Integration of Gasification and Solid Oxide Fuel Cells (SOFCs) for Combined Heat and Power (CHP)
Abstract
:1. Introduction
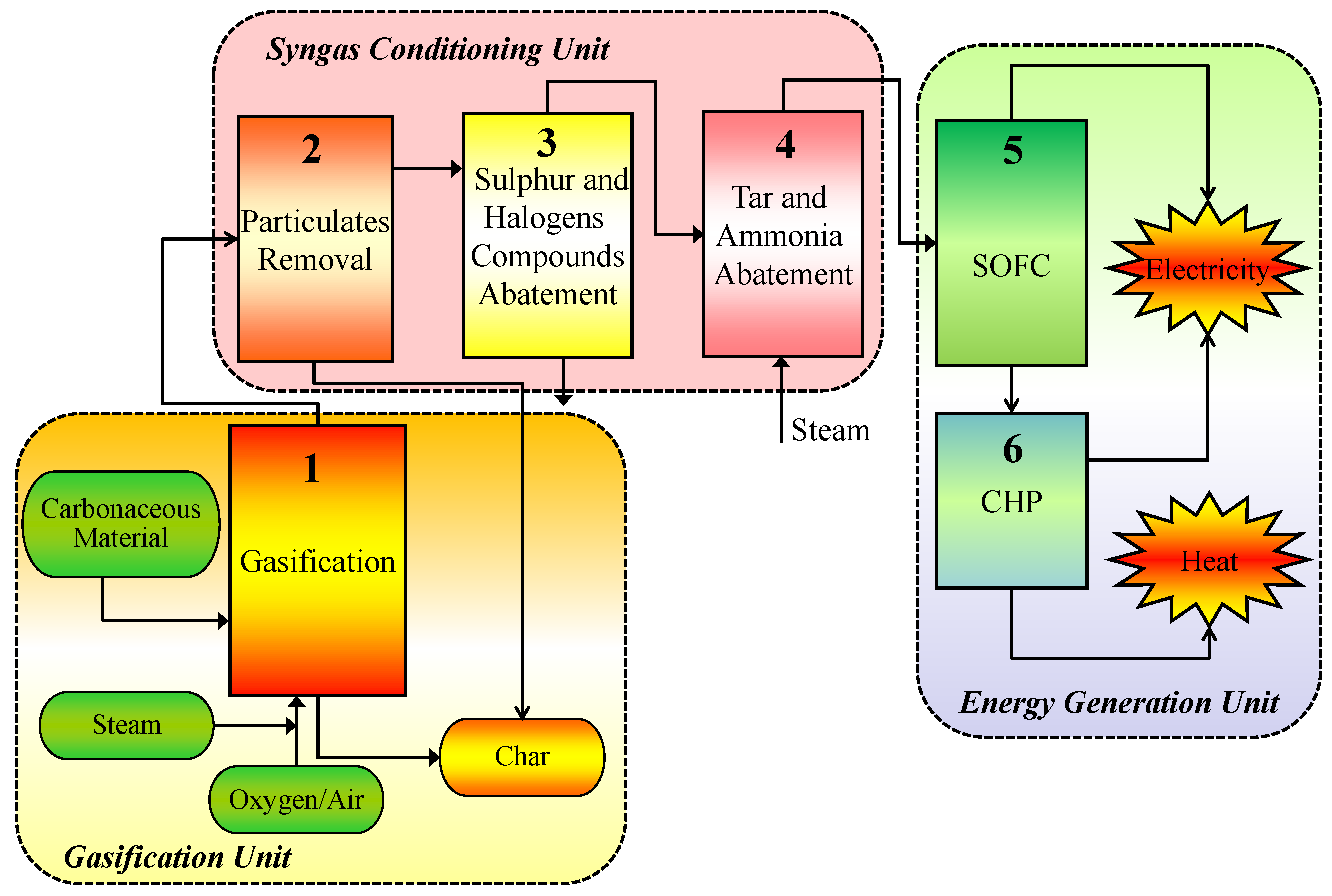
2. Biomass Gasification for Syngas Production
2.1. Feedstock Composition and Gasification Operating Conditions
2.2. Gasifier Type and Design
3. Syngas Cleaning and Conditioning
3.1. SOFC Requirements for Syngas Utilization
3.2. Hot Gas Cleaning and Conditioning Processes
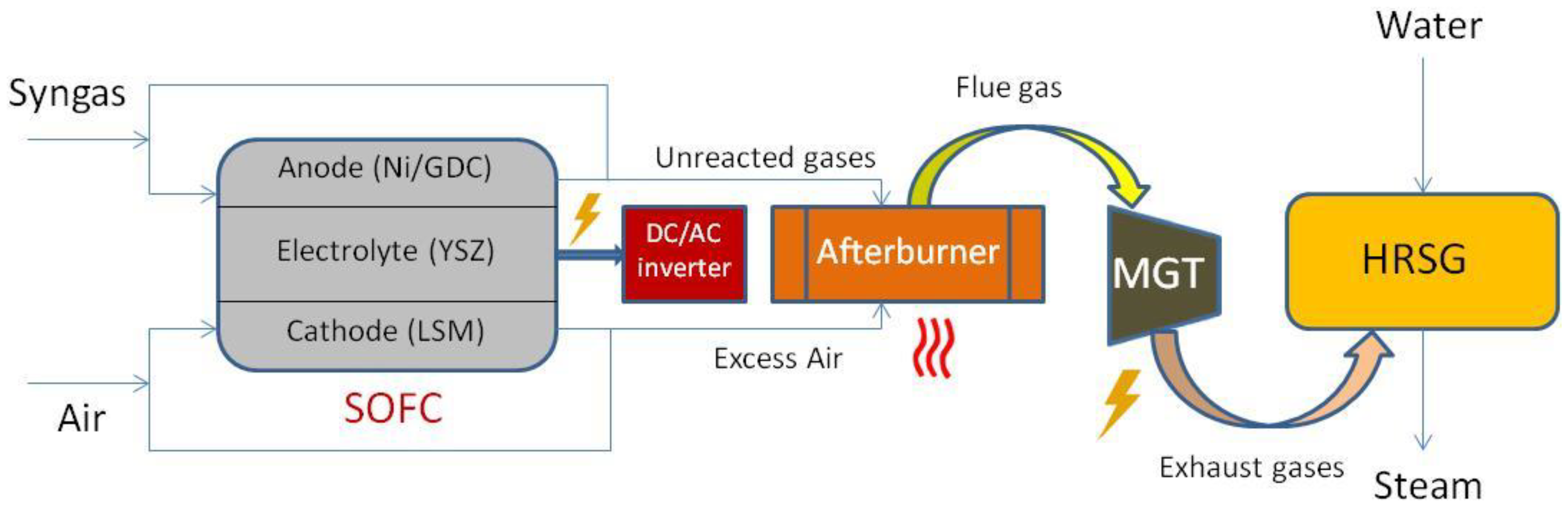
4. Solid Oxide Fuel Cells (SOFCs) Combined Heat and Power (CHP) Production
4.1. Introduction
4.2. Development of New Materials
4.3. CHP Technologies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pinto, F.; André, R.N. The role of gasification in achieving almost zero emissions in energy production from coal. In Fossil Fuels: Sources, Environmental Concerns and Waste Management Practices; Kumar, R., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2013; pp. 145–198. ISBN 978-1-62808-412-2. [Google Scholar]
- Din, Z.U.; Zainal, Z.A. The fate of SOFC anodes under biomass producer gas contaminants. Renew. Sustain. Energy Rev. 2017, 72, 1050–1066. [Google Scholar] [CrossRef]
- Ren, J.; Cao, J.-P.; Zhao, X.-Y.; Yang, F.-L.G.; Wei, X.-Y. Recent advances in syngas production from biomass catalytic gasification: A critical review on reactors, catalysts, catalytic mechanisms and mathematical models. Renew. Sustain. Energy Rev. 2019, 116, 109426. [Google Scholar] [CrossRef]
- Pinto, F.; André, R.N.; Carolino, C.; Miranda, M. Hot treatment and upgrading of syngas obtained by co-gasification of coal and wastes. Fuel Process. Technol. 2014, 126, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Watson, J.; Zhang, Y.; Si, B.; Chen, W.-T.; de Souza, R. Gasification of biowaste: A critical review and outlooks. Renew. Sustain. Energy Rev. 2018, 83, 1–17. [Google Scholar] [CrossRef]
- Pinto, F.; André, R.N.; Lopes, H.; Franco, C.; Carolino, C.; Costa, R.; Miranda, M.; Gulyurtlu, I. Comparison of a pilot scale gasification installation performance when air or oxygen is used as gasification medium 1. Tars and gaseous hydrocarbons formation. Fuel 2012, 101, 102–114. [Google Scholar] [CrossRef]
- Pinto, F.; André, R.N.; Lopes, H.; Franco, C.; Carolino, C.; Costa, R.; Miranda, M.; Gulyurtlu, I. Comparison of a pilot scale gasification installation performance when air or oxygen is used as gasification medium 2—Sulphur and nitrogen compounds abatement. Fuel 2012, 97, 770–782. [Google Scholar] [CrossRef]
- Pinto, F.; André, R.N.; Miranda, M.; Neves, D.; Varela, F.; Santos, J. Effect of gasification agent on co-gasification of rice production wastes mixtures. Fuel 2016, 180, 407–416. [Google Scholar] [CrossRef]
- Hauch, A.; Küngas, R.; Blennow, P.; Hansen, A.B.; Hansen, J.B.; Mathiesen, B.V.; Mog, M.B. Recent advances in solid oxide cell technology for electrolysis. Science 2020, 370, 6513. [Google Scholar] [CrossRef]
- Pinto, F.; Gominho, J.; André, R.N.; Miranda, M.; Gonçalves, D.; Varela, F.; Neves, D.; Santos, J.; Lourenço, A.; Pereira, H. Effect of rice husk torrefaction on syngas production and quality. Energy Fuels 2017, 31, 5183–5192. [Google Scholar] [CrossRef]
- Thomson, R.; Kwong, P.; Ahmad, E.; Nigam, K.D.P. Clean syngas from small commercial biomass gasifiers; a review of gasifier development, recent advances and performance evaluation. Int. J. Hydrogen Energy 2020, 45, 21087–21111. [Google Scholar] [CrossRef]
- Indrawan, N.; Kumar, A.; Moliere, M.; Sallam, K.A.; Huhnke, R.L. Distributed power generation via gasification of biomass and municipal solid waste: A review. J. Energy Inst. 2020, 93, 2293–2313. [Google Scholar] [CrossRef]
- Vilches, T.B.; Lind, F.; Ryden, M.; Thunman, H. Experience of more than 1000 h of operation with oxygen carriers and solid biomass at large scale. Appl. Energy 2017, 190, 1174–1183. [Google Scholar] [CrossRef]
- Basu, P. Biomass Gasification and Pyrolysis—Practical Design and Theory, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Phounglamcheik, A.; Babler, M.U.; Donag, P.; Amovic, M.; Ljunggren, R.; Engvall, K. Pyrolysis of wood in a rotary kiln pyrolyzer: Modeling and pilot plant trials. Energy Procedia 2017, 105, 908–913. [Google Scholar] [CrossRef]
- Aries Clean Energy. Clean Energy Brochure. Available online: https://ariescleanenergy.com/downloads/ (accessed on 15 December 2020).
- Technology BE. Bioresidues Energy Technology. Available online: www.betpl.net (accessed on 15 December 2020).
- Holzenergie Wegscheid. Available online: www.holzenergie-wegscheid.de (accessed on 15 December 2020).
- Ltd IEP. Infinite Energy. Available online: www.infiniteenergyindia.com (accessed on 15 December 2020).
- Lipro Energy. Available online: https://lipro-energy.de/en/innovative-technology/ (accessed on 15 December 2020).
- URBAS Woodgas CHP-Technology. Available online: https://www.urbas.at/en/energietechnik/ (accessed on 15 December 2020).
- Spanner Re2 Wood Cogeneration Plants. Available online: https://www.holz-kraft.com/images/press_release/2016/161128_spanner_uk-renewablecompanyaward1.pdf (accessed on 15 December 2020).
- Ronda Engineering: Ecogas Generator. Available online: http://www.ecogasgenerator.com/ (accessed on 15 December 2020).
- Xylowatt. Available online: www.xylowatt.com (accessed on 15 December 2020).
- Brynda, J.; Skoblia, S.; Beno, Z.; Pohorely, M.; Mosko, J. Application of the GP750 Gasifier for Combined Heat and Power Production. In Paper Presented at Biomass to Power and Heat. 2017. Available online: https://www.researchgate.net/publication/317544057_Application_of_the_GP750_Gasifier_for_Combined_Heat_and_Power_Production (accessed on 15 December 2020).
- CPC—Community Power Corporation. Available online: www.gocpc.com (accessed on 15 December 2020).
- Bio&Watt Energy from Biomass. Bio&Watt. Available online: www.bioewatt.com (accessed on 15 December 2020).
- Klaus, R. CHP with Biomass-Updraft Gasifier in the Range of 400 to 1000 kWel. ReGaWatt, 2019. Available online: https://www.regawatt.de (accessed on 15 December 2020).
- Burkhardt Heat and Power with Wood Pellets. Available online: https://burkhardt-gruppe.de/en/homepage/ (accessed on 15 December 2020).
- Systems PE. Biomass and Waste Gasification. Available online: http://www.prmenergy.com/ (accessed on 15 December 2020).
- Torbed—Energy Technology Application Description. Mortimer Technology Holdings Ltd. Available online: www.torftech.com (accessed on 15 December 2020).
- Ast NV. AHT Pyrogas. Available online: http://www.aht-pyrogas.de/en/ (accessed on 15 December 2020).
- Engineering, B. Available online: www.br-eg.com (accessed on 15 December 2020).
- Barisano, D. Biomass Gasification for Power Production. Country Report—Italy. Innsbruck, Enea. 2017. Available online: http://www.gasificationofbiomass.org/download.php?file=files/file/2017/Innsbruck/CR/Italy.pdf (accessed on 15 December 2020).
- Group TFE. Terruzzi Fercalx Energy Group. 2019. Available online: http://www.terruzzifercalxgroup.com/en/indexen.php (accessed on 15 December 2020).
- Chanderpur Works. Available online: www.chanderpur.com (accessed on 15 December 2020).
- EQTEC. EQTEC Gasifier Technology. Available online: www.eqtec.com (accessed on 15 December 2020).
- MEVA Energy. Available online: http://mevaenergy.com/technology/ (accessed on 15 December 2020).
- Cortus Energy. Rolf Ljunggren, 9th International Seminar on Gasification, Malmö. 19 October 2016. Available online: https://energiforskmedia.blob.core.windows.net/media/21796/cortus-woodroll-presentation-isg-2016-10-19.pdf (accessed on 15 December 2020).
- Sentis, L.; Rep, M.; Barisano, D.; Bocci, E.; Hamedani, S.R.; Pallozzi, V.; Tascioni, R. Techno-Economic Analysis of UNIFHY Hydrogen; The Fuel Cells and Hydrogen Joint Undertaking (FCH JU): Brussels, Belgium, 2016. [Google Scholar]
- Van der Meijden, C.M.; Veringa, H.J.; Rabou, L.P. The production of synthetic natural gas (SNG): A comparison of three wood gasification systems for energy balance and overall efficiency. Biomass Bioenergy 2000, 34, 302–311. [Google Scholar] [CrossRef]
- Konemann, J.-W. Development of biomass and waste gasification towards green chemicals. In Proceedings of the 2017 Syngas Technologies Conference, Global Syngas Technologies Council, Colorado Springs, CO, USA, 15–18 October 2017; pp. 1–19. [Google Scholar]
- Bhoi, P.R.; Huhnke, R.L.; Kumar, A.; Thapa, S.; Indrawan, N. Scale-up of a downdraft gasifier system for commercial scale mobile power generation. Renew. Energy 2018, 118, 25–33. [Google Scholar]
- Indrawan, N.; Kumar, A.; Kumar, S. Recent advances in power generation through biomass and municipal solid waste gasification. In Coal and Biomass Gasification: Recent Advances and Future Challenges; De, S., Moholkar, V., Bhaskar, T., Agarwal, A., Eds.; Springer: Singapore, 2018; pp. 369–402. [Google Scholar]
- Henriksen, U.; Ahrenfeldt, J.; Jensen, T.K.; Gøbel, B.; Bentzen, J.D.; Hindsgaul, C.; Sørensen, L.H. The design, construction and operation of a 75kW two-stage gasifier. Energy 2006, 31, 1542–1553. [Google Scholar] [CrossRef] [Green Version]
- Heidenreich, S.; Foscolo, P.U. New concepts in biomass gasification. Prog. Energy Combust. Sci. 2015, 46, 72–95. [Google Scholar] [CrossRef]
- Rauch, R.; Hofbauer, H.; Bosch, K.; Siefert, I.; Aichernig, C.; Tremmel, H. Steam gasification of biomass at CHP plant in guessing-status of the demonstration plant. In Proceedings of the Second World Conference and Technology Exhibition, Rome, Italy, 10–14 May 2004; pp. 1–4. [Google Scholar]
- Xiao, X.; Meng, X.; Le, D.D.; Takarada, T. Two-stage steam gasification of waste biomass in fluidized bed at low temperature: Parametric investigations and performance optimization. Bioresour. Technol. 2011, 102, 1975–1981. [Google Scholar] [CrossRef]
- Pei, H.; Wang, X.; Dai, X.; Jin, B.; Huang, Y. A novel two-stage biomass gasification concept: Design and operation of a 1.5 MWth demonstration plant. Bioresour. Technol. 2018, 267, 102–109. [Google Scholar] [CrossRef]
- Haspel, A.E. Ionic Gasification Breakthrough Technology for On-Site Waste-To-Energy Conversion. Cogent Energy Systems 2019. Available online: https://www.cogentenergysystems.com/ (accessed on 15 December 2020).
- SynCraft Two Stage Gasifier Engineering Gmbh. 2018. Available online: https://en.syncraft.at/wood-power-plants/overview (accessed on 15 December 2020).
- Kruse, A. Supercritical water gasification. Biofuels Bioprod. Biorefining Innov. Sustain. Econ. 2008, 2, 415–437. [Google Scholar] [CrossRef]
- Yanik, J.J.; Ebale, S.; Kruse, A.; Saglam, M.; Yüksel, M. Biomass gasification in supercritical water: Part 1. Effect of the nature of biomass. Fuel 2007, 86, 2410–2415. [Google Scholar] [CrossRef]
- Lanzini, A.; Ferrero, D.; Papurello, D.; Santarelli, M. Reporting degradation from different fuel contaminants in Ni-anode SOFCs. Fuel Cells 2017, 17, 423–433. [Google Scholar] [CrossRef]
- Bocci, E.; Di Carlo, A.; McPhail, S.J.; Gallucci, K.; Foscolo, P.U.; Moneti, M.; Villarini, M.; Carlini, M. Biomass to fuel cells state of the art: A review of the most innovative technology solutions. Int. J. Hydrogen Energy 2014, 39, 21876–21895. [Google Scholar] [CrossRef]
- Hofmann, P.H.; Panopoulos, K.D.; Aravind, P.V.; Siedlecki, M.; Schweiger, A.; Karl, J.; Ouweltjes, J.P.; Kakaras, E. Operation of solid oxide fuel cell on biomass product gas with tar levels >10 g Nm-3. Int. J. Hydrogen Energy 2009, 34, 9203–9212. [Google Scholar] [CrossRef]
- Milne, T.A.; Evans, R.J. Biomass gasifier “Tars”: Their Nature, Formation and Conservation; NREL/TP-570-25357; National Renewable Energy Laboratory: Goldern, CO, USA, 1998.
- Mondal, P.; Dang, G.S.; Garg, M.O. Syngas production through gasification and clean-up for downstream applications—Recent developments. Fuel Process. Technol. 2011, 92, 1395–1410. [Google Scholar] [CrossRef]
- Asadullah, M. Biomass gasification gas cleaning for downstream applications: A comparative critical review. Renew. Sustain. Energy Rev. 2014, 40, 118–132. [Google Scholar] [CrossRef]
- Roy, D.; Samanta, S.; Ghosh, S. Performance optimization through response surface methodology of an integrated biomass gasification based combined heat and power plant employing solid oxide fuel cell and externally fired gas turbine. Energy Convers. Manag. 2020, 222, 113182–113200. [Google Scholar] [CrossRef]
- Toonssen, R.; Sollai, S.; Aravind, P.V.; Woudstra, N.; Verkooijen, A.H.M. Alternative system designs of biomass gasification SOFC/GT hybrid systems. Int. J. Hydrogen Energy 2011, 36, 10414–10425. [Google Scholar] [CrossRef]
- Karmakar, M.K.; Chandra, P.; Chatterjee, P.K. A review on the fuel gas cleaning technologies in gasification process. J. Environ. Chem. Eng. 2015, 3, 689–702. [Google Scholar]
- Dayton, D. A Review of the Literature on Catalytic Biomass Tar Destruction—Milestone Completion Report; NREL/TP-510-32815; National Renewable Energy Laboratory: Goldern, CO, USA, 2002.
- Wang, L.; Weller, C.L.; Jones, D.D.; Hanna, M.A. Contemporary issues in thermal gasification of biomass and its application to electricity and fuel production. Biomass Bioenergy 2008, 32, 573–581. [Google Scholar] [CrossRef]
- Rapagnà, S.; Provendier, H.; Petit, C.; Kiennemann, A.; Foscolo, P.U. Development of catalysts suitable for hydrogen or syn-gas production from biomass gasification. Biomass Bioenergy 2002, 22, 377–388. [Google Scholar] [CrossRef]
- Cal, M.P.; Strickler, B.W.; Lizzio, A.A.; Gangwal, S.K. High temperature hydrogen sulfide adsorption on activated carbon II. Effects of gas temperature, gas pressure and sorbent Regeneration. Carbon 2000, 38, 1767–1774. [Google Scholar]
- Zevenhoven, R.; Kilpinen, P. Control of Pollutants in Flue Gases and Fuel Gases; Espoo University of Technology: Finland: Helsinki, 2001. [Google Scholar]
- Atakul, H.; Wakker, J.P. Removal of H2S from fuel gases at high temperatures using MnO/y-A12O3. Fuel 1995, 74, 187–191. [Google Scholar] [CrossRef]
- Pinto, F.; Lopes, H.; André, R.N.; Gulyurtlu, I.; Cabrita, I. Effect of catalysts in the quality of syngas and by-products obtained by co- gasification of coal and wastes. 1. Tars and nitrogen compounds abatement. Fuel 2007, 86, 2052–2063. [Google Scholar] [CrossRef]
- Devi, L.; Ptasinski, K.J.; Janssen, F.J.J.G. A review of the primary measures for tar elimination in biomass gasification processes. Biomass Bioenergy 2003, 24, 125–140. [Google Scholar] [CrossRef]
- Wang, W.; Padban, N.; Ye, Z.; Olofsson, G.; Andersson, A.; Bjerle, I. Catalytic hot gas cleaning of fuel gas from an air-blown pressurized fluidized-bed gasifier. Ind. Eng. Chem. Res. 2000, 39, 4075–4081. [Google Scholar] [CrossRef]
- Pfeifer, C.; Hofbauer, H. Development of catalytic tar decomposition downstream from a dual fluidized bed biomass steam gasifier. Powder Technol. 2008, 180, 9–16. [Google Scholar] [CrossRef]
- Gangwal, S.K.; Gupta, R.P.; Portzer, J.W.; Turk, B.S. Simultaneous removal of H2S and NH3 using metal oxide sorbents. In Proceedings of the Advanced Coal-Based Power & Environmental Systems ‘97 Conference, Pittsburgh, PA, USA, 22–24 July 1997; Research Triangle Institute, K. Jothimurugesan, Department of Chemical Engineering Hampton University: Hampton, VA, USA, 1998. [Google Scholar]
- Hoogers, G.; Bauen, A.; Chen, E.; Hart, D.; Hinsberger, M.; Hogarth, M.; Stone, R.; Thompsett, D. Fuel Cell Technology Handbook, 1st ed.; Hoogers, G., Ed.; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Marcantonio, V.; Bocci, E.; Ouweltjes, J.P.; Del Zotto, L.; Monarca, D. Evaluation of sorbents for high temperature removal of tars, hydrogen sulphide, hydrogen chloride and ammonia from biomass-derived syngas by using Aspen Plus. Int. J. Hydrogen Energy 2020, 45, 6651–6662. [Google Scholar] [CrossRef]
- Ramadhani, F.; Hussain, M.A.; Mokhlis, H. A Comprehensive review and technical guideline for optimal design and operations of fuel cell-based cogeneration systems. Processes 2019, 7, 950. [Google Scholar] [CrossRef] [Green Version]
- Shailesh, D.V. U.S. DOE Office of Fossil Energy Solid Oxide Fuel Cell (SOFC) Program. Available online: https://netl.doe.gov/sites/default/files/2020-03/AMR-SOFC-Program-Overview-4-29-2019.pdf (accessed on 11 January 2021).
- Niakolas, D.K. Review Sulfur poisoning of Ni-based anodes for Solid Oxide Fuel Cells in H/C-based fuels. Appl. Catal. A Gen. 2014, 486, 123–142. [Google Scholar] [CrossRef]
- Moretti, C.; Corona, B.; Rühlin, V.; Götz, T.; Martin Junginger, M.; Brunner, T.; Obernberger, I.; Li, S. Combining biomass gasification and solid oxid fuel cell for heat and power generation: An early-stage life cycle assessment. Energies 2020, 13, 2773. [Google Scholar] [CrossRef]
- Radenahmad, N.; Azad, A.T.; Saghir, M.; Taweekun, J.; Bakar, M.S.A.; Reza, M.S.; Azad, A.K. A review on biomass derived syngas for SOFC based combined heat and power application. Renew. Sustain. Energy Rev. 2020, 119, 109560. [Google Scholar] [CrossRef]
- Karvountzi, G.C.; Price, C.M.; Duby, P.F. Comparison of molten carbonate and solid oxide fuel cells for integration in a hybrid system for cogeneration or tri-generation. ASME Adv. Energy Syst. Div. AES 2004, 44, 139–150. [Google Scholar]
- Varbanov, P.; Klemes, J. Analysis and integration of fuel cell combined cycles for development of low-carbon energy technologies. Energy 2008, 33, 1508–1517. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Elsaid, K.; Wilberforce, T.; Kamil, M.; Sayed, E.T.; Olabi, A. Environmental aspects of fuel cells: A review. Sci. Total Environ. 2021, 752, 141803. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Situmorang, Y.A.; An, P.; Yang, J.; Hao, X.; Rizkiana, J.; Abudula, A.; Guan, G. A biomass-based small-scale power generation system with energy/exergy recuperation. Energy Convers. Manag. 2021, 227, 113623. [Google Scholar]
- Gandiglio, M.; De Sario, F.; Lanzini, A.; Bobba, S.; Santarelli, M.; Blengini, G.A. Life cycle assessment of a biogas-fed solid oxide fuel cell (SOFC) integrated in a wastewater treatment plant. Energies 2019, 12, 1611. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Li, Q.; Tan, W.; Qiu, H.; Su, C. Solid oxide fuel cells in combination with biomass gasification for electric power generation. Chin. J. Chem. Eng. 2020, 28, 1156–1161. [Google Scholar] [CrossRef]
- Jacobson, A.J. Materials for solid oxide fuel cells. Chem. Mater. 2010, 22, 660–674. [Google Scholar] [CrossRef]
- Wongchanapai, S.; Iwai, H.; Saito, M.; Yoshida, H. Performance evaluation of an integrated small-scale SOFC-biomass gasification power generation system. J. Power Sources 2012, 216, 314–322. [Google Scholar] [CrossRef]
- Wu, Z.; Zhu, P.; Yao, J.; Zhang, S.; Ren, J.; Yang, F.; Zhang, Z. Combined biomass gasification, SOFC, IC engine, and waste heat recovery system for power and heat generation: Energy, exergy, exergoeconomic, environmental (4E) evaluations. Appl. Energy 2020, 279, 115794. [Google Scholar]
- Gholamian, E.; Zare, V.; Mousavi, S.M. Integration of biomass gasification with a solid oxide fuel cell in a combined cooling, heating and power system: A thermodynamic and environmental analysis, International. J. Hydrog. Energy 2016, 41, 20396–20406. [Google Scholar] [CrossRef]
- Pantaleo, A.M.; Camporeale, S.; Fortunato, B. Small scale biomass CHP: Techno-economic performance of steam vs. gas turbines with bottoming ORC. Energy Procedia 2015, 82, 825–832. [Google Scholar] [CrossRef] [Green Version]
- Bang-Møller, C.; Rokni, M.; Elmegaard, B.; Ahrenfeldt, J.; Henriksen, U.B. Decentralized combined heat and power production by two-stage biomass gasification and solid oxide fuel cells. Energy 2013, 58, 527–537. [Google Scholar] [CrossRef]
- Borji, M.; Atashkari, K.; Ghorbani, S.; Nariman-Zadeh, N. Parametric analysis and Pareto optimization of an integrated autothermal biomass gasification, solid oxide fuel cell and micro gas turbine CHP system. Int. J. Hydrogen Energy 2015, 40, 14202–14223. [Google Scholar] [CrossRef]
- Bang-Møller, C.; Rokni, M. Thermodynamic performance study of biomass gasification, solid oxide fuel cell and micro gas turbine hybrid systems. Energy Convers. Manag. 2010, 51, 2330–2339. [Google Scholar] [CrossRef]
- Fryda, L.; Panopoulos, K.D.; Kakaras, E. Integrated CHP with autothermal biomass gasification and SOFC–MGT. Energy Convers. Manag. 2008, 49, 281–290. [Google Scholar] [CrossRef]
- Roy, D.; Samantab, S.; Ghosh, S. Techno-economic and environmental analyses of a biomass based system employing solid oxide fuel cell, externally fired gas turbine and organic Rankine cycle. J. Clean. Prod. 2019, 225, 36–57. [Google Scholar] [CrossRef]
| Reaction | ∆H (kJ/mol) | ||
|---|---|---|---|
| Oxidation | C(s) + O2 ⇆ CO2 | −392.5 | (1) |
| C(s) + ½ O2 ⇆ CO | −110.5 | (2) | |
| H + O2 ⇆ H2O | −242.0 | (3) | |
| CH4 + ½ O2 ⇆ CO + 2H2 | −35.7 | (4) | |
| CH4 + 2O2 ⇆ CO2 + 2H2O | −802.3 | (5) | |
| Boudouard | C(s) + CO2 ⇆ 2 CO | 172.0 | (6) |
| Water Gas: primary secondary | C(s) + H2O ⇆ CO + H2 | 131.4 | (7) |
| C(s) + 2 H2O ⇆ CO2 + 2 H2 | 90.4 | (8) | |
| Water–gas shift | CO + H2O ⇆ CO2 + H2 | −41.0 | (9) |
| Steam Reforming | CH4 + H2O ⇆ CO + 3 H2 | 205.9 | (10) |
| CH4 + 2 H2O ⇆ CO2 + 4 H2 | 164.7 | (11) | |
| CnHm + n H2O ⇆ n CO + (n + m/2) H2 | 210.1 | (12) | |
| CnHm + n/2 H2O ⇆ n/2 CO + (m-n) H2 + n/2 CH4 | 4.2 | (13) | |
| CO2 Reforming | CH4 + CO2 ⇆ 2 CO + 2 H2 | 247.0 | (14) |
| CnHm + n CO2 ⇆ 2n CO + m/2 H2 | 292.4 | (15) | |
| CnHm + n/4 CO2 ⇆ n/2 CO + (m-3n/2) H2 + (3n/4) CH4 | 45.3 | (16) | |
| Methanation | C(s) + 2 H2 ⇆ CH4 | −74.6 | (17) |
| CO + 3 H2 ⇆ CH4 + H2O | −205.9 | (18) | |
| 2CO + 2 H2 ⇆ CH4 + CO2 | −247.3 | (19) | |
| CO2 + 4 H2 ⇆ CH4 + 2H2O | −164.9 | (20) | |
| Decompositions of tar/hydrocarbons | pCxHy ⇆ qCnHm + rH2 | (21) | |
| CnHm ⇆ nC + m/2H2 | (22) | ||
| Gasifier Technology | Manufacturers | Reference |
|---|---|---|
| Downdraft gasifier | Aries Clean Energy | [16] |
| Bioresidues Energy Technologies | [17] | |
| Holzenergie wegscheid | [18] | |
| Infinite Energy | [19] | |
| LiPRO Energy GmbH & Co. KG Germany | [20] | |
| URBAS | [21] | |
| Joos downdraft gasifier–Spanner Re2 | [22] | |
| Ronda Engineering Srl | [23] | |
| Xylowatt | [24] | |
| TARPO | [25] | |
| Community Power Corporation | [26] | |
| BIO&WATT Gasification s.r.l. | [27] | |
| Updraft gasifier | ReGaWatt GmbH | [28] |
| Updraft co-current–Burkhardt Energie | [29] | |
| PRM Energy Systems | [30] | |
| Torbed | [31] | |
| Stratified combined updraft/downdraft | A.H.T Pyrogas | [32] |
| BR Energy Group | [33] | |
| Pyrox | [34] | |
| Terruzzi Fercalx Energy Group | [35] | |
| Chanderpur Works | [36] | |
| Fluidized bed gasifier | Bubbling fluidised bed gasifier–EQTEC | [37] |
| Aries Clean Energy | [16] | |
| Entrained flow gasifier | MEVA Energy | [38] |
| Woodroll® entrained flow gasifier–Cortus Energy | [39] |
| Technology | Main Characteristics | Reference |
|---|---|---|
| UNIQUE | Integrates gasification, gas cleaning (catalytic filter candle in the freeboard) and conditioning in one single unit. Air or steam/O2 are used as gasification medium. Tar levels of 1 g/Nm3 are reported. | [40] |
| Milena | Pyrolysis (CFB) and combustion (i.e., BFB) are performed in two separate, sequential reactors that are integrated in one refractory lined container. Air is used as gasification medium. The dilution of the syngas by N2 (from the air stream) and CO2 and H2O (from the combustion section) is minimized. | [41,42] |
| Internal cyclonic downdraft | Deals with low-density biomass and MSW. Uses internal separate combustion to generate high-temperature combustion flows. Air is used as gasification medium to produce syngas with tar content (300–400 mg/Nm3). | [43,44] |
| Multi-stage (Viking gasifier) | Combine pyrolysis and gasification. Air is used to produce syngas with low tar content (<15 mg/Nm3). Cold gas efficiency (CGE) of 93% and electrical efficiency of 25% are obtained. | [45] |
| Güssing fluidized bed | Dual fluidized bed reactors and nickel-based catalytic filters are inserted in the gasifier freeboard, using air/steam as gasifying agent, produces low tar (2–5 g/Nm3) and particulate matter. | [46,47] |
| LT-Circulating Fluidized Bed (CFB) | Suitable to deal with difficult biomass (straw, manure fibers, sewage sludge). Consists of two gasification stages. In the first one is used CFB at 630 °C and the second one uses BFB at 730 °C. CGE from 87% to 93% is reported, | [46] |
| Two-Stage Fluidized Catalytic | In the first stage a fluidized bed with silica sand is used and in the second stage a fix bed with catalyst for tar reforming is used. Tar content of 60 mg/Nm3 was reported. | [48] |
| Two-Stage Fluidized-Entrained Flow | In the first stage the fluidized bed operates at 650 °C and in the second stage the entrained reactor operates at 1300 °C. Syngas is produced free of tar and a CGE up to 64% is obtained. | [49] |
| Drop through plasma Heliostorm™ gasifier | Ionic Gasification, very high temperature of 10,000 °C is obtained in the core reaction zone. Carbon vaporizes at this temperature, breaking down into gaseous carbon atoms. Feedstock breaks down to individual atoms, leading to clean syngas without by-products, tar or harmful components. | [50] |
| Floating bed gasifier | In conventional fixed bed reactors the force of gravity and the gas flow act downwards and increases compression, while in the floating fixed bed reactor from SYNCRAFT these two forces act in opposition. Thus, feedstock inside the gasifier remains loosened and well permeable. The innovative floating fixed bed technology, leads to the highest degree of efficiency and allows the maximum raw material flexibility with economic advantages. | [51] |
| Supercritical water | Deals with wet and high moisture content feedstocks, without the need of pre-drying. Operates at conditions above those of supercritical water (22 MPa and 374 °C). | [52,53] |
| Key Indicator | |
|---|---|
| Tar before gas cleaning | 5–50 mg/Nm3 of tar in producer gas leaving |
| Syngas heat content (LHV) | Depends on gasification agent from 4–7 MJ/Nm3 (with air) to 10–12 MJ/Nm3 (with O2, steam, CO2) |
| Syngas quality | Depends much on feedstock, technology and gasification conditions |
| Cold gas efficiency | Usually in the range 75 to 90%. |
| Carbon conversion efficiency | Usually > 90% |
| Biomass requirements (size and moisture content) | Usually moisture contents below 10–15% Particle size depends on technology (much smaller for entrained flows) Other feedstocks pre-treatments: torrefaction and pelletization |
| Gasification temperature | For fluidized bed gasifiers: 800–850 °C For entrained flow gasifiers: 1100–1300 °C |
| Exit gas temperature | Depends on technology usually >800 °C |
| Scalability and modularity | For the minimum size units: 100–500 kWe Some units are scalable to 2 MWe or more. |
| Catalyst/bed systems | Usually low cost minerals like: dolomite or olivine |
| Technical complexity | Technical complexity depends on the technology and increase CAPEX and OPEX. General units have medium complexity and are semi autonomous, except plasma gasifiers that are highly complex. |
| Impurity | Particulate | Total Sulphur | Total Chlorine | NH3 | Tar | Alkali Metals | Others |
|---|---|---|---|---|---|---|---|
| SOFC | 10–100 ppm | <1 ppm | <1 ppm | - | <2000 ppm | <1 ppm | - |
| MCFC | 10–100 ppm | <0.5–1 ppm | 1 ppm | 1–3% vol | <2000 ppm | <1 ppm | |
| AFC | <100 ppm | Poison | Poison | - | Poison | Poison | Poisons: CH4, CO, CO2 |
| PEFC | <100 ppm | Poison | <1 ppm | <1 ppm | Poison | Poison | CO < 10 ppm |
| PAFC | <100 ppm | <50 ppm | Poison | Poison | Poison | Poison | CO < 500 ppm |
| Impurity | Syngas Conditioning Processes |
|---|---|
| Particulate | Hot cyclone Up to 1000 °C—90–95% |
| Hot membrane—99% | |
| Cyclones + sintered metal filter (up to 400 °C) | |
| Cyclones + ceramic filter (up to 800 °C) | |
| Alkali metals | Adsorption on solid sorbent, getter material. Above 500 °C, the calcium-based sorbents starts decomposing |
| Alkali getters at ≥600 °C (activated alumina at 800 °C) | |
| Tar | Hot catalytic/reforming (with Fe or Ni-based catalyst more effective then olivine or dolomite). Up to 1000 °C—80–95% |
| 1st Step: catalytic cracking using dolomite at 900 °C in secondary bed or thermal cracking at 1200 °C. 2nd step: cracking using Ni based catalyst at 800 °C (after HCl and H2S removal) | |
| Total sulphur | ZnO sorbent at 350–400 °C/Activated Carbon |
| Zn Titanate sorbent at ~600 °C | |
| Physical and chemical adsorption, ZnO/FeO sorbents. Catalysts like CoMo for COS conversion, Ni- and Fe-based catalysts. At 600–650 °C | |
| Total chlorine | Sorbents at high temperature Activated carbon, alumina and common alkali oxides |
| NaHCO3/Na2CO3 up to 550–600 °C | |
| NH3 | Thermal catalytic decomposition of NH3 using Ni and Zeolite as catalysts at 700–800 °C |
| Anode Used Materials | Ni-YSZ | Ni-GDC |
|---|---|---|
| Advantages |
|
|
| Disadvantages |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, P.; Pinto, F.; André, R.N.; Marques, P. Integration of Gasification and Solid Oxide Fuel Cells (SOFCs) for Combined Heat and Power (CHP). Processes 2021, 9, 254. https://doi.org/10.3390/pr9020254
Costa P, Pinto F, André RN, Marques P. Integration of Gasification and Solid Oxide Fuel Cells (SOFCs) for Combined Heat and Power (CHP). Processes. 2021; 9(2):254. https://doi.org/10.3390/pr9020254
Chicago/Turabian StyleCosta, Paula, Filomena Pinto, Rui Neto André, and Paula Marques. 2021. "Integration of Gasification and Solid Oxide Fuel Cells (SOFCs) for Combined Heat and Power (CHP)" Processes 9, no. 2: 254. https://doi.org/10.3390/pr9020254
APA StyleCosta, P., Pinto, F., André, R. N., & Marques, P. (2021). Integration of Gasification and Solid Oxide Fuel Cells (SOFCs) for Combined Heat and Power (CHP). Processes, 9(2), 254. https://doi.org/10.3390/pr9020254





