Biodesulfurization of Dibenzothiophene and Its Alkylated Derivatives in a Two-Phase Bubble Column Bioreactor by Resting Cells of Rhodococcus erythropolis IGTS8
Abstract
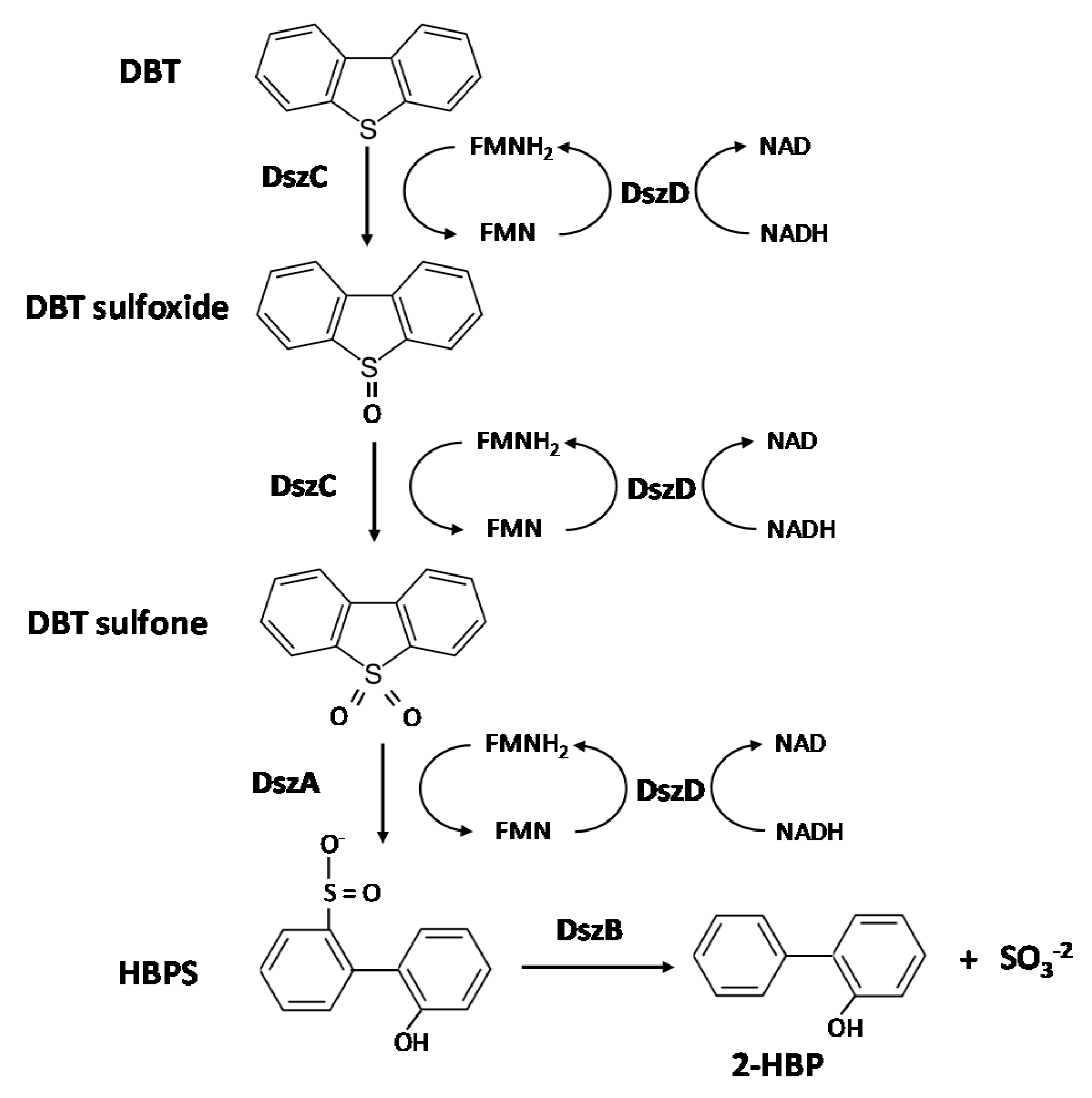
:1. Introduction
2. Materials and Methods
2.1. Microorganism
2.2. Materials
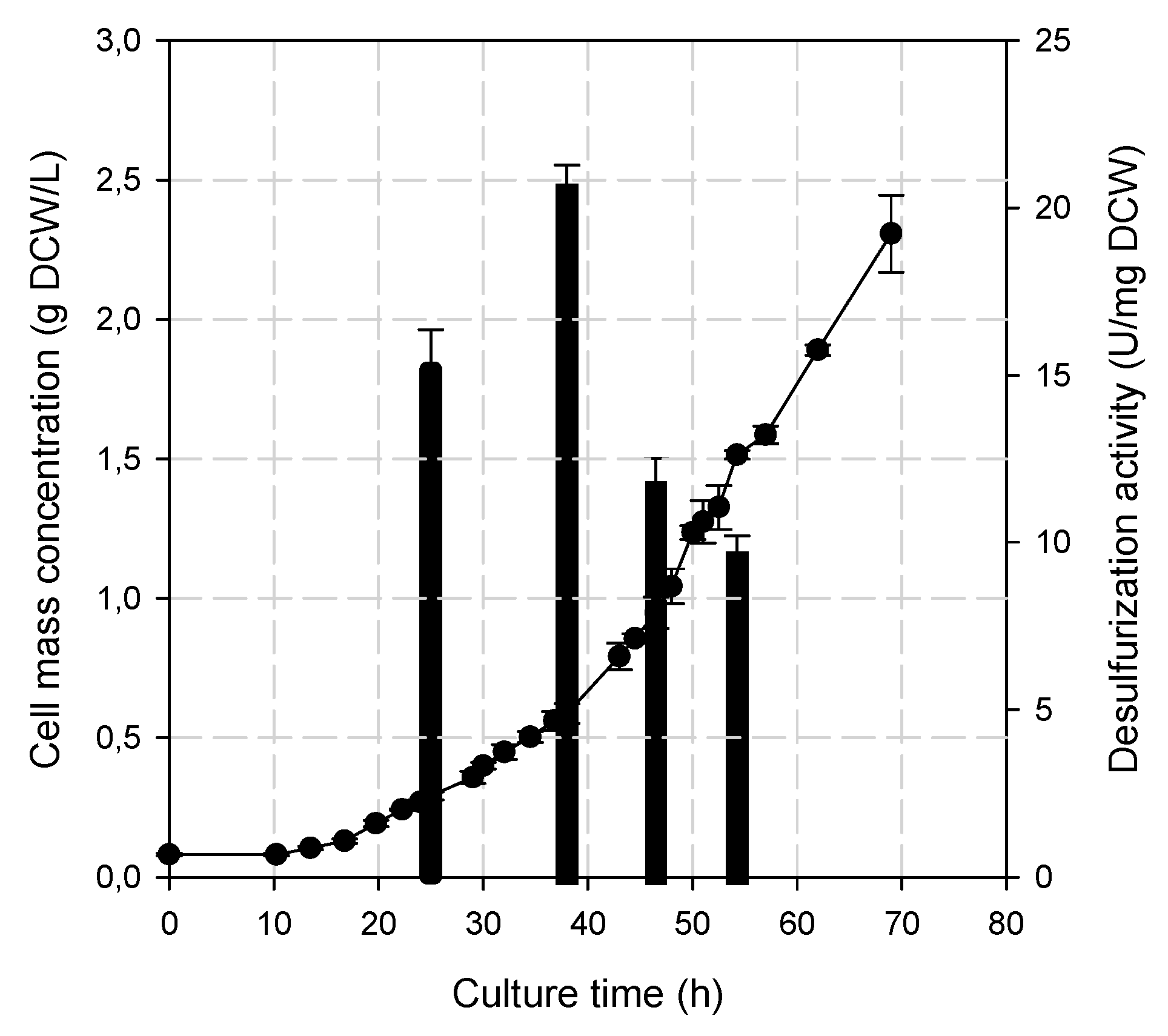
2.3. Biocatalyst Production
2.4. Bubble-Column-Type Bioreactor System for Biphasic Desulfurization
2.5. Resting Cells’ Biodesulfurization Reactions
2.6. Analytical Methods
2.7. Evaluation of Biodesulfurization Process
3. Results and Discussion
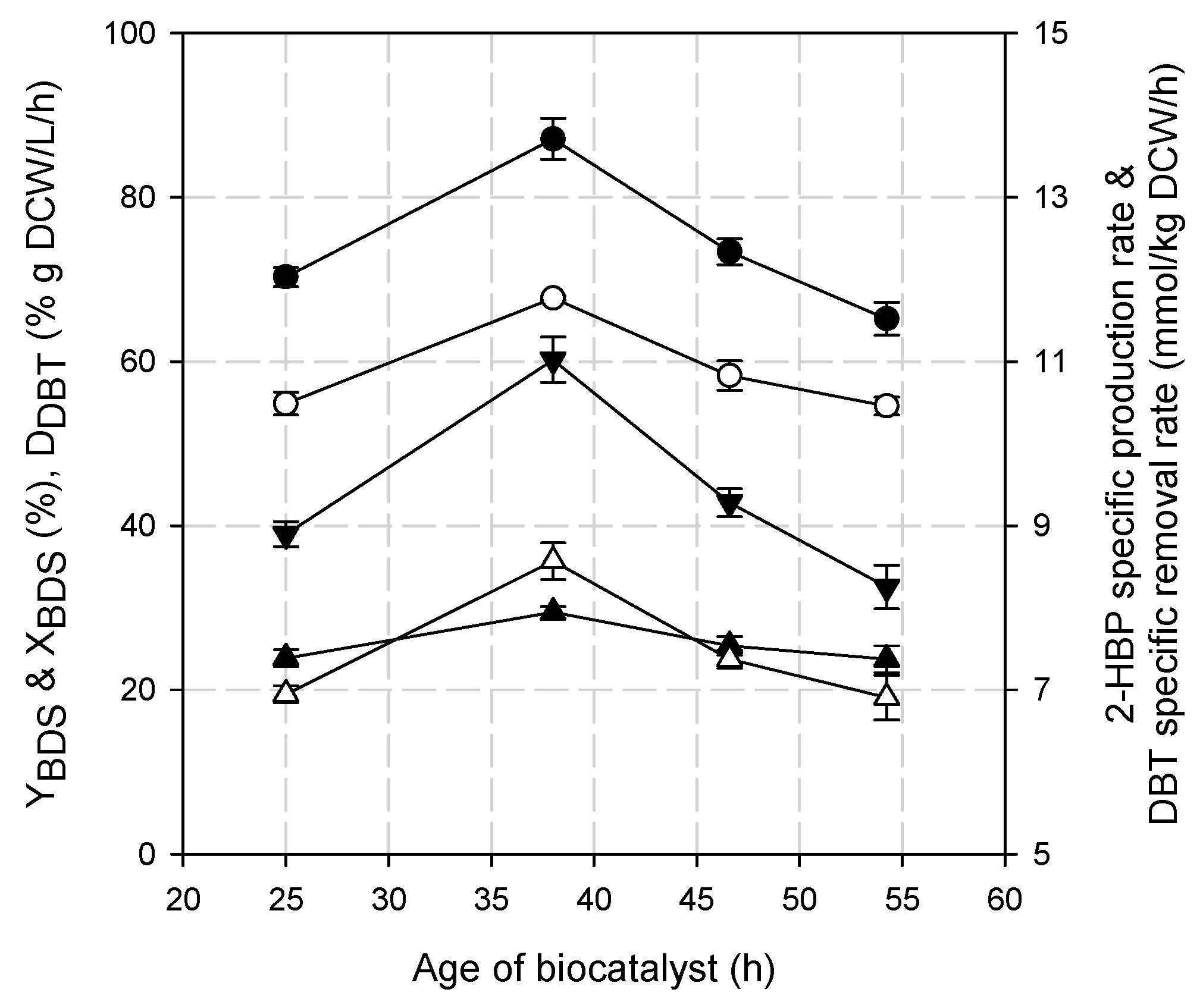
3.1. Effect of the Biocatalyst’s Age on DBT Biodesulfurization
3.2. Effect of Phase Ratio on the Desulfurization of DBT
3.3. Effect of Cell Concentration on DBT Desulfurization at Constant OFP
3.4. Effect of DBT Concentration
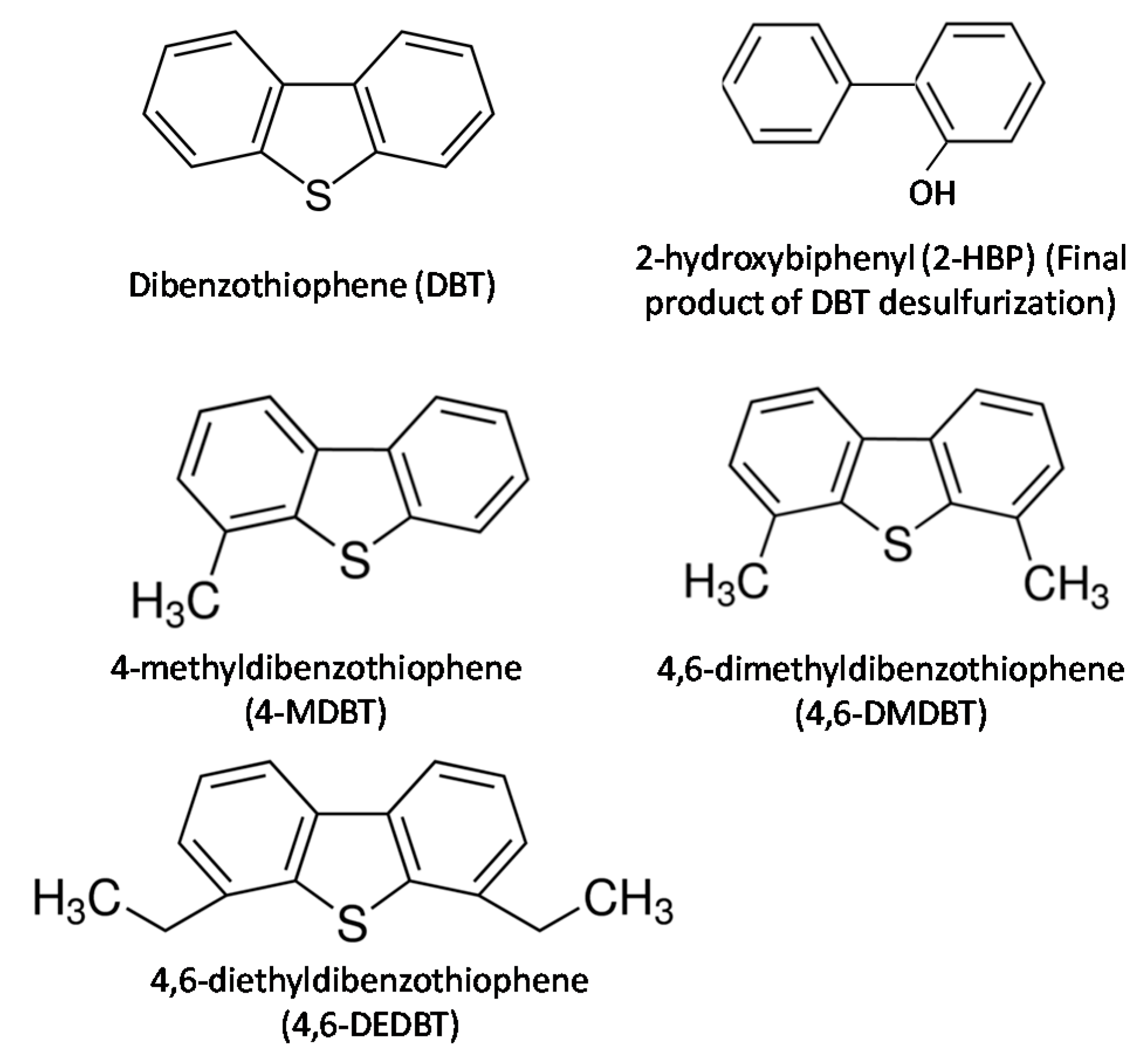
3.5. Biodesulfurization of Different OSCs
3.6. Multisubstrate Biodesulfurization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohabeli, G.; Ball, A.S. Biodesulfurization of diesel fuels: Past, present and future perspectives. Int. Biodeter. Biodegr. 2016, 110, 163–180. [Google Scholar] [CrossRef]
- Directive 2012/33/EU of the European Parliament and of the Council (12 November 2012). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32012L0033&from=EN (accessed on 26 September 2021).
- Hossain, M.N.; Park, H.C.; Choi, H.S. A comprehensive review on catalytic oxidative desulfurization of liquid fuel oil. Catalysts 2019, 9, 229. [Google Scholar] [CrossRef] [Green Version]
- Sadare, O.O.; Obazu, F.; Daramola, M.O. Biodesulfurization of petroleum distillates-Current status, opportunities and future challenges. Environments 2017, 4, 85. [Google Scholar] [CrossRef] [Green Version]
- Babich, I.V.; Moulijn, J.A. Science and technology of novel processes for deep desulfurization of oil refinery streams: A review. Fuel 2003, 82, 607–631. [Google Scholar] [CrossRef]
- Kilbane, J.J.; Stark, B. Biodesulfurization: A model system for microbial physiology research. World J. Microbiol. Biotechnol. 2016, 32, 137. [Google Scholar] [CrossRef]
- Maghsoudi, S.; Vossoughi, M.; Kheirolomoomc, A.; Tanaka, E.; Katoh, S. Biodesulfurization of hydrocarbons and diesel fuels by Rhodococcus sp. strain P32C1. Biochem. Eng. J. 2001, 8, 151–156. [Google Scholar] [CrossRef]
- Goindi, H.K.; Saini, V.S.; Verma, P.S.; Adhikari, D.K. Dibenzothiophene desulfurization in hydrocarbon environment by Staphylococcus sp. resting cells. Biotechnol. Lett. 2002, 24, 779–781. [Google Scholar] [CrossRef]
- Luo, M.F.; Xing, J.M.; Gou, Z.X.; Li, S.; Liu, H.Z.; Chen, J.Y. Desulfurization of dibenzothiophene by lyophilized cells of Pseudomonas delafieldii R-8 in the presence of dodecane. Biochem. Eng. J. 2003, 13, 1–6. [Google Scholar] [CrossRef]
- Del Olmo, C.H.; Alcon, A.; Santos, V.E.; Garcia-Ochoa, F. Modeling the production of a Rhodococcus erythropolis IGTS8 biocatalyst for DBT biodesulfurization: Influence of media composition. Enzym. Microb. Technol. 2005, 37, 157–166. [Google Scholar] [CrossRef]
- Caro, A.; Boltes, K.; Letón, P.; García-Calvo, E. Dibenzothiophene biodesulfurization in resting cell conditions by aerobic bacteria. Biochem. Eng. J. 2007, 35, 191–197. [Google Scholar] [CrossRef]
- Arabian, D.; Najafi, H.; Farhadi, F.; Dehkordi, A.M. Biodesulfurization of simulated light fuel oil by a native isolated bacteria Bacillus cereus HN. J. Pet. Sci. Technol. 2014, 4, 31–40. [Google Scholar] [CrossRef]
- Boltes, K.; del Aguila, R.A.; García-Calvo, E. Effect of mass transfer on biodesulfurization kinetics of alkylated forms of dibenzothiophene by Pseudomonas putida CECT5279. J. Chem. Technol. Biotechnol. 2013, 88, 422–431. [Google Scholar] [CrossRef]
- Gunam, I.B.W.; Yamamura, K.; Sujaya, I.N.; Antara, N.S.; Aryanta, W.R.; Tanaka, M.; Tomita, F.; Sone, T.; Asano, K. Biodesulfurization of dibenzothiophene and its derivatives using resting and immobilized cells of Sphingomonas subarctica T7b. J. Microbiol. Biotechnol. 2013, 23, 473–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maass, D.; de Oliveira, D.; de Souza, A.A.U.; Souza, S.M.A.G.U. Biodesulfurization of a system containing synthetic fuel using Rhodococcus erythropolis ATCC 4277. Appl. Biochem. Biotechnol. 2014, 174, 2079–2085. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Yang, H.; Zhan, X.; Wang, W. Enhancement of dibenzothiophene biodesulfurization by weakening the feedback inhibition effects based on a systematic understanding of the biodesulfurization mechanism by Gordonia sp. through the potential “4S” pathway. RSC Adv. 2016, 6, 82872–82881. [Google Scholar] [CrossRef]
- Dejaloud, A.; Vahabzadeh, F.; Habibi, A. Ralstonia eutropha as a biocatalyst for desulfurization of dibenzothiophene. Bioprocess Biosyst. Eng. 2017, 40, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Dejaloud, A.; Habibi, A.; Vahabzadeh, F. DBT desulfurization by Rhodococcus erythropolis PTCC 1767 in aqueous and biphasic systems. Chem. Pap. 2020, 74, 3605–3615. [Google Scholar] [CrossRef]
- Akhtar, N.; Akhtar, K.; Ghauri, M.A. Biodesulfurization of thiophenic compounds by a 2-hydroxybiphenyl-resistant Gordonia sp. HS126-4N carrying dszABC Genes. Curr. Microbiol. 2018, 75, 597–603. [Google Scholar] [CrossRef]
- Jatoi, A.S.; Aziz, S.; Soomro, S.A. Experimental and numerical investigation of DBT degradation via Rhodococcus spp. (SL-9) through the use of biological assisted method. Biomass Convers. Biorefinery 2021. [Google Scholar] [CrossRef]
- Martínez, I.; El-Said Mohamed, M.; Santos, V.E.; García, J.L.; García-Ochoa, F.; Díaz, E. Metabolic and process engineering for biodesulfurization in Gram-negative bacteria. J. Biotechnol. 2017, 262, 47–55. [Google Scholar] [CrossRef]
- Escobar, S.; Rodriguez, A.; Gomez, E.; Alcon, A.; Santos, V.E.; Garcia-Ochoa, F. Influence of oxygen transfer on Pseudomonas putida effects on growth rate and biodesulfurization capacity. Bioprocess Biosyst. Eng. 2016, 39, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Gomez, E.; Alcón, A.; Escobar, S.; Santos, V.E.; Garcia-Ochoa, F. Effect of fluid dynamic conditions on growth rate and biodesulfurization capacity of Rhodococcus erythropolis IGTS8. Biochem. Eng. J. 2015, 99, 138–146. [Google Scholar] [CrossRef]
- Zhang, S.H.; Chen, H.; Li, W. Kinetic analysis of biodesulfurization of model oil containing multiple alkyl dibenzothiophenes. Appl. Microbiol. Biotechnol. 2013, 97, 2193–2200. [Google Scholar] [CrossRef]
- Martínez, I.; Santos, V.E.; Gómez, E.; García-Ochoa, F. Biodesulfurization of dibenzothiophene by resting cells of Pseudomonas putida CECT5279: Influence of the oxygen transfer rate in the scale-up from shaken flask to stirred tank reactor. J. Chem. Technol. Biotechnol. 2016, 91, 184–189. [Google Scholar] [CrossRef]
- Boltes, K.; Caro, A.; Leton, P.; Rodriguez, A.; Garcia-Calvo, E. Gas–liquid mass transfer in oil–water emulsions with an airlift bio-reactor. Chem. Eng. Process 2008, 47, 2408–2412. [Google Scholar] [CrossRef]
- Zhou, Y.; Han, L.-R.; He, H.-W.; Sang, B.; Yu, D.-L.; Feng, J.-T.; Zhang, X. Effects of agitation, aeration and temperature on production of a novel glycoprotein GP-1 by Streptomyces kanasenisi ZX01 and scale-up based on volumetric oxygen transfer coefficient. Molecules 2018, 23, 125. [Google Scholar] [CrossRef] [Green Version]
- Youssef, A.A.; Al-Dahhan, M.H.; Dudukovic, M.P. Bubble columns with internals: A review. Int. J. Chem. React. Eng. 2013, 11, 169–223. [Google Scholar] [CrossRef]
- Stanbury, P.F.; Whitaker, A.; Hall, S.J. Chapter 9—Aeration and agitation. In Principles of Fermentation Technology, 3rd ed.; Stanbury, P.F., Whitaker, A., Hall, S.J., Eds.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 537–618. [Google Scholar] [CrossRef]
- Humbird, D.; Davis, R.; McMillan, J.D. Aeration costs in stirred-tank and bubble column bioreactors. Biochem. Eng. J. 2017, 127, 161–166. [Google Scholar] [CrossRef]
- Calzada, J.; Alcón, A.; Santos, V.E.; Garcia-Ochoa, F. Mixtures of Pseudomonas putida CECT 5279 cells of different ages: Optimization as biodesulfurization catalyst. Process Biochem. 2011, 46, 1323–1328. [Google Scholar] [CrossRef]
- Calzada, J.; Zamarro, M.T.; Alcón, A.; Santos, V.E.; Díaz, E.; García, J.L.; Garcia-Ochoa, F. Analysis of dibenzothiophene desulfurization in a recombinant Pseudomonas putida strain. Appl. Environ. Microbiol. 2009, 75, 875–877. [Google Scholar] [CrossRef] [Green Version]
- Alves, L.; Salgueiro, R.; Rodrigues, C.; Mesquita, E.; Matos, J.; Gírio, F.M. Desulfurization of dibenzothiophene, benzothiophene, and other thiophene analogs by a newly isolated bacterium, Gordonia alkanivorans strain 1B. Appl. Biochem. Biotechnol. 2005, 120, 199–208. [Google Scholar] [CrossRef]
- Ismail, W.; El-Sayed, W.S.; Abdul Raheem, A.S.; Mohamed, M.E.; El Nayal, A.M. Biocatalytic desulfurization capabilities of a mixed culture during non-destructive utilization of recalcitrant organosulfur compounds. Front. Microbiol. 2016, 7, 266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kayser, K.J.; Cleveland, L.; Park, H.S.; Kwak, J.H.; Kolhatkar, A.; Kilbane, J.J., II. Isolation and characterization of a moderate thermophile, Mycobacterium phlei GTIS10, capable of dibenzothiophene desulfurization. Appl. Microbiol. Biotechnol. 2002, 59, 737–745. [Google Scholar] [CrossRef]
- Li, L.; Ye, L.; Guo, Z.; Zhang, W.; Liao, X.; Lin, Y.; Liang, S. A kinetic model to optimize and direct the dose ratio of Dsz enzymes in the 4S desulfurization pathway in vitro and in vivo. Biotechnol. Lett. 2019, 41, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.E.; Al-Yacoub, Z.H.; Vedakumar, J.V. Biocatalytic desulfurization of thiophenic compounds and crude oil by newly isolated bacteria. Front. Microbiol. 2015, 6, 112. [Google Scholar] [CrossRef] [Green Version]
- Adlakha, J.; Singh, P.; Ram, S.K.; Kumar, M.; Singh, M.P.; Singh, D.; Sahai, V.; Srivastava, P. Optimization of conditions for deep desulfurization of heavy crude oil and hydrodesulfurized diesel by Gordonia sp. IITR100. Fuel 2016, 184, 761–769. [Google Scholar] [CrossRef]
- Malani, R.S.; Batghare, A.H.; Bhasarkar, J.B.; Moholkar, V.S. Kinetic modelling and process engineering aspects of biodesulfurization of liquid fuels: Review and analysis. Bioresour. Technol. Rep. 2021, 14, 100668. [Google Scholar] [CrossRef]
- Abin-Fuentes, A.; Leung, J.C.; El-Said Mohamed, M.; Wang, D.I.C.; Prather, K.L.J. Rate-limiting step analysis of the microbial desulfurization of dibenzothiophene in a model oil system. Biotechnol. Bioeng. 2014, 110, 876–884. [Google Scholar] [CrossRef] [Green Version]
- Nassar, H.N.; Abu Amr, S.S.; El-Gendy, N.S. Biodesulfurization of refractory sulfur compounds in petro-diesel by a novel hydrocarbon tolerable strain Paenibacillus glucanolyticus HN4. Environ. Sci. Pollut. Res. 2021, 28, 8102–8116. [Google Scholar] [CrossRef]
- Khosravinia, S.; Mahdavi, M.A.; Gheshlaghi, R.; Dehghani, H.; Rasekh, B. Construction and characterization of a new recombinant vector to remove sulfate repression of dsz promoter transcription in biodesulfurization of dibenzothiophene. Front. Microbiol. 2018, 9, 1578. [Google Scholar] [CrossRef]
- Schilling, B.M.; Alvarez, L.M.; Wang, D.I.C.; Cooney, C.L. Continuous desulfurization of dibenzothiophene with R. rhodochrous IGTS8 (ATCC 53968). Biotechnol. Prog. 2002, 18, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Abin-Fuentes, A.; Mohamed, M.E.S.; Wang, D.I.; Prather, K.L. Exploring the mechanism of biocatalyst inhibition in microbial desulfurization. Appl. Environ. Microbiol. 2013, 79, 7807–7817. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, M.; Horiuchi, K.; Yoshikawa, O.; Hirasawa, K.; Ishii, Y.; Fujino, K.; Sugiyama, H.; Maruhashi, K. Kinetic analysis of microbial desulfurization of model and light gas oils containing multiple alkyl dibenzothiophenes. Biosci. Biotech. Bioch. 2001, 65, 298–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mingfang, L.; Zhongxuan, G.; Jianmin, X.; Huizhou, L.; Jiayong, C. Microbial desulfurization of model and straight-run diesel oils. J. Chem. Technol. Biot. 2003, 78, 873–876. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, W.J.; Chen, J.M.; Cai, Y.B.; Li, W. Desulfurization of various organic sulfur compounds and the mixture of DBT+4,6-DMDBT by Mycobacterium sp. ZD-19. Bioresour. Technol. 2008, 99, 3630–3634. [Google Scholar] [CrossRef] [PubMed]




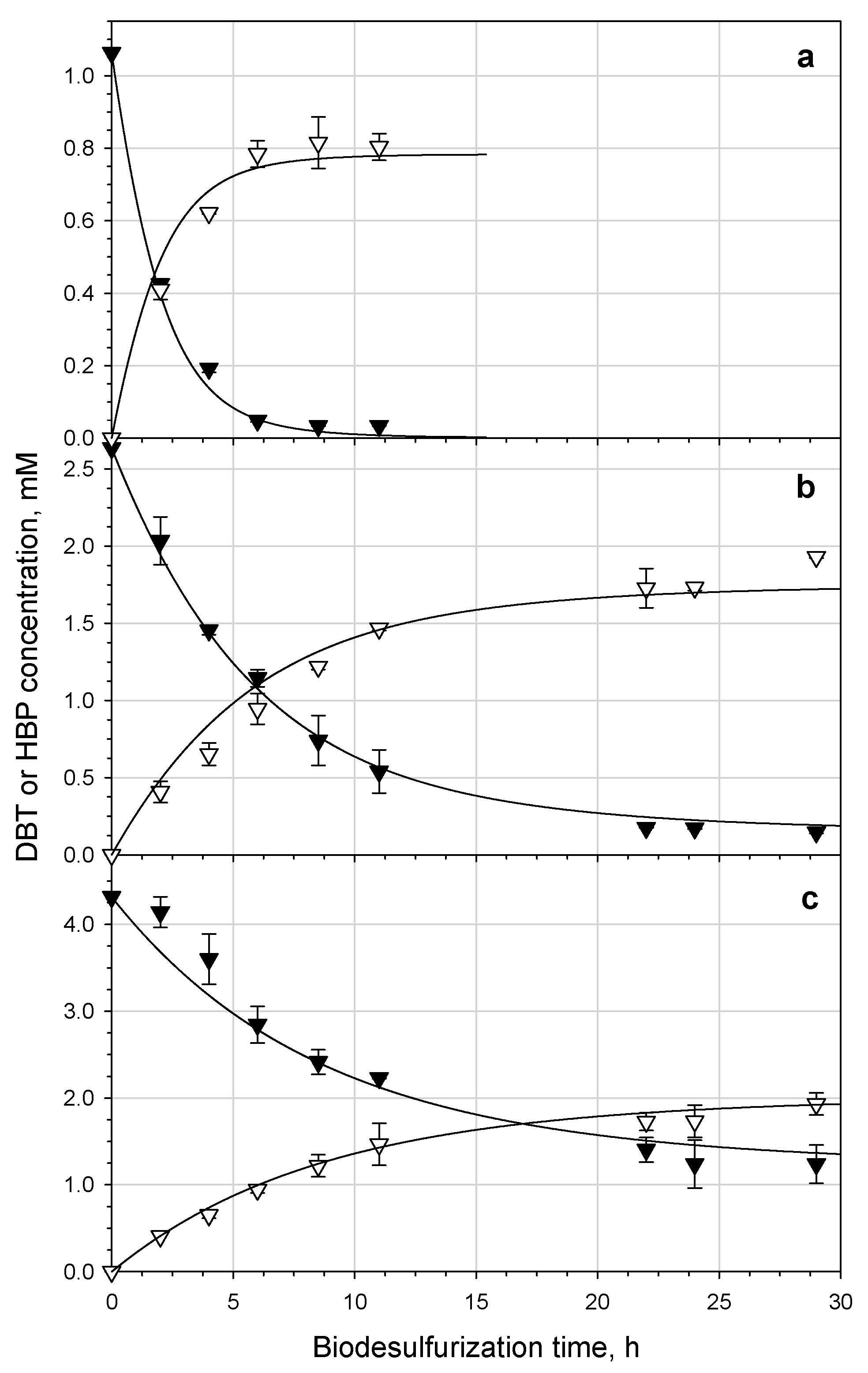
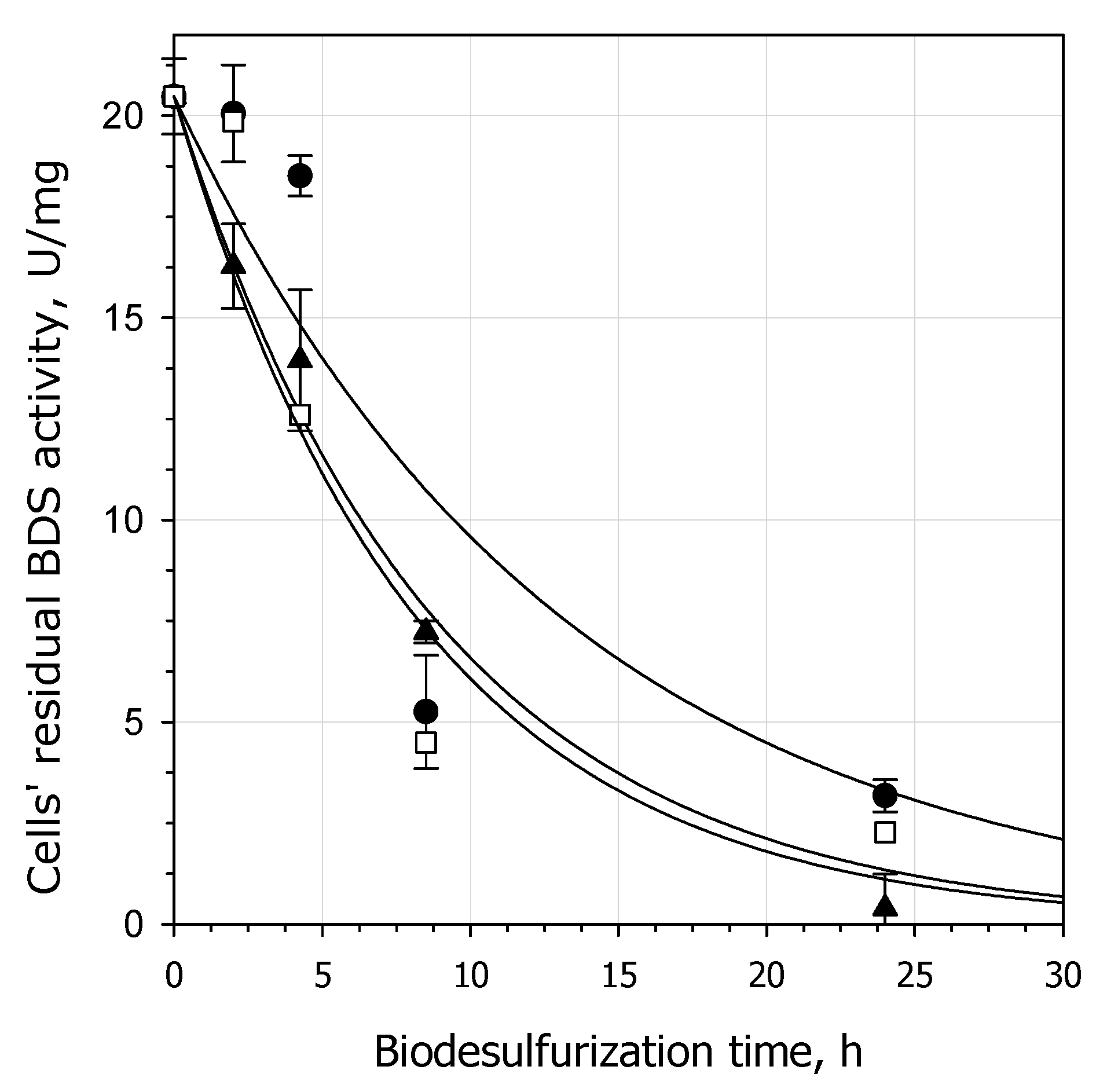


| Initial DBT Concentration (mM) | K (mM DBT) | kd (h−1) | a (mM DBT/h) | b (mM HBP/mM DBT) |
|---|---|---|---|---|
| 1.1 | 1.151 ± 0.065 | 0.099 ± 0.009 | 0.990 ± 0.032 | 0.738 ± 0.022 |
| 2.6 | 0.573 ± 0.009 | 0.705 ± 0.016 | ||
| 4.3 | 0.449 ± 0.011 | 0.652 ± 0.024 |
| OSCs | YBDS (%) | Specific Desulfurization Rate (Mmoles Cx-DBT/kg DCW/h) |
|---|---|---|
| 4-MDBT | 72.2 ± 2.6 | 9.4 ± 0.5 |
| 4,6-DMDBT | 60.0 ± 0.7 | 7.1 ± 0.9 |
| 4,6-DEDBT | 21.1 ± 4.9 | 2.0 ± 0.7 |
| OSC | K (mM OSC) | kd (h−1) | a (mM OSC/h) |
|---|---|---|---|
| 4-MDBT | 8.87 ± 1.03 | 0.099 ± 0.009 | 1.50 + 0.05 |
| 4,6-DMDBT | 8.57 ± 0.68 | 1.27 + 0.09 | |
| 4,6-DEDBT | 55.30 ± 4.41 | 1.21 + 0.19 |
| Concentration of Substrate | YBDS (%) | Specific Desulfurization Rate (Mmoles Cx-DBT/kg DCW/h) | |
|---|---|---|---|
| 5 mM DBT | 60.4 ± 0.2 | 11.19 ± 0.03 | |
| 3.5 mM DBT + 1.5 mM 4,6 DMDBT | |||
| DBT | 72.7 ± 2.0 | 9.31 ± 0.25 | |
| 4,6 DMDBT | 51.6 ± 1.0 | 3.0 ± 0.06 | |
| Overall | 66.2 ± 1.7 | 12.31 ± 0.19 | |
| 2.5 mM DBT + 2.5 mM 4,6 DMDBT | |||
| DBT | 80.9 ± 2.3 | 7.35 ± 0.21 | |
| 4,6 DMDBT | 52.2 ± 6.4 | 4.87 ± 0.60 | |
| Overall | 66.6 ± 4.4 | 12.22 ± 0.48 | |
| 1.5 mM DBT + 3.5 mM 4,6 DMDBT | |||
| DBT | 76.8 ± 0.8 | 3.66 ± 0.04 | |
| 4,6 DMDBT | 56.2 ± 3.2 | 7.38 ± 0.42 | |
| Overall | 62.4 ± 2.5 | 11.04 ± 0.28 | |
| 5 mM 4,6 DMDBT | 56.9 ± 0.4 | 11.00 ± 0.07 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prasoulas, G.; Dimos, K.; Glekas, P.; Kalantzi, S.; Sarris, S.; Templis, C.; Vavitsas, K.; Hatzinikolaou, D.G.; Papayannakos, N.; Kekos, D.; et al. Biodesulfurization of Dibenzothiophene and Its Alkylated Derivatives in a Two-Phase Bubble Column Bioreactor by Resting Cells of Rhodococcus erythropolis IGTS8. Processes 2021, 9, 2064. https://doi.org/10.3390/pr9112064
Prasoulas G, Dimos K, Glekas P, Kalantzi S, Sarris S, Templis C, Vavitsas K, Hatzinikolaou DG, Papayannakos N, Kekos D, et al. Biodesulfurization of Dibenzothiophene and Its Alkylated Derivatives in a Two-Phase Bubble Column Bioreactor by Resting Cells of Rhodococcus erythropolis IGTS8. Processes. 2021; 9(11):2064. https://doi.org/10.3390/pr9112064
Chicago/Turabian StylePrasoulas, George, Konstantinos Dimos, Panayiotis Glekas, Styliani Kalantzi, Stamatis Sarris, Chrysovalantis Templis, Konstantinos Vavitsas, Dimitris G. Hatzinikolaou, Nikolaos Papayannakos, Dimitris Kekos, and et al. 2021. "Biodesulfurization of Dibenzothiophene and Its Alkylated Derivatives in a Two-Phase Bubble Column Bioreactor by Resting Cells of Rhodococcus erythropolis IGTS8" Processes 9, no. 11: 2064. https://doi.org/10.3390/pr9112064
APA StylePrasoulas, G., Dimos, K., Glekas, P., Kalantzi, S., Sarris, S., Templis, C., Vavitsas, K., Hatzinikolaou, D. G., Papayannakos, N., Kekos, D., & Mamma, D. (2021). Biodesulfurization of Dibenzothiophene and Its Alkylated Derivatives in a Two-Phase Bubble Column Bioreactor by Resting Cells of Rhodococcus erythropolis IGTS8. Processes, 9(11), 2064. https://doi.org/10.3390/pr9112064










