Ice Growth Suppression in the Solution Flows of Antifreeze Protein and Sodium Chloride in a Mini-Channel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
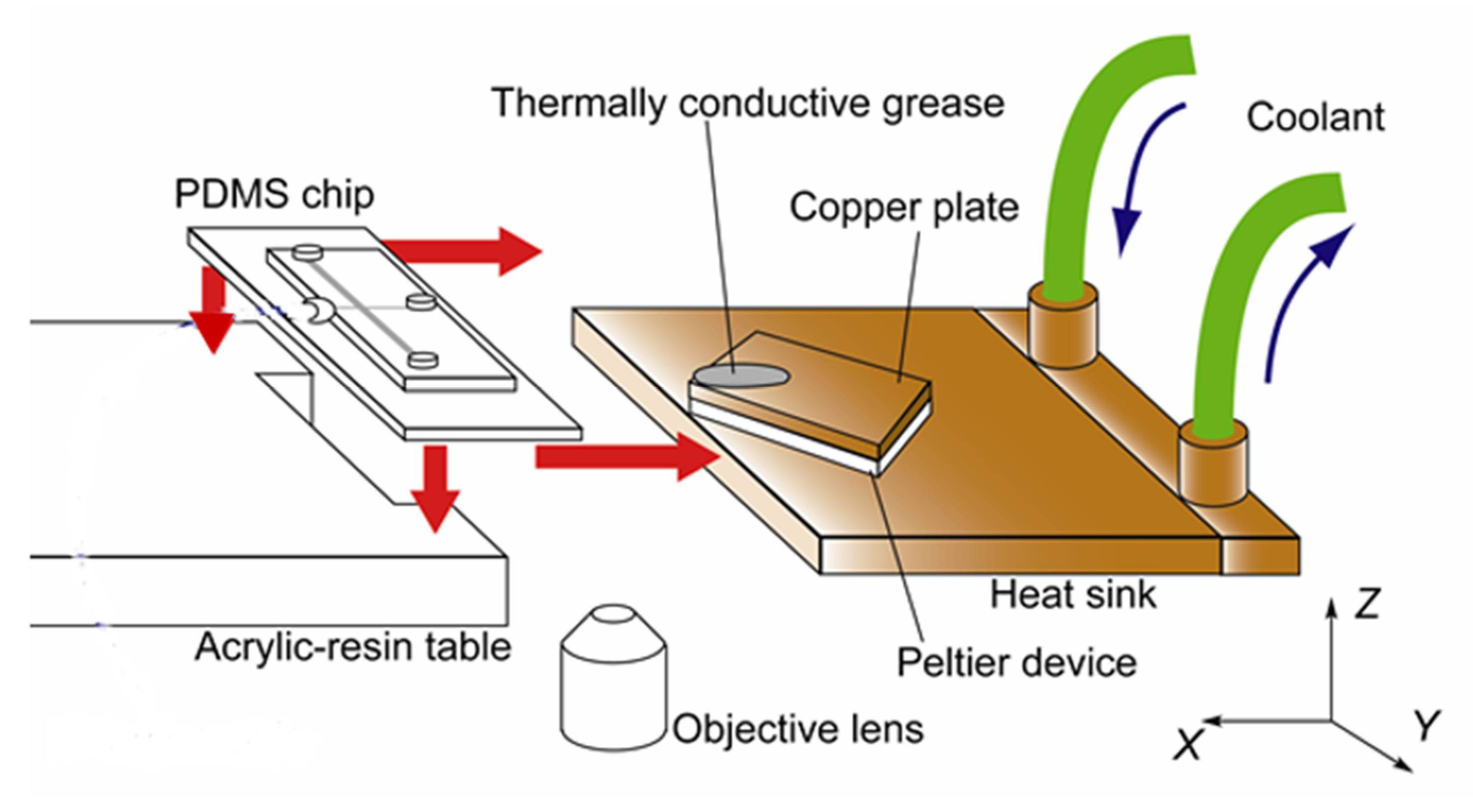
2.2. Apparatus
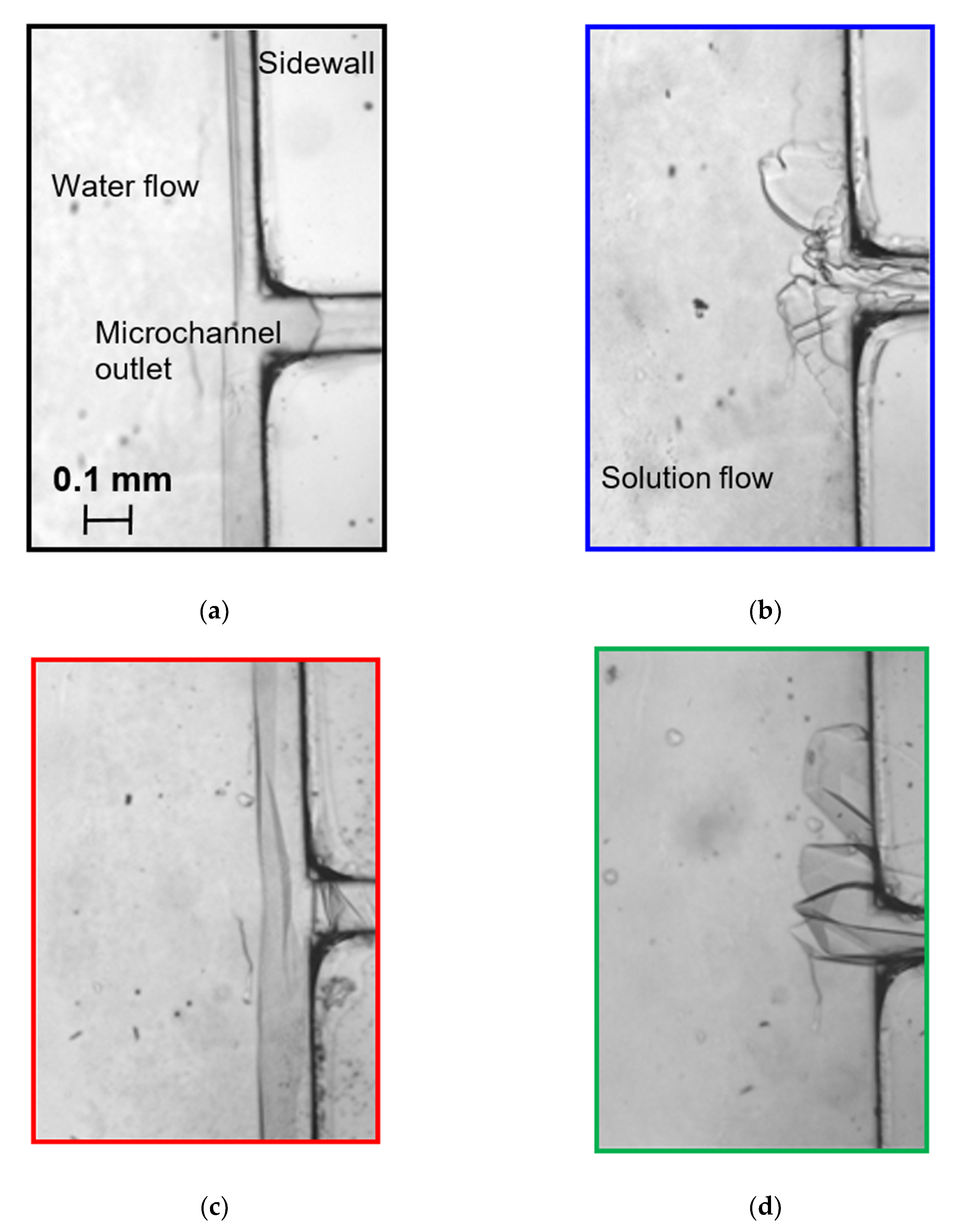
2.3. Mini-Channel
2.4. Experimental Condition
3. Results
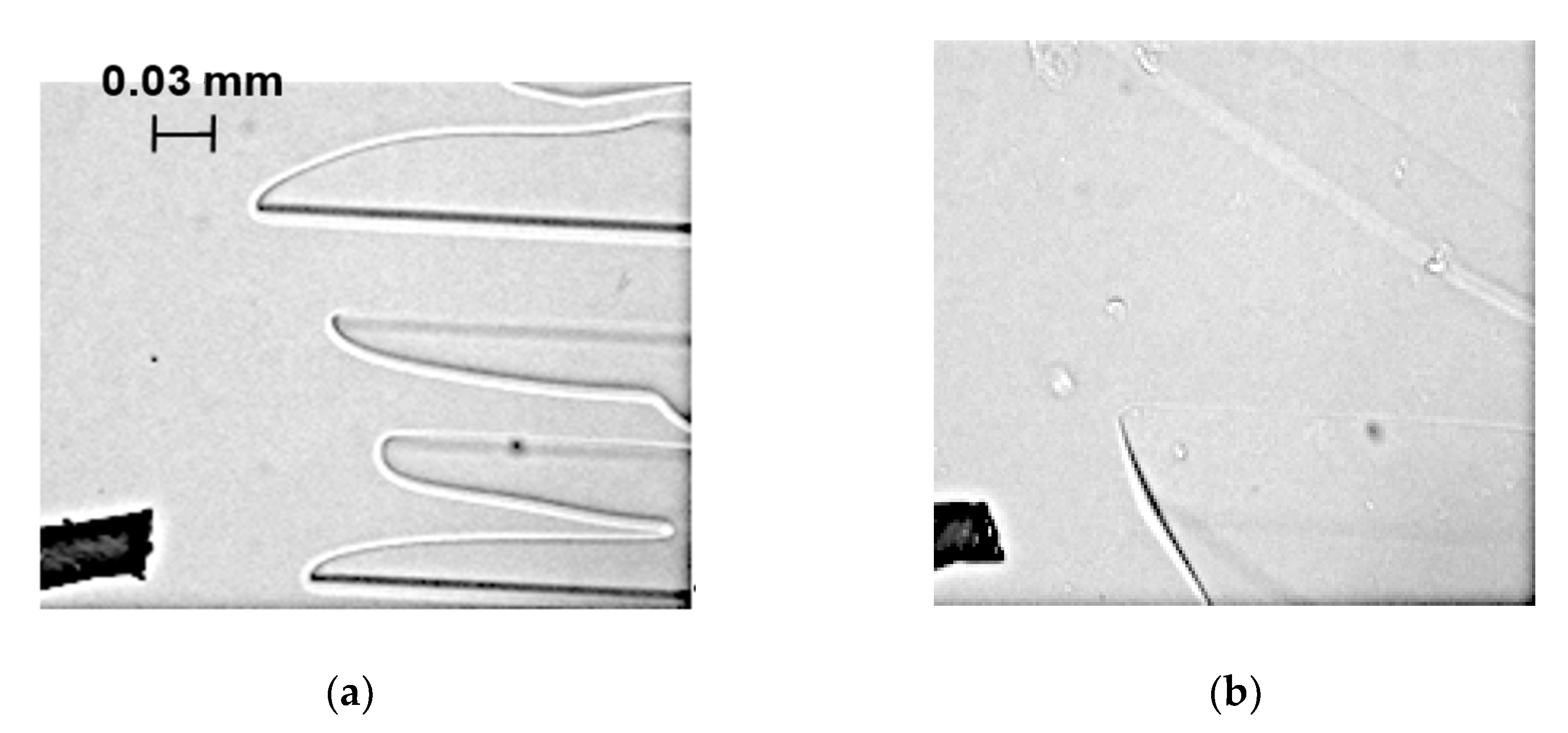
3.1. Interface Morphology
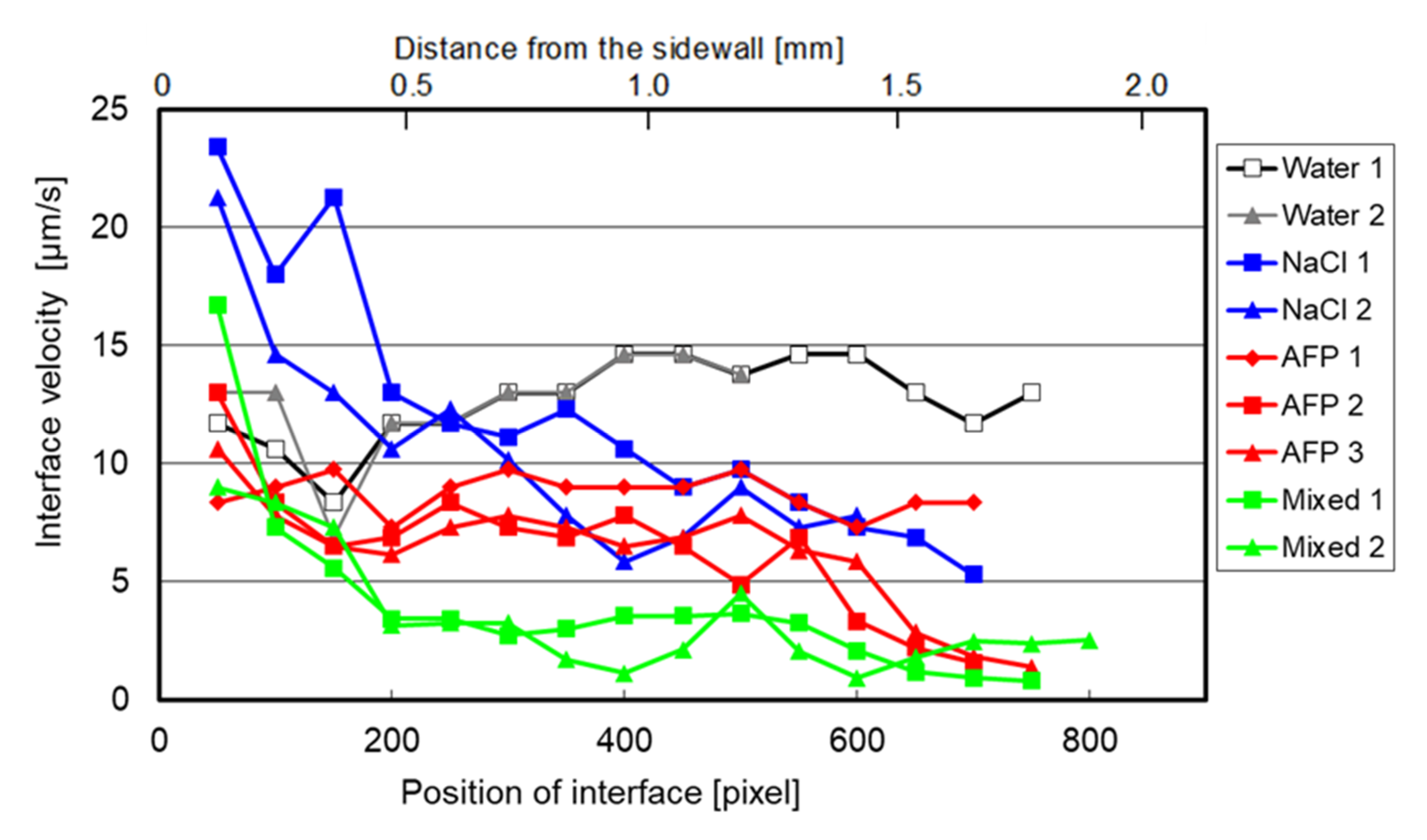
3.2. Interface Velocity
4. Discussion
5. Conclusions
- (1)
- The overlapping of serrated interfaces, the inclined interfaces, and the interfaces with sharp and flat tips were observed in the cases of the salt-solution, protein-solution, and mixed-solution flows, respectively.
- (2)
- The average interface velocity in the case of the mixed-solution flow was the lowest, and it decreased by 64%, compared with that of water flow.
- (3)
- The synergistic effects of the ions and antifreeze protein, which were discussed concerning the ice growth in the osmometer and unidirectional freezing, were confirmed in the case of ice growth along the mixed-solution flow in the mini-channel.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Shitzer, A. Cryosurgery: Analysis and experimentation of cryoprobes in phase changing media. J. Heat Transf. 2011, 133, 011005. [Google Scholar] [CrossRef]
- Berendsen, T.A.; Bruinsma, B.G.; Puts, C.F.; Saeidi, N.; Usta, O.B.; Uygun, B.E.; Izamis, M.-L.; Toner, M.; Yarmush, M.L.; Uybun, K. Supercooling enables long-term transplantation survival following 4 days of liver preservation. Nat. Med. 2014, 20, 790–793. [Google Scholar] [CrossRef]
- Ishikawa, J.; Oshima, M.; Iwasaki, F.; Suzuki, R.; Park, J.; Nakao, K.; Matsuzawa-Adachi, Y.; Mizutsuki, T.; Kobayashi, A.; Abe, Y.; et al. Hypothermic temperature effects on organ survival and restoration. Sci. Rep. 2015, 5, 09563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haymet, A.D.J.; Ward, L.G.; Harding, M.M. Winter flounder “antifreeze” proteins: Synthesis and ice growth inhibition of analogues that probe the relative importance of hydrophobic and hydrogen-bonding interactions. J. Am. Chem. Soc. 1999, 121, 941–948. [Google Scholar] [CrossRef]
- Chao, H.; Houston, M.E., Jr.; Hodges, R.S.; Kay, C.M.; Sykes, B.D.; Loewen, M.C.; Davies, P.L.; Sönnichsen, F.D. A diminished role for hydrogen bonds in antifreeze protein binding to ice. Biochemistry 1997, 36, 14652–14660. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, Y.; Inohara, N.; Yokoyama, E. Growth patterns and interfacial kinetic supercooling at ice/water interfaces at which anti-freeze glycoprotein molecules are adsorbed. J. Cryst. Growth 2005, 275, 167–174. [Google Scholar] [CrossRef]
- Butler, M.F. Freeze concentration of solutes at the ice/solution interface studied by optical interferometry. Cryst. Growth Des. 2002, 2, 541–548. [Google Scholar] [CrossRef]
- Hagiwara, Y.; Yamamoto, D. Temperature distribution and local heat flux in the unidirectional freezing of antifreeze-protein solution. Int. J. Heat Mass Transf. 2012, 55, 2384–2393. [Google Scholar] [CrossRef]
- Hagiwara, Y.; Aomatsu, H. Supercooling enhancement by adding antifreeze protein and ions to water in a narrow space. Int. J. Heat Mass Transfer 2015, 86, 55–64. [Google Scholar] [CrossRef]
- Miyamoto, T.; Nishi, N.; Waku, T.; Tanaka, N.; Hagiwara, Y. Effects of short-time preheating on ice growth in antifreeze polypeptides solutions in a narrow space. Heat Mass Transf. 2018, 54, 2415–2424. [Google Scholar] [CrossRef]
- Wilson, P.W.; Heneghan, A.F.; Haymet, A.D.J. Ice nucleation in nature: Supercooling point (SCP) measurements and the role of heterogeneous nucleation. Cryobiology 2003, 46, 88–98. [Google Scholar] [CrossRef]
- Grandum, S.; Yabe, A.; Nakagomi, K.; Tanaka, M.; Takemura, F.; Kobayashi, Y.; Ikemoto, M.; Frivik, P. Investigation of the characteristics of ice slurry containing antifreeze protein for ice storage applications. Trans. Jpn. Soc. Mech. Ser. B 1997, 63, 1770–1776. (In Japanese) [Google Scholar] [CrossRef] [Green Version]
- Takeshita, Y.; Waku, T.; Wilson, P.W.; Hagiwara, Y. Effects of winter flounder antifreeze protein on the growth of ice particles in an ice slurry flow in mini-channels. Biomolecules 2019, 9, 70. [Google Scholar] [CrossRef] [Green Version]
- Knight, C.A.; Cheng, C.C.; DeVries, A.L. Adsorption of α-helical antifreeze peptides on specific ice crystal surface planes. Biophys. J. 1991, 59, 409–418. [Google Scholar] [CrossRef] [Green Version]
- Davies, P.L.; Baardsnes, J.; Kuiper, M.J.; Walker, V.K. Structure and function of antifreeze proteins. Phil. Trans. R. Soc. Lond. B 2002, 357, 927–935. [Google Scholar] [CrossRef] [Green Version]
- Jorov, A.; Zhorov, B.S.; Yang, D.S.C. Theoretical study of interaction of winter flounder antifreeze protein with ice. Protein Sci. 2004, 13, 1524–1537. [Google Scholar] [CrossRef] [Green Version]
- Caro, C.G.; Pedley, T.I.; Seed, W.A. Mechanics of the circulation. In Cardiovascular Physiology; Guyton, A.C., Ed.; Medical and Technical Publishers: London, UK, 1974; Chapter 1. [Google Scholar]
- Ellerby, D.J.; Ennos, A.R. Resistances to fluid flow of model xylem vessels with simple and scalariform perforation plates. J. Exp. Bot. 1998, 49, 979–985. [Google Scholar] [CrossRef]
- Onishi, Y. An Experimental Study on Mechanism of Ice Growth Inhibition of Antifreeze Protein in Flowing Water in Mini-Channel. Master Thesis, Kyoto Institute of Technology, Kyoto, Japan, 21 February 2012. (In Japanese). [Google Scholar]
- Baust, J.B.; Gao, D.; Baust, J.M. Cryopreservation: An emerging paradigm change. Organogenesis 2009, 5, 90–96. [Google Scholar] [CrossRef] [Green Version]
- Hagiwara, Y.; Yamamoto, D. Cooperative effect of winter flounder antifreeze protein and ions on the unidirectional freezing of their solution in a narrow space. In Physics and Chemistry of Ice 2010; Hokkaido University Press: Sapporo, Japan, 2011; pp. 313–319. [Google Scholar]
- Hagiwara, Y.; Sakurai, R.; Nakanishi, R. Temperature of the solution of winter flounder antifreeze protein near ice surfaces in a narrow space. J. Cryst. Growth 2010, 312, 314–322. [Google Scholar] [CrossRef]
- Madura, J.D.; Baran, K.; Wierzbicki, A. Molecular recognition and binding of thermal hysteresis proteins to ice. J. Mol. Recognit. 2000, 13, 101–113. [Google Scholar] [CrossRef]
- Hagiwara, Y. Peculiar freezing fronts of solutions for antifreeze protein or antifreeze polypeptide in a narrow space (in Japanese). J. Jpn. Assoc. Cryst. Growth 2018, 45, 2-03. [Google Scholar]
- Evans, R.P.; Hobbs, R.S.; Goddard, S.V.; Fletcher, G.L. The importance of dissolved salts to the in vivo efficacy of antifreeze proteins. Comp. Biochem. Physiol. Part A 2007, 148, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Kristiansen, E.; Pedersen, S.A.; Zachariassen, K.E. Salt-induced enhancement of antifreeze protein activity: A salting-out effect. Cryobiology 2008, 57, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, K.; Furukawa, Y. Time development of a solute diffusion field and morphological instability on a planar interface in the directional growth of ice crystals. J. Cryst. Growth 2000, 209, 167–174. [Google Scholar] [CrossRef]
- Inglis, S.R.; McGann, M.J.; Price, W.S.; Harding, M.M. Diffusion NMR studies on fish antifreeze proteins and synthetic analogues. FEBS Lett. 2006, 580, 3911–3915. [Google Scholar] [CrossRef] [Green Version]
- Vanýsek, P. Ionic conductivity and diffusion at infinite dilution. In CRC Handbook of Chemistry and Physics, Internet Version 2005; Lide, D.R., Ed.; CRC Press: Boca Raton, FL, USA, 2005; Section 5; pp. 93–94. Available online: http://www.hbcpnetbase.com (accessed on 29 December 2020).


| Case | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| HPLC6 (mg/mL) | 0 | 0 | 0.25 | 0.25 |
| NaCl (wt%) | 0 | 0.45 | 0 | 0.45 |
| Magnification | ×5 |
| Binning | 2 × 2 |
| Pixel number | 672 × 512 |
| Area size (μm2) | 1572 × 1198 |
| Pixel resolution (μm2) | 2.34 × 2.34 |
| Frame rate (fps) | 1 |
| Exposure time (s) | 0.05 |
| Case | 1 (Water) | 2 (Salt Solution) | 3 (AFP Solution) | 4 (Mixed Solution) |
|---|---|---|---|---|
| (μm/s) | 12.6 | 11.3 | 7.1 | 4.6 |
| 1 | 0.90 | 0.56 | 0.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taira, K.; Waku, T.; Hagiwara, Y. Ice Growth Suppression in the Solution Flows of Antifreeze Protein and Sodium Chloride in a Mini-Channel. Processes 2021, 9, 306. https://doi.org/10.3390/pr9020306
Taira K, Waku T, Hagiwara Y. Ice Growth Suppression in the Solution Flows of Antifreeze Protein and Sodium Chloride in a Mini-Channel. Processes. 2021; 9(2):306. https://doi.org/10.3390/pr9020306
Chicago/Turabian StyleTaira, Kazuya, Tomonori Waku, and Yoshimichi Hagiwara. 2021. "Ice Growth Suppression in the Solution Flows of Antifreeze Protein and Sodium Chloride in a Mini-Channel" Processes 9, no. 2: 306. https://doi.org/10.3390/pr9020306
APA StyleTaira, K., Waku, T., & Hagiwara, Y. (2021). Ice Growth Suppression in the Solution Flows of Antifreeze Protein and Sodium Chloride in a Mini-Channel. Processes, 9(2), 306. https://doi.org/10.3390/pr9020306






