Immunological Analytical Techniques for Cosmetics Quality Control and Process Monitoring
Abstract
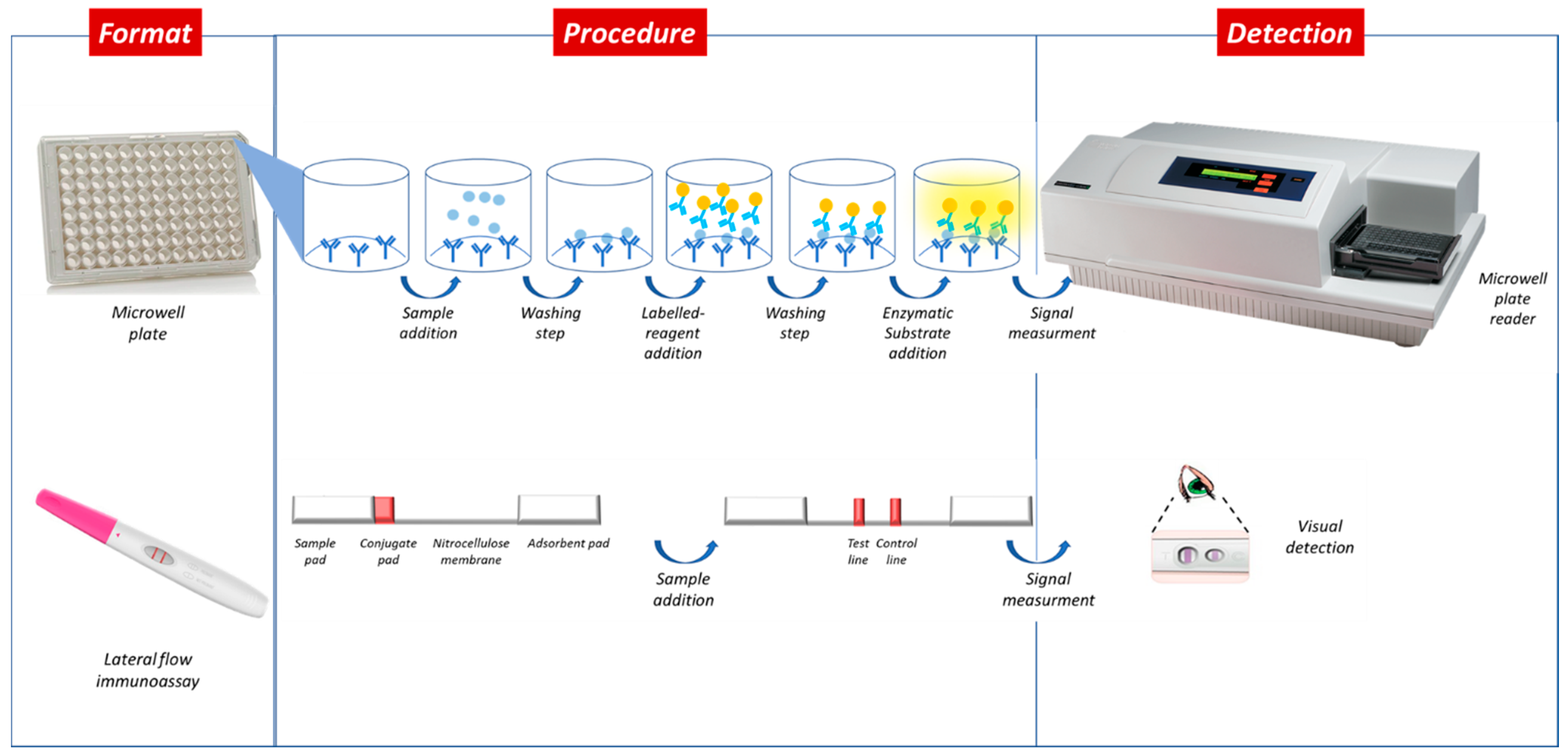
:1. Introduction
2. Detection of Allergens in Cosmetics
3. Detection of Prohibited and Restricted Cosmetic Ingredients
4. Detection of Toxins, Bacteria, and Antibiotics
5. Detection of Marker Indicative for Counterfeiting
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Dorato, S. General concepts: Current legislation on cosmetics in various countries. In Analysis of Cosmetic Products, 2nd ed.; Salvador, A., Chisvert, A., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2018; pp. 3–37. [Google Scholar]
- Mildau, G. General review of official methods of analysis of cosmetics. In Analysis of Cosmetic Products, 2nd ed.; Salvador, A., Chisvert, A., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2018; pp. 67–83. [Google Scholar]
- Mitsui, T. Introduction. In New Cosmetic Science, 1st ed.; Mitsui, T., Ed.; Elsevier Science B.V.: Amsterdam, The Netherlands, 1997; pp. 3–7. [Google Scholar]
- Chiari, B.G.; Almeida, M.G.J.; Corrêa, M.A.; Isaac, V.L.B. Cosmetics’ quality control. In Latest Research into Quality Control, 1st ed.; Akyar, I., Ed.; InTech: Rijeka, Croatia, 2012; pp. 337–364. [Google Scholar]
- Celeiro, M.; Garcia-Jares, C.; Llompart, M.; Lores, M. Recent Advances in Sample Preparation for Cosmetics and Personal Care Products Analysis. Molecules 2021, 26, 4900. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Li, G.; Xu, X.; Zhu, L.; Huang, R.; Chen, X. Application of nano-ELISA in food analysis: Recent advances and challenges. TrAC Trends Anal. Chem. 2019, 113, 140–156. [Google Scholar] [CrossRef]
- Sengupta, P.; Wang, C.W.; Ma, Z.F. Enzyme-Linked Immunosorbent Assay (ELISA) Technique for Food Analysis. In Techniques to Measure Food Safety and Quality, 1st ed.; Khan, M.S., Rahman, M.S., Eds.; Springer: Cham, Switzerland, 2021; pp. 91–115. [Google Scholar]
- Calabretta, M.M.; Zangheri, M.; Calabria, D.; Lopreside, A.; Montali, L.; Marchegiani, E.; Trozzi, I.; Guardigli, M.; Mirasoli, M.; Michelini, E. Paper-Based Immunosensors with Bio-Chemiluminescence Detection. Sensors 2021, 21, 4309. [Google Scholar] [CrossRef]
- Calabria, D.; Calabretta, M.M.; Zangheri, M.; Marchegiani, E.; Trozzi, I.; Guardigli, M.; Michelini, E.; Di Nardo, F.; Anfossi, L.; Baggiani, C.; et al. Recent Advancements in Enzyme-Based Lateral Flow Immunoassays. Sensors 2021, 21, 3358. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, M.; Kong, N.; Liu, J. Lab-on-paper micro-and nano-analytical devices: Fabrication, modification, detection and emerging applications. Microchim. Acta 2016, 183, 1521–1542. [Google Scholar] [CrossRef]
- Allergens in Cosmetics. Available online: https://www.fda.gov/cosmetics/cosmetic-ingredients/allergens-cosmetics (accessed on 12 August 2021).
- Rey, A.; Corbi, E.; Pérès, C.; David, N. Determination of suspected fragrance allergens extended list by two-dimensional gas chromatography–mass spectrometry in ready-to-inject samples. J. Chromatogr. A 2015, 1404, 95–103. [Google Scholar] [CrossRef]
- Tranchida, P.Q.; Maimone, M.; Franchina, F.A.; Bjerk, T.R.; Zini, C.A.; Purcaro, G.; Mondello, L. Four-stage (low-) flow modulation comprehensive gas chromatography quadrupole mass spectrometry for the determination of recently-highlighted cosmetic allergens. J. Chromatogr. A 2016, 1439, 144–151. [Google Scholar] [CrossRef]
- Devos, C.; Ochiai, N.; Sasamoto, K.; Sandra, P.; David, F. Full evaporation dynamic headspace in combination with selectable one-dimensional/two-dimensional gas chromatography–mass spectrometry for the determination of suspected fragrance allergens in cosmetic products. J. Chromatogr. A 2012, 1255, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Cordero, C.; Rubiolo, P.; Reichenbach, S.E.; Carretta, A.; Cobelli, L.; Giardina, M.; Bicchi, C. Method translation and full metadata transfer from thermal to differential flow modulated comprehensive two dimensional gas chromatography: Profiling of suspected fragrance allergens. J. Chromatogr. A 2017, 1480, 70–82. [Google Scholar] [CrossRef]
- Celeiro, M.; Lamas, J.P.; Vila, M.; Garcia-Jares, C.; Homem, V.; Ratola, N.; Llompart, M. Determination of multiclass personal care products in continental waters by solid-phase microextraction followed by gas chromatography-tandem mass spectrometry. J. Chromatogr. A 2019, 1607, 460398. [Google Scholar] [CrossRef]
- Desmedt, B.; Canfyn, M.; Pype, M.; Baudewyns, S.; Hanot, V.; Courselle, P.; Deconinck, E. HS–GC–MS method for the analysis of fragrance allergens in complex cosmetic matrices. Talanta 2015, 131, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Outeiral, J.; Elcoroaristizabal, S.; Amigo, J.M.; Vidal, M. Development and validation of a method for the determination of regulated fragrance allergens by High-Performance Liquid Chromatography and parallel factor analysis 2. J. Chromatogr. A 2017, 1526, 82–92. [Google Scholar] [CrossRef]
- Food Allergens Reliable Analytical Solutions for Your Allergen Management. Available online: https://food.r-biopharm.com/analytes/food-allergens/ (accessed on 12 August 2021).
- Ross, G.M.; Bremer, M.G.; Nielen, M.W. Consumer-friendly food allergen detection: Moving towards smartphone-based immunoassays. Anal. Bioanal. Chem. 2018, 410, 5353–5371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, G.; Salentijn, G.I.; Nielen, M.W. A critical comparison between flow-through and lateral flow immunoassay formats for visual and smartphone-based multiplex allergen detection. Biosensors 2019, 9, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fast and Reliable Test Kits for Food Allergen Detection. Available online: https://www.romerlabs.com/en/products/test-kits/food-allergen-test-kits/ (accessed on 12 August 2021).
- 3M™ Allergen Protein ELISA Kit. Available online: https://www.3m.com/3M/en_US/p/d/b5005041032/ (accessed on 12 August 2021).
- AlerTox ELISA. Available online: https://www.hygiena.com/food-safety-solutions/allergen-detection/alertox-elisa/ (accessed on 12 August 2021).
- Duffort, O.A.; Polo, F.; Lombardero, M.; Díaz-Perales, A.; Sánchez-Monge, R.; García-Casado, G.; Barber, D. Immunoassay to quantify the major peach allergen Pru p 3 in foodstuffs. Differential allergen release and stability under physiological conditions. J. Agric. Food Chem. 2002, 50, 7738–7741. [Google Scholar] [CrossRef]
- Ashley, J.; D’Aurelio, R.; Piekarska, M.; Temblay, J.; Pleasants, M.; Trinh, L.; Tothill, I.E. Development of a β-Lactoglobulin sensor based on SPR for milk allergens detection. Biosensors 2018, 8, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sollid, L.M. Coeliac disease: Dissecting a complex inflammatory disorder. Nat. Rev. Immunol. 2002, 2, 647–655. [Google Scholar] [CrossRef]
- Battais, F.; Richard, C.; Jacquenet, S.; Denery-Papini, S.; Moneret-Vautrin, D.A. Wheat Grain Allergies: An Update on Wheat Allergens. Eur. Ann. Allergy Clin. Immunol. 2008, 40, 67–76. [Google Scholar]
- Battais, F.; Courcoux, P.; Popineau, Y.; Kanny, G.; Moneret-Vautrin, D.-A.; Denery-Papini, S. Food Allergy to Wheat: Differences in Immunoglobulin E-Binding Proteins as a Function of Age or Symptoms. J. Cereal Sci. 2005, 42, 109–117. [Google Scholar] [CrossRef]
- Mäki, M.; Collin, P. Coeliac disease. The lancet 1997, 349(9067), 1755–1759. [Google Scholar] [CrossRef]
- Sharma, G.M.; Rallabhandi, P.; Williams, K.M.; Herrmann, M.; Sadrieh, N. Gluten quantitation in cosmetic products by enzyme-linked immunosorbent assay. J. AOAC Int. 2016, 99, 586–590. [Google Scholar] [CrossRef]
- Tranquet, O.; Lupi, R.; Echasserieau-Laporte, V.; Pietri, M.; Larré, C.; Denery-Papini, S. Characterization of antibodies and development of an indirect competitive immunoassay for detection of deamidated gluten. J. Agric. Food Chem. 2015, 63, 5403–5409. [Google Scholar] [CrossRef]
- Available online: https://ec.europa.eu/growth/sectors/cosmetics/legislation_en (accessed on 12 August 2021).
- Zhang, Y.; Xie, S.S.; Wu, Y.C.; Liang, Q.W.; Xie, X.T.; Luo, Z.H.; Liu, M.S. Research progress of detection technology for illegal addition of prohibited substances in cosmetics. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; Volume 559, p. 012030. [Google Scholar]
- Denver, N.; Khan, S.; Homer, N.Z.; MacLean, M.R.; Andrew, R. Current strategies for quantification of estrogens in clinical research. J. Steroid Biochem. Mol. Biol. 2019, 192, 105373. [Google Scholar] [CrossRef]
- Tian, W.; Wang, L.; Lei, H.; Sun, Y.; Xiao, Z. Antibody production and application for immunoassay development of environmental hormones: A review. Chem. Biol. Technol. Agric. 2018, 5, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Liu, A.; Anfossi, L.; Shen, L.; Li, C.; Wang, X. Non-competitive immunoassay for low-molecular-weight contaminant detection in food, feed and agricultural products: A mini-review. Trends Food Sci. Technol. 2018, 71, 181–187. [Google Scholar] [CrossRef]
- Wang, M.; Guo, L.; Yu, M.; Zhao, H. The application of a lateral flow immunographic assay to rapidly test for dexamethasone in commercial facial masks. Anal. Bioanal. Chem. 2019, 411, 5703–5710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safety and Technical Standard for Cosmetics. Available online: http://www.sesec.eu/app/uploads/2016/02/Cosmetics-Safety-and-Technical-Standards-2015-Version-Foreword-and-summary.pdf (accessed on 10 October 2021).
- UNION, P. Regulation (EC) No 1223/2009 of the European parliament and of the council. Off. J. Eur. Union L 2009, 342, 59. [Google Scholar]
- Zhang, S.; Yao, T.; Wang, S.; Feng, R.; Chen, L.; Zhu, V.; Yang, G. Upconversion luminescence nanoparticles-based immunochromatographic assay for quantitative detection of triamcinolone acetonide in cosmetics. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 214, 302–308. [Google Scholar] [CrossRef]
- Yu, H.Y.; Liao, H.M. Triamcinolone permeation from different liposome formulations through rat skin in vitro. Int. J. Pharm. 1996, 127, 1–7. [Google Scholar] [CrossRef]
- Matysova, L.; Hajkova, R.; Šícha, J.; Solich, P. Determination of methylparaben, propylparaben, triamcinolone acetonide and its degradation product in a topical cream by RP-HPLC. Anal. Bioanal. Chem. 2003, 376, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Liang, X.; Wu, X.; He, X.; Kuang, Y.; Hu, X. Screening and quantification of 18 glucocorticoid adulterants from herbal pharmaceuticals and health foods by HPLC and confirmed by LC-Q-TOF-MS/MS. Food Addit. Contam. Part A 2018, 35, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Barré, F.P.; Flinders, B.; Garcia, J.P.; Jansen, I.; Huizing, L.R.; Porta, T.; Cillero-Pastor, B. Derivatization strategies for the detection of triamcinolone acetonide in cartilage by using matrix-assisted laser desorption/ionization mass spectrometry imaging. Anal. Chem. 2016, 88, 12051–12059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, Y.S.; Kwon, I.K.; Lee, Y.; Lee, K.B. Quantitative monitoring of corticosteroids in cosmetic products manufactured in Korea using LC–MS/MS. Forensic Sci. Int. 2012, 220, e23–e28. [Google Scholar] [CrossRef]
- Malone, E.M.; Elliott, C.T.; Kennedy, D.G.; Regan, L. Screening and quantitative confirmatory method for the analysis of glucocorticoids in bovine milk using liquid chromatography-tandem mass spectrometry. J. AOAC Int. 2010, 93, 1656–1665. [Google Scholar] [CrossRef] [Green Version]
- Cui, X.; Wu, P.; Lai, D.; Zheng, S.; Chen, Y.; Eremin, S.A.; Zhao, S. Development of a highly specific fluorescence immunoassay for detection of diisobutyl phthalate in edible oil samples. J. Agric. Food Chem. 2015, 63, 9372–9378. [Google Scholar] [CrossRef]
- Zhang, M.; Yu, X.; Wang, Y.; Hu, Y.; Liu, S. A highly sensitive indirect competitive enzyme-linked immunosorbent assay (ic-ELISA) by antigen coating for diethyl phthalate analysis in foods. Food Anal. Methods 2013, 6, 1223–1228. [Google Scholar] [CrossRef]
- Tang, M.; Wu, Y.; Deng, D.; Wei, J.; Zhang, J.; Yang, D.; Li, G. Development of an optical fiber immunosensor for the rapid and sensitive detection of phthalate esters. Sens. Actuators B Chem. 2018, 258, 304–312. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, S.; Zhuang, H.; Hu, Y. Determination of dimethyl phthalate in environment water samples by a highly sensitive indirect competitive ELISA. Appl. Biochem. Biotechnol. 2012, 166, 436–445. [Google Scholar] [CrossRef]
- Chen, Y.; He, Q.; Shen, D.; Jiang, Z.; Eremin, S.A.; Zhao, S. Fluorescence polarization immunoassay based on a new monoclonal antibody for the detection of the Diisobutyl phthalate in Yoghurt. Food Control 2019, 105, 38–44. [Google Scholar] [CrossRef]
- He, F.; Tian, Y.; Xu, Z.; Luo, L.; Yang, J.; Wang, H.; Shen, Y. Development of an immunochromatographic assay as a screen for detection of total phthalate acid esters in cooking oil. J. Toxicol. Environ. Health 2019, 81, 80–88. [Google Scholar] [CrossRef]
- Zhang, M.C.; Wang, Q.E.; Zhuang, H.S. A novel competitive fluorescence immunoassay for the determination of dibutyl phthalate. Anal. Bioanal. Chem. 2006, 386, 1401–1406. [Google Scholar] [CrossRef]
- Zhang, M.; Cong, Y.; Sheng, Y.; Liu, B. A direct competitive enzyme-linked immunosorbent assay by antibody coated for diethyl phthalate analysis. Anal. Biochem. 2010, 406, 24–28. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, Y.; Liu, S.; Cong, Y.; Liu, B.; Wang, L. Rapid monitoring of dipropyl phthalate in food samples using a chemiluminescent enzyme immunoassay. Food Anal. Methods 2012, 5, 1105–1113. [Google Scholar] [CrossRef]
- Gimeno, P.; Maggio, A.F.; Bousquet, C.; Quoirez, A.; Civade, C.; Bonnet, P.A. Analytical method for the identification and assay of 12 phthalates in cosmetic products: Application of the ISO 12787 international standard “Cosmetics–Analytical methods–Validation criteria for analytical results using chromatographic techniques”. J. Chromatogr. A 2012, 1253, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, Z.; Zhao, S.; Duan, J.; Tao, H.; Wang, W.; Liu, S. Determination of total phthalate in cosmetics using a simple three-phase sample preparation method. Anal. Bioanal. Chem. 2018, 410, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Ding, S.; You, H.; Zhang, Y.; Wang, Y.; Yang, X.; Yuan, J. An immunoassay for dibutyl phthalate based on direct hapten linkage to the polystyrene surface of microtiter plates. PLoS ONE 2011, 6, e29196. [Google Scholar] [CrossRef]
- Lv, B.; Jiang, Q.; Zhu, C. A review of heavy metals immunoassay detection. Adv. J. Food Sci. Technol. 2015, 8, 559–565. [Google Scholar] [CrossRef]
- Su, S.P.; Xu, F.; Cao, H.; Zhai, M.S.; Yu, J.S. Perspective in the rapid methods for the detection of heavy metals. Appl. Chem. Ind. 2013, 42, 355–359. [Google Scholar]
- Verma, N.; Singh, M. Biosensors for heavy metals. Biometals 2005, 18, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Khosraviani, M.; Pavlov, A.R.; Flowers, G.C.; Blake, D.A. Detection of heavy metals by immunoassay: Optimization and validation of a rapid, portable assay for ionic cadmium. Environ. Sci. Technol. 1998, 32, 137–142. [Google Scholar] [CrossRef]
- Chakrabarti, P.; Hatcher, F.M.; Blake, R.C.; Ladd, P.A.; Blake, D.A. Enzyme immunoassay to determine heavy metals using antibodies to specific metal-EDTA complexes: Optimization and validation of an immunoassay for soluble indium. Anal. Biochem. 1994, 217, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, H.; Shu, Q.; Wang, W.; Wang, Z.; Yang, S.; Wang, L.; Fu, Z. An ultra-facile and label-free immunoassay strategy for detection of copper (II) utilizing chemiluminescence self-enhancement of Cu (II)-ethylenediaminetetraacetate chelate. Biosens. Bioelectron. 2016, 85, 157–163. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, H.; Pschenitza, M.; Niessner, R.; Li, Y.; Knopp, D.; Deng, A. Highly sensitive and specific determination of mercury (II) ion in water, food and cosmetic samples with an ELISA based on a novel monoclonal antibody. Anal. Bioanal. Chem. 2012, 403, 2519–2528. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, M.; Yao, X.; Deng, A.; Li, J. Highly sensitive electroluminescence immunoassay for Hg (II) ions based on the use of CdSe quantum dots, the methylmercury-6-mercaptonicotinic acid-ovalbumin conjugate, and a specific monoclonal antibody. Microchim. Acta 2015, 182, 469–477. [Google Scholar] [CrossRef]
- Michalek, I.M.; John, S.M.; Caetano dos Santos, F.L. Microbiological contamination of cosmetic products–observations from Europe, 2005–2018. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 2151–2157. [Google Scholar] [CrossRef]
- Becks, V.; Lorenzoni, N. Pseudomonas aeruginosa outbreak in a neonatal intensive care unit: A possible link to contaminated hand lotion. Am. J. Infect. Control 1995, 23, 396–398. [Google Scholar] [CrossRef]
- Behravan, J.; Bazzaz, F.; Malaekeh, P. Survey of bacteriological contamination of cosmetic creams in Iran (2000). Int. J. Dermatol. 2005, 44, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Campana, R.; Scesa, C.; Patrone, V.; Vittoria, E.; Baffone, W. Microbiological study of cosmetic products during their use by consumers: Health risk and efficacy of preservative systems. Lett. Appl. Microbiol. 2006, 43, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Moore, I. Manufacturing Cosmetic Ingredients according to Good Manufacturing Practice Principles. In Global Regulatory Issues for the Cosmetics Industry; William Andrew Publishing: Norwich, NY, USA, 2009; pp. 79–92. [Google Scholar]
- US Pharmacopeial Convention. USP: National Formulary; USP: Rockville, MD, USA, 2006. [Google Scholar]
- European Pharmacopeia Secretariat. European Pharmacopeia; European Pharmacopeia Secretariat: Strasbourg, France, 1998. [Google Scholar]
- Okeke, I.N.; Lamikanra, A. Bacteriological quality of skin-moisturizing creams and lotions distributed in a tropical developing country. J. Appl. Bacteriol. 2001, 91, 922–928. [Google Scholar] [CrossRef]
- Alamer, S.; Eissa, S.; Chinnappan, R.; Herron, P.; Zourob, M. Rapid colorimetric lactoferrin-based sandwich immunoassay on cotton swabs for the detection of foodborne pathogenic bacteria. Talanta 2018, 185, 275–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, N.; Song, Y.; Zeinhom, M.M.; Chang, Y.C.; Sheng, L.; Li, H.; Lin, Y. Nanozyme-mediated dual immunoassay integrated with smartphone for use in simultaneous detection of pathogens. ACS Appl. Mater. Interfaces 2017, 9, 40671–40680. [Google Scholar] [CrossRef]
- Xue, L.; Huang, F.; Hao, L.; Cai, G.; Zheng, L.; Li, Y.; Lin, J. A sensitive immunoassay for simultaneous detection of foodborne pathogens using MnO2 nanoflowers-assisted loading and release of quantum dots. Food Chem. 2020, 322, 126719. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, X.; Zhang, Y.; Wang, R.; Ji, Y.; Sun, J.; Wang, J. Functional nanozyme mediated multi-readout and label-free lateral flow immunoassay for rapid detection of Escherichia coli O157: H7. Food Chem. 2020, 329, 127224. [Google Scholar] [CrossRef]
- English, D.; Scalici, C.; Hamilton, J.; Destro, C.; Jimenez, L. Evaluation of the TECRA™ visual immunoassay for detecting Staphylococcus aureus in cosmetic/pharmaceutical raw materials and finished products. J. Rapid Methods Autom. Microbiol. 1999, 7, 193–203. [Google Scholar] [CrossRef]
- Xie, W.; Chen, C.; Huang, Y.; Fu, H. Determination of chloramphenicol in cosmetics by GC-MS. Chin. J. Chromatogr. 2006, 24, 659. [Google Scholar]
- Wang, P.; Li, J.; Zheng, H. Simultaneous determination of seven sulfonamides and metronidazole and chloramphenicol in cosmetics by high performance liquid chromatography. Chin. J. Chromatogr. 2007, 25, 743–746. [Google Scholar]
- Aiassa, V.; Zoppi, A.; Becerra, M.C.; Albesa, I.; Longhi, M.R. Enhanced inhibition of bacterial biofilm formation and reduced leukocyte toxicity by chloramphenicol: β-cyclodextrin: N-acetylcysteine complex. Carbohydr. Polym. 2016, 152, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Lofrano, G.; Libralato, G.; Adinolfi, R.; Siciliano, A.; Iannece, P.; Guida, M.; Carotenuto, M. Photocatalytic degradation of the antibiotic chloramphenicol and effluent toxicity effects. Ecotoxicol. Environ. Saf. 2016, 123, 65–71. [Google Scholar] [CrossRef]
- US FDA. Code of Federal Regulations. 556.283 Florfenicol; US Food and Drug Administration: Washington, DC, USA, 2002.
- European Union. Commission Regulation (EU) No 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. Off. J. Eur. Union 2010, L15, 1–32. [Google Scholar]
- The Japan Food Chemical Research Foundation. Japan Positive List System. Available online: https://www.ffcr.or.jp/en/zanryu/the-japanese-positive/the-japanese-positive-list-system-for-agricultural-chemical-residues-in-foods-enforcement-on-may-29-.html (accessed on 12 August 2021).
- Ministry of Agriculture. No. 235 Bulletin of the Ministry of Agriculture of the People’s Republic of China; Ministry of Agriculture: Pechino, China, 2002.
- Vosough, M.; Esfahani, H.M. Fast HPLC-DAD quantification procedure for selected sulfonamids, metronidazole and chloramphenicol in wastewaters using second-order calibration based on MCR-ALS. Talanta 2013, 113, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Rimkus, G.G.; Hoffmann, D. Enantioselective analysis of chloramphenicol residues in honey samples by chiral LC-MS/MS and results of a honey survey. Food Addit. Contam. Part A 2017, 34, 950–961. [Google Scholar] [CrossRef] [PubMed]
- Fedeniuk, R.W.; Mizuno, M.; Neiser, C.; O’Byrne, C. Development of LC–MS/MS methodology for the detection/determination and confirmation of chloramphenicol, chloramphenicol 3-O-β-d-glucuronide, florfenicol, florfenicol amine and thiamphenicol residues in bovine, equine and porcine liver. J. Chromatogr. B 2015, 991, 68–78. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, R.; Li, J.; Wang, X.; Xu, L.; Li, Y.; Li, P. Highly Specific Chemiluminescence Immunoassay for the Determination of Chloramphenicol in Cosmetics. Int. J. Anal. Chem. 2019, 2019, 7131907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xun, Y.P.; Yan, H.; Liu, J.; Shi, L.L.; Chen, P.; Du, H.W. Development of a high-throughput Immunoassay for rapid detection of multiple antibiotic residues in cosmetics. In Advanced Materials Research; Tang, Y., Xu, Q., Min, Y., Eds.; Trans Tech Publications Ltd.: Bach, Switzerland, 2015; Volume 1073, pp. 357–361. [Google Scholar]
- Zangheri, M.; Di Nardo, F.; Calabria, D.; Marchegiani, E.; Anfossi, L.; Guardigli, M.; Roda, A. Smartphone biosensor for point-of-need chemiluminescence detection of ochratoxin A in wine and coffee. Anal. Chim. Acta 2021, 1163, 338515. [Google Scholar] [CrossRef]
- Le Loir, Y.; Baron, F.; Gautier, M. [i] Staphylococcus aureus [/i] and food poisoning. Genet. Mol. Res. GMR 2003, 2, 63–76. [Google Scholar]
- Cheng, H.P.; Chuang, H.S. Rapid and Sensitive Nano-Immunosensors for Botulinum. ACS Sens. 2019, 4, 1754–1760. [Google Scholar] [CrossRef]
- Dayan-Kenigsberg, J.; Bertocchi, A.; Garber, E.A. Rapid detection of ricin in cosmetics and elimination of artifacts associated with wheat lectin. J. Immunol. Methods 2008, 336, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Sasikumar, B.; Swetha, V.P.; Parvathy, V.A.; Sheeja, T.E. Advances in adulteration and authenticity testing of herbs and spices. In Advances in Food Authenticity Testing; Downey, G., Ed.; Woodhead Publishing: Sawston, UK, 2016; pp. 585–624. [Google Scholar]
- Mandli, J.; Fatimi, I.E.; Seddaoui, N.; Amine, A. Enzyme immunoassay (ELISA/immunosensor) for a sensitive detection of pork adulteration in meat. Food Chem. 2018, 255, 380–389. [Google Scholar] [CrossRef]
- Chávez, N.A.; Salinas, E.; Jauregui, J.; Palomares, L.A.; Macias, K. Detection of bovine milk adulterated with cheese whey by western blot immunoassay. Food Agric. Immunol. 2008, 19, 265–272. [Google Scholar] [CrossRef]
- Sharma, R.; Verma, A.; Shinde, N.; Mann, B.; Gandhi, K.; Wichers, J.H.; van Amerongen, A. Adulteration of cow’s milk with buffalo’s milk detected by an on-site carbon nanoparticles-based lateral flow immunoassay. Food Chem. 2021, 351, 129311. [Google Scholar] [CrossRef]
- Pizzo, J.S.; Galuch, M.B.; Santos, P.D.; Santos, O.O.; Visentainer, L.; Eberlin, M.N.; Visentainer, J.V. Assessment of adulteration of cosmetics based on vegetable oils by GC-FID and lipid profile using direct infusion electrospray ionization mass spectrometry (ESI-MS). J. Braz. Chem. Soc. 2018, 29, 2457–2465. [Google Scholar] [CrossRef]
- Paikrao, H.M.; Shende, V.A. Investigation of adulteration in kumkum. Mater. Today Proc. 2020, 29, 801–806. [Google Scholar] [CrossRef]
- Lee, T.H.; Wani, W.A.; Koay, Y.S.; Kavita, S.; Tan, E.T.T.; Shreaz, S. Recent advances in the identification and authentication methods of edible bird’s nest. Food Res. Int. 2017, 100 Pt 1, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.C.; Kang, K.A.; Lim, C.M.; Park, J.H.; Jung, K.S.; Hyun, J.W. Water extract of edible bird’s nest attenuated the oxidative stress-induced matrix metalloproteinase-1 by regulating the mitogen-activated protein kinase and activator protein-1 pathway in human keratinocytes. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 347–354. [Google Scholar] [CrossRef]
- Vimala, B.; Hussain, H.; Nazaimoon, W.M.W. Effects of edible bird’s nest on tumour necrosis factor-alpha secretion, nitric oxide production and cell viability of lipopolysaccharide-stimulated RAW 264.7 macrophages. Food Agric. Immunol. 2012, 23, 303–314. [Google Scholar] [CrossRef]
- Xie, Y.; Zeng, H.; Huang, Z.; Xu, H.; Fan, Q.; Zhang, Y.; Zheng, B. Effect of maternal administration of edible bird’s nest on the learning and memory abilities of suckling offspring in mice. Neural Plast. 2018, 2018, 7697261. [Google Scholar] [CrossRef] [Green Version]
- Ma, F.; Liu, D. Sketch of the edible bird’s nest and its important bioactivities. Food Res. Int. 2012, 48, 559–567. [Google Scholar] [CrossRef]
- Yang, M.; Cheung, S.H.; Li, S.C.; Cheung, H.Y. Establishment of a holistic and scientific protocol for the authentication and quality assurance of edible bird’s nest. Food Chem. 2014, 151, 271–278. [Google Scholar] [CrossRef]
- Seow, E.K.; Ibrahim, B.; Muhammad, S.A.; Lee, L.H.; Cheng, L.H. Differentiation between house and cave edible bird’s nests by chemometric analysis of amino acid composition data. LWT-Food Sci. Technol. 2016, 65, 428–435. [Google Scholar] [CrossRef]
- Kong, H.K.; Wong, K.H.; Lo, S.C. Identification of peptides released from hot water insoluble fraction of edible bird’s nest under simulated gastro-intestinal conditions. Food Res. Int. 2016, 85, 19–25. [Google Scholar] [CrossRef]
- Guo, L.; Wu, Y.; Liu, M.; Wang, B.; Ge, Y.; Chen, Y. Authentication of edible bird’s nests by TaqMan-based real-time PCR. Food Control 2014, 44, 220–226. [Google Scholar] [CrossRef]
- Quek, M.C.; Chin, N.L.; Tan, S.W.; Yusof, Y.A.; Law, C.L. Molecular identification of species and production origins of edible bird’s nest using FINS and SYBR green I based real-time PCR. Food Control 2018, 84, 118–127. [Google Scholar] [CrossRef]
- Zhang, S.; Lai, X.; Liu, X.; Li, Y.; Li, B.; Huang, X.; Yang, G. Competitive enzyme-linked immunoassay for sialoglycoprotein of edible bird’s nest in food and cosmetics. J. Agric. Food Chem. 2012, 60, 3580–3585. [Google Scholar] [CrossRef]
- Tukiran, N.A.; Ismail, A.; Mustafa, S.; Hamid, M. Enzyme immunoassay for the detection of porcine gelatine in edible bird’s nests. Food Addit. Contam. Part A 2015, 32, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zheng, L.; Xie, Y.; Zeng, H.; Fan, Q.; Zheng, B.; Zhang, Y. Identification and determination of glycoprotein of edible bird’s nest by nanocomposites based lateral flow immunoassay. Food Control 2019, 102, 214–220. [Google Scholar] [CrossRef]




| Target Analyte | Immunological Method | Limit of Detection | Cosmetic Target | Sample Pre-Treatment | Ref |
|---|---|---|---|---|---|
| Peach allergen (Pru p 3) | Non-competitive colorimetric ELISA | 0.1 ng/mL | Shampoo, air-freshener, toothpaste, and soap | Samples were diluted in dilution buffer (PBS containing 1% BSA and 0.1% Tween 20) at a ratio of at least 1:10 to avoid potential effects from the sample matrix | [25] |
| β-lactoglobulin (BLG) | Label-free immunosensor based on surface plasmon resonance (SPR) | 0.16 µg/mL | Controlling implants in final rinse samples of cleaning in-place (CIP) systems of food and cosmetic producers | - | [26] |
| Gluten | Three commercial colorimetric ELISA kits were tested: two non-competitive and one competitive | Non-competitive 5 and 0.3 ppm; competitive ELISA 10 ppm. | Cosmetics in both liquid form (e.g., shampoo, conditioner) and solid form (e.g., blusher, lipstick) | The cosmetics, in liquid form (e.g., shampoo, conditioner), were directly weighed, whereas the solid cosmetics (e.g., blusher, lipstick) were mixed into homogeneous form before weighing. The cosmetic samples were weighed in 50 mL tubes depending on the ELISA kit and solubilized/suspended in extraction buffer. | [31] |
| Deamidated gluten | Competitive indirect colorimetric ELISA | 25 ng/mL | Maize, rice, and soya flour | Soluble proteins were extracted by suspending flour at 100 mg/mL in PBS for 1 h, at room temperature. Under continuous agitation. Supernatants were collected after centrifugation (10 min/2500 g), diluted at 1:20 in PBS containing 0.1% skimmed milk | [32] |
| Dexamethasone | Competitive colloidal-gold LFIA | 100 ng/mL | Facial masks | One gram of each sample was weighed and transferred to a 10-mL tube. The DE was extracted using 5 mL of acetonitrile and saturated salt water (2/3, v/v) as the extraction reagent and ultrasonic waves were applied for 10 min. The extracts were centrifuged at 5000 r/min for 10 min and the supernatant was transferred to a new tube. The supernatant was evaporated to dryness under nitrogen, and the extract was re-dissolved in PBS for analysis by LFIA. | [38] |
| Triamcinolone acetonide | LFIA method for quantifying TCA by exploiting a probe based on up-conversion of luminescence nanoparticles | 20 μg/kg | Cream, mask, and essence | 0.1 g of the sample was diluted 20 times with distilled water. 80 μL of the diluted sample was used in UCNPs-ICA. | [41] |
| Phthalates dbp | Indirect competitive colorimetric ELISA | 0.426 ng/mL | Nail polish | Nail polish was weighed at 0.5 g. After that, 5.0 mL of acetonitrile was added to the nail polish andultrasonically vibrated for 30 min. Another extraction procedure in a stoppered glass tube was carried out at room temperature overnight. The extract (2 mL) was evaporated using a centrifugal evaporator to remove acetonitrile and then dissolved in 2 mL of assay buffer (PBS containing 0.1% BSA). | [59] |
| Mercury(II) ion, | Indirect competitive colorimetric ELISA | 0.08 ng mL−1 | Facial cleansers and night creams | Sample (0.5 or 1 g) was soaked with 30% HNO3 overnight at room temperature, followed by boiling until it was dissolved. After cooling, the solution was centrifuged and the supernatant adjusted to a pH value of about 7.0 with 1 mol L−1 NaOH and diluted with pure water appropriately for ELISA. | [66] |
| Mercury(II) ion | Electrochemiluminescent (ECL) competitive immunoassay | 6.2 pg/mL | Hand cream | Microwave digestion was used to extract the hand cream. Briefly, 1 g of the sample was mixed with 3.0 mL HNO3 and 2.0 mL H2O2 in turn. After heating at 100 °C for 20 min, the sample was cooled to room temperature and filtered for use. | [67] |
| Staphylococcus aureus | Non-competitive colorimetric ELISA | Not showed | Irradiated Starch, Simethicone, Emulsion, Antiflatulent Liquid, Denture Adhesive, Carboxymethyl- cellulose, Medicated Dentifrice, Fluoride Dentifrice, Diaper Rash, Ointment, Gel Dentifrice, Silica | Each sample was mixed and heated for 15 min at 95C in a boiling water bath to increase lysis efficiency. | [80] |
| Chloramphenicol | Direct competitive chemiluminescent ELISA | 0.0021 ng/mL | Primer lotion | Cosmetics samples (2.00 g ± 0.02 g) were weighed precisely and transferred into 50 mL polypropylene centrifuge tubes. Then, 20 mL of PBS was added and vortexed for 30 s, and then ultra-sonication was conducted for 3 min for the extraction of CAP. Each sample was centrifuged at 12,000× g for 10 min at 4 °C, and the supernatant was collected and filtered through a 0.22 µm membrane. | [92] |
| ciprofloxacin, tetracycline (TC) and sulfamethoxydiazine (SMD) | Competitive Multi-Dot-ELISA. The device is based on a nitrocellulose membrane in which the different antigens were immobilized in localized areas. After the incubation with the sample and the specific antibodies labeled with HRP, by adding a colorimetric enzymatic substrate, it was possible to visually detect the formation of colored spots. The position of these spots made is possible to distinguish among the different target analytes | CPFX: 2.50 μg/mL, TC: 2.50 μg/mL SMD: 1.25 μg/mL | 15 commercial cosmetic products were randomly selected from supermarkets and beauty parlors | All samples were diluted and mixed gently with 50% methanol and then ultrasonically oscillated for 30 min. Following the centrifugation, the supernatant fluid was collected for testing. | [93] |
| Botulinum neurotoxin (BoNT) produced by Clostridium botulinum | Immunosensor based on the use of functionalized fluorescent nanoparticles conjugated to anti- BoNT antibodies. After incubation with BoNT-containing samples, the detection is performed by adding antibody-conjugated AuNPs. | 10 pg/mL | BOTOX and Dysport currently used in cosmetics treatment | Diluted in 1 mL of digestion buffer containing 50 mM HEPES (pH 7.4), 5 mM NaCl, 0.1% Tween 20, 0.05% ZnCl2, and 2 mM DTT. | [95] |
| Ricin | Competitive colorimetric LFIA | 0.005 μg/mL | Eye make-up, shampoo, body lotion | Eye make-up samples were diluted 1:1 with 10 mMPBS/0.1% Tween-20/5% non-fat milk (PBSTM) and analyzed using the LFDs according to manufacturer’s instructions. The viscous shampoo and body lotion samples were first diluted 1:9 and 1:19, respectively, with PBS and then diluted 1:1 with PBSTM to generate the 50% PBSTM solution for analysis with the LFDs. | [97] |
| Sialoglycoprotein typical of edible bird’s nest | Competitive colorimetric ELISA | LoDs were 10–18 μg/g depending on the different cosmetic matrices analyzed | Facial mask, eye cream, whitening serum, face cream, essence | The homogenized sample (1 g) was placed in a 20 mL volumetric flask. The flask was then filled with PBS, ultrasonicated for 2 min, and centrifuged at 10,000 rpm for 1 min. The aqueous phase was diluted to a suitable concentration with PBS for ELISA. | [114] |
| Porcine gelatins, which is a common adulterant found in EBN used to increase the net weight before selling | Competitive colorimetric ELISA | 0.10 μg/g | Not tested on cosmetic matrix | - | [115] |
| Characteristic glycoprotein of EBN | Non-competitive colorimetric LFIA (traditional colloidal gold is replaced by the nanocomposite Au@ SiO2) | 7.02 ng/mL | Not tested on cosmetic matrix | [116] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zangheri, M.; Calabretta, M.M.; Calabria, D.; Fiori, J.; Guardigli, M.; Michelini, E.; Melandri, S.; Maris, A.; Mirasoli, M.; Evangelisti, L. Immunological Analytical Techniques for Cosmetics Quality Control and Process Monitoring. Processes 2021, 9, 1982. https://doi.org/10.3390/pr9111982
Zangheri M, Calabretta MM, Calabria D, Fiori J, Guardigli M, Michelini E, Melandri S, Maris A, Mirasoli M, Evangelisti L. Immunological Analytical Techniques for Cosmetics Quality Control and Process Monitoring. Processes. 2021; 9(11):1982. https://doi.org/10.3390/pr9111982
Chicago/Turabian StyleZangheri, Martina, Maria Maddalena Calabretta, Donato Calabria, Jessica Fiori, Massimo Guardigli, Elisa Michelini, Sonia Melandri, Assimo Maris, Mara Mirasoli, and Luca Evangelisti. 2021. "Immunological Analytical Techniques for Cosmetics Quality Control and Process Monitoring" Processes 9, no. 11: 1982. https://doi.org/10.3390/pr9111982
APA StyleZangheri, M., Calabretta, M. M., Calabria, D., Fiori, J., Guardigli, M., Michelini, E., Melandri, S., Maris, A., Mirasoli, M., & Evangelisti, L. (2021). Immunological Analytical Techniques for Cosmetics Quality Control and Process Monitoring. Processes, 9(11), 1982. https://doi.org/10.3390/pr9111982











