Three-Dimensional Numerical Simulations and Antifouling Mechanism of Microorganisms on Microstructured Surfaces
Abstract
:1. Introduction
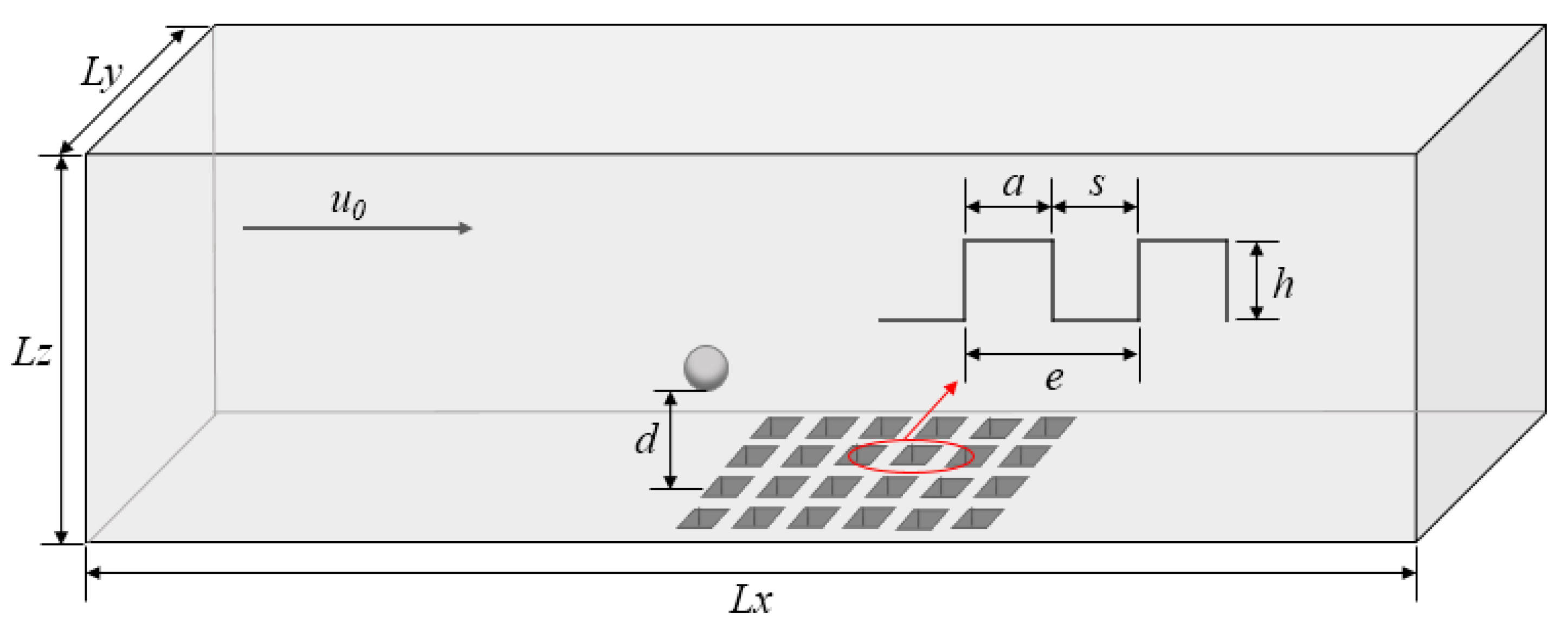
2. Mathematical Physical Model
2.1. Model Parameters and Boundary Conditions
2.2. Governing Equation and Calculation Method
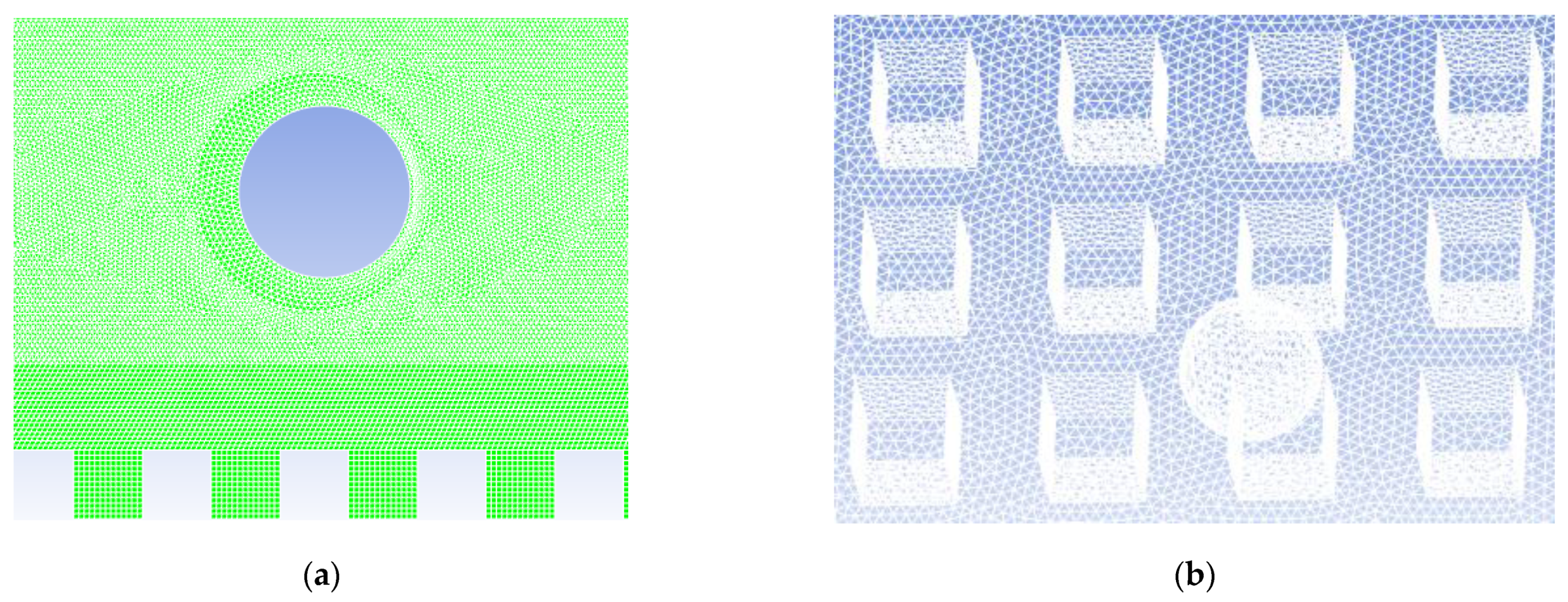
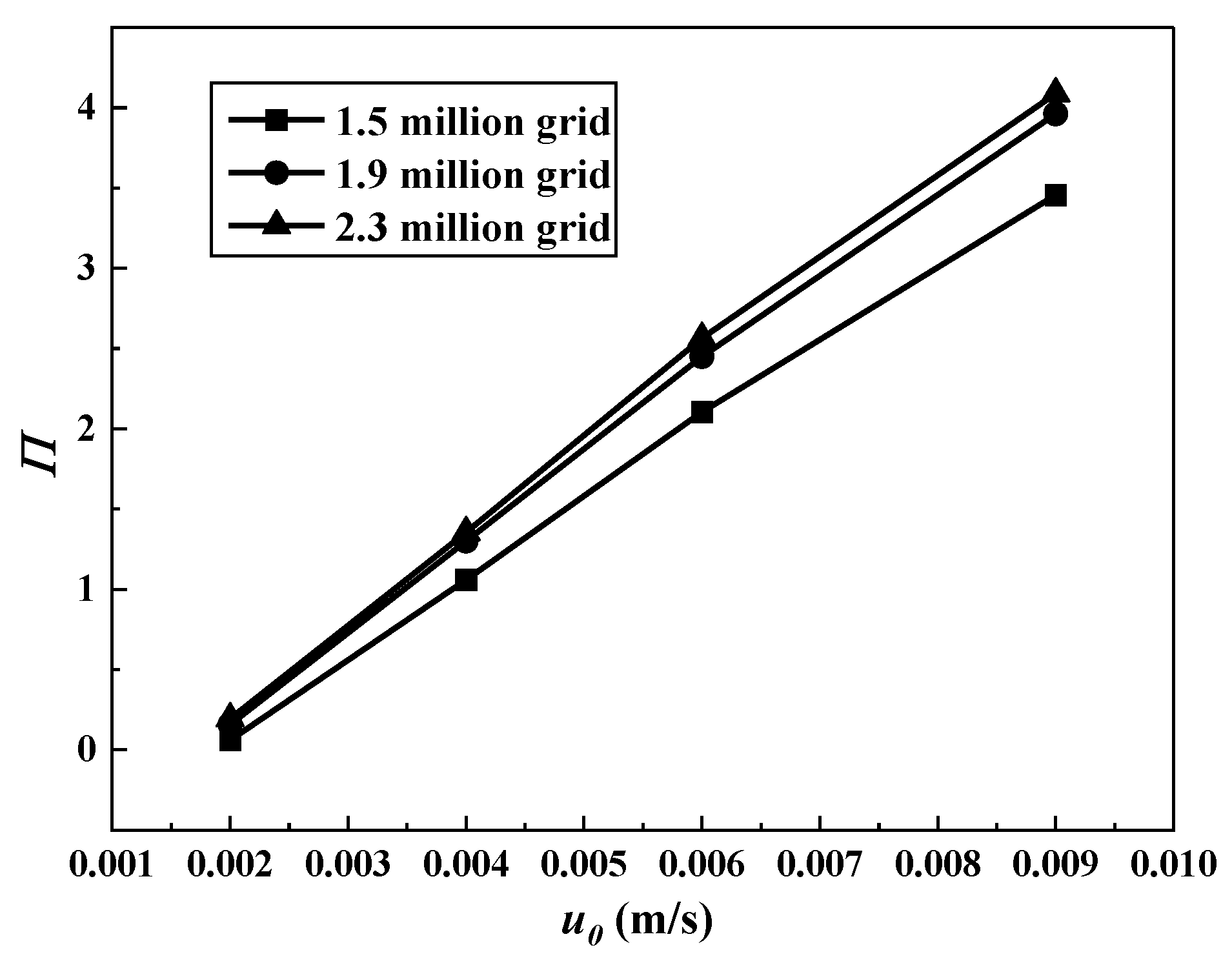
2.3. Mesh Generation and Independence Test
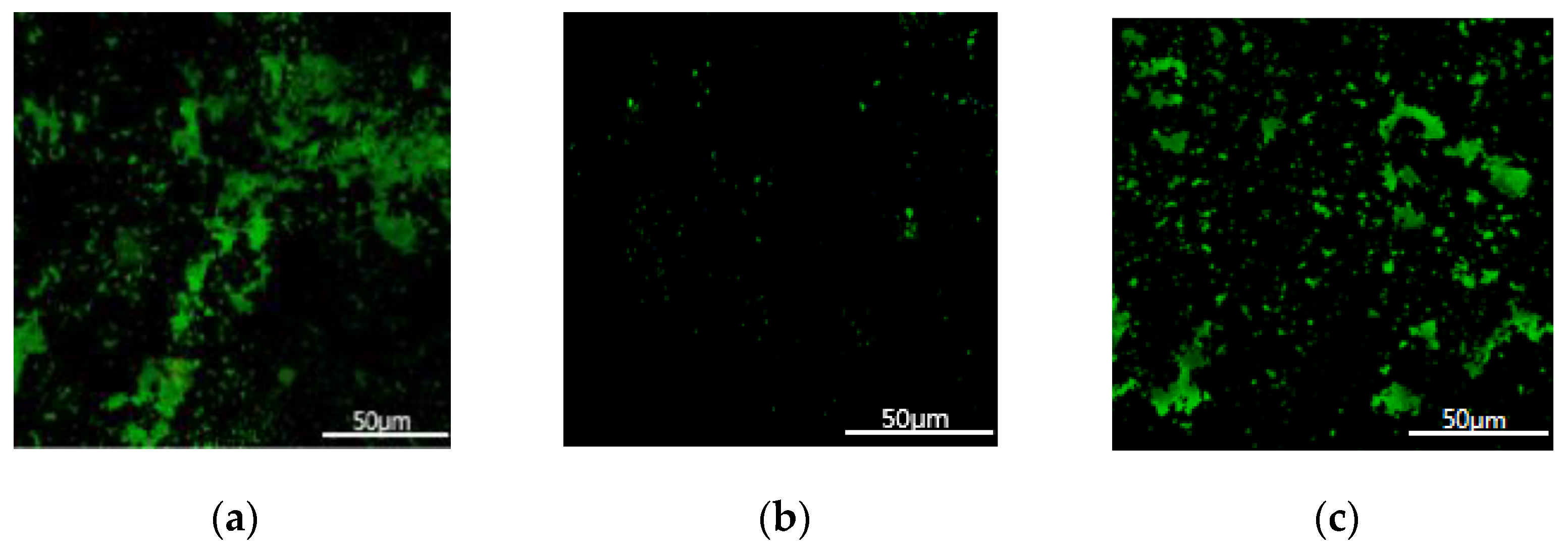
3. Experiment and Model Validation
4. Simulation Results and Discussion
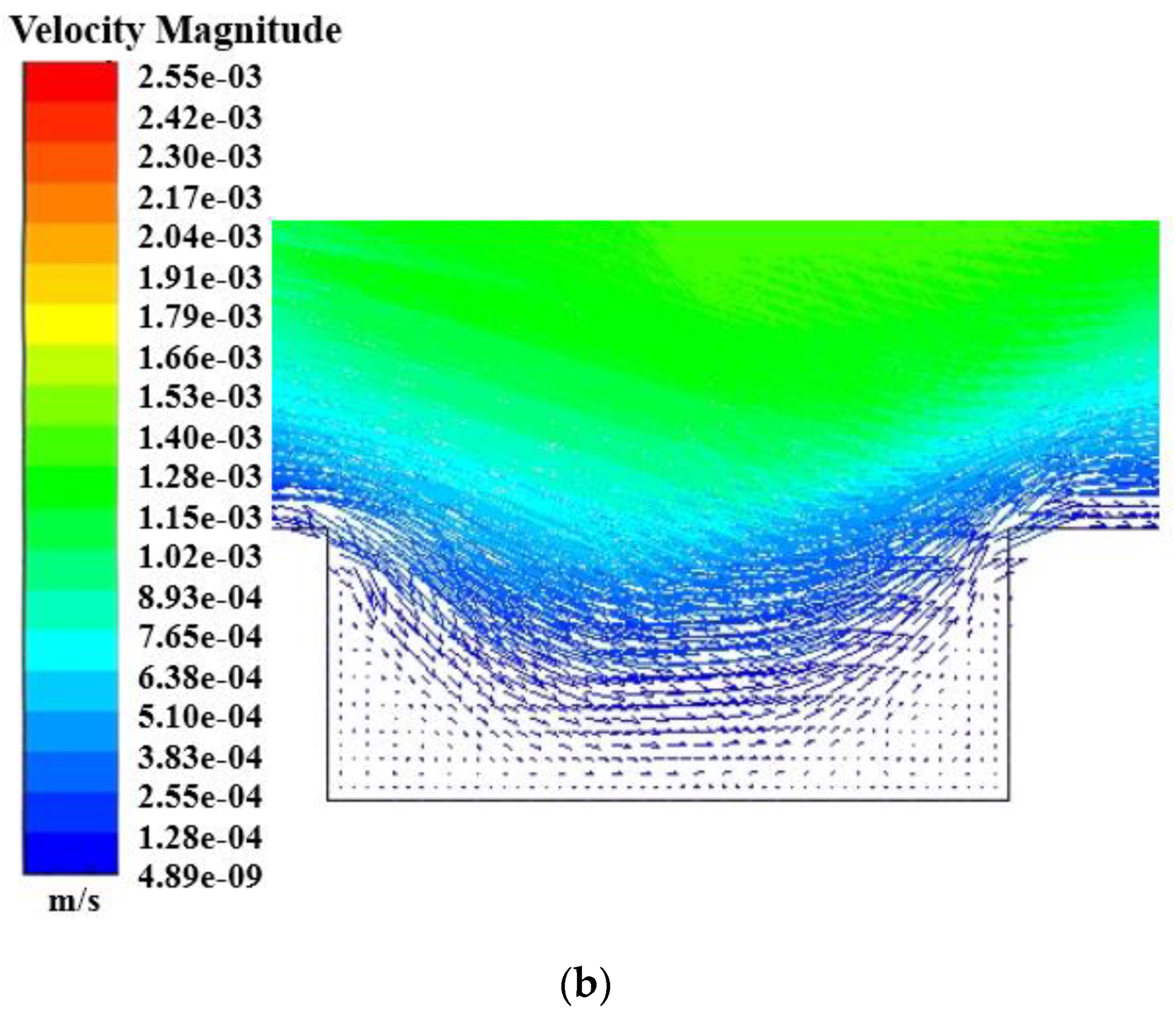
4.1. Characteristics of Fluid Kinematics in Microbial Motion
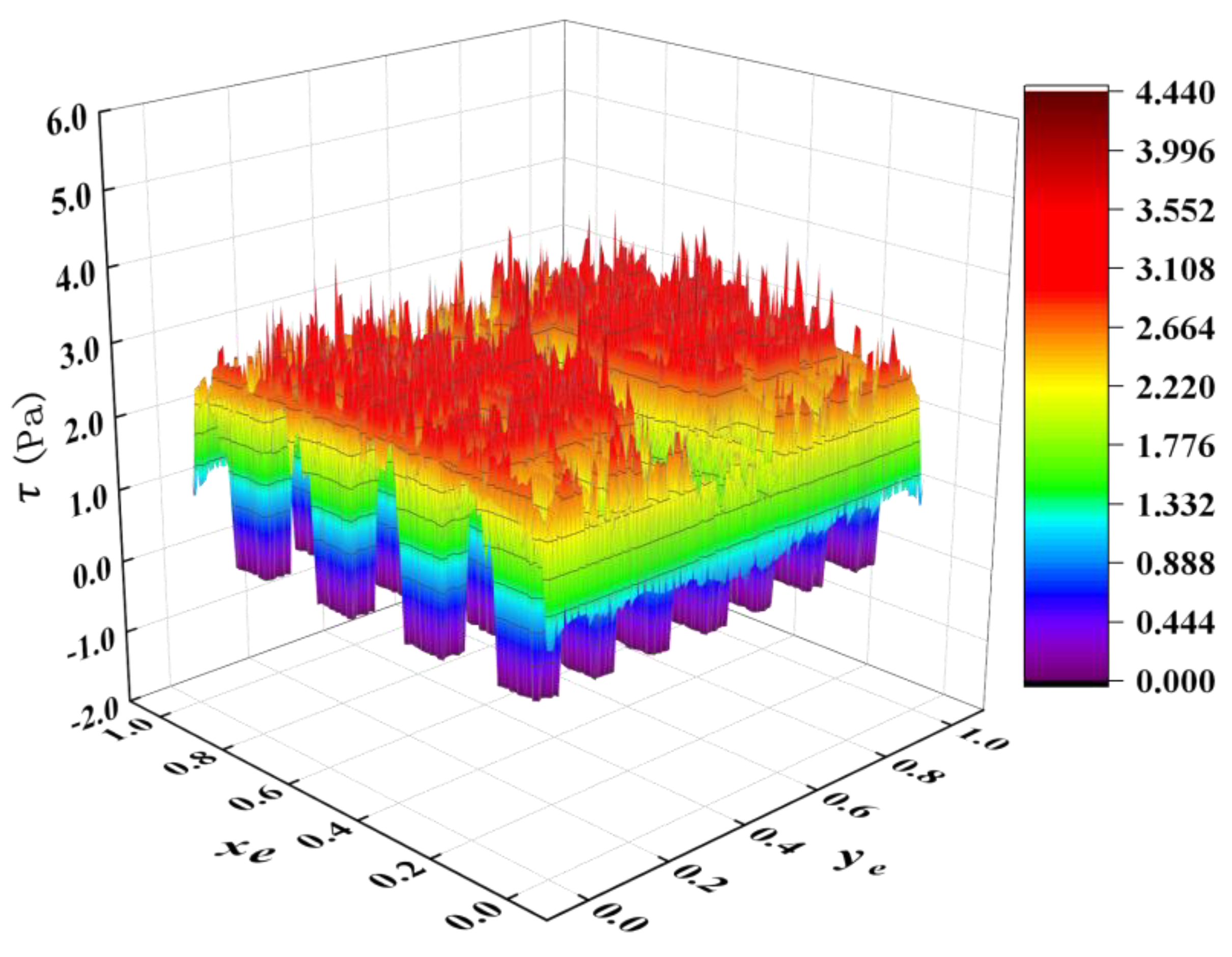
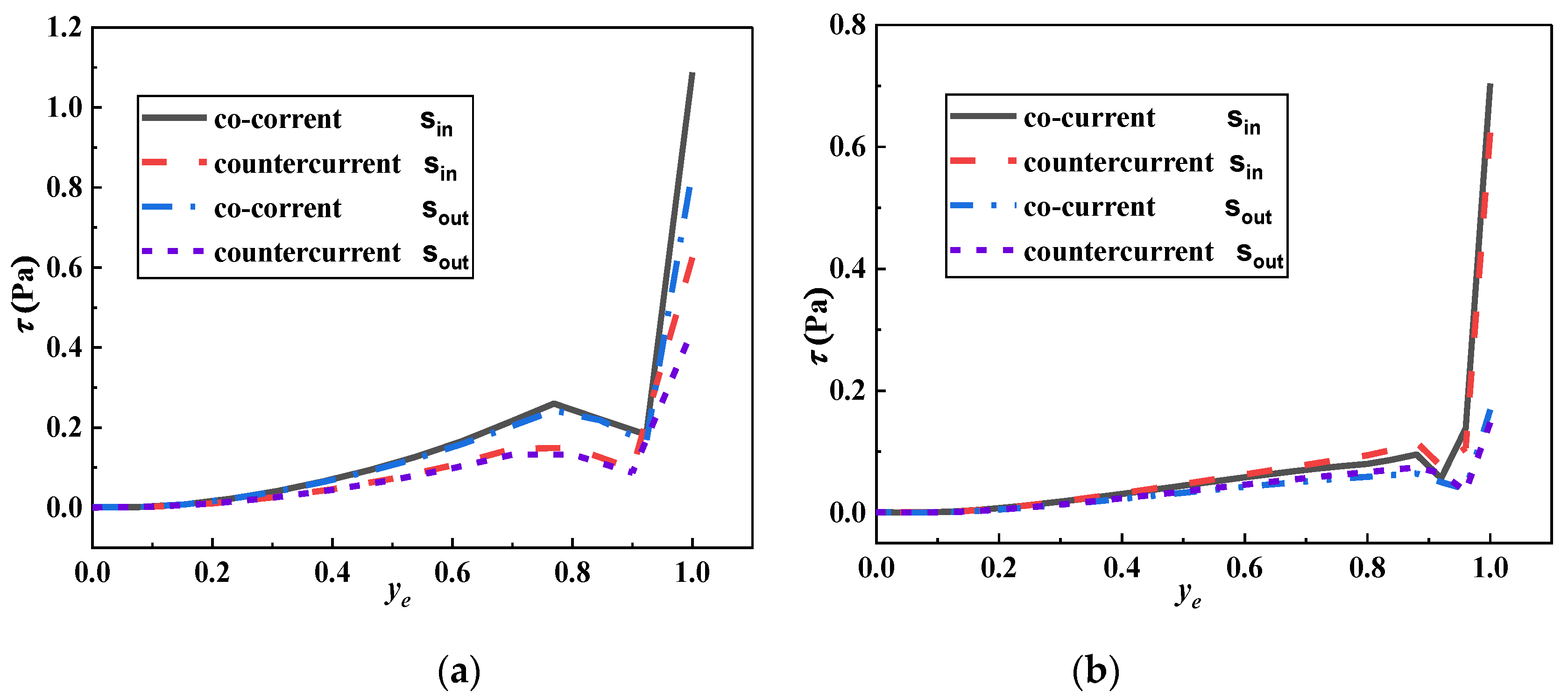
4.2. Shear Stress Distribution of Microbial Movement
5. Conclusions
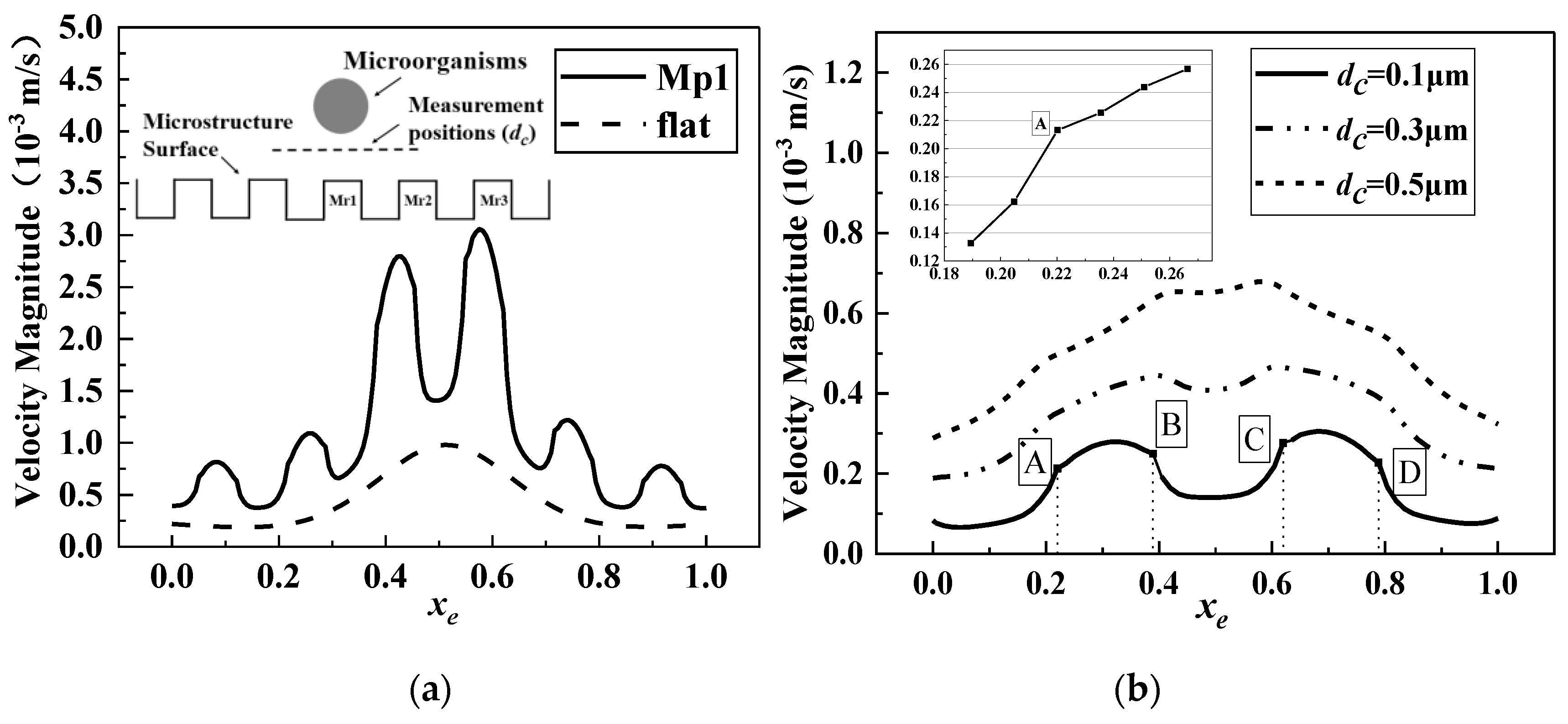
- When microorganisms moved at different heights on the microstructured surface, the influences of their movements on the flow velocity were related to the heights at which they were located. When d > r, the velocity below the microorganisms was greater than that above the surrounding microstructure, and when d < r, the flow velocity below the microorganisms was significantly lower than that above the surrounding microstructure. Therefore, a low-velocity region was formed around the microorganisms in the process of approaching the microstructured surface.
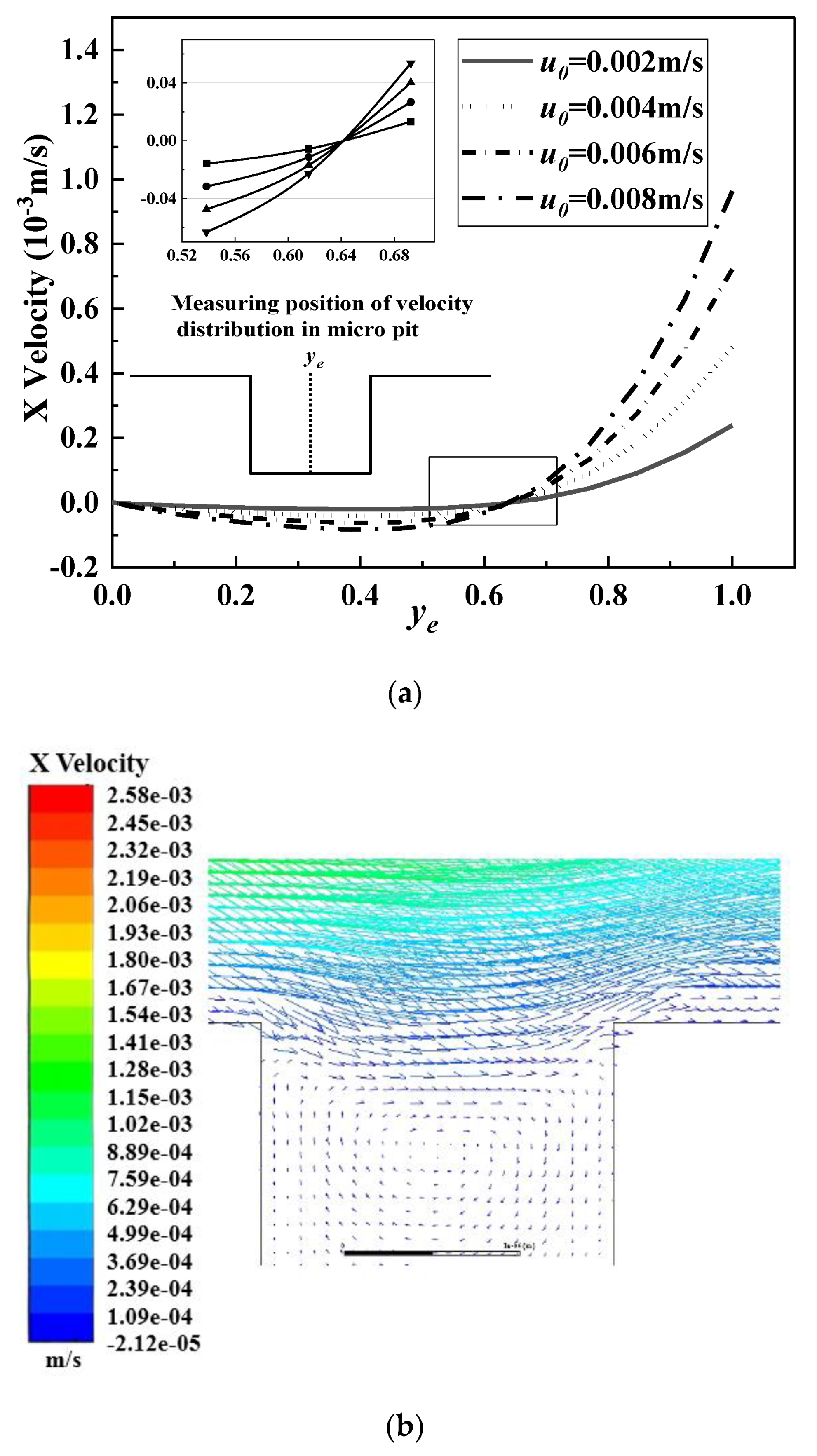
- The increase of the initial velocity in the simulation channel significantly increased the vortex motion in the micropits. As the formation of biofouling conditioning layers occurred in the nanoscale, they easily entered the micropits and appeared everywhere on the microstructured surface. However, the existence of the micro-vortex flow caused particles to rotate and even spin out of the micropits; thereby, reducing the formation of conditioning layers in the microstructure.
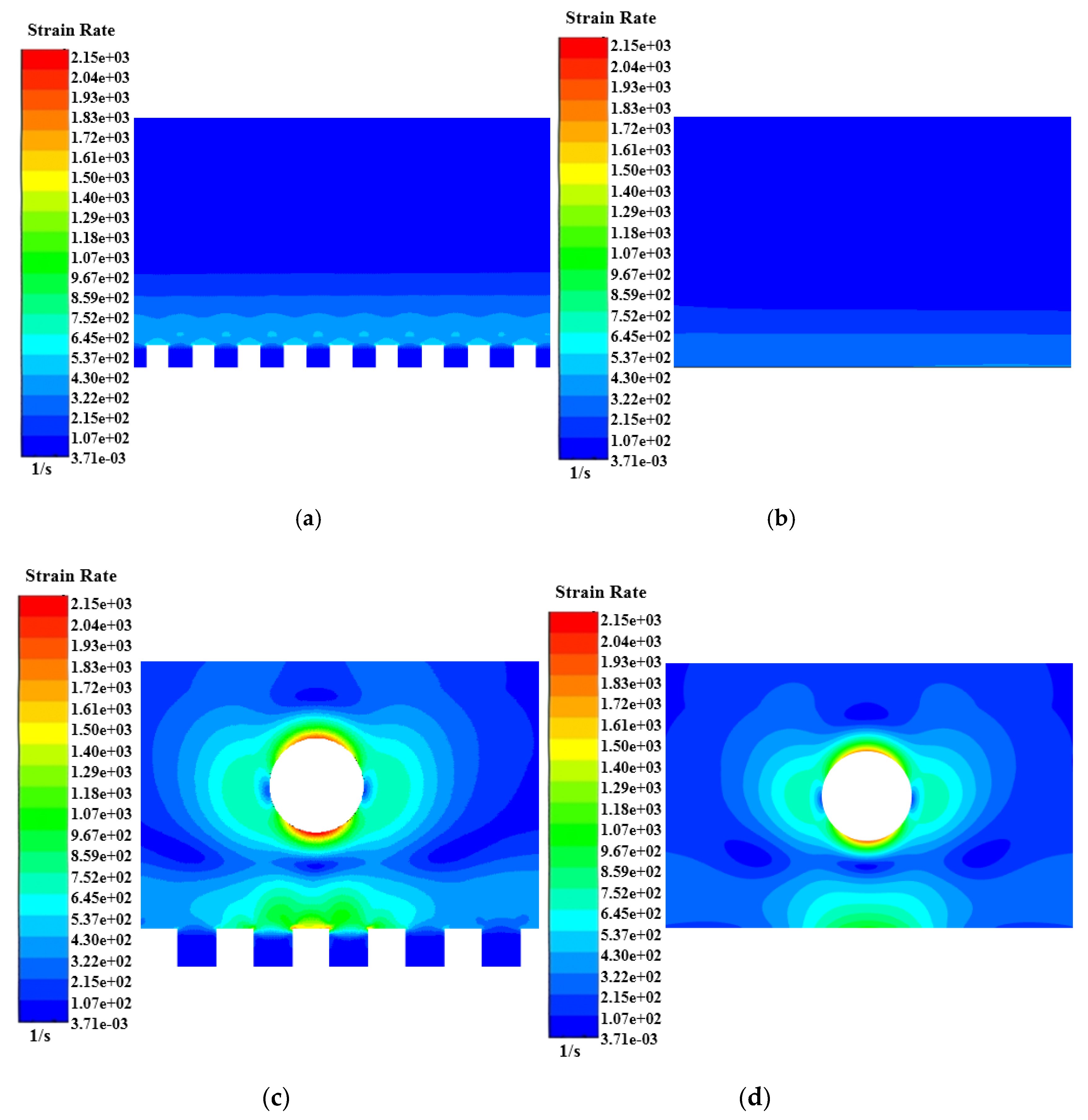
- The movement of microorganisms near the wall caused a low shear stress distribution on adjacent microridges. In previous studies, the consideration of relatively high shear stress on microstructured surfaces caused difficulty in microbial attachments. However, in the current simulation, the addition of moving microorganisms significantly weakened the shear stress when the microbes approached the wall, indicating that its impact has been overestimated in previous studies. There is no conclusive evidence that high shear stress is the main antifouling factor for microstructured surfaces. The shear stress gradient of the microstructured surface fluctuated sharply at ridge steps, signifying that microridge edges have a better effect in reducing biological attachment. The sudden change of the shear stress (large ) was found to be an important parameter to reduce microbial attachment.
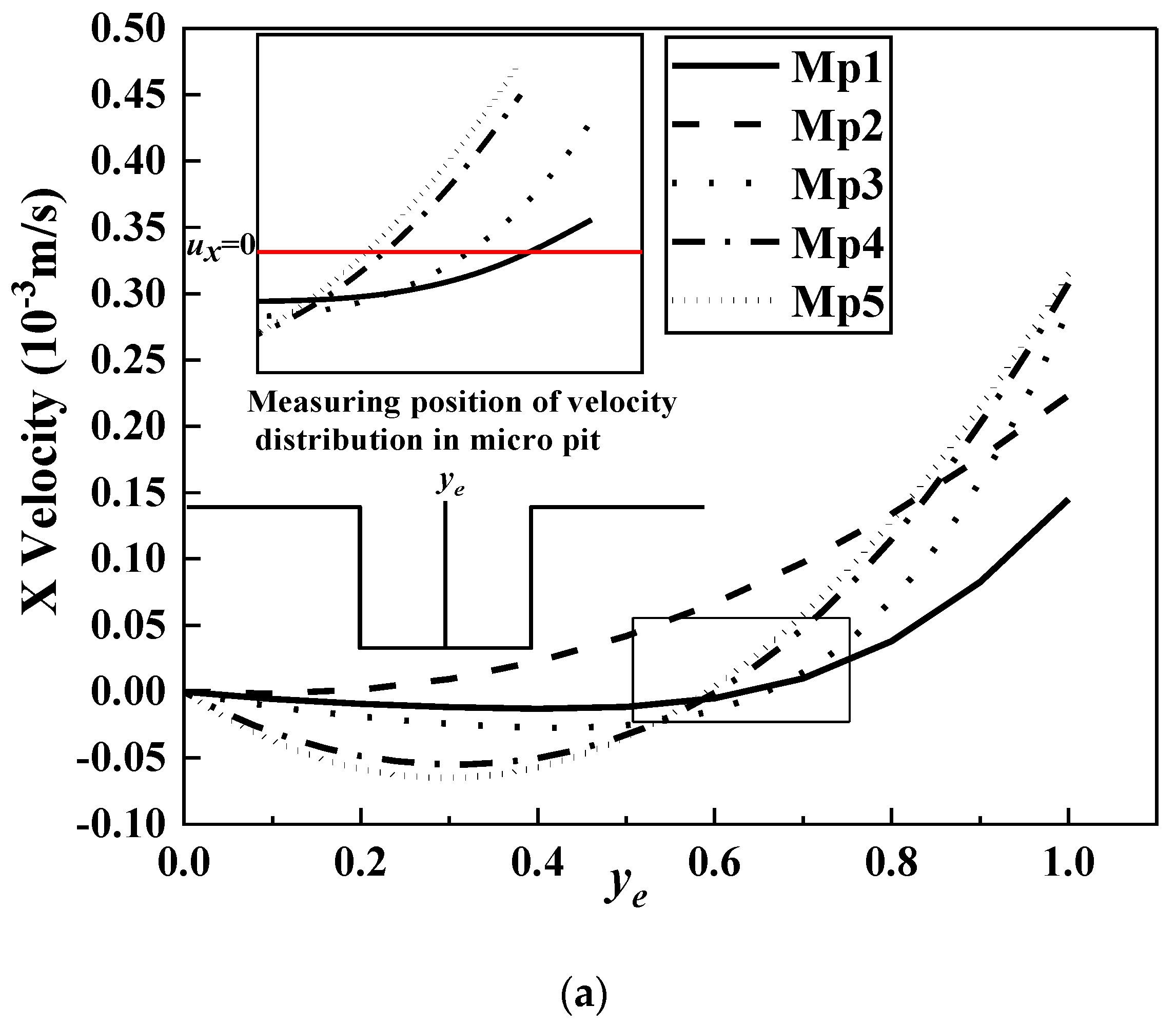
- The average shear stresses of the wall Sin and Sout in the downstream and counterflow directions decreased with the increase in micropit depth and width. The Mp5 microridge had a larger shear stress gradient, suggesting its superior antifouling performance to other surfaces; however, this inference was only applicable when r > s/2, that is, when the main fouling organisms cannot enter the micropit. Further investigation of the shear stress in the micro pits shows that when r < s/2, the Mp1 micropit was the most difficult to adhere to. Spherical microorganisms were only considered in the current simulation; however, in actual situations, the shape and characteristic size of microorganisms are quite different. Therefore, in future simulations, microorganisms with different shapes and sizes will be considered to optimize antifouling models of microstructured surfaces.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gu, Y.Q.; Mou, J.G.; Dai, D.S.; Zheng, S.H.; Jiang, L.F.; Wu, D.H.; Ren, Y.; Liu, F.Q. Characteristics on drag reduction of bionic jet surface based on earthworm’s back orifice jet. Acta Phys. Sin. 2015, 64, 024701. [Google Scholar] [CrossRef]
- Murthy, P.S.; Venugopalan, V.P.; Nair, K.V.K.; Subramoniam, T. Larval Settlement and Surfaces: Implications in Development of Antifouling Strategies. Mar. Ind. Biofouling 2009, 4, 233–263. [Google Scholar] [CrossRef]
- Lou, T.; Bai, X.Q.; Yuan, C.Q.; Yang, Z.C. Advances in Surface Microstructure Antifouling Technology for Ship Hull. Surf. Technol. 2019, 48, 115–126. [Google Scholar] [CrossRef]
- Gregory, S.W.; David, W.G.; Lin, S.; Li, X.; Bronwen, W.C.; Sverre, M.; Jolanta, A.W. A gecko skin micro/nano structure-a low adhesion, superhydrophobic, anti-wetting, self-cleaning, biocompatible, antibacterial surface. Acta Biomater. 2015, 21, 109–122. [Google Scholar] [CrossRef]
- Brennan, A.B.; Baney, R.H.; Carman, M.L.; Estes, T.G.; Feinberg, A.W.; Wilson, L.H.; Schumacher, J.F. Surface Topography for Non-Toxic Bioadhesion Control. U.S. Patent 7143709 B2, 5 December 2006. [Google Scholar]
- Kommeren, A.S. Structured Antifouling Coatings for the Marine Environment; Technische Universiteit Eindhoven: Eindhoven, The Netherlands, 2017. [Google Scholar]
- Callow, M.E.; Fletcher, R.L. The influence of low surface energy materials on bioadhesion a review. Int. Biodeterior. Biodegrad. 1994, 34, 333–348. [Google Scholar] [CrossRef]
- Fletcher, M.; Loeb, G. Influence of substratum characteristics on the attachment of a marine pseudomonad to solid surfaces. Appl. Environ. Microb. 1979, 37, 67–72. [Google Scholar] [CrossRef] [Green Version]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Berntsson, K.M.; Andreasson, H.; Jonsson, P.R.; Larsson, L.; Ring, K.; Petronis, S.; Gatenholm, P. Reduction of barnacle recruitment on micro-textured surfaces: Analysis of effective topographic characteristics and evaluation of skin friction. Biofouling 2000, 16, 245–261. [Google Scholar] [CrossRef]
- Omran, M.; Akarri, S.; Torsaeter, O. The Effect of Wettability and Flow Rate on Oil Displacement Using Polymer-Coated Silica Nanoparticles: A Microfluidic Study. Processes 2020, 8, 991. [Google Scholar] [CrossRef]
- Schumacher, J.F.; Carman, M.L.; Estes, T.G.; Feinberg, A.W.; Wilson, L.H.; Callow, M.E.; Callow, J.A.; Finlay, J.A.; Brennan, A.B. Engineered antifouling microtopographies-effect of feature size, geometry, and roughness on settlement of zoospores of the green alga ulva. Biofouling 2007, 23, 55–62. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Lin, C.G.; Zhang, J.W.; Wang, L.; Xu, F.L.; Zhou, J.; Duan, D.X.; Liu, H.H. Antifouling performance of surface microtopographies based on sea star luidia quinaria. Key Eng. Mater. 2013, 562–565, 1290–1295. [Google Scholar] [CrossRef]
- Scardino, A.J.; Guenther, J.; Nys, R.D. Attachment point theory revisited: The fouling response to a microtextured matrix. Biofouling 2008, 24, 45–53. [Google Scholar] [CrossRef]
- Long, C.J.; Schumacher, J.F.; Robinson, P.A.C.; Finlay, J.A.; Callow, M.E.; Callow, J.A.; Brennan, A.B. A model that predicts the attachment behavior of ulva linza zoospores on surface topography. Biofouling 2010, 26, 411–419. [Google Scholar] [CrossRef]
- Magin, C.M.; Long, C.J.; Cooper, S.P.; Ista, L.K.; Lopez, G.P.; Brennan, A.B. Engineered antifouling microtopographies: The role of Reynolds number in a model that predicts attachment of zoospores of ulva and cells of cobetia marina. Biofouling 2010, 26, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, J.F.; Long, C.J.; Callow, M.E.; Finlay, J.A.; Callow, J.A.; Brennan, A.B. Engineered nanoforce gradients for inhibition of settlement (attachment) of swimming algal spores. Langmuir 2008, 24, 4931–4937. [Google Scholar] [CrossRef] [PubMed]
- Decker, J.T.; Kirschner, C.M.; Long, C.J.; Finlay, J.A.; Callow, M.E.; Callow, J.A.; Brennan, A.B. Engineered Antifouling Microtopographies: An Energetic Model That Predicts Cell Attachment. Langmuir 2013, 29, 13023–13030. [Google Scholar] [CrossRef]
- Jian, Z.; Xin, H.Y.; Tu, S.-T. Lattice Boltzmann Simulation on Droplet Flow through 3D Metal Foam. Processes 2019, 7, 877. [Google Scholar] [CrossRef] [Green Version]
- Won, Y.J.; Jung, S.Y.; Jang, J.H.; Lee, J.W.; Chae, H.R.; Choi, D.C.; Hyun, A.K.; Lee, C.H.; Park, P.K. Correlation of membrane fouling with topography of patterned membranes for water treatment. J. Membr. Sci. 2016, 498, 14–19. [Google Scholar] [CrossRef]
- Jung, S.Y.; Hyun, A.K. Transport and deposition of colloidal particles on a patterned membrane surface: Effect of cross-flow velocity and the size ratio of particle to surface pattern. J. Membr. Sci. 2019, 572, 309–319. [Google Scholar] [CrossRef]
- Mohammed Ridha, B.A.F.; Felicia, W.Y.M.; Narayana, N.S.; Hosseini, F.M.; Eunice Phang, S.W.; Chua, B.L.; Chow, Y.H.; Yong, L.C.; Al-Obaidi, A.S.M. Comparisons of Flow Patterns over a Hierarchical and a Non-hierarchical Surface in Relation to Biofouling Control. USA MATEC Web Conf. 2018, 02014–02029. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.K.; Won, Y.J.; Yoo, J.H.; Ahn, K.H.; Lee, C.H. Flow analysis and fouling on the patterned membrane surface. J. Membr. Sci. 2013, 427, 320–325. [Google Scholar] [CrossRef]
- Darnton, N.C.; Turner, L.; Rojevsky, S.; Berg, H.C. Dynamics of bacterial swarming. Biophys. J. 2010, 98, 2082–2090. [Google Scholar] [CrossRef] [Green Version]
- Halder, P.; Nasabi, M.; Jayasuriya, N.; Shimeta, J.; Deighton, M.; Bhattacharya, S.; Mitchell, A.; Bhuiyan, M.A. An assessment of the dynamic stability of microorganisms on patterned surfaces in relation to biofouling control. Biofouling 2014, 30, 695–707. [Google Scholar] [CrossRef]
- Smayda, T.J. The suspension and sinking of phytoplankton in the sea. Oceanogr. Mar. Biol. Ann. Rev. 1970, 8, 353–414. [Google Scholar]
- Schwuchow, J.M.; Kern, V.D.; Wagner, T.; Sack, F.D. The density of apical cells of dark-grown protonemata of the moss Ceratodon purpureus. Protoplasma 2000, 211, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Rosenhahn, A.; Sendra, G.H. Surface Sensing and Settlement Strategies of Marine Biofouling Organisms. Biointerphases 2012, 7, 63–75. [Google Scholar] [CrossRef]



| Micropit Parameters | Mp1 | Mp2 | Mp3 | Mp4 | Mp5 |
|---|---|---|---|---|---|
| h (μm) | 2 | 2 | 5 | 5 | 5 |
| s (μm) | 2 | 5 | 5 | 8 | 10 |
| Shear Stress | Mr0 | Mr1 | Mr2 | Mr3 | Mr4 |
|---|---|---|---|---|---|
| (d = 0.5 μm) | 1.467 | 0.651 | 0.678 | 1.884 | 1.468 |
| (d = 0.5 μm) | 2.005 | 1.059 | 0.951 | 2.768 | 2.005 |
| (d = 5 μm) | 0.061 | 0.110 | 0.308 | 0.277 | 0.105 |
| (d = 5 μm) | 0.075 | 0.187 | 0.446 | 0.274 | 0.153 |
| Shear Stress Gradients | Mr0 | Mr1 | Mr2 | Mr3 | Mr4 |
|---|---|---|---|---|---|
| (d = 0.5 μm) | 80.240 | 58.898 | 62.846 | 96.839 | 98.532 |
| (d = 5 μm) | 6.662 | 19.532 | 21.658 | 10.756 | 5.710 |
| Each Point at the Ridge | A0 | B0 | A1 | C0 | D0 | C1 |
|---|---|---|---|---|---|---|
| 60.461 | −65.822 | 15.795 | 17.186 | −19.479 | 15.086 |
| Average Shear Stress | Mp1 | Mp2 | Mp3 | Mp4 | Mp5 |
|---|---|---|---|---|---|
| 0.178 | 0.125 | 0.094 | 0.084 | 0.069 | |
| 0.156 | 0.062 | 0.058 | 0.040 | 0.035 | |
| 0.117 | 0.112 | 0.089 | 0.080 | 0.069 | |
| 0.096 | 0.033 | 0.053 | 0.042 | 0.037 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Wang, S.; Li, C.; Li, H. Three-Dimensional Numerical Simulations and Antifouling Mechanism of Microorganisms on Microstructured Surfaces. Processes 2021, 9, 319. https://doi.org/10.3390/pr9020319
Yang H, Wang S, Li C, Li H. Three-Dimensional Numerical Simulations and Antifouling Mechanism of Microorganisms on Microstructured Surfaces. Processes. 2021; 9(2):319. https://doi.org/10.3390/pr9020319
Chicago/Turabian StyleYang, Hongyue, Songling Wang, Chunxi Li, and Hengfan Li. 2021. "Three-Dimensional Numerical Simulations and Antifouling Mechanism of Microorganisms on Microstructured Surfaces" Processes 9, no. 2: 319. https://doi.org/10.3390/pr9020319





