Bioassay-Guided Discovery of Potential Partial Extracts with Cytotoxic Effects on Liver Cancer Cells from Vietnamese Medicinal Herbs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Instrumentation
2.3. Collecting, Preparing, and Extracting Plant Materials
2.4. Cell Cultures
2.5. Cytotoxicity Screening ASSAY
2.6. Preparation of Partial Extracts
2.7. Total Flavonoids Content (TFC)
2.8. DPPH Free Radical Scavenging Assay
2.9. Determination of 50% Cytotoxic Concentration (CC50)
2.10. Statistical Analysis
3. Results
3.1. Cytotoxicity Screening
3.2. Evaluation of TFC, Antioxidant Activity, and CC50 of the Extracts from Luvunga scandens Leaves
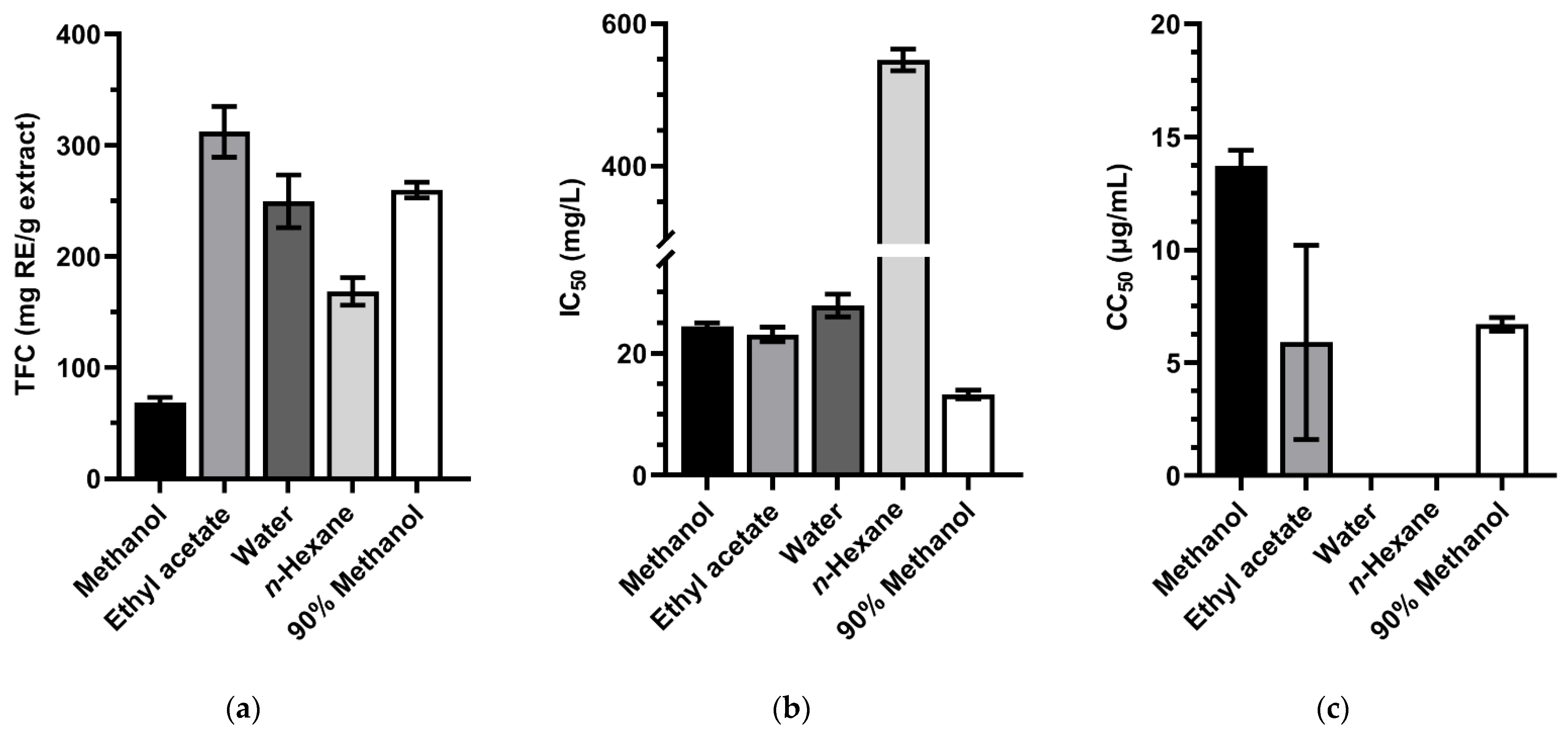
3.3. Evaluation of TFC, Antioxidant Activity, and CC50 of the Extracts from Hyptis suaveolens Roots
| No. | Scientific Name | Plant Family | Traditional Use | Plant Part | Cytotoxicity Effect (%) 1 | ||
|---|---|---|---|---|---|---|---|
| Huh-7 Cells | Hep3B Cells | HepG2 Cells | |||||
| 1 | Piper sarmentosum Roxb. | Piperaceae | Rheumatism arthralgia, rheumatic bone pain, wet joint pain and traumatic injury treatment [16,17]. Abdominal distension, gastricism, stomachache, toothache, headache [16]. Breast cancer [25], cervical carcinoma, and colon adenocarcinoma [26]. | Roots | 19.70 ± 5.33 | 29.01 ± 4.61 | 15.25 ± 11.78 |
| Leaves | 6.78 ± 2.60 | 24.81 ± 12.75 | 12.22 ± 12.37 | ||||
| Stems | 18.36 ± 3.48 | 22.41 ± 8.18 | 7.12 ± 13.18 | ||||
| 2 | Glinus oppositifolius (L.) Aug.DC. | Molluginaceae | Fever, liver diseases, abdominal pain and jaundice treatment [16]. | Stems | 20.40 ± 7.60 | 20.61 ± 3.53 | 12.82 ± 11.14 |
| 3 | Vernonia amygdalina Delile | Compositae | Stomach disorder, skin wound, diarrhea, scabies, ascariasis, tonsillitis, fever and worms infection [27]. Anticancer [28]. | Leaves | 27.06 ± 12.09 | 21.82 ± 4.57 | 0.00 ± 13.42 |
| 4 | Curcuma aromatica Salisb. | Zingiberaceae | Bruises, corn, sprains, dysentery and gastric ailments treatment [29]. Healing wounds and fractured bones [29]. Anti-tumor [30] and anti-carcinogenic [31]. | Roots | 13.06 ± 5.66 | 19.76 ± 4.94 | 0.00 ± 3.79 |
| Leaves | 11.59 ± 3.88 | 21.00 ± 7.59 | 7.05 ± 13.41 | ||||
| 5 | Phyllanthus amarus Schumach. & Thonn. | Phyllanthaceae | Cooling, diuretic, stomachic, febrifuge and antiseptic. Hepatitis, jaundice, fever, snake bites treatment [16]. | Roots | 21.48 ± 3.50 | 31.69 ± 7.33 | 17.09 ± 10.96 |
| Stems | 16.88 ± 4.17 | 20.13 ± 8.33 | 3.63 ± 6.01 | ||||
| 6 | Crinum sp. | Crinum | Emetic, rheumatism, earache [32]. Swelling, urinary tract problems and anticancer [33]. | Leaves | 12.59 ± 1.96 | 15.41 ± 5.13 | 0.35 ± 4.94 |
| 7 | Zingiber zerumbet (L.) Roscoe ex Sm. | Zingiberaceae | Stomach pains, diarrhea, inflammation, flatulence, fever, poisoning, allergies, and bacterial infections [34]. Joint inflammation and pain [35]. Anticancer [36]. | Roots | 6.59 ± 3.43 | 18.11 ± 7.26 | 0.00 ± 7.72 |
| Leaves | 9.90 ± 3.85 | 14.18 ± 9.86 | 6.79 ± 8.37 | ||||
| Stems | 11.21 ± 2.89 | 17.22 ± 3.53 | 4.53 ± 3.90 | ||||
| 8 | Hyptis suaveolens (L.) Poit. | Lamiaceae | Headache and fever treatment, stomach pains, diarrhea, flatulence, wound healing, hemostasis, snake bites [16]. Cancers and tumors treatment [37]. | Roots | 75.25 ± 4.36 | 84.77 ± 1.23 | 73.84 ± 3.05 |
| Stems | 24.10 ± 3.67 | 40.23 ± 5.47 | 35.39 ± 6.11 | ||||
| Leaves | 29.47 ± 4.38 | 40.05 ± 14.55 | 31.77 ± 13.74 | ||||
| 9 | Dicliptera chinensis (L.) Juss. | Acanthaceae | Hepatoprotection [38]. Stomachache, enteritis and diarrhea treatment [39]. | Stems | 18.12 ± 2.75 | 22.95 ± 6.91 | 9.64 ± 4.90 |
| Leaves | 20.36 ± 5.32 | 22.01 ± 6.39 | 4.46 ± 4.56 | ||||
| 10 | Curcuma zedoaria (Christm.) Roscoe | Zingiberaceae | Aid digestion, relief for colic, blood purifier [16]. Stomach diseases, leucoderma, toothache and promoting menstruation [17,40]. Anticancer [41]. | Roots | 12.00 ± 1.39 | 24.86 ± 0.57 | 11.64 ± 15.02 |
| Leaves | 24.78 ± 8.82 | 45.00 ± 13.46 | 19.50 ± 2.94 | ||||
| 11 | Eurycoma longifolia Jack | Simaroubaceae | Fever, jaundice, cachexia, and dropsy treatment [42,43]. Antimalarial, anti-tumor, and anticancer activity [44,45]. | Stems | 26.56 ± 4.54 | 29.50 ± 1.45 | 21.45 ± 6.17 |
| Leaves | 19.32 ± 10.10 | 33.10 ± 4.35 | 15.89 ± 12.97 | ||||
| 12 | Solanum torvum Sw. | Solanaceae | Liver diseases, spleen enlargement, stomachache and toothache treatment [16,46]. Haemostasis and anti-inflammation [47]. | Fruits | 21.81 ± 2.04 | 39.68 ± 5.16 | 17.38 ± 3.85 |
| Rhizomes | 24.52 ± 2.37 | 34.81 ± 1.71 | 18.99 ± 7.83 | ||||
| Leaves | 30.64 ± 3.67 | 77.39 ± 6.53 | 24.67 ± 2.70 | ||||
| 13 | Melastoma malabathricum L. | Melastomataceae | Liver diseases, hepatitis [16]. | Fruits | 11.82 ± 1.70 | 25.53 ± 3.74 | 4.40 ± 7.71 |
| Stems | 25.34 ± 5.72 | 33.67 ± 2.44 | 11.96 ± 5.28 | ||||
| Leaves | 13.91 ± 8.95 | 33.70 ± 9.73 | 6.69 ± 8.85 | ||||
| 14 | Luvunga scandens (Roxb.) Buch.-Ham | Rutaceae | Ascites, cirrhosis [15]. | Leaves | 36.34 ± 11.48 | 54.30 ± 12.19 | 73.35 ± 1.21 |
| 15 | Carallia brachiata (Lour.) Merr. | Rhizophoraceae | Inflammation of throat and mouth [15]. Liver diseases (folk experience). | Rhizomes | 26.11 ± 6.18 | 25.73 ± 2.08 | 12.37 ± 3.03 |
| Leaves | 16.57 ± 9.42 | 31.06 ± 0.31 | 12.43 ± 14.15 | ||||
| 16 | Helicteres hirsuta Lour. | Malvaceae | Ulcer, anti-malaria, dysentery, flu, smallpox [14]. Liver diseases (folk experience) [48]. | Leaves | 23.08 ± 9.32 | 28.47 ± 12.11 | 12.93 ± 8.18 |
| 17 | Miliusa velutina (A.DC.) Hook.f. & Thomson | Annonaceae | Eye inflammation, stomach pains, sinusitis, skin diseases [15]. Liver diseases (folk experience). | Stems | 14.22 ± 5.61 | 21.93 ± 4.97 | 0.10 ± 2.27 |
| Leaves | 15.58 ± 9.08 | 31.22 ± 12.21 | 7.02 ± 2.41 | ||||
| 18 | Grewia nervosa (Lour.) Panigrahi | Malvaceae | Cough, anti-malaria, digestive disorders [15]. Liver diseases (folk experience). | Fruits | 14.82 ± 5.63 | 24.85 ± 5.79 | 7.95 ± 4.64 |
| Stems | 31.00 ± 6.84 | 45.50 ± 1.04 | 6.73 ± 12.07 | ||||
| Leaves | 12.86 ± 4.09 | 20.18 ± 9.89 | 4.17 ± 1.78 | ||||
| 19 | Oroxylum indicum (L.) Kurz | Bignoniaceae | Cough, ulcer, abdominal pain [17]. Liver diseases (folk experience). | Stems | 33.90 ± 7.97 | 32.36 ± 10.17 | 23.80 ± 12.57 |
| Leaves | 14.80 ± 8.18 | 25.65 ± 10.84 | 7.17 ± 9.15 | ||||
| 20 | Cleome gynandra L. | Cleomaceae | Arthritis, pimples, fever, anti-malaria, dysentery [16]. Liver diseases (folk experience). | Stems | 34.55 ± 7.12 | 22.76 ± 8.05 | 11.11 ± 3.60 |
| Leaves | 17.37 ± 6.44 | 25.80 ± 2.46 | 8.96 ± 4.57 | ||||
| 21 | Lasia spinosa (L.) Thwaites | Araceae | Liver diseases, hepatitis, ascites, cirrhosis [15]. Liver failure, liver tonic [16]. | Fruits | 23.48 ± 12.26 | 31.81 ± 10.98 | 2.71 ± 6.98 |
| Rhizomes | 13.82 ± 11.69 | 38.22 ± 12.09 | 12.64 ± 4.24 | ||||
| Stems | 18.17 ± 7.59 | 26.31 ± 8.87 | 4.65 ± 6.69 | ||||
| Leaves | 7.68 ± 10.18 | 25.06 ± 6.46 | 7.75 ± 12.37 | ||||
| 22 | Ipomoea pes-tigridis L. | Convolvulaceae | Pimples, hemoptysis [15]. Liver diseases (folk experience). | Roots | 29.25 ± 10.95 | 18.24 ± 8.19 | 7.11 ± 6.30 |
| Stems | 14.97 ± 6.72 | 28.99 ± 9.28 | 14.32 ± 11.86 | ||||
| Leaves | 11.42 ± 6.41 | 24.15 ± 3.43 | 13.17 ± 8.12 | ||||
| 23 | Cleome rutidosperma DC. | Cleomaceae | Hepatitis (folk experience). | Stems | 11.97 ± 8.95 | 27.19 ± 7.27 | 1.06 ± 9.51 |
| Leaves | 29.47 ± 1.37 | 24.64 ± 3.64 | 18.61 ± 6.58 | ||||
| 24 | Crotalaria pallida Aiton | Leguminosae | Anticancer [16]. | Stems | 23.80 ± 5.87 | 16.82 ± 5.59 | 11.64 ± 5.77 |
| Leaves | 10.01 ± 7.22 | 21.80 ± 6.98 | 15.84 ± 4.57 | ||||
3.4. Evaluation of TFC, Antioxidant Activity, and CC50 of the Extracts from Solanum torvum Leaves
4. Discussion
4.1. Total Flavonoids Content, Antioxidant Activity, and Cytotoxic Effect of the Extracts from Luvunga scandens Leaves
4.2. Total Flavonoids Content, Antioxidant Activity, and Cytotoxic Effect of the Extracts from Hyptis suaveolens Roots
4.3. Total Flavonoids Content, Antioxidant Activity, and Cytotoxic Effect of the Extracts from Solanum torvum Leaves
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| HCC | Hepatocellular carcinoma. |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide. |
| TFC | Total flavonoids content. |
| Huh-7 HCC cells | Huh-7 human hepatocellular carcinoma cells. |
| DPPH | 1,1-Diphenyl-2-picrylhydrazyl. |
| IC50 | The half maximal inhibitory concentration. |
| CC50 | The 50% cytotoxic concentration. |
| DMEM | Dulbecco’s modified Eagle’s medium. |
| RE | Rutin equivalent. |
| L. scandens leaves | Luvunga scandens (Roxb.) Buch.-Ham. ex Wight & Arn. leaves. |
| H. suaveolens roots | Hyptis suaveolens (L.) Poit. roots. |
| S. torvum leaves | Solanum torvum Sw. leaves. |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- GLOBOCAN. Vietnam Fact Sheets. Available online: https://gco.iarc.fr/today/data/factsheets/populations/704-viet-nam-fact-sheets.pdf (accessed on 17 September 2021).
- Castelli, G.; Pelosi, E.; Testa, U. Liver Cancer: Molecular Characterization, Clonal Evolution and Cancer Stem Cells. Cancers 2017, 9, 127. [Google Scholar] [CrossRef] [Green Version]
- Fattovich, G.; Stroffolini, T.; Zagni, I.; Donato, F. Hepatocellular carcinoma in cirrhosis: Incidence and risk factors. Gastroenterology 2004, 127, S35–S50. [Google Scholar] [CrossRef] [PubMed]
- European Association For The Study Of The Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.M.; Sinn, D.H.; Jung, S.-H.; Gwak, G.-Y.; Paik, Y.-H.; Choi, M.S.; Lee, J.H.; Koh, K.C.; Paik, S.W. The recommended treatment algorithms of the BCLC and HKLC staging systems: Does following these always improve survival rates for HCC patients? Liver Int. 2016, 36, 1490–1497. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Arimoto, A.; Wakasa, T.; Kita, R.; Kimura, T.; Osaki, Y. Surgical resection for hepatocellular carcinoma: Clinical outcomes and safety in elderly patients. Eur. J. Gastroenterol. Hepatol. 2013, 25, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Ishizawa, T.; Mise, Y.; Aoki, T.; Hasegawa, K.; Beck, Y.; Sugawara, Y.; Kokudo, N. Surgical technique: New advances for expanding indications and increasing safety in liver resection for HCC. J. Hepatobiliary Pancreat. Sci. 2010, 17, 389–393. [Google Scholar] [CrossRef] [Green Version]
- Anwanwan, D.; Singh, S.K.; Singh, S.; Saikam, V.; Singh, R. Challenges in liver cancer and possible treatment approaches. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188314. [Google Scholar] [CrossRef]
- Li, Y.; Martin, R.C.G. Herbal Medicine and Hepatocellular Carcinoma: Applications and Challenges. Evid.-Based Complement. Altern. Med. 2011, 2011, 541209. [Google Scholar] [CrossRef] [Green Version]
- Rojas Rojas, T.; Bourdy, G.; Ruiz, E.; Cerapio, J.P.; Pineau, P.; Gardon, J.; Doimi, F.; Deparis, X.; Deharo, E.; Bertani, S. Herbal Medicine Practices of Patients With Liver Cancer in Peru: A Comprehensive Study Toward Integrative Cancer Management. Integr Cancer Ther. Integr. Cancer Ther. 2018, 17, 52–64. [Google Scholar] [CrossRef] [Green Version]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Heberlein, J.; Kirsten, W.H. Natural Product Shows Effectiveness in Combating Colorectal Cancer. Available online: https://ncifrederick.cancer.gov/about/theposter/content/natural-product-shows-effectiveness-combating-colorectal-cancer (accessed on 21 October 2021).
- Chi, V.V. Dictionary of Vietnamese Medicinal Plants; Medical Publishing House: Hanoi, Vietnam, 2012. [Google Scholar]
- Chi, V.V. Medicinal plants in An Giang province; The An Giang Committee of Science and Technology: An Giang, Vietnam, 1991. [Google Scholar]
- Bich, D.H.; Chung, D.Q.; Chuong, B.X.; Dong, N.T.; Dam, D.T.; Hien, P.V.; Lo, V.N.; Mai, P.D.; Man, P.K.; Nhu, D.T.; et al. Plants and Animals Used as Medicine in Vietnam; Science and Technics Publishing House: Hanoi, Vietnam, 2004; Volume 1. [Google Scholar]
- Loi, D.T. Vietnamese Medicinal Plants and Remedies; Medical Publshing House: Hanoi, Vietnam, 2004. [Google Scholar]
- Nothias, L.-F.; Nothias-Esposito, M.; da Silva, R.; Wang, M.; Protsyuk, I.; Zhang, Z.; Sarvepalli, A.; Leyssen, P.; Touboul, D.; Costa, J.; et al. Bioactivity-Based Molecular Networking for the Discovery of Drug Leads in Natural Product Bioassay-Guided Fractionation. J. Nat. Prod. 2018, 81, 758–767. [Google Scholar] [CrossRef] [Green Version]
- Kuo, C.-Y.; Weng, T.-S.; Kumar, K.J.S.; Tseng, Y.-H.; Tung, T.-W.; Wang, S.-Y.; Wang, H.-C. Ethanol Extracts of Dietary Herb, Alpinia nantoensis, Exhibit Anticancer Potential in Human Breast Cancer Cells. Integr Cancer Ther. Integr. Cancer. Ther. 2019, 18, 1534735419866924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Christ, B.; Müller, K. Zur serienmäßigen Bestimmung des Gehaltes an Flavonol-Derivaten in Drogen. Archiv der Pharmazie 1960, 293, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Pękal, A.; Pyrzynska, K. Evaluation of aluminium complexation reaction for flavonoid content assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef] [Green Version]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Li, C.-H.; Yen, C.-H.; Chen, Y.-F.; Lee, K.-J.; Fang, C.-C.; Zhang, X.; Lai, C.-C.; Huang, S.-F.; Lin, H.-K.; Arthur Chen, Y.-M. Characterization of the GNMT-HectH9-PREX2 tripartite relationship in the pathogenesis of hepatocellular carcinoma. Int. J. Cancer 2017, 140, 2284–2297. [Google Scholar] [CrossRef] [PubMed]
- Hematpoor, A.; Paydar, M.; Liew, S.Y.; Sivasothy, Y.; Mohebali, N.; Looi, C.Y.; Wong, W.F.; Azirun, M.S.; Awang, K. Phenylpropanoids isolated from Piper sarmentosum Roxb. induce apoptosis in breast cancer cells through reactive oxygen species and mitochondrial-dependent pathways. Chem. Biol. Interact. 2018, 279, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Sakilan, J.; Demayo, C.; Opanasopit, P. Phytochemical analysis and determination of antimicrobial, antioxidant and anticancer activity of the leaf ethanolic extracts of Piper Sarmentosum Roxb in Lapuyan Zamboanga Del Sur, Philippines. Int. J. Pharm. Sci. Res. 2019, 10, 5715–5722. [Google Scholar] [CrossRef]
- Abebe, W. Traditional pharmaceutical practice in gondar region, northwestern Ethiopia. J. Ethnopharmacol. 1984, 11, 33–47. [Google Scholar] [CrossRef]
- Siew, Y.-Y.; Yew, H.-C.; Neo, S.-Y.; Seow, S.-V.; Lew, S.-M.; Lim, S.-W.; Lim, C.S.E.-S.; Ng, Y.-C.; Seetoh, W.-G.; Ali, A. Evaluation of anti-proliferative activity of medicinal plants used in Asian Traditional Medicine to treat cancer. J. Ethnopharmacol. 2019, 235, 75–87. [Google Scholar] [CrossRef]
- Sikha, A.; Harini, A. Pharmacological activities of wild turmeric (Curcuma aromatica Salisb): A review. J. Pharmacogn. Phytochem. 2015, 3, 01–04. [Google Scholar]
- Liu, B.; Gao, Y.-Q.; Wang, X.-M.; Wang, Y.-C.; Fu, L.-Q. Germacrone inhibits the proliferation of glioma cells by promoting apoptosis and inducing cell cycle arrest. Mol. Med. Rep. 2014, 10, 1046–1050. [Google Scholar] [CrossRef] [PubMed]
- Booker, A.; Frommenwiler, D.; Johnston, D.; Umealajekwu, C.; Reich, E.; Heinrich, M. Chemical variability along the value chains of turmeric (Curcuma longa): A comparison of nuclear magnetic resonance spectroscopy and high performance thin layer chromatography. J. Ethnopharmacol. 2014, 152, 292–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, L.H.; Döpke, W.; Wagner, J.; Mügge, C. Alkaloids from Crinum amabile. Phytochemistry 1998, 48, 371–376. [Google Scholar] [CrossRef]
- Fennell, C.; Van Staden, J. Crinum species in traditional and modern medicine. J. Ethnopharmacol. 2001, 78, 15–26. [Google Scholar] [CrossRef]
- Sidahmed, H.M.A.; Hashim, N.M.; Abdulla, M.A.; Ali, H.M.; Mohan, S.; Abdelwahab, S.I.; Taha, M.M.E.; Fai, L.M.; Vadivelu, J. Antisecretory, gastroprotective, antioxidant and anti-Helicobcter pylori activity of zerumbone from Zingiber zerumbet (L.) Smith. PLoS ONE 2015, 10, e0121060. [Google Scholar] [CrossRef] [Green Version]
- Jaidka, M.; Kaur, R.; Sepat, S. Scientific cultivation of ginger (Zingiber officinalis). In Advances in Vegetable Agronomy; Director Indian Agricultural Research Institute: New Delhi, India, 2018. [Google Scholar]
- Koga, A.Y.; Beltrame, F.L.; Pereira, A.V. Several aspects of Zingiber zerumbet: A review. Rev. Bras. Farmacogn. 2016, 26, 385–391. [Google Scholar] [CrossRef]
- Hartwell, J.L. Plants used against cancer. A survey. Lloydia 1969, 32, 78–107. [Google Scholar]
- Zhang, K.; Gao, Y.; Zhong, M.; Xu, Y.; Li, J.; Chen, Y.; Duan, X.; Zhu, H. Hepatoprotective effects of Dicliptera chinensis polysaccharides on dimethylnitrosamine-induced hepatic fibrosis rats and its underlying mechanism. J. Ethnopharmacol. 2016, 179, 38–44. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, Q.; Gao, Y.; Cao, H.; Lian, Y.; Li, Z.; Xu, J.; Zhong, M.; Li, J.; Wei, R. Polysaccharides from Dicliptera chinensis ameliorate liver disturbance by regulating TLR-4/NF-κB and AMPK/Nrf2 signalling pathways. J. Cell. Mol. Med. 2020, 24, 6397–6409. [Google Scholar] [CrossRef] [Green Version]
- Saikia, N.; Nath, S. Ethnomedicinal observations of some species of the genus Curcuma Linn. growing in Assam. J. Econ. Taxon. Bot. 2003, 27, 430–433. [Google Scholar]
- Lourembam, R.M.; Yadav, A.S.; Kundu, G.C.; Mazumder, P.B. Curcuma Zedoaria (Christm.) Roscoe inhibits proliferation of MDA-MB231 cells via caspase-cascade apoptosis. Orient. Pharm. Exp. Med. 2019, 19, 235–241. [Google Scholar] [CrossRef]
- Ang, H.; Ikeda, S.; Gan, E. Evaluation of the potency activity of aphrodisiac in Eurycoma longifolia Jack. Phytother. Res. 2001, 15, 435–436. [Google Scholar] [CrossRef]
- Jamal, J.A. Malay traditional medicine: An overview of scientific and technological advancement. Tech. Monit. 2006, 1, 37–49. [Google Scholar]
- Jiwajinda, S.; Santisopasri, V.; Murakami, A.; Kawanaka, M.; Kawanaka, H.; Gasquet, M.; Eilas, R.; Balansard, G.; Ohigashi, H. In vitro anti-tumor promoting and anti-parasitic activities of the quassinoids from Eurycoma longifolia, a medicinal plant in Southeast Asia. J. Ethnopharmacol. 2002, 82, 55–58. [Google Scholar] [CrossRef]
- Bhat, R.; Karim, A. Tongkat Ali (Eurycoma longifolia Jack): A review on its ethnobotany and pharmacological importance. Fitoterapia 2010, 81, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Siemonsma, J.; Piluek, K. Plant Resources of South-East Asia (PROSEA) No. 8: Vegetables. Bogor: Prosea Found. 1994. Available online: https://agris.fao.org/agris-search/search.do?recordID=XF2015019284 (accessed on 22 June 2021).
- Lu, Y.; Luo, J.; Huang, X.; Kong, L. Four new steroidal glycosides from Solanum torvum and their cytotoxic activities. Steroids 2009, 74, 95–101. [Google Scholar] [CrossRef]
- Ban, N.T. Checklist of Plant Species of Vietnam; Agriculture Publishing House: Hanoi, Vietnam, 2003; Volume 2. [Google Scholar]
- Phongpaichit, S.; Nikom, J.; Rungjindamai, N.; Sakayaroj, J.; Hutadilok-Towatana, N.; Rukachaisirikul, V.; Kirtikara, K. Biological activities of extracts from endophytic fungi isolated from Garcinia plants. FEMS Microbiol. Immunol. 2007, 51, 517–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Permanasari, A.A.; Wahyuni, T.S.; Adianti, M.; Hafid, A.F.; Widyawaruyanti, A. Anti-hepatocellular Carcinoma Activity Using Huh7it Cells of Several Plants From East Kalimantan, Indonesia. In Proceedings of the AFPS, Bali, Indonesia, 23–27 October 2019. [Google Scholar]
- Kingston, D.G.I.; Rao, M.M.; Zucker, W.V. Plant Anticancer Agents. IX. Constituents of Hyptis tomentosa. J. Nat. Prod. 1979, 42, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Gurunagarajan, S.; Pemaiah, B. Comparative studies on cytotoxic effect of Hyptis suaveolens Poit. and Leonotis nepeatefolia R. Br. against EAC cell lines. J. Pharm. Res. 2011, 4, 1222–1224. [Google Scholar]
- Babalola, O.O.; Ojo, O.E.; Oloyede, F.A. Hepatoprotective activity of aqueous extract of the leaves of Hyptis suaveolens (L.) Poit on acetaminophen Induced hepatotoxicity in rabbits. Res. J. Chem. Sci. 2011, 1, 85–88. [Google Scholar]
- Alok, S.; Sanjay, J.; Monika, S.; Alok, M.; Padmini, S.; Prabodh, S. In-vitro evaluation of antioxidant activity of leaves of Hyptis suaveolens (L.) Poit. Int. J. Pharm. Res. 2010, 1, 86–93. [Google Scholar]
- Oscar, S.-A.; Antonio, C.-N.; Marina, G.-V.; Elsa, R.-S.; Gabriel, V.-A. Phytochemical screening, antioxidant activity and in vitro biological evaluation of leave extracts of Hyptis suaveolens (L.) from south of Mexico. S. Afr. J. Bot. 2020, 128, 62–66. [Google Scholar] [CrossRef]
- Balachandran, C.; Emi, N.; Arun, Y.; Yamamoto, Y.; Ahilan, B.; Sangeetha, B.; Duraipandiyan, V.; Inaguma, Y.; Okamoto, A.; Ignacimuthu, S.; et al. In vitro anticancer activity of methyl caffeate isolated from Solanum torvum Swartz. fruit. Chem.-Biol. Interact. 2015, 242, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Waghulde, H.; Kamble, S.; Patankar, P.; Jaiswal, B.; Pattanayak, S.; Bhagat, C.; Mohan, M. Antioxidant activity, phenol and flavonoid contents of seeds of Punica granatum (Punicaceae) and Solanum torvum (Solanaceae). Pharmacologyonline 2011, 1, 193–202. [Google Scholar]
- Arif, M.; Fareed, S. Pharmacognostical studies and evaluation of total phenolic and flavonoid contents of traditionally utilized fruits of Solanum torvum Sw. Indian J. Nat. Prod. Resour. 2011, 2, 218–224. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, H.M.; Nguyen, N.Y.T.; Chau, N.T.N.; Nguyen, A.B.T.; Tran, V.K.T.; Hoang, V.; Le, T.M.; Wang, H.-C.; Yen, C.-H. Bioassay-Guided Discovery of Potential Partial Extracts with Cytotoxic Effects on Liver Cancer Cells from Vietnamese Medicinal Herbs. Processes 2021, 9, 1956. https://doi.org/10.3390/pr9111956
Nguyen HM, Nguyen NYT, Chau NTN, Nguyen ABT, Tran VKT, Hoang V, Le TM, Wang H-C, Yen C-H. Bioassay-Guided Discovery of Potential Partial Extracts with Cytotoxic Effects on Liver Cancer Cells from Vietnamese Medicinal Herbs. Processes. 2021; 9(11):1956. https://doi.org/10.3390/pr9111956
Chicago/Turabian StyleNguyen, Hien Minh, Nhi Yen Thi Nguyen, Nghia Trong Ngoc Chau, Anh Bao Thi Nguyen, Van Kieu Thi Tran, Viet Hoang, Tri Minh Le, Hui-Chun Wang, and Chia-Hung Yen. 2021. "Bioassay-Guided Discovery of Potential Partial Extracts with Cytotoxic Effects on Liver Cancer Cells from Vietnamese Medicinal Herbs" Processes 9, no. 11: 1956. https://doi.org/10.3390/pr9111956





