Biomaterials and Their Biomedical Applications: From Replacement to Regeneration
Abstract
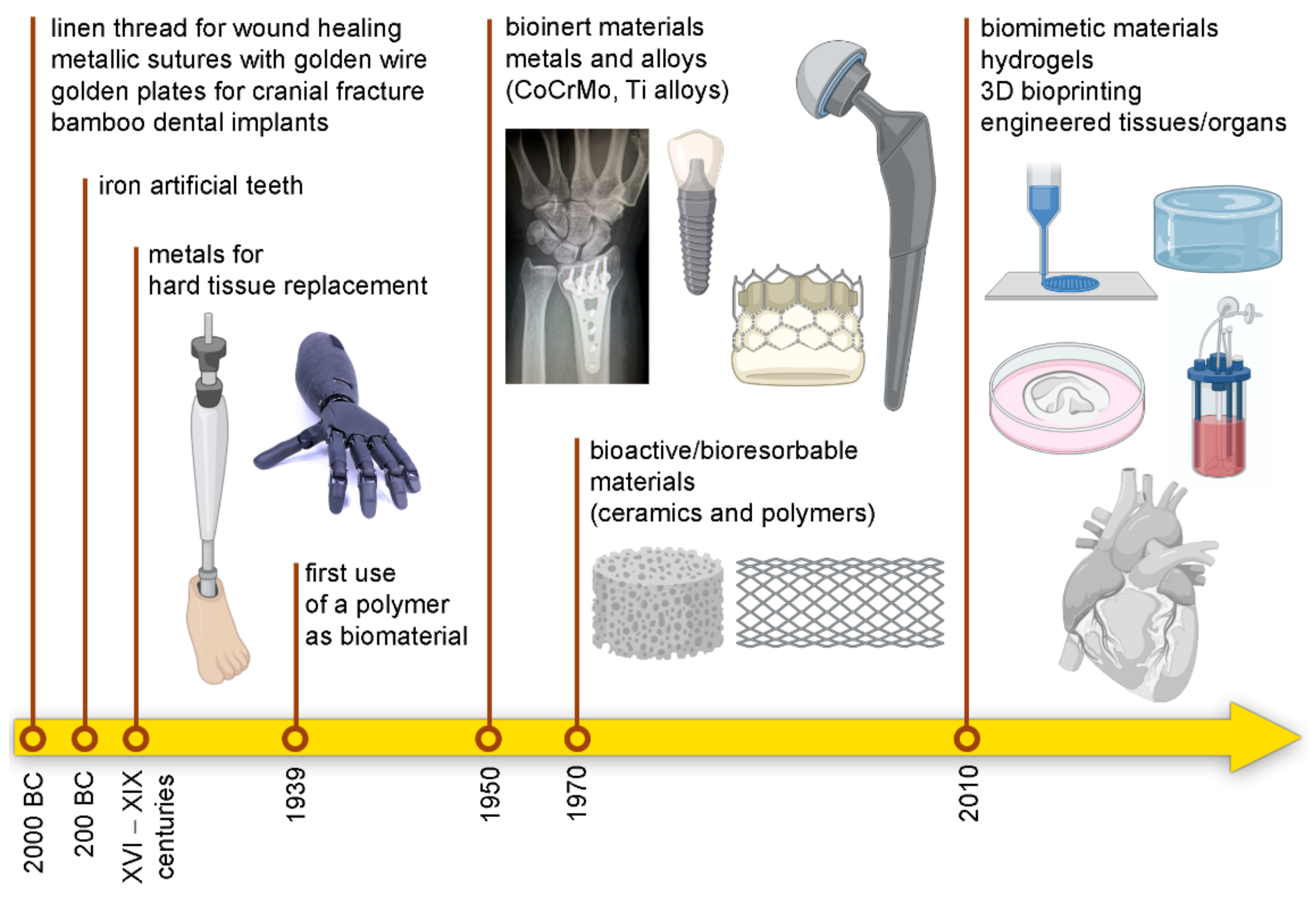
:1. When the History Began
2. Biocompatibility as the Crucial Item
3. Evolving Definitions of Biomaterials
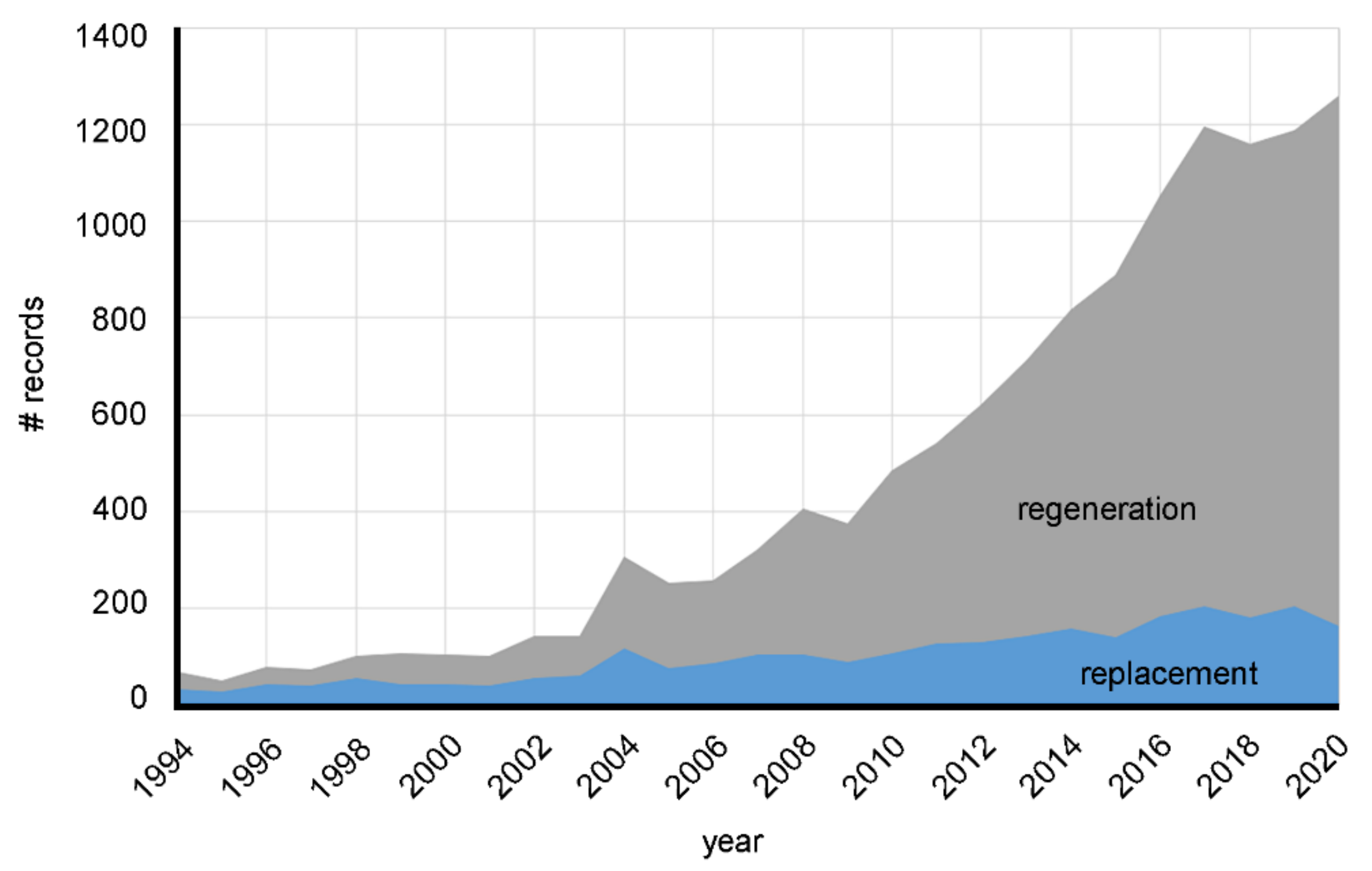
4. Biomaterials in the Scientific Literature
4.1. Biomaterials for Tissue Engineering
4.2. Three-Dimensional Printing of Biomaterials
4.3. Smart Biomaterials
4.4. Biomaterials Functionalized with Vesicles
5. Biomaterials and Biomedical Devices
5.1. Total Hip Prosthesis
5.2. Total Artificial Heart (TAH)
6. What Are Biomaterials Intended for from 2021 Onwards?
7. Some Words to Conclude
Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ratner, B.D.; Zhang, G. A History of Biomaterials. In Biomaterials Science. An Introduction to Materials in Medicine, 4th ed.; Ratner, B.D., Hoffman, A.S., Schoen, F.J., Lemons, J.E., Eds.; Elsevier Academic Press: London, UK, 2020. [Google Scholar]
- Gentleman, E.; Ball, M.D.; Stevens, M.M. Medical Sciences—Vol.II—Biomaterials. Available online: https://www.eolss.net/ebooklib/home.aspx (accessed on 29 September 2021).
- Muffly, T.M.; Tizzano, A.P.; Walters, M.D. The history and evolution of sutures in pelvic surgery. J. R. Soc. Med. 2011, 104, 107–112. [Google Scholar] [CrossRef]
- Bobbio, A. The first endosseous alloplastic implant in the history of man. Bull. Hist. Dent. 1972, 20, 1–6. [Google Scholar]
- Anjard, R. Mayan dental wonders. J. Oral Implantol. 1981, 9, 423–426. [Google Scholar] [PubMed]
- Ali, F. History of dental implants, in memoriam: Dr. Per-Ingvar Branemark, the man who made people smile. Int. J. Adv. Res. Ideas Innov. Technol. 2019, 5, 123–124. [Google Scholar]
- Crubézy, E.; Murail, P.; Girard, L.; Bernadou, J.-P. False teeth of the Roman world. Nat. Cell Biol. 1998, 391, 29. [Google Scholar] [CrossRef] [PubMed]
- Barker, C.F.; Markmann, J.F. Historical Overview of Transplantation. Cold Spring Harb. Perspect. Med. 2013, 3, a014977. [Google Scholar] [CrossRef]
- Biocompatibility Assessment Resource Center. FDA. Available online: https://www.fda.gov/medical-devices/premarket-submissions/biocompatibility-assessment-resource-center (accessed on 2 July 2021).
- ISO 10993-1:2009 Biological Evaluation of Medical Devices—Part 1. Available online: https://www.iso.org/standard/44908.html (accessed on 29 September 2021).
- Lee, K.C.; Eisig, S.B.; Perrino, M.A. Foreign Body Giant Cell Reaction to a Proplast/Teflon Interpositional Implant: A Case Report and Literature Review. J. Oral Maxillofac. Surg. 2018, 76, 1719–1724. [Google Scholar] [CrossRef] [PubMed]
- Moizhess, T.G. Carcinogenesis induced by foreign bodies. Biochemistry 2008, 73, 763–775. [Google Scholar] [CrossRef]
- Feldman, D. Polymer History. Des. Monomers Polym. 2008, 11, 1–15. [Google Scholar] [CrossRef]
- Castro, J.C. Albert Einstein and his abdominal aortic aneurysm. Gac. Med. Mex. 2011, 147, 74–76. [Google Scholar]
- LeVeen, H.H.; Rarberio, J.R. Tissue reaction to plastics used in surgery with special reference to teflon. Ann. Surg. 1949, 129, 74–84. [Google Scholar] [CrossRef]
- Vallet-Regí, M. Ceramics for medical applications. J. Chem. Soc. Dalton Trans. 2001, 97–108. [Google Scholar] [CrossRef]
- Hench, L.L. Bioactive Ceramics. Ann. N. Y. Acad. Sci. 1988, 523, 54–71. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; Jones, J.R. Bioactive Glasses: Frontiers and Challenges. Front. Bioeng. Biotechnol. 2015, 3, 194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordström, E.G.; Muñoz, O.L.S. Physics of bone bonding mechanism of different surface bioactive ceramic materials in vitro and in vivo. Bio-Med. Mater. Eng. 2001, 11, 221–231. [Google Scholar]
- Baino, F.; Hamzehlou, S.; Kargozar, S. Bioactive Glasses: Where Are We and Where Are We Going? J. Funct. Biomater. 2018, 9, 25. [Google Scholar] [CrossRef] [Green Version]
- Marin, E.; Boschetto, F.; Pezzotti, G. Biomaterials and biocompatibility: An historical overview. J. Biomed. Mater. Res. Part A 2020, 108, 1617–1633. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Biomaterials in orthopedic surgery. Am. J. Surg. 1967, 114, 31–41. [Google Scholar] [CrossRef]
- Society for Biomaterials (SFB). Available online: https://biomaterials.org/ (accessed on 15 June 2021).
- Park, J.B. Introduction. In Biomaterials Science and Engineering; Springer: Boston, MA, USA, 1984; ISBN 978-1-4612-9710-9. [Google Scholar]
- Bergmann, C.P. Stumpf Aisha Biomaterials. In Dental Ceramics; Springer: Berlin, Germany, 2013; pp. 9–13. ISBN 978-3-642-38224-6. [Google Scholar]
- Williams, D.F.; David, F. European Society for Biomaterials Definitions in biomaterials. In Proceedings of the Consensus Conference of the European Society for Biomaterials, Chester, UK, 3–5 March 1986; Elsevier: Amsterdam, The Netherlands; Chester, UK; Volume 4.
- The European Society for Biomaterials 9th European Conference on Biomaterials Chester, UK 9–11th September, 1991 in conjunction with 2nd Consensus Conference on Definitions in Biomaterials 7–8th September, 1991 and IUPAC working party on blood compatibility. J. Mater. Sci. Mater. Electron. 1991, 2, 62. [CrossRef]
- World Health Organization. Ageing and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 14 June 2021).
- Yannas, I.V.; Burke, J.F. Design of an artificial skin. I. Basic design principles. J. Biomed. Mater. Res. 1980, 14, 65–81. [Google Scholar] [CrossRef]
- Badylak, S.; Lantz, G.C.; Coffey, A.; Geddes, L.A. Small intestinal submucosa as a large diameter vascular graft in the dog. J. Surg. Res. 1989, 47, 74–80. [Google Scholar] [CrossRef]
- Vacanti, C.A.; Vacanti, J.P. Functional Organ Replacement, The New Technology of Tissue Engineering. Surg. Technol. Int. 1991, 1, 43–49. [Google Scholar]
- Langer, R.; Vacanti, J.P. Tissue Engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skalak, R.; Fox, C.F. Tissue engineering. In Proceedings of the Workshop Held at Granlibakken, Lake Tahoe, CA, USA, 26–29 February 1988; Liss: New York, NY, USA, 1988. [Google Scholar]
- Griffith, L.G.; Naughton, G. Tissue Engineering—Current Challenges and Expanding Opportunities. Science 2002, 295, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Kowalczewski, C.J.; Saul, J.M. Biomaterials for the Delivery of Growth Factors and Other Therapeutic Agents in Tissue Engineering Approaches to Bone Regeneration. Front. Pharmacol. 2018, 9, 513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, X.; Zhao, M.; Lash, B.; Martino, M.M.; Julier, Z. Growth Factor Engineering Strategies for Regenerative Medicine Applications. Front. Bioeng. Biotechnol. 2020, 7. [Google Scholar] [CrossRef]
- Chen, L.; Liu, J.; Guan, M.; Zhou, T.; Duan, X.; Xiang, Z. Growth Factor and Its Polymer Scaffold-Based Delivery System for Cartilage Tissue Engineering. Int. J. Nanomed. 2020, 15, 6097–6111. [Google Scholar] [CrossRef] [PubMed]
- Palecek, S.P.; Loftus, J.C.; Ginsberg, M.H.; Lauffenburger, D.A.; Horwitz, A.F. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature 1997, 385, 537–540. [Google Scholar] [CrossRef]
- Korossis, S.; Bolland, F.; Kearney, J.N.; Fisher, J.; Ingham, E. Bioreactors in tissue engineering. Top. Tissue Eng. 2005, 2, 1–23. [Google Scholar]
- Selden, C.; Fuller, B. Role of Bioreactor Technology in Tissue Engineering for Clinical Use and Therapeutic Target Design. Bioengineering 2018, 5, 32. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.; Chauhan, V.M.; Ghaemmaghami, A.M.; Aylott, J.W. New generation of bioreactors that advance extracellular matrix modelling and tissue engineering. Biotechnol. Lett. 2018, 41, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Todros, S.; Spadoni, S.; Maghin, E.; Piccoli, M.; Pavan, P. A Novel Bioreactor for the Mechanical Stimulation of Clinically Relevant Scaffolds for Muscle Tissue Engineering Purposes. Processes 2021, 9, 474. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics. J. Am. Ceram. Soc. 2005, 81, 1705–1728. [Google Scholar] [CrossRef]
- Fernandes, J.S.; Gentile, P.; Pires, R.A.; Reis, R.L.; Hatton, P.V. Multifunctional bioactive glass and glass-ceramic biomaterials with antibacterial properties for repair and regeneration of bone tissue. Acta Biomater. 2017, 59, 2–11. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Wu, C.; Chang, J. Bioceramics to regulate stem cells and their microenvironment for tissue regeneration. Mater. Today 2018, 24, 41–56. [Google Scholar] [CrossRef]
- Safari, B.; Aghanejad, A.; Roshangar, L.; Davaran, S. Osteogenic effects of the bioactive small molecules and minerals in the scaffold-based bone tissue engineering. Colloids Surfaces B Biointerfaces 2020, 198, 111462. [Google Scholar] [CrossRef]
- Sethu, S.N.; Namashivayam, S.; Devendran, S.; Nagarajan, S.; Tsai, W.-B.; Narashiman, S.; Ramachandran, M.; Ambigapathi, M. Nanoceramics on osteoblast proliferation and differentiation in bone tissue engineering. Int. J. Biol. Macromol. 2017, 98, 67–74. [Google Scholar] [CrossRef]
- Wang, M. Developing bioactive composite materials for tissue replacement. Biomaterials 2003, 24, 2133–2151. [Google Scholar] [CrossRef]
- Liu, S.; Qin, S.; He, M.; Zhou, D.; Qin, Q.; Wang, H. Current applications of poly(lactic acid) composites in tissue engineering and drug delivery. Compos. Part B Eng. 2020, 199, 108238. [Google Scholar] [CrossRef]
- Reddy, M.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef]
- Place, E.S.; George, J.H.; Williams, C.K.; Stevens, M.M. Synthetic polymer scaffolds for tissue engineering. Chem. Soc. Rev. 2009, 38, 1139–1151. [Google Scholar] [CrossRef]
- Salzano, E.; Di Serio, M.; Santacesaria, E. The evaluation of risks of ethoxylation reactors. Process. Saf. Prog. 2007, 26, 304–311. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Florit, M.; Pardo, A.; Domingues, R.M.A.; Graça, A.L.; Babo, P.S.; Reis, R.L.; Gomes, M.E. Natural-Based Hydrogels for Tissue Engineering Applications. Molecules 2020, 25, 5858. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; Yacoub, M.H. Hydrogel scaffolds for tissue engineering: Progress and challenges. Glob. Cardiol. Sci. Pr. 2013, 2013, 316–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2001, 54, 3–12. [Google Scholar] [CrossRef]
- Barbon, S.; Stocco, E.; Dalzoppo, D.; Todros, S.; Canale, A.; Boscolo-Berto, R.; Pavan, P.; Macchi, V.; Grandi, C.; De Caro, R.; et al. Halogen-Mediated Partial Oxidation of Polyvinyl Alcohol for Tissue Engineering Purposes. Int. J. Mol. Sci. 2020, 21, 801. [Google Scholar] [CrossRef] [Green Version]
- Barbon, S.; Contran, M.; Stocco, E.; Todros, S.; Macchi, V.; Caro, R.; Porzionato, A. Enhanced Biomechanical Properties of Polyvinyl Alcohol-Based Hybrid Scaffolds for Cartilage Tissue Engineering. Processes 2021, 9, 730. [Google Scholar] [CrossRef]
- Schmedlen, R.H.; Masters, K.S.; West, J.L. Photocrosslinkable polyvinyl alcohol hydrogels that can be modified with cell adhesion peptides for use in tissue engineering. Biomaterials 2002, 23, 4325–4332. [Google Scholar] [CrossRef]
- Guadalupe, E.; Ramos, D.; Shelke, N.B.; James, R.; Gibney, C.; Kumbar, S.G. Bioactive polymeric nanofiber matrices for skin regeneration. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Rana, D.; Zreiqat, H.; Benkirane-Jessel, N.; Ramakrishna, S.; Ramalingam, M. Development of decellularized scaffolds for stem cell-driven tissue engineering. J. Tissue Eng. Regen. Med. 2015, 11, 942–965. [Google Scholar] [CrossRef] [PubMed]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crapo, P.M.; Gilbert, T.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendibil, U.; Ruiz-Hernandez, R.; Retegi-Carrion, S.; Garcia-Urquia, N.; Olalde-Graells, B.; Abarrategi, A. Tissue-Specific Decellularization Methods: Rationale and Strategies to Achieve Regenerative Compounds. Int. J. Mol. Sci. 2020, 21, 5447. [Google Scholar] [CrossRef]
- Rabbani, M.; Zakian, N.; Alimoradi, N. Contribution of physical methods in decellularization of animal tissues. J. Med. Signals Sens. 2021, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Keane, T.; Swinehart, I.T.; Badylak, S.F. Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance. Methods 2015, 84, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Pérez, J.; Ahearne, M. The impact of decellularization methods on extracellular matrix derived hydrogels. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, L.; Taylor, A.; Faulk, D.M.; Keane, T.; Saldin, L.T.; Reing, J.; Swinehart, I.T.; Turner, N.; Ratner, B.D.; Badylak, S.F. The impact of detergents on the tissue decellularization process: A ToF-SIMS study. Acta Biomater. 2016, 50, 207–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zia, S.; Mozafari, M.; Natasha, G.; Tan, A.; Cui, Z.; Seifalian, A. Hearts beating through decellularized scaffolds: Whole-organ engineering for cardiac regeneration and transplantation. Crit. Rev. Biotechnol. 2015, 36, 705–715. [Google Scholar] [CrossRef]
- Crabbe, A.; Liu, Y.; Sarker, S.F.; Bonenfant, N.R.; Barrila, J.; Borg, Z.D.; Lee, J.J.; Weiss, D.J.; Nickerson, C.A. Recellularization of Decellularized Lung Scaffolds Is Enhanced by Dynamic Suspension Culture. PLoS ONE 2015, 10, e0126846. [Google Scholar] [CrossRef]
- Chen, F.; Yoo, J.J.; Atala, A. Acellular collagen matrix as a possible “off the shelf” biomaterial for urethral repair. Urology 1999, 54, 407–410. [Google Scholar] [CrossRef]
- Rasti, H.; Saghiri, N.; Baharara, J.; Mahdavi-Shahri, N.; Marjani, M.; Alavi, S.H.; Hoseini, B. Differentiation of Blastema Cells in Decellularized Bladder Scaffold in vitro. Zahedan J. Res. Med. Sci. 2015, 17. [Google Scholar] [CrossRef] [Green Version]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bose, S.; Ke, D.; Sahasrabudhe, H.; Bandyopadhyay, A. Additive manufacturing of biomaterials. Prog. Mater. Sci. 2017, 93, 45–111. [Google Scholar] [CrossRef] [PubMed]
- Touri, M.; Kabirian, F.; Saadati, M.; Ramakrishna, S.; Mozafari, M. Additive Manufacturing of Biomaterials—The Evolution of Rapid Prototyping. Adv. Eng. Mater. 2019, 21. [Google Scholar] [CrossRef]
- Atala, A. Introduction: 3D Printing for Biomaterials. Chem. Rev. 2020, 120, 10545–10546. [Google Scholar] [CrossRef]
- Shirazi, S.F.S.; Gharehkhani, S.; Mehrali, M.; Yarmand, H.; Metselaar, H.S.C.; Kadri, N.A.; ABU Osman, N.A. A review on powder-based additive manufacturing for tissue engineering: Selective laser sintering and inkjet 3D printing. Sci. Technol. Adv. Mater. 2015, 16, 033502. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Rawal, P.; Tripathi, D.M.; Ramakrishna, S.; Kaur, S. Prospects for 3D bioprinting of organoids. Bio-Design Manuf. 2021, 4, 627–640. [Google Scholar] [CrossRef]
- Boso, D.; Carraro, E.; Maghin, E.; Todros, S.; Dedja, A.; Giomo, M.; Elvassore, N.; De Coppi, P.; Pavan, P.; Piccoli, M. Porcine Decellularized Diaphragm Hydrogel: A New Option for Skeletal Muscle Malformations. Biomedicines 2021, 9, 709. [Google Scholar] [CrossRef] [PubMed]
- Elkhoury, K.; Morsink, M.; Sanchez-Gonzalez, L.; Kahn, C.; Tamayol, A.; Arab-Tehrany, E. Biofabrication of natural hydrogels for cardiac, neural, and bone Tissue engineering Applications. Bioact. Mater. 2021, 6, 3904–3923. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, P.S.; Bhattacharya, C.; Afewerki, S.; Langer, R.S. Smart Biomaterials: Recent Advances and Future Directions. ACS Biomater. Sci. Eng. 2018, 4, 3809–3817. [Google Scholar] [CrossRef]
- Tibbitt, M.W.; Langer, R. Living Biomaterials. Accounts Chem. Res. 2017, 50, 508–513. [Google Scholar] [CrossRef]
- Holzapfel, B.M.; Reichert, J.C.; Schantz, J.-T.; Gbureck, U.; Rackwitz, L.; Nöth, U.; Jakob, F.; Rudert, M.; Groll, J.; Hutmacher, D.W. How smart do biomaterials need to be? A translational science and clinical point of view. Adv. Drug Deliv. Rev. 2013, 65, 581–603. [Google Scholar] [CrossRef]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef] [Green Version]
- Kasiński, A.; Zielińska-Pisklak, M.; Oledzka, E.; Sobczak, M. Smart Hydrogels—Synthetic Stimuli-Responsive Antitumor Drug Release Systems. Int. J. Nanomed. 2020, 15, 4541–4572. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, Y. Rational Design of Smart Hydrogels for Biomedical Applications. Front. Chem. 2021, 8. [Google Scholar] [CrossRef]
- Parhi, R. Cross-Linked Hydrogel for Pharmaceutical Applications: A Review. Adv. Pharm. Bull. 2017, 7, 515–530. [Google Scholar] [CrossRef]
- Zhao, X. Multi-scale multi-mechanism design of tough hydrogels: Building dissipation into stretchy networks. Soft Matter 2013, 10, 672–687. [Google Scholar] [CrossRef] [Green Version]
- Buwalda, S.J.; Vermonden, T.; Hennink, W.E. Hydrogels for Therapeutic Delivery: Current Developments and Future Directions. Biomacromolecules 2017, 18, 316–330. [Google Scholar] [CrossRef]
- Amukarimi, S.; Mozafari, M. 4D bioprinting of tissues and organs. Bioprinting 2021, 23, e00161. [Google Scholar] [CrossRef]
- Ionov, L. 4D Biofabrication: Materials, Methods, and Applications. Adv. Health Mater. 2018, 7, e1800412. [Google Scholar] [CrossRef]
- Yang, G.H.; Yeo, M.; Koo, Y.W.; Kim, G.H. 4D Bioprinting: Technological Advances in Biofabrication. Macromol. Biosci. 2019, 19, e1800441. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, J.; Lu, X.; Li, Y. Shape Memory Biomaterials and Their Clinical Applications. In Biomedical Materials; Narayan, R., Ed.; Springer: Berlin, Germany, 2020; pp. 195–255. ISBN 978-3-030-49205-2. [Google Scholar]
- Diba, M.; Spaans, S.; Ning, K.; Ippel, B.D.; Yang, F.; Loomans, B.; Dankers, P.Y.W.; Leeuwenburgh, S.C.G. Self-Healing Biomaterials: From Molecular Concepts to Clinical Applications. Adv. Mater. Interfaces 2018, 5. [Google Scholar] [CrossRef]
- Mirfakhrai, T.; Madden, J.D.; Baughman, R.H. Polymer artificial muscles. Mater. Today 2007, 10, 30–38. [Google Scholar] [CrossRef]
- Nemat-Nasser, S.; Thomas, C. Ionomeric polymer-metal composites. In Electroactive Polymer (EAP) Actuators as Artificial Muscles: Reality, Potential and Challenges; SPIE: Bellingham, WA, USA, 2001; pp. 171–230. [Google Scholar]
- Li, J.; Xin, M.; Ma, Z.; Shi, Y.; Pan, L. Nanomaterials and their applications on bio-inspired wearable electronics. Nanotechnology 2021, 32, 472002. [Google Scholar] [CrossRef]
- Brennan, M.; Layrolle, P.; Mooney, D.J. Biomaterials Functionalized with MSC Secreted Extracellular Vesicles and Soluble Factors for Tissue Regeneration. Adv. Funct. Mater. 2020, 30. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhang, Y.; Chen, Q. Mesenchymal Stem Cell (MSC)-Derived Extracellular Vesicles: Potential Therapeutics as MSC Trophic Mediators in Regenerative Medicine. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2019, 303, 1735–1742. [Google Scholar] [CrossRef]
- Wang, C.; Wang, M.; Xu, T.; Zhang, X.; Lin, C.; Gao, W.; Xu, H.; Lei, B.; Mao, C. Engineering Bioactive Self-Healing Antibacterial Exosomes Hydrogel for Promoting Chronic Diabetic Wound Healing and Complete Skin Regeneration. Theranostics 2019, 9, 65–76. [Google Scholar] [CrossRef]
- Mardpour, S.; Ghanian, M.H.; Sadeghi-Abandansari, H.; Mardpour, S.; Nazari, A.; Shekari, F.; Baharvand, H. Hydrogel-Mediated Sustained Systemic Delivery of Mesenchymal Stem Cell-Derived Extracellular Vesicles Improves Hepatic Regeneration in Chronic Liver Failure. ACS Appl. Mater. Interfaces 2019, 11, 37421–37433. [Google Scholar] [CrossRef]
- Zhang, K.; Zhao, X.; Chen, X.; Wei, Y.; Du, W.; Wang, Y.; Liu, L.; Zhao, W.; Han, Z.; Kong, D.; et al. Enhanced Therapeutic Effects of Mesenchymal Stem Cell-Derived Exosomes with an Injectable Hydrogel for Hindlimb Ischemia Treatment. ACS Appl. Mater. Interfaces 2018, 10, 30081–30091. [Google Scholar] [CrossRef] [PubMed]
- Merola, M.; Affatato, S. Materials for Hip Prostheses: A Review of Wear and Loading Considerations. Materials 2019, 12, 495. [Google Scholar] [CrossRef] [Green Version]
- Aherwar, A.; Singh, A.K.; Patnaik, A. Current and future biocompatibility aspects of biomaterials for hip prosthesis. AIMS Environ. Sci. 2015, 3, 23–43. [Google Scholar] [CrossRef]
- Knight, S.R.; Aujla, R.; Biswas, S.P. Total Hip Arthroplasty—over 100 years of operative history. Orthop. Rev. 2011, 3, e16. [Google Scholar] [CrossRef] [Green Version]
- Muster, D. Themistocles Gluck, Berlin 1890: A pioneer of multidisciplinary applied research into biomaterials for endoprostheses. Bull. Hist. Dent. 1990, 38, 3–6. [Google Scholar]
- Triclot, P. Metal-on-metal: History, state of the art (2010). Int. Orthop. 2011, 35, 201–206. [Google Scholar] [CrossRef] [Green Version]
- Moussa, A.A.; Fischer, J.; Yadav, R.; Khandaker, M. Minimizing Stress Shielding and Cement Damage in Cemented Femoral Component of a Hip Prosthesis through Computational Design Optimization. Adv. Orthop. 2017, 2017, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Reimeringer, M.; Nuño, N.; Desmarais-Trépanier, C.; Lavigne, M.; Vendittoli, P.-A. The influence of uncemented femoral stem length and design on its primary stability: A finite element analysis. Comput. Methods Biomech. Biomed. Eng. 2013, 16, 1221–1231. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Kim, J.-S.; Cho, S.-H. Strain distribution in the proximal human femur. An in vitro comparison in the intact femur and after insertion of reference and experimental femoral stems. J. Bone Jt. Surgery. Br. Vol. 2001, 83, 295–301. [Google Scholar] [CrossRef]
- Rezaei, F.; Hassani, K.; Solhjoei, N.; Karimi, A. Carbon/PEEK composite materials as an alternative for stainless steel/titanium hip prosthesis: A finite element study. Australas. Phys. Eng. Sci. Med. 2015, 38, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Avval, P.T.; Samiezadeh, S.; Klika, V.; Bougherara, H. Investigating stress shielding spanned by biomimetic polymer-composite vs. metallic hip stem: A computational study using mechano-biochemical model. J. Mech. Behav. Biomed. Mater. 2015, 41, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Sasso, E.D.; Bagno, A.; Scuri, S.T.; Gerosa, G.; Iop, L. The Biocompatibility Challenges in the Total Artificial Heart Evolution. Annu. Rev. Biomed. Eng. 2019, 21, 85–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerosa, G.; Scuri, S.; Iop, L.; Torregrossa, G. Present and future perspectives on total artificial hearts. Ann. Cardiothorac. Surg. 2014, 3, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Cooley, D.A. The total artificial heart. Nat. Med. 2003, 9, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Lindbergh, C.A. An apparatus for the culture of whole organs. J. Exp. Med. 1935, 62, 409–431. [Google Scholar] [CrossRef]
- Akutsu, T. Components of artificial hearts. Bull. N. Y. Acad. Med. 1972, 48, 362–378. [Google Scholar]
- Kolff, W. The Invention of the Artificial Heart. Int. J. Artif. Organs 1990, 13, 396–403. [Google Scholar] [CrossRef] [Green Version]
- Cooley, D.A.; Liotta, D.; Hallman, G.L.; Bloodwell, R.D.; Leachman, R.D.; Milam, J.D. Orthotopic cardiac prosthesis for two-staged cardiac replacement. Am. J. Cardiol. 1969, 24, 723–730. [Google Scholar] [CrossRef]
- Cook, J.A.; Shah, K.B.; Quader, M.A.; Cooke, R.H.; Kasirajan, V.; Rao, K.K.; Smallfield, M.C.; Tchoukina, I.; Tang, D.G. The total artificial heart. J. Thorac. Dis. 2015, 7, 2172. [Google Scholar]
- Han, J.J. Aeson—The Carmat total artificial heart is approved for enrollment in the United States. Artif. Organs 2021, 45, 445–446. [Google Scholar] [CrossRef]
- Copeland, J.G.; Smith, R.G.; Arabia, F.A.; Nolan, P.E.; Sethi, G.K.; Tsau, P.H.; McClellan, D.; Slepian, M.J. Cardiac Replacement with a Total Artificial Heart as a Bridge to Transplantation. N. Engl. J. Med. 2004, 351, 859–867. [Google Scholar] [CrossRef] [Green Version]
- Mohacsi, P.; Leprince, P. The CARMAT total artificial heart. Eur. J. Cardio-Thoracic Surg. 2014, 46, 933–934. [Google Scholar] [CrossRef] [Green Version]
- Jansen, P.; Van Oeveren, W.; Capel, A.; Carpentier, A. In vitro haemocompatibility of a novel bioprosthetic total artificial heart. Eur. J. Cardio-Thoracic Surg. 2012, 41, e166–e172. [Google Scholar] [CrossRef] [Green Version]
- Gross, J.M. Calcification of bioprosthetic heart valves and its assessment. J. Thorac. Cardiovasc. Surg. 2001, 121, 428–430. [Google Scholar] [CrossRef] [Green Version]
- Zouhair, S.; Sasso, E.D.; Tuladhar, S.R.; Fidalgo, C.; Vedovelli, L.; Filippi, A.; Borile, G.; Bagno, A.; Marchesan, M.; De Rossi, G.; et al. A Comprehensive Comparison of Bovine and Porcine Decellularized Pericardia: New Insights for Surgical Applications. Biomolecules 2020, 10, 371. [Google Scholar] [CrossRef] [Green Version]
- Jana, S. Endothelialization of cardiovascular devices. Acta Biomater. 2019, 99, 53–71. [Google Scholar] [CrossRef]
- Todesco, M.; Zardin, C.; Iop, L.; Palmosi, T.; Capaldo, P.; Romanato, F.; Gerosa, G.; Bagno, A. Hybrid membranes for the production of blood contacting surfaces: Physicochemical, structural and biomechanical characterization. Biomater. Res. 2021, 25, 1–12. [Google Scholar] [CrossRef]
- Todesco, M.; Pontara, E.; Cheng, C.; Gerosa, G.; Pengo, V.; Bagno, A. Preliminary hemocompatibility assessment of an innovative material for blood contacting surfaces. J. Mater. Sci. Mater. Med. 2021, 32, 1–7. [Google Scholar] [CrossRef]
- Ratner, B.D. Biomaterials: Been There, Done That, and Evolving into the Future. Annu. Rev. Biomed. Eng. 2019, 21, 171–191. [Google Scholar] [CrossRef]
- Sharp, P.A.; Langer, R. Promoting Convergence in Biomedical Science. Science 2011, 333, 527. [Google Scholar] [CrossRef]
- Davenport, T.; Kalakota, R. The potential for artificial intelligence in healthcare. Futur. Health J. 2019, 6, 94–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macchiarini, P.; Jungebluth, P.; Go, T.; Asnaghi, M.A.; Rees, L.E.; Cogan, T.A.; Dodson, A.; Martorell, J.; Bellini, S.; Parnigotto, P.P.; et al. Clinical transplantation of a tissue-engineered airway. Lancet 2008, 372, 2023–2030. [Google Scholar] [CrossRef]
- Delaere, P.R.; Van Raemdonck, D. The trachea: The first tissue-engineered organ? J. Thorac. Cardiovasc. Surg. 2014, 147, 1128–1132. [Google Scholar] [CrossRef] [Green Version]
- Taylor, D.A.; Parikh, R.B.; Sampaio, L.C. Bioengineering Hearts: Simple yet Complex. Curr. Stem Cell Rep. 2017, 3, 35–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fioretta, E.S.; Motta, S.E.; Lintas, V.; Loerakker, S.; Parker, K.K.; Baaijens, F.P.T.; Falk, V.; Hoerstrup, S.P.; Emmert, M.Y. Next-generation tissue-engineered heart valves with repair, remodelling and regeneration capacity. Nat. Rev. Cardiol. 2020, 18, 92–116. [Google Scholar] [CrossRef]
- Mirdamadi, E.S.; Kalhori, D.; Zakeri, N.; Azarpira, N.; Solati-Hashjin, M. Liver Tissue Engineering as an Emerging Alternative for Liver Disease Treatment. Tissue Eng. Part B Rev. 2020, 26, 145–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katari, R.; Edgar, L.; Wong, T.; Boey, A.; Mancone, S.; Igel, D.; Callese, T.; Voigt, M.; Tamburrini, R.; Zambon, J.P.; et al. Tissue-Engineering Approaches to Restore Kidney Function. Curr. Diabetes Rep. 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Tebyanian, H.; Karami, A.; Nourani, M.R.; Motavallian, E.; Barkhordari, A.; Yazdanian, M.; Seifalian, A. Lung tissue engineering: An update. J. Cell. Physiol. 2019, 234, 19256–19270. [Google Scholar] [CrossRef] [PubMed]
- Horst, M.; Eberli, D.; Gobet, R.; Salemi, S. Tissue Engineering in Pediatric Bladder Reconstruction—The Road to Success. Front. Pediatr. 2019, 7, 91. [Google Scholar] [CrossRef] [Green Version]
- Petite, H.; Viateau, V.; Bensaïd, W.; Meunier, A.; De Pollak, C.; Bourguignon, M.; Oudina, K.; Sedel, L.; Guillemin, G. Tissue-engineered bone regeneration. Nat. Biotechnol. 2000, 18, 959–963. [Google Scholar] [CrossRef]
- Sierra-Sánchez, A.; Kim, K.H.; Blasco-Morente, G.; Arias-Santiago, S. Cellular human tissue-engineered skin substitutes investigated for deep and difficult to heal injuries. NPJ Regen. Med. 2021, 6, 1–23. [Google Scholar] [CrossRef]
- Keeney, M.; Lai, J.H.; Yang, F. Recent progress in cartilage tissue engineering. Curr. Opin. Biotechnol. 2011, 22, 734–740. [Google Scholar] [CrossRef]
- Law, J.X.; Liau, L.L.; Aminuddin, B.S.; Ruszymah, B.H.I. Tissue-engineered trachea: A review. Int. J. Pediatr. Otorhinolaryngol. 2016, 91, 55–63. [Google Scholar] [CrossRef]
- Tibbitt, M.W.; Rodell, C.B.; Burdick, J.A.; Anseth, K.S. Progress in material design for biomedical applications. Proc. Natl. Acad. Sci. USA 2015, 112, 14444–14451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosoyama, K.; Lazurko, C.; Muñoz, M.; McTiernan, C.D.; Alarcon, E.I. Peptide-Based Functional Biomaterials for Soft-Tissue Repair. Front. Bioeng. Biotechnol. 2019, 7, 205. [Google Scholar] [CrossRef]
- Aguado, B.A.; Grim, J.C.; Rosales, A.M.; Watson-Capps, J.J.; Anseth, K.S. Engineering precision biomaterials for personalized medicine. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef]

| Pros and Cons | Examples | Applications | |
|---|---|---|---|
| Metals | Pros:
| stainless steel, CoCrMo, titanium, Ti6Al4V, nitinol, nickel, platinum, tantalum | orthopedic, orthodontic, cardiovascular |
| Ceramics | Pros:
| alumina, zirconia, hydroxyapatite, beta tri-calcium phosphate, pyrolytic carbon | orthopedic, orthodontic, cardiovascular |
| Polymers | Pros:
| polymethylmethacrilate (PMMA), ultra-high molecular weight polyethylene (UHMWPE), polylactic acid (PLA), poly tetrafluoroethylene (PTFE), nylon, polyethylene, polyurethane, celluloid, cellophane, polycaprolactone (PCL), polyglycolic acid (PGA), polylactic acid (PLA), poly-lactic-co-glycolic acid (PLGA), poly(ethers) including polyethylene glycol (PEG), polyvinyl alcohol (PVA) and polyurethanes (PUs) | orthopedic, orthodontic, cardiovascular, breast implants, scaffold for soft tissues |
| Biologically-derived materials | Pros:
| porcine/bovine pericardium | bioprosthetic heart valves, total artificial heart |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todros, S.; Todesco, M.; Bagno, A. Biomaterials and Their Biomedical Applications: From Replacement to Regeneration. Processes 2021, 9, 1949. https://doi.org/10.3390/pr9111949
Todros S, Todesco M, Bagno A. Biomaterials and Their Biomedical Applications: From Replacement to Regeneration. Processes. 2021; 9(11):1949. https://doi.org/10.3390/pr9111949
Chicago/Turabian StyleTodros, Silvia, Martina Todesco, and Andrea Bagno. 2021. "Biomaterials and Their Biomedical Applications: From Replacement to Regeneration" Processes 9, no. 11: 1949. https://doi.org/10.3390/pr9111949
APA StyleTodros, S., Todesco, M., & Bagno, A. (2021). Biomaterials and Their Biomedical Applications: From Replacement to Regeneration. Processes, 9(11), 1949. https://doi.org/10.3390/pr9111949








