Gold(I) Complexes with P-Donor Ligands and Their Biological Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Lines and Culture Conditions
2.3. Synthesis of Complexes (1)–(3)
2.3.1. [Au(TrippyPhos)Cl] (1)
2.3.2. [Au(BippyPhos)Cl]·0.5CH2Cl2 (2)
2.3.3. [Au(meCgPPh)Cl] (3)
2.4. Experimental Methods
2.4.1. Nuclear Magnetic Resonance (NMR) Spectroscopy
2.4.2. Fast Atom Bombardment Mass Spectrometry (FAB-MS)
2.4.3. Elemental Analyses
2.4.4. X-ray Diffraction Data
2.4.5. Lipophilicity and Solution Stability
2.4.6. MTT Cell Viability Assay
2.4.7. Thioredoxin Reductase Activity Assay
3. Results and Discussion
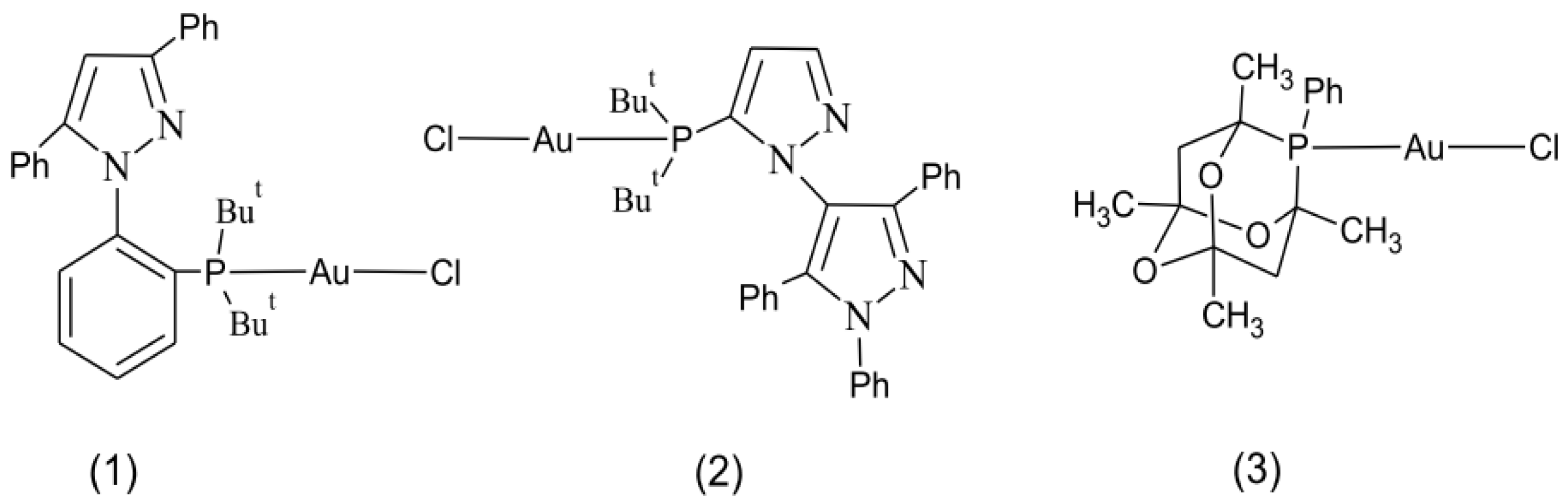
3.1. Synthesis and Characterization
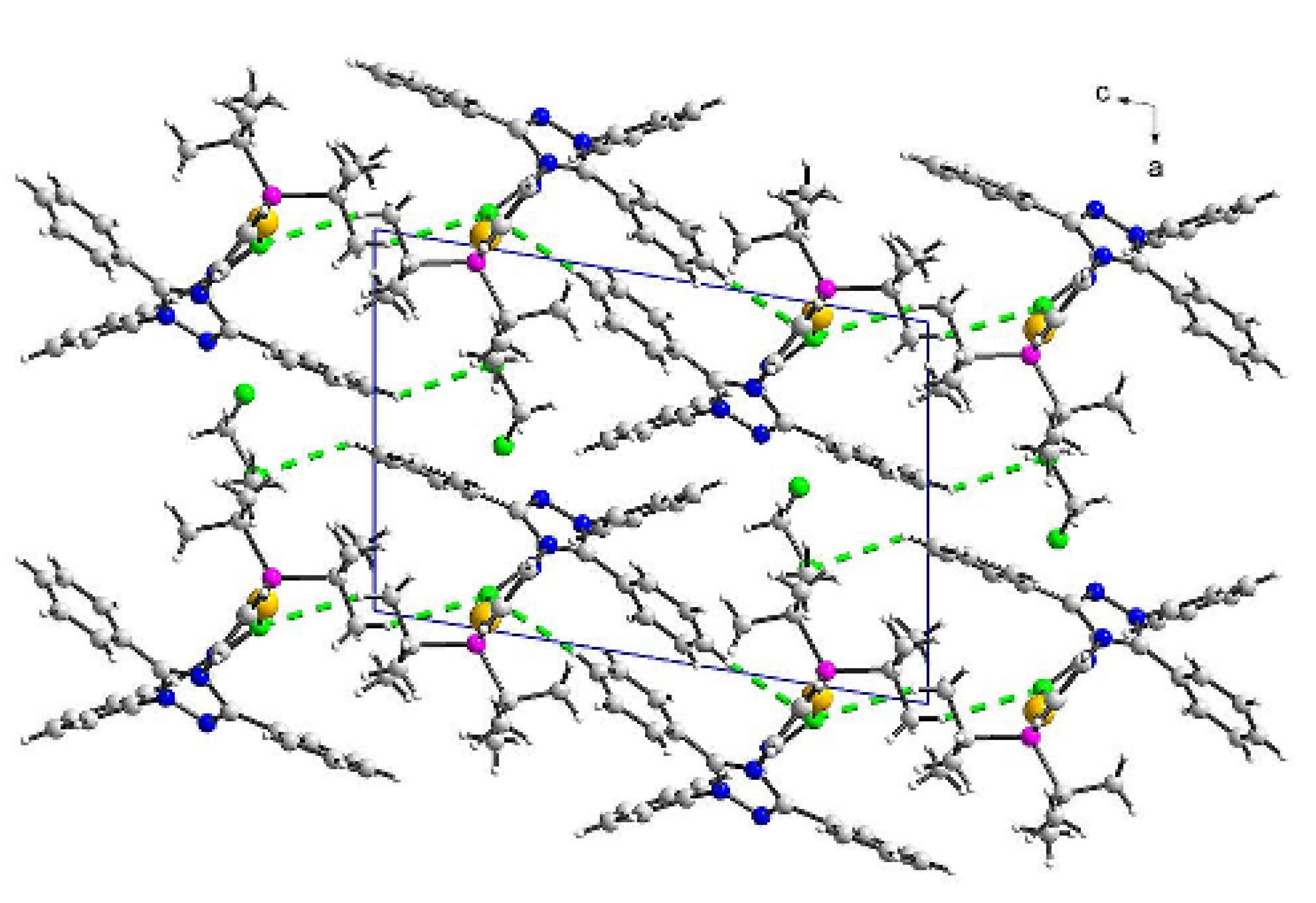
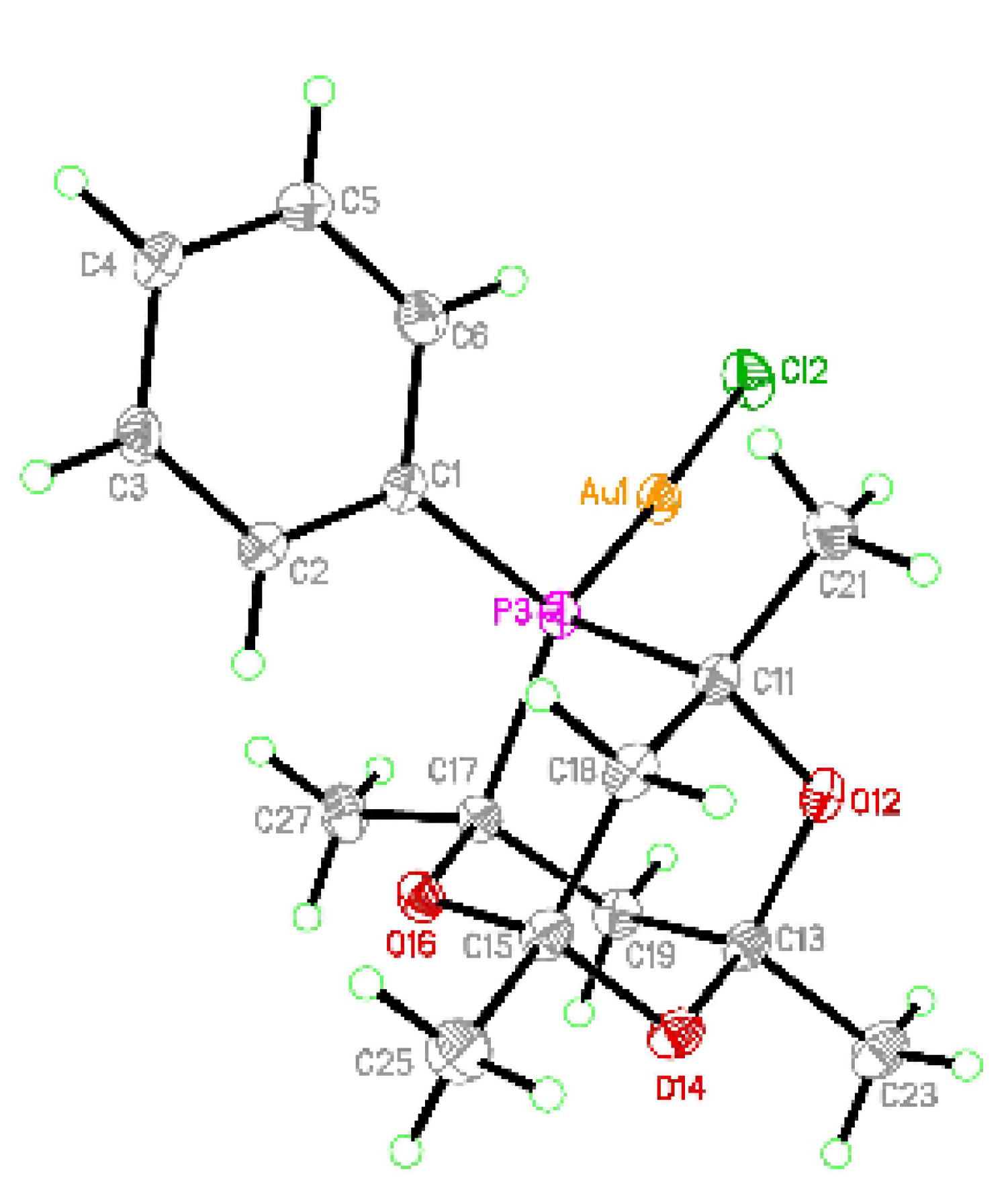
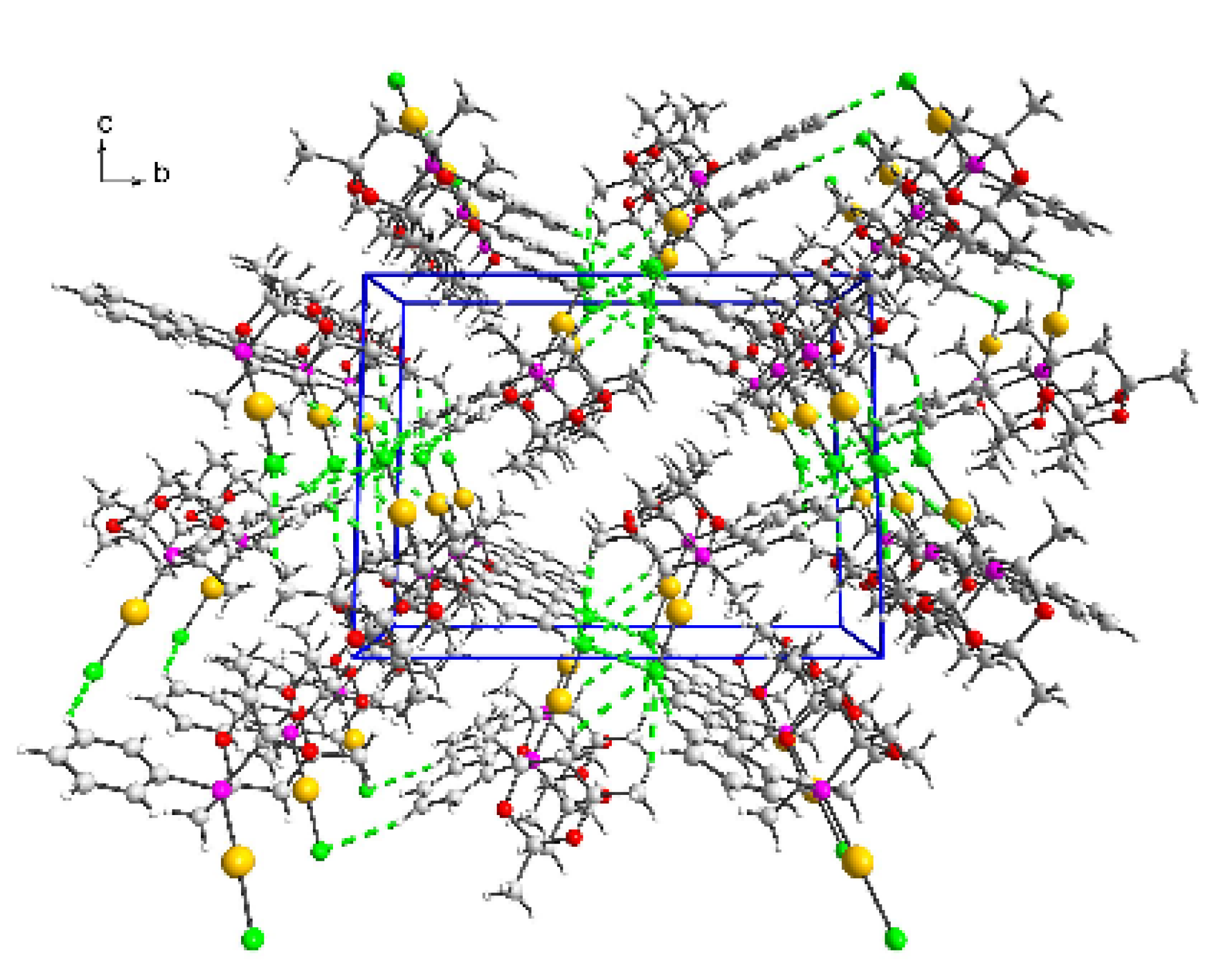
3.2. Crystal Structures of (2) and (3)
3.3. Solution Stability and Lipophilicity
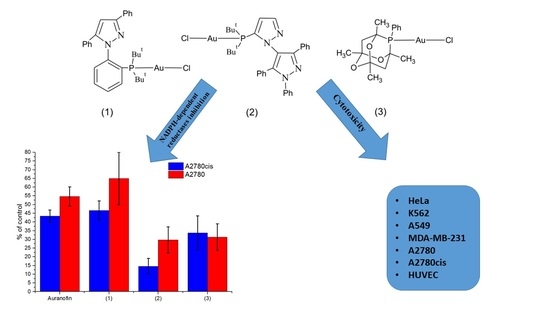
3.4. Cytotoxic Effect of Phosphine Gold(I) Complexes
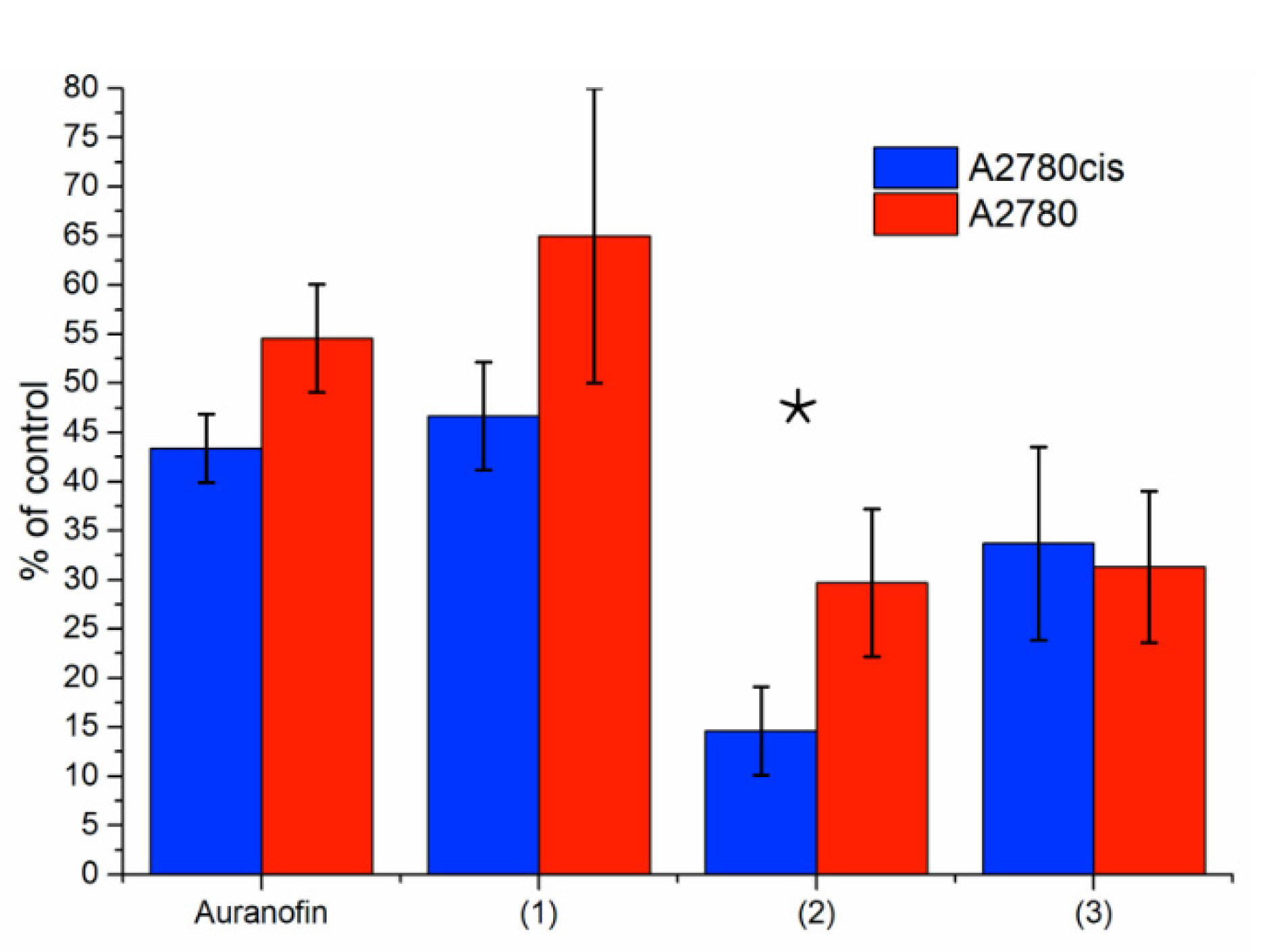
3.5. Thioredoxin Reductase Activity in A2780 and A2780cis Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, S.-H.; Lee, J.H.; Berek, J.S.; Hu, M.C.T. Auranofin displays anticancer activity against ovarian cancer cells through FOXO3 activation independent of p53. Int. J. Oncol. 2014, 45, 1691–1698. [Google Scholar] [CrossRef] [Green Version]
- Marzano, C.; Gandin, V.; Folda, A.; Scutari, G.; Bindoli, A.; Rigobello, M.P. Inhibition of thioredoxin reductase by auranofin induces apoptosis in cisplatin-resistant human ovarian cancer cells. Free Radic. Biol. Med. 2007, 42, 872–881. [Google Scholar] [CrossRef]
- Gromer, S.; Arscott, L.D.; Williams, C.H., Jr.; Schirmer, R.H.; Becker, K. Human placenta thioredoxin reductase. Isolation of the selenoenzyme, steady state kinetics, and inhibition by therapeutic gold compounds. J. Biol. Chem. 1998, 273, 20096–20101. [Google Scholar] [CrossRef] [Green Version]
- Rigobello, M.P.; Bindoli, A. Mitochondrial thioredoxin reductase purification, inhibitor studies, and role in cell signaling. Methods Enzymol. 2010, 474, 109–122. [Google Scholar]
- Karlenius, T.C.; Tonissen, K.F. Thioredoxin and Cancer: A Role for Thioredoxin in all States of Tumor Oxygenation. Cancers 2010, 2, 209–232. [Google Scholar] [CrossRef] [Green Version]
- Saccoccia, F.; Angelucci, F.; Boumis, G.; Carotti, D.; Desiato, G.; Miele, A.E.; Bellelli, A. Thioredoxin reductase and its inhibitors. Curr. Protein Pept. Sci. 2014, 15, 621–646. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Qian, J.; Zhu, P.; Hua, R.; Liu, J.; Hang, J.; Meng, C.; Shan, W.; Miao, J.; Ling, Y. Novel Phenylmethylenecyclohexenone Derivatives as Potent TrxR Inhibitors Display High Antiproliferative Activity and Induce ROS, Apoptosis, and DNA Damage. ChemMedChem 2021, 16, 702–712. [Google Scholar] [CrossRef]
- Mohammadi, F.; Soltani, A.; Ghahremanloo, A.; Javid, H.; Hashemy, S.I. The thioredoxin system and cancer therapy: A review. Cancer Chemother. Pharmacol. 2019, 84, 925–935. [Google Scholar] [CrossRef]
- Lu, J.; Chew, E.-H.; Holmgren, A. Targeting thioredoxin reductase is a basis for cancer therapy by arsenic trioxide. Proc. Natl. Acad. Sci. USA 2007, 104, 12288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powis, G.; Kirkpatrick, D.L. Thioredoxin signaling as a target for cancer therapy. Curr. Opin. Pharmacol. 2007, 7, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Butler, L.M.; Zhou, X.; Xu, W.S.; Scher, H.I.; Rifkind, R.A.; Marks, P.A.; Richon, V.M. The histone deacetylase inhibitor SAHA arrests cancer cell growth, up-regulates thioredoxin-binding protein-2, and down-regulates thioredoxin. Proc. Natl. Acad. Sci. USA 2002, 99, 11700–11705. [Google Scholar] [CrossRef] [Green Version]
- Rubbiani, R.; Kitanovic, I.; Alborzinia, H.; Can, S.; Kitanovic, A.; Onambele, L.A.; Stefanopoulou, M.; Geldmacher, Y.; Sheldrick, W.S.; Wolber, G.; et al. Benzimidazol-2-ylidene Gold(I) Complexes Are Thioredoxin Reductase Inhibitors with Multiple Antitumor Properties. J. Med. Chem. 2010, 53, 8608–8618. [Google Scholar] [CrossRef] [PubMed]
- Hickey, J.L.; Ruhayel, R.A.; Barnard, P.J.; Baker, M.V.; Berners-Price, S.J.; Filipovska, A. Mitochondria-targeted chemotherapeutics: The rational design of gold(I) N-heterocyclic carbene complexes that are selectively toxic to cancer cells and target protein selenols in preference to thiols. J. Am. Chem. Soc. 2008, 130, 12570–12571. [Google Scholar] [CrossRef]
- Kaps, L.; Biersack, B.; Müller-Bunz, H.; Mahal, K.; Münzner, J.; Tacke, M.; Mueller, T.; Schobert, R. Gold(I)–NHC complexes of antitumoral diarylimidazoles: Structures, cellular uptake routes and anticancer activities. J. Inorg. Biochem. 2012, 106, 52–58. [Google Scholar] [CrossRef]
- Gandin, V.; Fernandes, A.P.; Rigobello, M.P.; Dani, B.; Sorrentino, F.; Tisato, F.; Björnstedt, M.; Bindoli, A.; Sturaro, A.; Rella, R.; et al. Cancer cell death induced by phosphine gold(I) compounds targeting thioredoxin reductase. Biochem. Pharmacol. 2010, 79, 90–101. [Google Scholar] [CrossRef] [Green Version]
- Parrilha, G.L.; Ferraz, K.S.O.; Lessa, J.A.; de Oliveira, K.N.; Rodrigues, B.L.; Ramos, J.P.; Souza-Fagundes, E.M.; Ott, I.; Beraldo, H. Metal complexes with 2-acetylpyridine-N(4)-orthochlorophenylthiosemicarbazone: Cytotoxicity and effect on the enzymatic activity of thioredoxin reductase and glutathione reductase. Eur. J. Med. Chem. 2014, 84, 537–544. [Google Scholar] [CrossRef]
- Abbehausen, C.; Peterson, E.J.; de Paiva, R.E.F.; Corbi, P.P.; Formiga, A.L.B.; Qu, Y.; Farrell, N.P. Gold(I)-Phosphine-N-Heterocycles: Biological Activity and Specific (Ligand) Interactions on the C-Terminal HIVNCp7 Zinc Finger. Inorg. Chem. 2013, 52, 11280–11287. [Google Scholar] [CrossRef] [PubMed]
- Yeo, C.I.; Ooi, K.K.; Tiekink, E.R.T. Gold-Based Medicine: A Paradigm Shift in Anti-Cancer Therapy? Molecules 2018, 23, 1410. [Google Scholar] [CrossRef] [Green Version]
- Marzo, T.; Cirri, D.; Gabbiani, C.; Gamberi, T.; Magherini, F.; Pratesi, A.; Guerri, A.; Biver, T.; Binacchi, F.; Stefanini, M.; et al. Auranofin, Et(3)PAuCl, and Et(3)PAuI Are Highly Cytotoxic on Colorectal Cancer Cells: A Chemical and Biological Study. ACS Med. Chem. Lett. 2017, 8, 997–1001. [Google Scholar] [CrossRef]
- De Nisi, A.; Bergamini, C.; Leonzio, M.; Sartor, G.; Fato, R.; Naldi, M.; Monari, M.; Calonghi, N.; Bandini, M. Synthesis, cytotoxicity and anti-cancer activity of new alkynyl-gold(i) complexes. Dalton Trans. 2016, 45, 1546–1553. [Google Scholar] [CrossRef]
- Zou, T.; Lum, C.T.; Lok, C.-N.; Zhang, J.-J.; Che, C.-M. Chemical biology of anticancer gold(III) and gold(I) complexes. Chem. Soc. Rev. 2015, 44, 8786–8801. [Google Scholar] [CrossRef]
- Maggini, S. Classification of P,N-binucleating ligands for hetero- and homobimetallic complexes. Coord. Chem. Rev. 2009, 253, 1793–1832. [Google Scholar] [CrossRef]
- Munzeiwa, W.A.; Omondi, B.; Nyamori, V.O. Architecture and synthesis of P,N-heterocyclic phosphine ligands. Beilstein J. Org. Chem. 2020, 16, 362–383. [Google Scholar] [CrossRef] [Green Version]
- Berners-Price, S.J.; Sadler, P.J. Phosphines and Metal Phosphine Complexes: Relationship of Chemistry to Anticancer and Other Biological Activity. In Bioinorganic Chemistry; Springer: Berlin/Heidelberg, Germany, 1988; pp. 27–102. [Google Scholar]
- Murray, B.S.; Babak, M.V.; Hartinger, C.G.; Dyson, P.J. The development of RAPTA compounds for the treatment of tumors. Coord. Chem. Rev. 2016, 306, 86–114. [Google Scholar] [CrossRef]
- Phillips, A.D.; Gonsalvi, L.; Romerosa, A.; Vizza, F.; Peruzzini, M. Coordination chemistry of 1,3,5-triaza-7-phosphaadamantane (PTA): Transition metal complexes and related catalytic, medicinal and photoluminescent applications. Coord. Chem. Rev. 2004, 248, 955–993. [Google Scholar] [CrossRef]
- Bravo, J.; Bolaño, S.; Gonsalvi, L.; Peruzzini, M. Coordination chemistry of 1,3,5-triaza-7-phosphaadamantane (PTA) and derivatives. Part II. The quest for tailored ligands, complexes and related applications. Coord. Chem. Rev. 2010, 254, 555–607. [Google Scholar] [CrossRef]
- Miranda, S.; Vergara, E.; Mohr, F.; de Vos, D.; Cerrada, E.; Mendía, A.; Laguna, M. Synthesis, Characterization, and in Vitro Cytotoxicity of Some Gold(I) and Trans Platinum(II) Thionate Complexes Containing Water-Soluble PTA and DAPTA Ligands. X-ray Crystal Structures of [Au(SC4H3N2)(PTA)], trans-[Pt(SC4H3N2)2(PTA)2], trans-[Pt(SC5H4N)2(PTA)2], and trans-[Pt(SC5H4N)2(DAPTA)2]. Inorg. Chem. 2008, 47, 5641–5648. [Google Scholar] [PubMed]
- Rotta-Loria, N.L.; Chisholm, A.J.; MacQueen, P.M.; McDonald, R.; Ferguson, M.J.; Stradiotto, M. Exploring the Influence of Phosphine Ligation on the Gold-Catalyzed Hydrohydrazination of Terminal Alkynes at Room Temperature. Organometallics 2017, 36, 2470–2475. [Google Scholar] [CrossRef]
- Bats, J.W.; Hamzic, M.; Hashmi, A.S. RefCODE:GASLUK, CCDC 1538193: Experimental Crystal Structure Determination. 2017. Available online: https://www.research.manchester.ac.uk/portal/en/datasets/ccdc-1429470-experimental-crystal-structure-determination(b59b2c1f-b73c-486e-bfc7-53c2107344ef).html (accessed on 18 November 2021).
- Mann, F.G.; Wells, A.F.; Purdie, D. The constitution of complex metallic salts. Part VI. The constitution of the phosphine and arsine derivatives of silver and aurous halides. The configuration of the co-ordinated argentous and aurous complex. J. Chem. Soc. 1937, 1828–1836. [Google Scholar] [CrossRef]
- Oxford Diffraction. CrysAlis RED and CrysAlis CCD; Oxford Diffraction Ltd.: Abingdon, UK, 2006. Available online: https://journals.iucr.org/e/services/stdswrefs.html (accessed on 1 October 2021).
- Sheldrick, G.M. SHELXS97, SHELXL97 and CIFTAB; University of Göttingen Germany: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Brandenburg, K.D. Release 2.1e; Crystal Impact GbR: Bonn, Germany, 2001. [Google Scholar]
- Farrugia, L. ORTEP-3 for Windows—A version of ORTEP-III with a Graphical User Interface (GUI). J. Appl. Crystallogr. 1997, 30 Pt 1, 565. [Google Scholar] [CrossRef]
- Richert, M.; Walczyk, M.; Cieślak, M.J.; Kaźmierczak-Barańska, J.; Królewska-Golińska, K.; Wrzeszcz, G.; Muzioł, T.; Biniak, S. Synthesis, X-ray structure, physicochemical properties and anticancer activity of mer and fac Ru(iii) triphenylphosphine complexes with a benzothiazole derivative as a co-ligand. Dalton Trans. 2019, 48, 10689–10702. [Google Scholar] [CrossRef]
- Singer, R.A.; Doré, M.; Sieser, J.E.; Berliner, M.A. Development of nonproprietary phosphine ligands for the Pd-catalyzed amination reaction. Tetrahedron Lett. 2006, 47, 3727–3731. [Google Scholar] [CrossRef]
- Singer, R.A.; Caron, S.; McDermott, R.E.; Arpin, P.; Do, N.M. Alternative Biarylphosphines for Use in the Palladium-Catalyzed Amination of Aryl Halides. Synthesis 2003, 2003, 1727–1731. [Google Scholar] [CrossRef]
- Singer, R.A. BippyPhos: A Highly Versatile Ligand for Pd-Catalyzed C−N, C−O and C−C Couplings. Isr. J. Chem. 2020, 60, 294–302. [Google Scholar] [CrossRef]
- Crawford, S.M.; Lavery, C.B.; Stradiotto, M. BippyPhos: A Single Ligand With Unprecedented Scope in the Buchwald–Hartwig Amination of (Hetero)aryl Chlorides. Chem. A Eur. J. 2013, 19, 16760–16771. [Google Scholar] [CrossRef] [PubMed]
- Adjabeng, G.; Brenstrum, T.; Frampton, C.S.; Robertson, A.J.; Hillhouse, J.; McNulty, J.; Capretta, A. Palladium Complexes of 1,3,5,7-Tetramethyl-2,4,8-trioxa-6-phenyl-6-phosphaadamantane: Synthesis, Crystal Structure and Use in the Suzuki and Sonogashira Reactions and the α-Arylation of Ketones. J. Org. Chem. 2004, 69, 5082–5086. [Google Scholar] [CrossRef] [PubMed]
- Alvino, J.F.; Bennett, T.; Anderson, D.; Donoeva, B.; Ovoshchnikov, D.; Adnan, R.H.; Appadoo, D.; Golovko, V.; Andersson, G.; Metha, G.F. Far-infrared absorption spectra of synthetically-prepared, ligated metal clusters with Au6, Au8, Au9 and Au6Pd metal cores. RSC Adv. 2013, 3, 22140–22149. [Google Scholar] [CrossRef] [Green Version]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar]
- Schwarz, C.; Handelmann, J.; Baier, D.M.; Ouissa, A.; Gessner, V.H. Mono- and diylide-substituted phosphines (YPhos): Impact of the ligand properties on the catalytic activity in gold(i)-catalysed hydroaminations. Catal. Sci. Technol. 2019, 9, 6808–6815. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.-Q.; Lichte, D.; Rodstein, I.; Weber, P.; Seitz, A.-K.; Scherpf, T.; Gessner, V.H.; Gooßen, L.J. Ylide-Functionalized Phosphine (YPhos)–Palladium Catalysts: Selective Monoarylation of Alkyl Ketones with Aryl Chlorides. Org. Lett. 2019, 21, 7558–7562. [Google Scholar] [CrossRef]
- Zuccarello, G.; Mayans, J.G.; Escofet, I.; Scharnagel, D.; Kirillova, M.S.; Pérez-Jimeno, A.H.; Calleja, P.; Boothe, J.R.; Echavarren, A.M. Enantioselective Folding of Enynes by Gold(I) Catalysts with a Remote C2-Chiral Element. J. Am. Chem. Soc. 2019, 141, 11858–11863. [Google Scholar] [CrossRef]
- Hartlaub, S.F.; Lauricella, N.K.; Ryczek, C.N.; Furneaux, A.G.; Melton, J.D.; Piro, N.A.; Kassel, W.S.; Nataro, C. Late Transition Metal Compounds with 1,1′-Bis(phosphino)ferrocene Ligands. Eur. J. Inorg. Chem. 2017, 424–432. [Google Scholar] [CrossRef]
- Homs, A.; Escofet, I.; Echavarren, A.M. On the Silver Effect and the Formation of Chloride-Bridged Digold Complexes. Org. Lett. 2013, 15, 5782–5785. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Day, C.S.; Zhang, L.; Hauser, K.J.; Jones, A.C. A Unique Au–Ag–Au Triangular Motif in a Trimetallic Halonium Dication: Silver Incorporation in a Gold(I) Catalyst. Chem. A Eur. J. 2013, 19, 12264–12271. [Google Scholar] [CrossRef]
- Hu, J.-Y.; Zhang, J.; Wang, G.-X.; Sun, H.-L.; Zhang, J.-L. Constructing a Catalytic Cycle for C–F to C–X (X = O, S, N) Bond Transformation Based on Gold-Mediated Ligand Nucleophilic Attack. Inorg. Chem. 2016, 55, 2274–2283. [Google Scholar] [CrossRef]
- Hill, D.T. a. S. B.M. (2,3,4,6-Tetra-O-acetyl-1-thio-β-D-glucopyranosato-S) (triethylphosphine)gold. Cryst. Struct. Commun. 1980, 9, 679–686. [Google Scholar]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm 2002, 4, 378–392. [Google Scholar] [CrossRef]
- Kariuki, B.M.; Newman, P.D. Asymmetric Cationic Phosphines: Synthesis, Coordination Chemistry, and Reactivity. Inorg. Chem. 2018, 57, 9554–9563. [Google Scholar] [CrossRef]
- Cunningham, T.J.; Elsegood, M.R.J.; Kelly, P.F.; Smith, M.B.; Staniland, P.M. Coordination Studies of a New Nonsymmetric Ditertiary Phosphane Bearing a Single Phosphaadamantane Cage. Eur. J. Inorg. Chem. 2008, 2326–2335. [Google Scholar] [CrossRef]
- Kim, J.H.; Reeder, E.; Parkin, S.; Awuah, S.G. Gold(I/III)-Phosphine Complexes as Potent Antiproliferative Agents. Sci. Rep. 2019, 9, 12335. [Google Scholar] [CrossRef] [PubMed]
- Leeson, P.D.; Springthorpe, B. The influence of drug-like concepts on decision-making in medicinal chemistry. Nat. Rev. Drug Discov. 2007, 6, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Waring, M.J. Lipophilicity in drug discovery. Expert Opin. Drug Discov. 2010, 5, 235–248. [Google Scholar] [CrossRef]
- Hopkins, A.L.; Keserü, G.M.; Leeson, P.D.; Rees, D.C.; Reynolds, C.H. The role of ligand efficiency metrics in drug discovery. Nat. Rev. Drug Discov. 2014, 13, 105–121. [Google Scholar] [CrossRef]
- Chmiel, T.; Mieszkowska, A.; Kempińska-Kupczyk, D.; Kot-Wasik, A.; Namieśnik, J.; Mazerska, Z. The impact of lipophilicity on environmental processes, drug delivery and bioavailability of food components. Microchem. J. 2019, 146, 393–406. [Google Scholar] [CrossRef]
- Gabathuler, R. Approaches to transport therapeutic drugs across the blood-brain barrier to treat brain diseases. Neurobiol. Dis. 2010, 37, 48–57. [Google Scholar] [CrossRef]
- Deo, K.M.; Sakoff, J.; Gilbert, J.; Zhang, Y.; Aldrich Wright, J.R. Synthesis, characterisation and influence of lipophilicity on cellular accumulation and cytotoxicity of unconventional platinum(iv) prodrugs as potent anticancer agents. Dalton Trans. 2019, 48, 17228–17240. [Google Scholar] [CrossRef] [PubMed]
- Landini, I.; Lapucci, A.; Pratesi, A.; Massai, L.; Napoli, C.; Perrone, G.; Pinzani, P.; Messori, L.; Mini, E.; Nobili, S. Selection and characterization of a human ovarian cancer cell line resistant to auranofin. Oncotarget 2017, 8, 96062–96078. [Google Scholar] [CrossRef]
- Shaw, C.F. Gold-Based Therapeutic Agents. Chem. Rev. 1999, 99, 2589–2600. [Google Scholar] [CrossRef]
- Coffer, M.T.; Shaw, C.F.; Eidsness, M.K.; Watkins, J.W.; Elder, R.C. Reactions of auranofin and chloro(triethylphosphine)gold with bovine serum albumin. Inorg. Chem. 1986, 25, 333–339. [Google Scholar] [CrossRef]
- Coffer, M.T.; Shaw, C.F.; Hormann, A.L.; Mirabelli, C.K.; Crooke, S.T. Thiol competition for Et3PAuS-albumin: A nonenzymatic mechanism for Et3PO formation. J. Inorg. Biochem. 1987, 30, 177–187. [Google Scholar] [CrossRef]
- Schmidt, C.; Albrecht, L.; Balasupramaniam, S.; Misgeld, R.; Karge, B.; Brönstrup, M.; Prokop, A.; Baumann, K.; Reichl, S.; Ott, I. A gold(i) biscarbene complex with improved activity as a TrxR inhibitor and cytotoxic drug: Comparative studies with different gold metallodrugs. Metallomics 2019, 11, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Tolbatov, I.; Cirri, D.; Marchetti, L.; Marrone, A.; Coletti, C.; Re, N.; La Mendola, D.; Messori, L.; Marzo, T.; Gabbiani, C.; et al. Mechanistic Insights Into the Anticancer Properties of the Auranofin Analog Au(PEt3)I: A Theoretical and Experimental Study. Front. Chem. 2020, 8. [Google Scholar] [CrossRef] [PubMed]


| Compound | δH(CH3) (tBu) | δC(1-6)(CH3) (tBu) | δC(7,8) (tBu) | δP–C9(Ph) | δ(N–C14(Ph)) | δP |
|---|---|---|---|---|---|---|
| (1) | 1.13d, 1.37d (+0.32, +0.28) | 31.5d, 32.1d (+1.1, +1.7) | 39.0 d, 39.3d (+6.4, +6.7) | 133.1 d (+3.0) | 142.2 s (+6.0) | 57.6 (+38.6) |
| TrippyPhos | 0.81, 1.09 | 30.4 s | 32.6 s | 130.1 | 136.2 | 19.0 |
| Compound | IC50 [μΜ] | ||||||
|---|---|---|---|---|---|---|---|
| HeLa | K562 | A549 | MDA-MB-231 | A2780 | A2780cis | HUVEC | |
| 1 | 1.7 ± 0.9 | 0.5 ± 0.4 | 0.8 ± 0.3 | 10.7 ± 3.0 | 1.42 ± 0.14 * | 7.05 ± 0.67 | 2.00±0.07 |
| 2 | 3.0 ± 0.8 | 1.5 ± 1.0 | 5.0 ± 2.6 | 13.4 ± 3.9 | 1.67 ± 0.51 | 3.03 ± 0.35 | 27±0.56 |
| 3 | 22 ± 5.3 | 15 ± 3.1 | 19 ± 2.9 | 5.50 ± 0.25 | 2.8 ± 0.43 | 2.23 ± 0.25 | 5.5±0.44 |
| auranofin | 0.8 ±0.1 | 0.3 ±0.04 | 3.0±0.3 | 5.43 ± 0.95 | 2.32 ± 0.19 | 3.68 ± 0.08 | 0.27±0.01 |
| cis-Pt | 20 ± 6.0 | 40 ± 7.0 | 30 ± 6.5 | 0.79 ± 0.24 | 4.45 ± 0.78 | 33.33 ± 0.94 | 30 ± 6.5 |
| Cell Line | NADPH-Dependent Reductases [Unit/108 Cells ± SD] | TrxR Activity [Unit/108 Cells ± SD] |
|---|---|---|
| A2780 | 0.69 ± 0.17 | 0.270 ± 0.078 |
| A2780cis | 1.28 ± 0.19 | 0.629 ± 0.102 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Richert, M.; Mikstacka, R.; Walczyk, M.; Cieślak, M.J.; Kaźmierczak-Barańska, J.; Królewska-Golińska, K.; Muzioł, T.M.; Biniak, S. Gold(I) Complexes with P-Donor Ligands and Their Biological Evaluation. Processes 2021, 9, 2100. https://doi.org/10.3390/pr9122100
Richert M, Mikstacka R, Walczyk M, Cieślak MJ, Kaźmierczak-Barańska J, Królewska-Golińska K, Muzioł TM, Biniak S. Gold(I) Complexes with P-Donor Ligands and Their Biological Evaluation. Processes. 2021; 9(12):2100. https://doi.org/10.3390/pr9122100
Chicago/Turabian StyleRichert, Monika, Renata Mikstacka, Mariusz Walczyk, Marcin Janusz Cieślak, Julia Kaźmierczak-Barańska, Karolina Królewska-Golińska, Tadeusz Mikołaj Muzioł, and Stanisław Biniak. 2021. "Gold(I) Complexes with P-Donor Ligands and Their Biological Evaluation" Processes 9, no. 12: 2100. https://doi.org/10.3390/pr9122100