Bimetallic Pt-Co Nanoparticle Deposited on Alumina for Simultaneous CO and Toluene Oxidation in the Presence of Moisture
Abstract
1. Introduction
2. Experimental Section
Synthesis of Pt/Al2O3 Catalyst
3. Results and Discussion
3.1. XRD Analysis
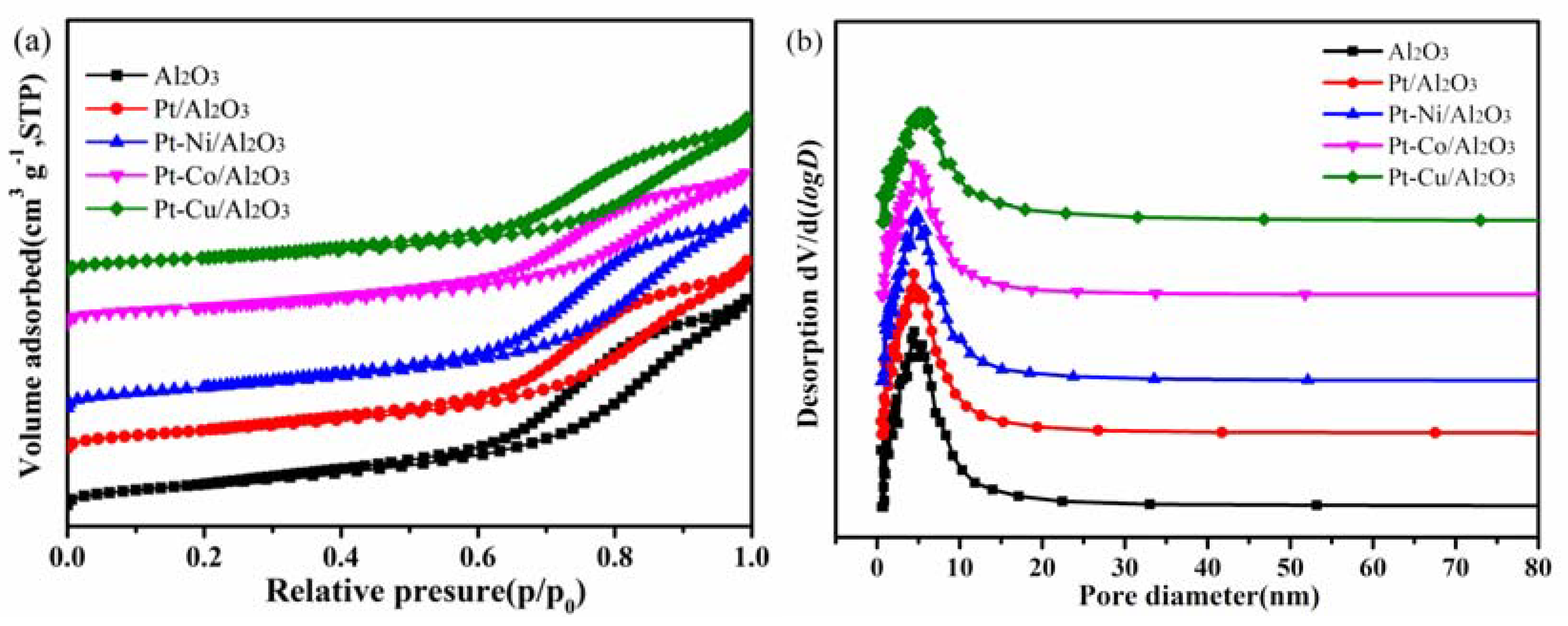
3.2. Surface Area Analysis
3.3. Microstructure
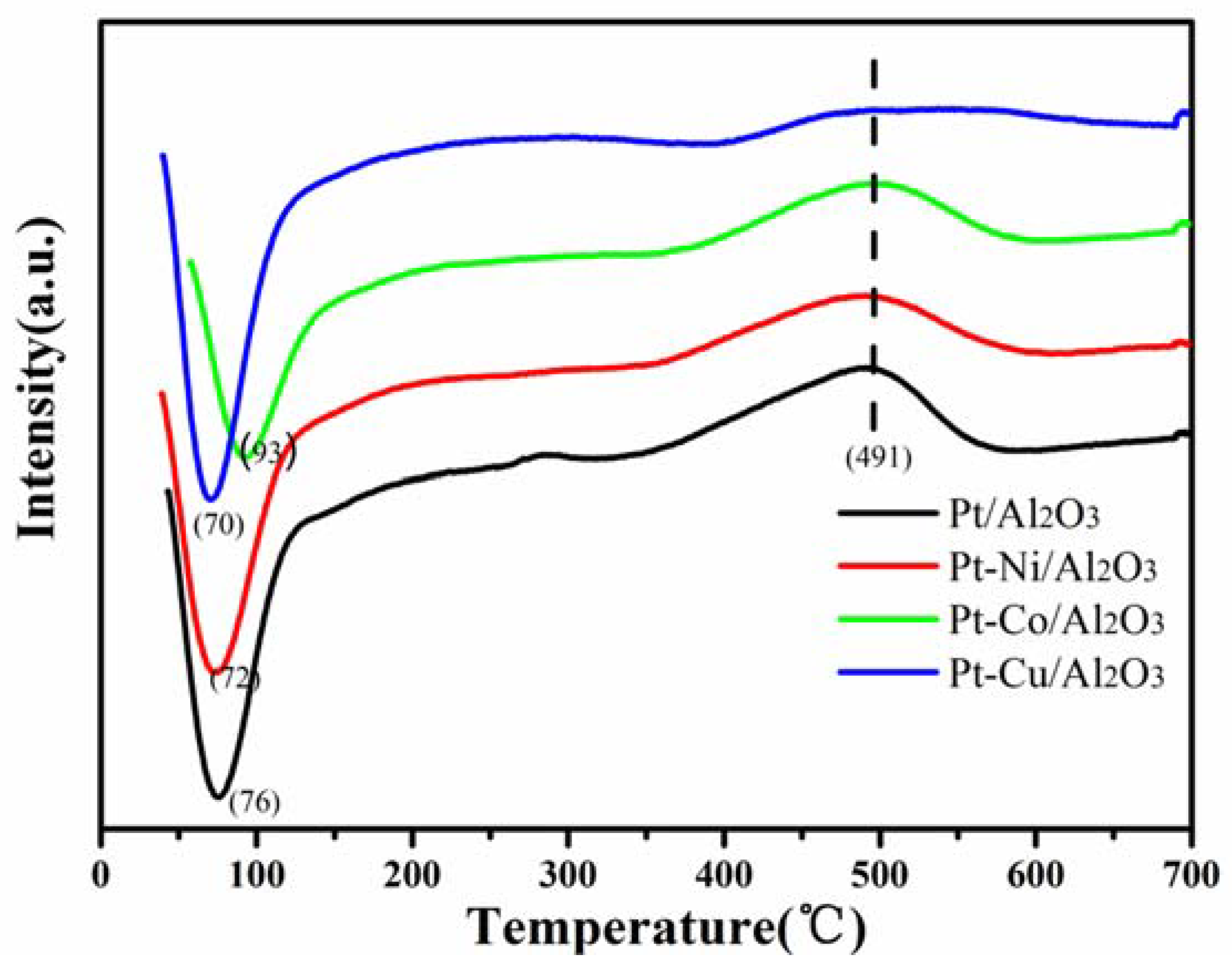
3.4. Temperature Programmed Reduction (TPR)
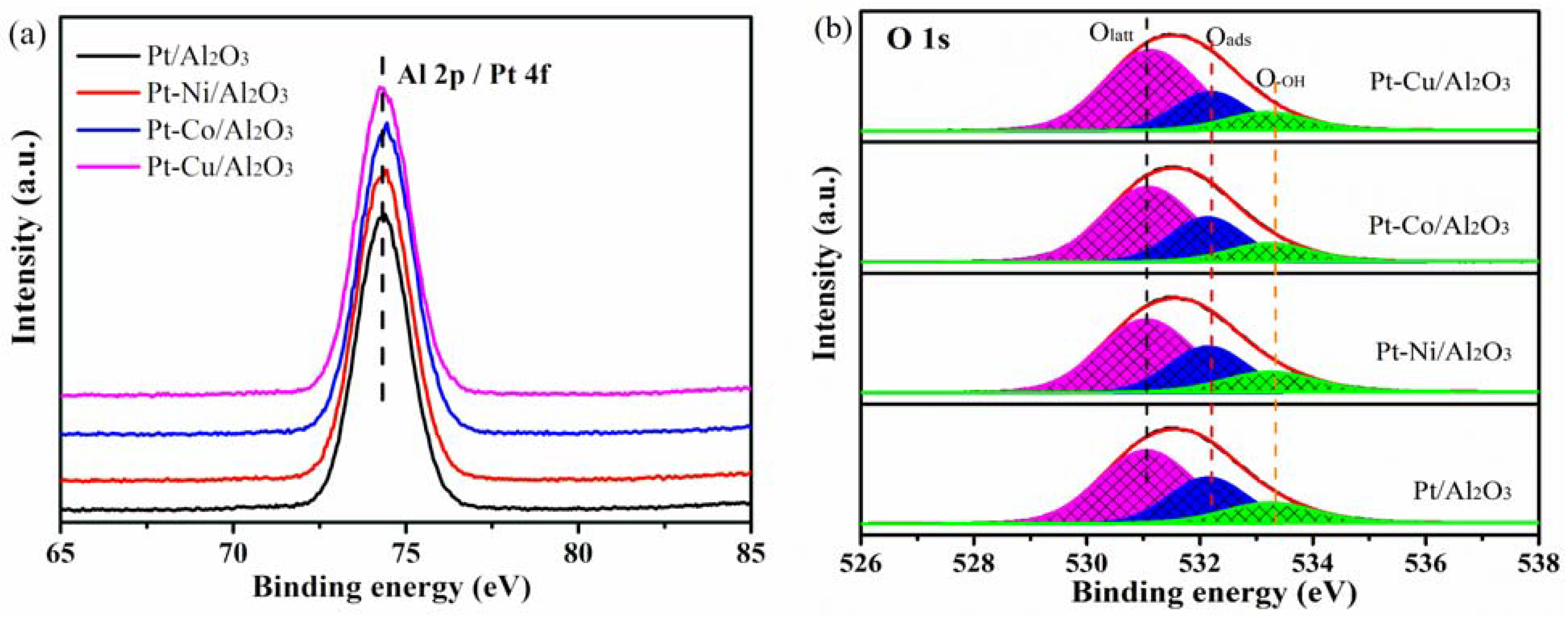
3.5. Surface Element Composition
3.6. Oxygen Temperature-Programmed Desorption (O2-TPD)
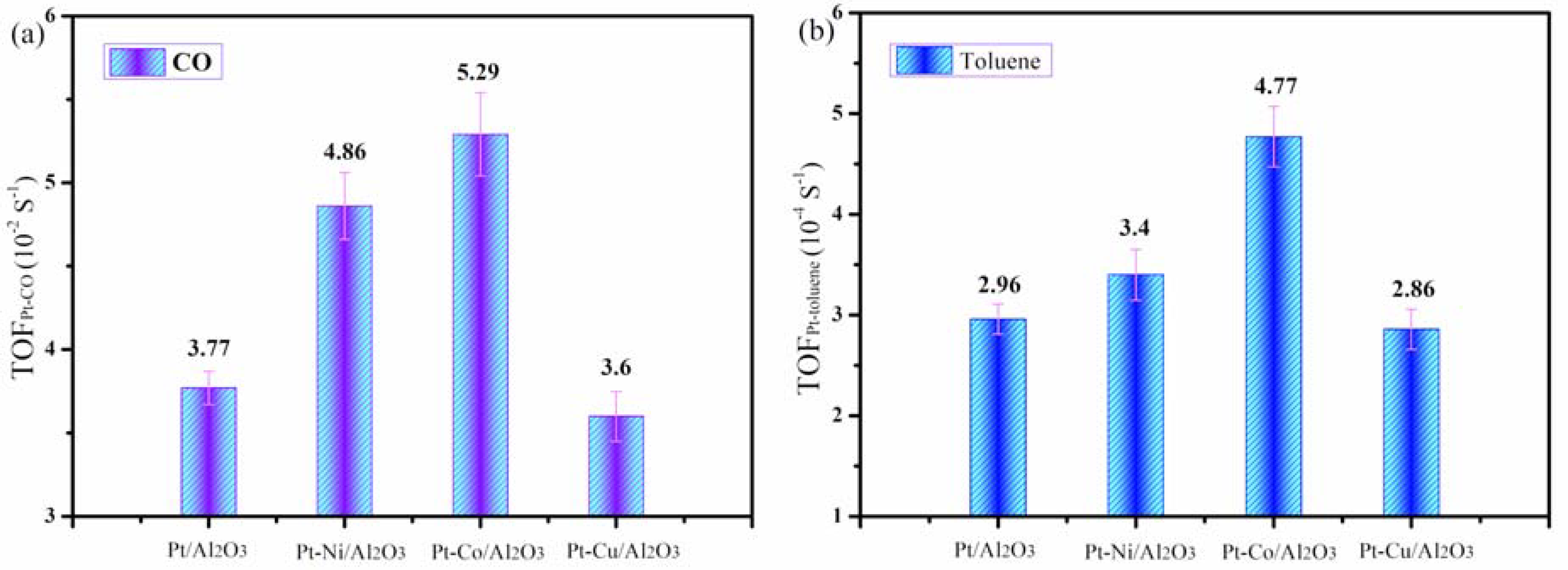
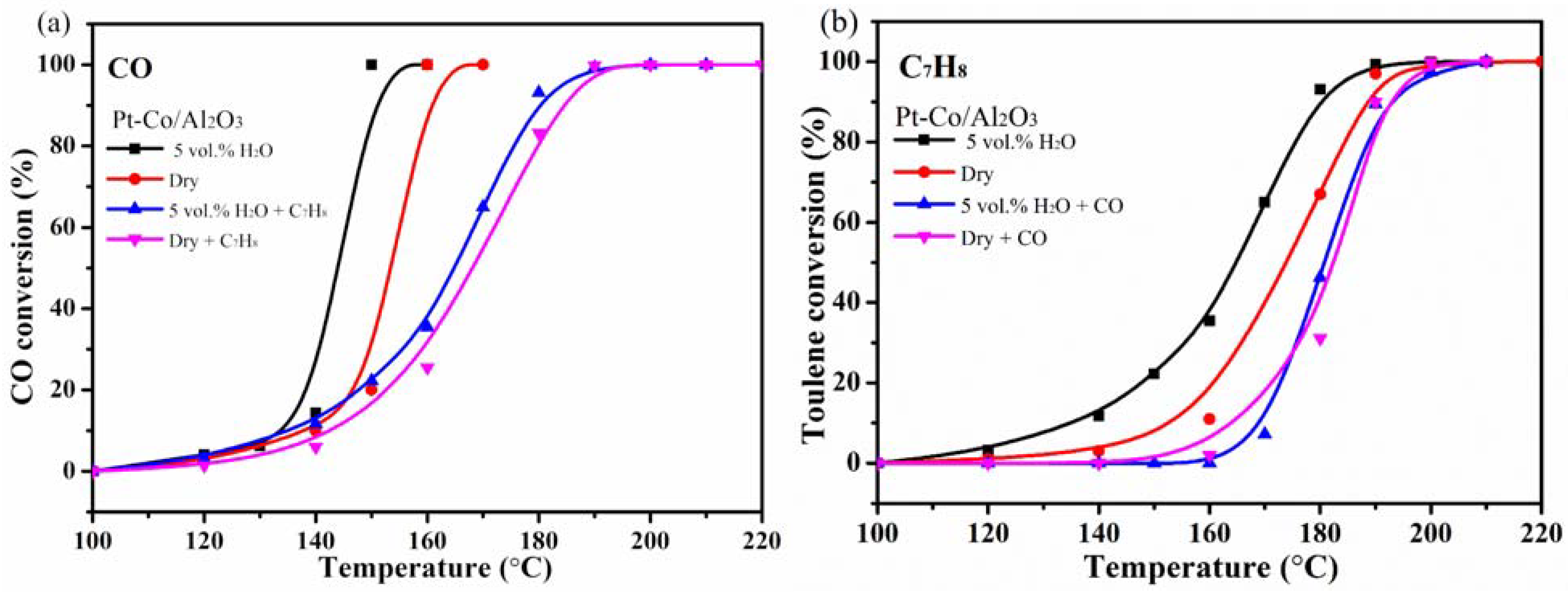
3.7. Catalytic Activity Measurement
3.8. The Effects of WHSV and Moisture
3.9. Catalytic Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Russell, A.; Epling, W.S. Diesel Oxidation Catalysts. Catal. Rev. 2011, 53, 337–423. [Google Scholar] [CrossRef]
- Feng, X.; Guo, J.; Wen, X.; Xu, M.; Chu, Y.; Yuan, S. Enhancing performance of Co/CeO2 catalyst by Sr doping for catalytic combustion of toluene. App. Surf. Sci. 2018, 445, 145–153. [Google Scholar] [CrossRef]
- Peng, R.; Li, S.; Sun, X.; Ren, Q.; Chen, L.; Fu, M.; Wu, J.; Ye, D. Size effect of Pt nanoparticles on the catalytic oxidation of toluene over Pt/CeO2 catalysts. Appl. Catal. B Environ. 2018, 220, 462–470. [Google Scholar] [CrossRef]
- Torrente-Murciano, L.; Solsona, B.; Agouram, S.; Sanchis, R.; López, J.M.; García, T.; Zanella, R. Low temperature total oxidation of toluene by bimetallic Au–Ir catalysts. Catal. Sci. Technol. 2017, 7, 2886–2896. [Google Scholar] [CrossRef]
- Mo, S.; Li, S.; Ren, Q.; Zhang, M.; Sun, Y.; Wang, B.; Feng, Z.; Zhang, Q.; Chen, Y.; Ye, D. Vertically-aligned Co3O4 arrays on Ni foam as monolithic structured catalysts for CO oxidation: Effects of morphological transformation. Nanoscale 2018, 10, 7746–7758. [Google Scholar] [CrossRef]
- Wang, H.; Lu, Y.; Han, Y.; Lu, C.; Wan, H.; Xu, Z.; Zheng, S. Enhanced catalytic toluene oxidation by interaction between copper oxide and manganese oxide in Cu-O-Mn/γ-Al2O3 catalysts. App. Surf. Sci. 2017, 420, 260–266. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Fang, S.; Yang, Y.; Zhao, X. UV–Vis-infrared light-driven thermocatalytic abatement of benzene on Fe doped OMS-2 nanorods enhanced by a novel photoactivation. Chem. Eng. J. 2018, 332, 205–215. [Google Scholar] [CrossRef]
- Cheng, L.; Men, Y.; Wang, J.; Wang, H.; An, W.; Wang, Y.; Duan, Z.; Liu, J. Crystal facet-dependent reactivity of α-Mn2O3 microcrystalline catalyst for soot combustion. Appl. Catal. B Environ. 2017, 204, 374–384. [Google Scholar] [CrossRef]
- Li, S.; Wang, D.; Wu, X.; Chen, Y. Recent advance on VOCs oxidation over layered double hydroxides derived mixed metal oxides. Chin. J. Catal. 2020, 41, 550–560. [Google Scholar] [CrossRef]
- Ma, L.; Seo, C.Y.; Chen, X.; Li, J.; Schwank, J.W. Sodium-promoted Ag/CeO2 nanospheres for catalytic oxidation of formaldehyde. Chem. Eng. J. 2018, 350, 419–428. [Google Scholar] [CrossRef]
- Kamal, M.S.; Razzak, S.A.; Hossain, M.M. Catalytic oxidation of volatile organic compounds (VOCs)—A review. Atmos. Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- Mo, S.; Li, S.; Xiao, H.; He, H.; Xue, Y.; Zhang, M.; Ren, Q.; Chen, B.; Chen, Y.; Ye, D. Low-temperature CO oxidation over integrated penthorum chinense-like MnCo2O4 arrays anchored on three-dimensional Ni foam with enhanced moisture resistance. Catal. Sci. Technol. 2018, 8, 1663–1676. [Google Scholar] [CrossRef]
- Mo, S.; Zhang, Q.; Li, S.; Ren, Q.; Zhang, M.; Xue, Y.; Peng, R.; Xiao, H.; Chen, Y.; Ye, D. Integrated Cobalt Oxide Based Nanoarray Catalysts with Hierarchical Architectures: In Situ Raman Spectroscopy Investigation on the Carbon Monoxide Reaction Mechanism. ChemCatChem 2018, 10, 3012–3026. [Google Scholar] [CrossRef]
- Mo, S.; Zhang, Q.; Sun, Y.; Zhang, M.; Li, J.; Ren, Q.; Fu, M.; Wu, J.; Chen, L.; Ye, D. Gaseous CO and toluene co-oxidation over monolithic core–shell Co3O4-based hetero-structured catalysts. J. Mater. Chem. A 2019, 7, 16197–16210. [Google Scholar] [CrossRef]
- Slavinskaya, E.M.; Stadnichenko, A.I.; Muravyov, V.V.; Kardash, T.Y.; Derevyannikova, E.A.; Zaikovskii, V.I.; Stonkus, O.A.; Lapin, I.N.; Svetlichnyi, V.A.; Boronin, A.I. Transformation of a Pt-CeO2 Mechanical Mixture of Pulsed-Laser-Ablated Nanoparticles to a Highly Active Catalyst for Carbon Monoxide Oxidation. ChemCatChem 2018, 10, 2232–2247. [Google Scholar] [CrossRef]
- Mo, S.; Zhang, Q.; Zhang, M.; Zhang, Q.; Li, J.; Fu, M.; Wu, J.; Chen, P.; Ye, D. Elucidating the special role of strong metal–support interactions in Pt/MnO2 catalysts for total toluene oxidation. Nanoscale Horiz. 2019, 4, 1425–1433. [Google Scholar] [CrossRef]
- Zhang, Y.; Cattrall, R.W.; McKelvie, I.D.; Kolev, S.D. Gold, an alternative to platinum group metals in automobile catalytic converters. Gold Bull. 2011, 44, 145–153. [Google Scholar] [CrossRef]
- Dadi, R.K.; Luss, D.; Balakotaiah, V. Bifurcation features of mixtures containing CO and hydrocarbons in diesel oxidation catalyst. Chem. Eng. J. 2016, 304, 941–952. [Google Scholar] [CrossRef]
- Buzková Arvajová, A.; Březina, J.; Pečinka, R.; Kočí, P. Modeling of two-step CO oxidation light-off on Pt/γ-Al2O3 in the presence of C3H6 and NOx. Appl. Catal. B Environ. 2018, 233, 167–174. [Google Scholar] [CrossRef]
- Ma, L.; Seo, C.Y.; Chen, X.; Sun, K.; Schwank, J.W. Indium-doped Co3O4 nanorods for catalytic oxidation of CO and C3H6 towards diesel exhaust. Appl. Catal. B Environ. 2018, 222, 44–58. [Google Scholar] [CrossRef]
- Zhang, Q.; Mo, S.; Li, J.; Sun, Y.; Zhang, M.; Chen, P.; Fu, M.; Wu, J.; Chen, L.; Ye, D. In situ DRIFT spectroscopy insights into the reaction mechanism of CO and toluene co-oxidation over Pt-based catalysts. Catal. Sci. Technol. 2019, 9, 4538–4551. [Google Scholar] [CrossRef]
- Wen, M.; Yang, D.; Wu, Q.S.; Lu, R.P.; Zhu, Y.Z.; Zhang, F. Inducing synthesis of amorphous EuFePt nanorods and their comprehensive enhancement of magnetism, thermostability and photocatalysis. Chem. Commun. 2010, 46, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Liu, M.; Liu, H.; Chen, Q.; Yin, Y.; Jin, M. Construction of Pd-M (M = Ni, Ag, Cu) alloy surfaces for catalytic applications. Nano Res. 2017, 11, 780–790. [Google Scholar] [CrossRef]
- Fang, H.; Yang, J.; Wen, M.; Wu, Q. Nanoalloy Materials for Chemical Catalysis. Adv. Mater. 2018, 30, 1705698–1705707. [Google Scholar] [CrossRef]
- Xu, L.; Chen, D.; Qu, J.; Wang, L.; Tang, J.; Liu, H.; Yang, J. Replacement reaction-based synthesis of supported palladium catalysts with atomic dispersion for catalytic removal of benzene. J. Mater. Chem. A 2018, 6, 17032–17039. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, Z.; Cao, L.; Chen, Y.; Zhu, E.; Lin, Z.; Li, M.; Yan, A.; Zettl, A.; Wang, Y.M.; et al. High-performance transition metal–doped Pt3Ni octahedra for oxygen reduction reaction. Science 2015, 348, 1230–1232. [Google Scholar] [CrossRef]
- Kim, H.Y.; Cho, S.; Sa, Y.J.; Hwang, S.M.; Park, G.G.; Shin, T.J.; Jeong, H.Y.; Yim, S.D.; Joo, S.H. Self-Supported Mesostructured Pt-Based Bimetallic Nanospheres Containing an Intermetallic Phase as Ultrastable Oxygen Reduction Electrocatalysts. Small 2016, 12, 5347–5353. [Google Scholar] [CrossRef]
- Sato, K.; Ito, A.; Tomonaga, H.; Kanematsu, H.; Wada, Y.; Asakura, H.; Hosokawa, S.; Tanaka, T.; Toriyama, T.; Yamamoto, T.; et al. Isolated electron-rich Pt at the surface of Pt-Co alloy nanoparticles on γ-Al₂O₃ support: Synergistic effect between Pt and Co for exhaust purification. ChemPlusChem 2019, 84, 447–456. [Google Scholar] [CrossRef]
- Shen, W.-J.; Okumura, M.; Matsumura, Y.; Haruta, M. The influence of the support on the activity and selectivity of Pd in CO hydrogenation. Appl. Catal. A Gen. 2001, 213, 225–232. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, Y.; Guo, Y.; Wang, H.; Wang, L.; Lou, Y.; Guo, Y.; Lu, G.; Wang, Y. The effects of the Pd chemical state on the activity of Pd/Al2O3 catalysts in CO oxidation. Catal. Sci. Technol. 2014, 4, 3973–3980. [Google Scholar] [CrossRef]
- Xu, X.; Fu, Q.; Wei, M.; Wu, X.; Bao, X. Comparative studies of redox behaviors of Pt–Co/SiO2 and Au–Co/SiO2 catalysts and their activities in CO oxidation. Catal. Sci. Technol. 2014, 4, 3151–3158. [Google Scholar] [CrossRef]
- Wong, A.; Liu, Q.; Griffin, S.; Nicholls, A.; Regalbuto, J.R. Synthesis of ultrasmall, homogeneously alloyed, bimetallic nanoparticles on silica supports. Science 2017, 358, 1427–1430. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Xiao, W.; Wang, S.; Ren, Z.; Ding, J.; Gao, P.-X. Boosting catalytic propane oxidation over PGM-free Co3O4 nanocrystal aggregates through chemical leaching: A comparative study with Pt and Pd based catalysts. Appl. Catal. B Environ. 2018, 226, 585–595. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.; Yu, E.; Cai, S.; Jia, H.; Chen, J.; Liang, P. In situ pyrolysis of Ce-MOF to prepare CeO2 catalyst with obviously improved catalytic performance for toluene combustion. Chem. Eng. J. 2018, 344, 469–479. [Google Scholar] [CrossRef]
- Wang, Y.-Z.; Zhao, Y.-X.; Gao, C.-G.; Liu, D.-S. Origin of the High Activity and Stability of Co3O4 in Low-temperature CO Oxidation. Catal. Lett. 2008, 125, 134–138. [Google Scholar] [CrossRef]
- Jha, A.; Mhamane, D.; Suryawanshi, A.; Joshi, S.M.; Shaikh, P.; Biradar, N.; Ogale, S.; Rode, C.V. Triple nanocomposites of CoMn2O4, Co3O4 and reduced graphene oxide for oxidation of aromatic alcohols. Catal. Sci. Technol. 2014, 4, 1771. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, Y.; Wu, X.; Zhan, Y.; Wang, X.; Au, C.-T.; Jiang, L. Synthesis of Co-Mn oxides with double-shelled nanocages for low-temperature toluene combustion. Catal. Sci. Technol. 2018, 8, 4494–4502. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, X.; Fan, W.; Wang, Y. Manganese-promoted cobalt oxide as efficient and stable non-noble metal catalyst for preferential oxidation of CO in H2 stream. Appl. Catal. B Environ. 2011, 102, 207–214. [Google Scholar] [CrossRef]
- Zhang, Q.; Mo, S.; Chen, B.; Zhang, W.; Huang, C.; Ye, D. Hierarchical Co3O4 nanostructures in-situ grown on 3D nickel foam towards toluene oxidation. Mol. Catal. 2018, 454, 12–20. [Google Scholar] [CrossRef]
- Luo, Y.; Zheng, Y.; Zuo, J.; Feng, X.; Wang, X.; Zhang, T.; Zhang, K.; Jiang, L. Insights into the high performance of Mn-Co oxides derived from metal-organic frameworks for total toluene oxidation. J. Hazard. Mater. 2018, 349, 119–127. [Google Scholar] [CrossRef]
- Mo, S.; Li, S.; Li, W.; Li, J.; Chen, J.; Chen, Y. Excellent low temperature performance for total benzene oxidation over mesoporous CoMnAl composited oxides from hydrotalcites. J. Mater. Chem. A 2016, 4, 8113–8122. [Google Scholar] [CrossRef]
- Mo, S.; Li, S.; Li, J.; Deng, Y.; Peng, S.; Chen, J.; Chen, Y. Rich surface Co(III) ions-enhanced Co nanocatalyst benzene/toluene oxidation performance derived from CoIICoIII layered double hydroxide. Nanoscale 2016, 8, 15763–15773. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Liu, Y.; Deng, J.; Yang, J.; Zhao, X.; Han, Z.; Zhang, K.; Dai, H. Insights into the active sites of ordered mesoporous cobalt oxide catalysts for the total oxidation of o -xylene. J. Catal. 2017, 352, 282–292. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, L.; Chen, M.; Shi, C. CoMnxOy nanosheets with molecular-scale homogeneity: An excellent catalyst for toluene combustion. Catal. Sci. Technol. 2018, 8, 459–471. [Google Scholar] [CrossRef]
- Tian, Z.-Y.; Tchoua Ngamou, P.H.; Vannier, V.; Kohse-Höinghaus, K.; Bahlawane, N. Catalytic oxidation of VOCs over mixed Co–Mn oxides. Appl. Catal. B Environ. 2012, 117–118, 125–134. [Google Scholar] [CrossRef]
- Daté, M.; Okumura, M.; Tsubota, S.; Haruta, M. Vital Role of Moisture in the Catalytic Activity of Supported Gold Nanoparticles. Angew. Chem. Int. Ed. 2004, 116, 2181–2184. [Google Scholar] [CrossRef]
- Wang, H.F.; Kavanagh, R.; Guo, Y.L.; Guo, Y.; Lu, G.Z.; Hu, P. Structural origin: Water deactivates metal oxides to CO oxidation and promotes low-temperature CO oxidation with metals. Angew. Chem. Int. Ed. 2012, 51, 6657–6661. [Google Scholar] [CrossRef]
- Chen, B.-B.; Shi, C.; Crocker, M.; Wang, Y.; Zhu, A.-M. Catalytic removal of formaldehyde at room temperature over supported gold catalysts. Appl. Catal. B Environ. 2013, 132–133, 245–255. [Google Scholar] [CrossRef]




| Sample | Pt Loading (wt.%) | M (Ni, Co and Cu) Loading (wt.%) | SBET (m2 g−1) | Vpore (cm3 g−1) | Pore Diameter (nm) | Pt a Dispersion (%) | TOFPt-CO b (10−2 S−1) | TOFPt-toluene b (10−4 S−1) |
|---|---|---|---|---|---|---|---|---|
| Pt-Al2O3 | 0.49 | — | 269.99 | 0.91 | 6.77 | 47.67 | 3.77 | 2.96 |
| Pt/Ni-Al2O3 | 0.47 | 0.41 | 295.81 | 0.95 | 6.43 | 51.07 | 4.86 | 3.40 |
| Pt/Co-Al2O3 | 0.48 | 0.43 | 226.25 | 0.73 | 6.44 | 59.97 | 5.29 | 4.77 |
| Pt/Cu-Al2O3 | 0.48 | 0.44 | 205.99 | 0.75 | 7.25 | 52.63 | 3.60 | 2.86 |
| Al2O3 | — | — | 303.34 | 1.00 | 6.62 | — | — | — |
| Sample | Olatt BE (eV) | Oads BE (eV) | O-OH BE (eV) | Olatt (%) | Oads (%) | OOH (%) |
|---|---|---|---|---|---|---|
| Pt/Al2O3 | 531.17 | 532.23 | 533.41 | 60.9 | 23 | 16.1 |
| Pt-Ni/Al2O3 | 531.06 | 532.24 | 533.4 | 59.7 | 27.0 | 13.3 |
| Pt-Co/Al2O3 | 531.08 | 532.13 | 533.23 | 57.5 | 27.3 | 15.2 |
| Pt-Cu/Al2O3 | 531.13 | 532.19 | 533.18 | 63.6 | 23.2 | 13.2 |
| Sample | Temperature (°C) | Simple Conditions | Mixture Conditions | ||||
|---|---|---|---|---|---|---|---|
| T10/°C | T50/°C | T99/°C | T10/°C | T50/°C | T99/°C | ||
| Pt/Al2O3 | CO | 144 | 169 | 180 | 155 | 180 | 200 |
| Toluene | 150 | 182 | 200 | 176 | 190 | 200 | |
| Pt-Ni/Al2O3 | CO | 140 | 155 | 160 | 139 | 172 | 220 |
| Toluene | 130 | 172 | 220 | 166 | 190 | 220 | |
| Pt-Co/Al2O3 | CO | 140 | 155 | 160 | 142 | 168 | 190 |
| Toluene | 153 | 174 | 195 | 165 | 182 | 200 | |
| Pt-Cu/Al2O3 | CO | 143 | 156 | 160 | 160 | 185 | 210 |
| Toluene | 158 | 185 | 210 | 182 | 198 | 220 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, P.; Li, J.; Mo, S.; Zhang, Q.; Shen, T.; Xie, Q. Bimetallic Pt-Co Nanoparticle Deposited on Alumina for Simultaneous CO and Toluene Oxidation in the Presence of Moisture. Processes 2021, 9, 230. https://doi.org/10.3390/pr9020230
Peng P, Li J, Mo S, Zhang Q, Shen T, Xie Q. Bimetallic Pt-Co Nanoparticle Deposited on Alumina for Simultaneous CO and Toluene Oxidation in the Presence of Moisture. Processes. 2021; 9(2):230. https://doi.org/10.3390/pr9020230
Chicago/Turabian StylePeng, Peng, Jun Li, Shengpeng Mo, Qi Zhang, Taiming Shen, and Qinglin Xie. 2021. "Bimetallic Pt-Co Nanoparticle Deposited on Alumina for Simultaneous CO and Toluene Oxidation in the Presence of Moisture" Processes 9, no. 2: 230. https://doi.org/10.3390/pr9020230
APA StylePeng, P., Li, J., Mo, S., Zhang, Q., Shen, T., & Xie, Q. (2021). Bimetallic Pt-Co Nanoparticle Deposited on Alumina for Simultaneous CO and Toluene Oxidation in the Presence of Moisture. Processes, 9(2), 230. https://doi.org/10.3390/pr9020230







