Evaluation of a New Cerium Oxide-Bismuth Oxide-Based Nanobiocomposite as a Biocatalyst for Biodiesel Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of COBO Nanoparticles
2.3. Grafting of PDA on COBO Nanoparticles
2.4. Immobilization of Lipase on COBO NCs
2.5. Characterization of Nanoparticles
2.6. Lipase Activity Assay
2.7. Effect of Temperature and pH on Lipase Activity
2.8. Recycling of NBCs
2.9. RSM Optimization of Biodiesel Production
2.10. Recovery of NBCs
2.11. Characterization of Biodiesel
3. Results
3.1. Characterization of NCs
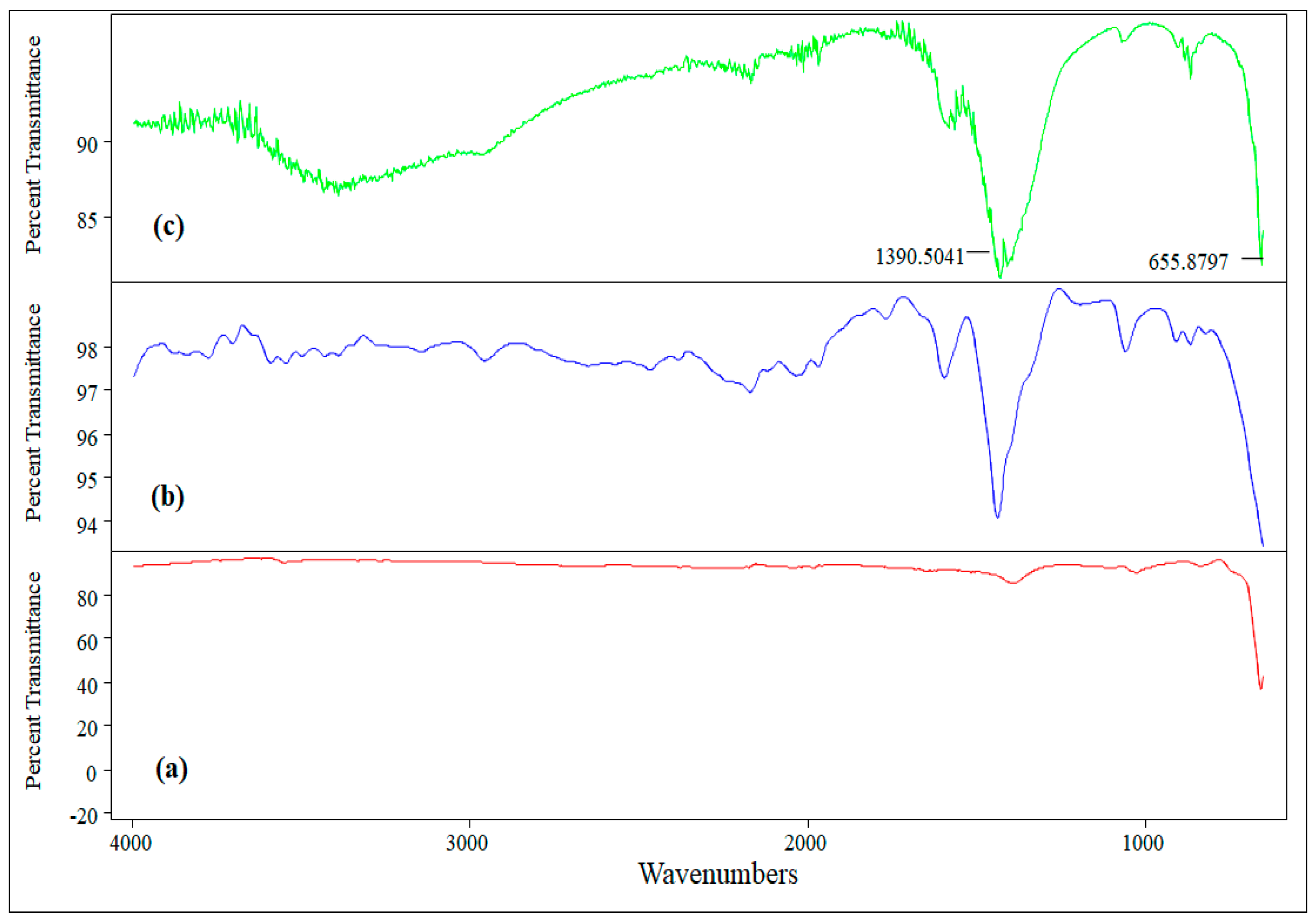
3.1.1. FTIR Spectroscopy
3.1.2. SEM
3.1.3. XRD
3.2. Surface Modification of NBCs
3.3. Lipase Activities of NBCs
3.4. Effect of pH and Temperature on Lipase Activity
3.5. Reusability of NBCs
3.6. Optimization of Biodiesel Production with NBC3 as Biocatalyst
3.7. Characterization of Biodiesel
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mumtaz, M.W.; Adnan, A.; Anwar, F.; Mukhtar, H.; Raza, M.A.; Ahmad, F.; Rashid, U. Response surface methodology: An emphatic tool for optimized biodiesel production using rice bran and sunflower oils. Energies 2012, 5, 3307–3328. [Google Scholar] [CrossRef] [Green Version]
- Talebian-Kiakalaieh, A.; Amin, N.A.S.; Mazaheri, H. A review on novel processes of biodiesel production from waste cooking oil. Appl. Energy 2013, 104, 683–710. [Google Scholar] [CrossRef]
- Sohail, S.; Mumtaz, M.W.; Mukhtar, H.; Touqeer, T.; Anjum, M.K.; Rashid, U.; Wan Ab Karim Ghani, W.A.; Choong, T.S.Y. Spirogyra Oil-Based Biodiesel: Response Surface Optimization of Chemical and Enzymatic Transesterification and Exhaust Emission Behavior. Catalysts 2020, 10, 1214. [Google Scholar] [CrossRef]
- Akoh, C.C.; Chang, S.-W.; Lee, G.-C.; Shaw, J.-F. Enzymatic approach to biodiesel production. J. Agric. Food Chem. 2007, 55, 8995–9005. [Google Scholar] [CrossRef]
- Touqeer, T.; Mumtaz, M.W.; Mukhtar, H.; Irfan, A.; Akram, S.; Shabbir, A.; Rashid, U.; Nehdi, I.A.; Choong, T.S.Y. Fe3O4-PDA-Lipase as Surface Functionalized Nano Biocatalyst for the Production of Biodiesel Using Waste Cooking Oil as Feedstock: Characterization and Process Optimization. Energies 2020, 13, 177. [Google Scholar] [CrossRef] [Green Version]
- Bi, Y.; Wang, Z.; Zhang, R.; Diao, Y.; Tian, Y.; Jin, Z. Improved catalytic properties of Thermomyces lanuginosus lipase immobilized onto newly fabricated polydopamine-functionalized magnetic Fe3O4 nanoparticles. Processes 2020, 8, 629. [Google Scholar] [CrossRef]
- Akram, S.; Wang, Z.; Chen, L.; Wang, Q.; Shen, G.; Han, N.; Chen, Y.; Ge, G. Low-temperature efficient degradation of ethyl acetate catalyzed by lattice-doped CeO2–CoOx nanocomposites. Catal. Commun. 2016, 73, 123–127. [Google Scholar] [CrossRef]
- Fatima, A.; Mumtaz, M.W.; Mukhtar, H.; Akram, S.; Touqeer, T.; Rashid, U.; Ul Mustafa, M.R.; Nehdi, I.A.; Saiman, M.I. Synthesis of lipase-immobilized CeO2 nanorods as heterogeneous nano-biocatalyst for optimized biodiesel production from Eruca sativa seed oil. Catalysts 2020, 10, 231. [Google Scholar] [CrossRef] [Green Version]
- Zulfiqar, A.; Mumtaz, M.W.; Mukhtar, H.; Najeeb, J.; Irfan, A.; Akram, S.; Touqeer, T.; Nabi, G. Lipase-PDA-TiO2 NPs: An emphatic nano-biocatalyst for optimized biodiesel production from Jatropha curcas oil. Renew. Energy 2021, 169, 1026–1037. [Google Scholar] [CrossRef]
- Li, F.; Hülsey, M.J.; Yan, N.; Dai, Y.; Wang, C.-H. Co-transesterification of waste cooking oil, algal oil and dimethyl carbonate over sustainable nanoparticle catalysts. Chem. Eng. J. 2021, 405, 127036. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, C.; Zhang, L.; Chang, Y.; Hao, Y. Converting waste cooking oil to biodiesel in China: Environmental impacts and economic feasibility. Renew. Sustain. Energy Rev. 2021, 140, 110661. [Google Scholar] [CrossRef]
- Jayakumar, G.; Irudayaraj, A.A.; Raj, A.D. Investigation on the synthesis and photocatalytic activity of activated carbon–cerium oxide (AC–CeO2) nanocomposite. Appl. Phys. A 2019, 125, 1–9. [Google Scholar] [CrossRef]
- Pujar, M.S.; Hunagund, S.M.; Desai, V.R.; Patil, S.; Sidarai, A.H. One-step synthesis and characterizations of cerium oxide nanoparticles in an ambient temperature via Co-precipitation method. AIP Conf. Proc. 2018, 1942, 050026. [Google Scholar]
- Wang, H.-W.; Hu, Z.-A.; Chang, Y.-Q.; Chen, Y.-L.; Lei, Z.-Q.; Zhang, Z.-Y.; Yang, Y.-Y. Facile solvothermal synthesis of a graphene nanosheet–bismuth oxide composite and its electrochemical characteristics. Electrochim. Acta 2010, 55, 8974–8980. [Google Scholar] [CrossRef]
- He, C.; Yu, Y.; Yue, L.; Qiao, N.; Li, J.; Shen, Q.; Yu, W.; Chen, J.; Hao, Z. Low-temperature removal of toluene and propanal over highly active mesoporous CuCeOx catalysts synthesized via a simple self-precipitation protocol. Appl. Catal. B Environ. 2014, 147, 156–166. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, C.; Tang, L.; Zhang, Y.; Zhang, G.-J. Magnetic-enhanced fluorescence sensing of tumor miRNA by combination of MNPs@ PDA with duplex specific nuclease. RSC Adv. 2021, 11, 2968–2975. [Google Scholar] [CrossRef]
- Moyo, L.; Iyuke, S.; Muvhiiwa, R.; Simate, G.; Hlabangana, N. Application of response surface methodology for optimization of biodiesel production parameters from waste cooking oil using a membrane reactor. S. Afr. J. Chem. Eng. 2021, 35, 1–7. [Google Scholar] [CrossRef]
- Helmi, M.; Tahvildari, K.; Hemmati, A.; Safekordi, A. Phosphomolybdic acid/graphene oxide as novel green catalyst using for biodiesel production from waste cooking oil via electrolysis method: Optimization using with response surface methodology (RSM). Fuel 2021, 287, 119528. [Google Scholar] [CrossRef]
- Mukhtar, H.; Khursheed, S.; Mumtaz, M.W.; Rashid, U.; Al-Resayes, S.I. Optimization of lipase biosynthesis from Rhizopus oryzae for biodiesel production using multiple oils. Chem. Eng. Technol. 2016, 39, 1707–1715. [Google Scholar] [CrossRef]
- Mukhtar, H.; Suliman, S.M.; Shabbir, A.; Mumtaz, M.W.; Rashid, U.; Rahimuddin, S.A. Evaluating the Potential of Oleaginous Yeasts as Feedstock for Biodiesel Production. Protein Pept. Lett. 2018, 25, 195–201. [Google Scholar] [CrossRef]
- Mumtaz, M.W.; Adnan, A.; Mahmood, Z.; Mukhtar, H.; Danish, M.; Ahmad, Z. Biodiesel production using Eruca sativa oil: Optimization and characterization. Pak. J. Bot. 2012, 44, 1111–1120. [Google Scholar]









| Source | Sequential p-Value | Lack of Fit p-Value | Adjusted R-Squared | Predicted R-Squared | |
|---|---|---|---|---|---|
| Linear | 0.0027 | 0.0016 | 0.4984 | 0.2398 | |
| 2FI | 0.8241 | 0.0009 | 0.4228 | −1.5370 | |
| Quadratic | <0.0001 | 0.7517 | 0.9753 | 0.9537 | Suggested |
| Cubic | 0.7517 | 0.9676 |
| Source | Sum of Squares | Df | Mean Square | F Value | p-Value Prob > F |
|---|---|---|---|---|---|
| Model | 1305.53 | 9 | 145.06 | 84.32 | <0.0001 |
| A-Reaction Time | 130.04 | 1 | 130.04 | 75.59 | <0.0001 |
| B-NBC Conc. | 471.13 | 1 | 471.13 | 273.86 | <0.0001 |
| C-Methanol: oil | 28.90 | 1 | 28.90 | 16.80 | 0.0021 |
| AB | 26.64 | 1 | 26.64 | 15.49 | 0.0028 |
| AC | 1.36 | 1 | 1.36 | 0.79 | 0.3946 |
| BC | 0.36 | 1 | 0.36 | 0.21 | 0.6566 |
| A2 | 17.46 | 1 | 17.46 | 10.15 | 0.0097 |
| B2 | 1.10 | 1 | 1.10 | 0.64 | 0.4422 |
| C2 | 173.50 | 1 | 173.50 | 100.85 | <0.0001 |
| Residual | 17.20 | 10 | 1.72 | ||
| Lack of Fit | 5.92 | 5 | 1.18 | 0.53 | 0.7517 |
| Pure Error | 11.28 | 5 | 2.26 | ||
| Cor Total | 1322.74 | 19 |
| Peak # | Retention Time (min) | FAME | % (w/w) |
|---|---|---|---|
| 1 | 16.868 | Hexadecanoic acid methy ester | 1.9 |
| 2 | 18.681 | 9-Octadecenoic acid methyl ester | 42.6 |
| 3 | 20.358 | 11-Eicosenoic acid methy ester | 6.5 |
| 4 | 22.129 | Erucic acid methyl ester | 47.7 |
| 5 | 23.458 | 15-Tetracosenoic acid methyl ester | 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahzadi, A.; Mumtaz, M.W.; Mukhtar, H.; Akram, S.; Touqeer, T.; Zambare, V.; Christopher, L. Evaluation of a New Cerium Oxide-Bismuth Oxide-Based Nanobiocomposite as a Biocatalyst for Biodiesel Production. Processes 2021, 9, 2012. https://doi.org/10.3390/pr9112012
Shahzadi A, Mumtaz MW, Mukhtar H, Akram S, Touqeer T, Zambare V, Christopher L. Evaluation of a New Cerium Oxide-Bismuth Oxide-Based Nanobiocomposite as a Biocatalyst for Biodiesel Production. Processes. 2021; 9(11):2012. https://doi.org/10.3390/pr9112012
Chicago/Turabian StyleShahzadi, Anam, Muhammad Waseem Mumtaz, Hamid Mukhtar, Sadia Akram, Tooba Touqeer, Vasudeo Zambare, and Lew Christopher. 2021. "Evaluation of a New Cerium Oxide-Bismuth Oxide-Based Nanobiocomposite as a Biocatalyst for Biodiesel Production" Processes 9, no. 11: 2012. https://doi.org/10.3390/pr9112012







