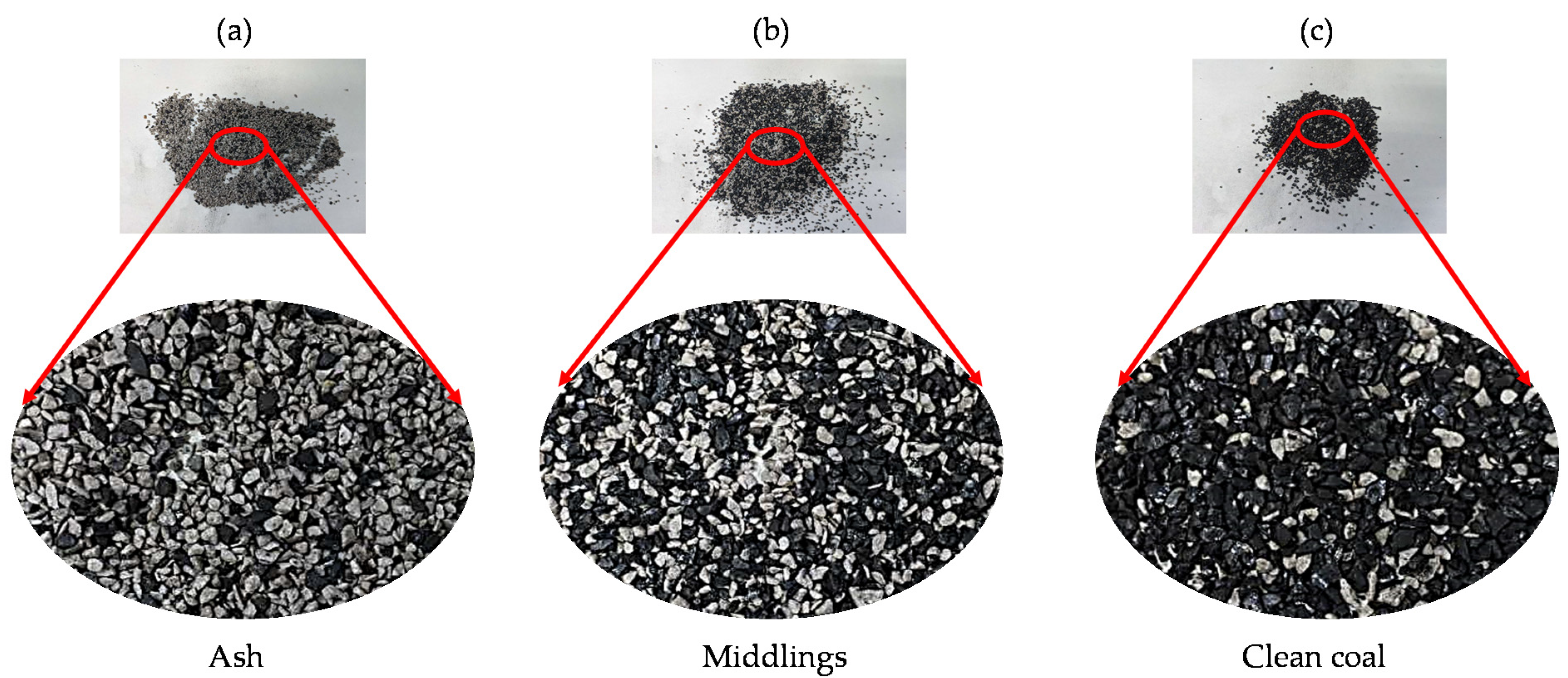

| Size (mm) | Proximate Analysis (wt.%) | Ultimate Analysis (wt.%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ash | M | V.M | F.C | S | C | H | N | O | |
| 0.5–1 | 28.92 | 2.35 | 21.71 | 47.02 | 4.71 | 49.82 | 0.31 | 7.07 | 9.17 |
| Coal Grade of Ash | Ash Content (Ad) Range/% | Coal Grade of Sulfur | Sulfur Content (Sd) Range/% |
|---|---|---|---|
| Ultra-low-ash coal | Ad ≤ 10.00 | Ultra-low-sulfur coal | Sd ≤ 0.50 |
| Low-ash coal | 10.00 < Ad ≤ 20.00 | Low-sulfur coal | 0.50 < Sd ≤ 1.00 |
| Medium ash coal | 20.00 < Ad ≤ 30.00 | Medium-low-sulfur coal | 1.00 < Sd ≤ 1.50 |
| High-ash coal | 30.00 < Ad ≤ 40.00 | Medium sulfur coal | 1.50 < Sd ≤ 2.00 |
| Ultra-high-ash coal | 40.00 < Ad ≤ 50.00 | High-sulfur coal | 2.00 < Sd ≤ 3.00 |
| Ultra-high-sulfur coal | Sd > 3.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Wang, Q. New Approach Study on Dry Coal Cleaning System with Two-Stage Corona Electrostatic Processes for High-Sulfur Low-Grade Fine Coals. Processes 2021, 9, 1915. https://doi.org/10.3390/pr9111915
Liu C, Wang Q. New Approach Study on Dry Coal Cleaning System with Two-Stage Corona Electrostatic Processes for High-Sulfur Low-Grade Fine Coals. Processes. 2021; 9(11):1915. https://doi.org/10.3390/pr9111915
Chicago/Turabian StyleLiu, Chengyuan, and Qingyue Wang. 2021. "New Approach Study on Dry Coal Cleaning System with Two-Stage Corona Electrostatic Processes for High-Sulfur Low-Grade Fine Coals" Processes 9, no. 11: 1915. https://doi.org/10.3390/pr9111915







