Environmental Method for Synthesizing Amorphous Silica Oxide Nanoparticles from a Natural Material
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of NPs
2.1.1. RHs Ball Milling
2.1.2. Optical Microscope Images
2.1.3. Thermal Decomposition Step
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Le, V.H.; Nhan, C.; Thuc, H.H.; Thuc, H.H.; Thuc, C.N.H.; Thuc, H.H. Synthesis of silica nanoparticles from Vietnamese rice husk by sol–gel method. Nanoscale Res. Lett. 2013, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Basu, H.; Singhal, R.K.; Pimple, M.V. Reddy AVRR Synthesis and characterization of silica microsphere and their application in removal of uranium and thorium from water. Int. J. Environ. Sci. Technol. 2015, 12, 1899–1906. [Google Scholar] [CrossRef]
- Jin, Y.; Davarpanah, A. Using Photo-Fenton and Floatation Techniques for the Sustainable Management of Flow-Back Produced Water Reuse in Shale Reservoirs Exploration. Water Air Soil Pollut. 2020. [Google Scholar] [CrossRef]
- Davarpanah, A. The feasible visual laboratory investigation of formate fluids on the rheological properties of a shale formation. Int. J. Environ. Sci. Technol. 2019. [Google Scholar] [CrossRef]
- Awaji, N.; Ohkubo, S.; Nakanishi, T.; Aoyama, T.; Sugita, Y.; Takasaki, K.; Komiya, S. Thermal oxide growth at chemical vapor deposited SiO2/Si interface during annealing evaluated by difference x-ray reflectivity. Appl. Phys. Lett. 1997, 71, 1954–1956. [Google Scholar] [CrossRef]
- Park, J.-H.; Oh, C.; Shin, S.-I.; Moon, S.-K.; Oh, S.-G. Preparation of hollow silica microspheres in W/O emulsions with polymers. J. Colloid. Interface Sci. 2003, 266, 107–114. [Google Scholar] [CrossRef]
- Wooldridge, M.S.; Torek, P.V.; Donovan, M.T.; Hall, D.L.; Miller, T.A.; Palmer, T.R.; Schrock, C.R. An experimental investigation of gas-phase combustion synthesis of SiO2 nanoparticles using a multi-element diffusion flame burner. Combust. Flame 2020, 131, 98–109. [Google Scholar] [CrossRef]
- Bonamartini, A.; Bondioli, F.; Maria, A.; Focher, B.; Leonelli, C.; Corradi, A.B.; Bondioli, F.; Ferrari, A.M.; Focher, B.; Leonelli, C. Synthesis of silica nanoparticles in a continuous-flow microwave reactor. Powder Technol. 2006, 167, 45–48. [Google Scholar]
- Affandi, S.; Setyawan, H.; Winardi, S.; Purwanto, A.; Balgis, R. A facile method for production of high-purity silica xerogels from bagasse ash. Adv. Powder Technol. 2009, 20, 468–472. [Google Scholar] [CrossRef]
- Cai, X.; Hong, R.Y.; Wang, L.S.; Wang, X.Y.; Li, H.Z.; Zheng, Y.; Wei, D.G. Synthesis of silica powders by pressured carbonation. Chem. Eng. J. 2009, 151, 380–386. [Google Scholar] [CrossRef]
- Fakhar, H.; Jiang, J. A zero-waste approach to blast furnace slag by synthesis of mesoporous nanosilica with high surface area. Int. J. Environ. Sci. Technol. 2020, 17, 309–318. [Google Scholar] [CrossRef]
- Davarpanah, A. Feasible analysis of reusing flowback produced water in the operational performances of oil reservoirs. Environ. Sci. Pollut. Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Davarpanah, A.; Mirshekari, B. Mathematical modeling of injectivity damage with oil droplets in the waste produced water re-injection of the linear flow. Eur. Phys. J. Plus. 2019. [Google Scholar] [CrossRef]
- Davarpanah, A.; Razmjoo, A.; Mirshekari, B. An overview of management, recycling, and wasting disposal in the drilling operation of oil and gas wells in Iran. Cogent. Environ. Sci. 2018. [Google Scholar] [CrossRef]
- Davarpanah, A.; Mirshekari, B. Effect of formate fluids on the shale stabilization of shale layers. Energy Rep. 2019. [Google Scholar] [CrossRef]
- Abolghasemi, R.; Haghighi, M.; Solgi, M.; Mobinikhaledi, A. Rapid synthesis of ZnO nanoparticles by waste thyme (Thymus vulgaris L.). Int. J. Environ. Sci. Technol. 2019, 16, 6985–6990. [Google Scholar] [CrossRef]
- Lim, J.S.; Abdul Manan, Z.; Wan Alwi, S.R.; Hashim, H. A review on utilisation of biomass from rice industry as a source of renewable energy. Renew. Sustain. Energy Rev. 2012, 16, 3084–3094. [Google Scholar] [CrossRef]
- Efremova, S.V. Rice hull as a renewable raw material and its processing routes. Russ. J. Gen. Chem. 2012, 82, 999–1005. [Google Scholar] [CrossRef]
- Fang, M.; Yang, L.; Chen, G.; Shi, Z.; Luo, Z.; Cen, K. Experimental study on rice husk combustion in a circulating fluidized bed. Fuel Process. Technol. 2004, 85, 1273–1282. [Google Scholar] [CrossRef]
- Timmer, C.P.; Block, S.; David, D. Long-Run Dynamics of Rice Consumption, 1960–2050. Rice in the Global Economy: Strategic Research and Policy Issues for Food Security; International Rice Research Institute: Los Banos, Philippines, 2010; pp. 139–174. [Google Scholar]
- Soares, L.W.; Braga, R.M.; Freitas, J.C.; Ventura, R.A.; Pereira, D.S.; Melo, D.M. The effect of rice husk ash as pozzolan in addition to cement Portland class G for oil well cementing. J. Pet. Sci. Eng. 2015, 131, 80–85. [Google Scholar] [CrossRef]
- Li, J.; Li, Q.; Qian, C.; Wang, X.; Lan, Y.; Wang, B.; Yin, W. Volatile organic compounds analysis and characterization on activated biochar prepared from rice husk. Int. J. Environ. Sci. Technol. 2019, 16, 7653–7662. [Google Scholar] [CrossRef]
- Zemnukhova, L.; Kharchenko, U.; Beleneva, I. Biomass derived silica containing products for removal of microorganisms from water. Int. J. Environ. Sci. Technol. 2015, 12, 1495–1502. [Google Scholar] [CrossRef]
- Pode, R. Potential applications of rice husk ash waste from rice husk biomass power plant. Renew. Sustain. Energy Rev. 2016, 53, 1468–1485. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Shaban, M. Recycling of different solid wastes in synthesis of high-order mesoporous silica as adsorbent for safranin dye. Int. J. Environ. Sci. Technol. 2019, 16, 7573–7582. [Google Scholar] [CrossRef]
- Davarpanah, A.; Mirshekari, B.; Behbahani, T.J.; Hemmati, M. Integrated production logging tools approach for convenient experimental individual layer permeability measurements in a multi-layered fractured reservoir. J. Pet. Explor. Prod. Technol. 2018, 8, 743–751. [Google Scholar] [CrossRef]
- Liou, T.H. Preparation and characterization of nano-structured silica from rice husk. Mater. Sci. Eng. A 2004, 364, 313–323. [Google Scholar] [CrossRef]
- Della, V.P.; Kühn, I.; Hotza, D.; Chen, H. Rice husk ash as an alternate source for active silica production. Mater. Lett. 2002, 57, 818–821. [Google Scholar] [CrossRef]
- Kumari, S.; Tyagi, M.; Jagadevan, S. Mechanistic removal of environmental contaminants using biogenic nano-materials. Int. J. Environ. Sci. Technol. 2019, 16, 7591–7606. [Google Scholar] [CrossRef]
- Chen, H. Biogenic Silica Nanoparticles Derived from Rice Husk Biomass and Their Applications; Texas State University: San Marcos, TX, USA, 2013. [Google Scholar]
- Shen, J.; Liu, X.; Zhu, S.; Zhang, H.; Tan, J. Effects of calcination parameters on the silica phase of original and leached rice husk ash. Mater. Lett. 2011, 65, 1179–1183. [Google Scholar] [CrossRef]
- Wang, W.; Martin, J.C.; Zhang, N.; Ma, C.; Han, A.; Sun, L. Harvesting silica nanoparticles from rice husks. J. Nanoparticle Res. 2011, 13, 6981–6990. [Google Scholar] [CrossRef]
- Hamad, M.A.; Khattab, I.A.; Fang, M.; Yang, L.; Chen, G.; Shi, Z.; Luo, Z.; Cen, K. Effect of the combustion process on the structure of rice hull silica. Thermochim. Acta 1981, 48, 343–349. [Google Scholar] [CrossRef]
- Xu, W.; Lo, T.Y.; Memon, S.A. Microstructure and reactivity of rich husk ash. Constr. Build. Mater. 2012, 29, 541–547. [Google Scholar] [CrossRef]
- Ghorbani, F.; Sanati, A.M.; Maleki, M. Production of silica nanoparticles from rice husk as agricultural waste by environmental friendly technique. Environ. Stud. Persian. Gulf. 2015, 2, 56–65. [Google Scholar]
- Mahmud, A.; Megat-Yusoff, P.S.M.; Ahmad, F.; Farezzuan, A.A. Acid leaching as efficient chemical treatment for rice husk in production of amorphous silica nanoparticles. ARPN J. Eng. Appl. Sci. 2016, 11, 13384–13388. [Google Scholar]
- Tari, F.; Shekarriz, M.; Zarrinpashne, S.; Ruzbehani, A. Catalytic and environmentally friendly removal of hydrogen sulfide from Claus-derived molten sulfur by nanosilica. Int. J. Environ. Sci. Technol. 2019, 16, 1691–1700. [Google Scholar] [CrossRef]




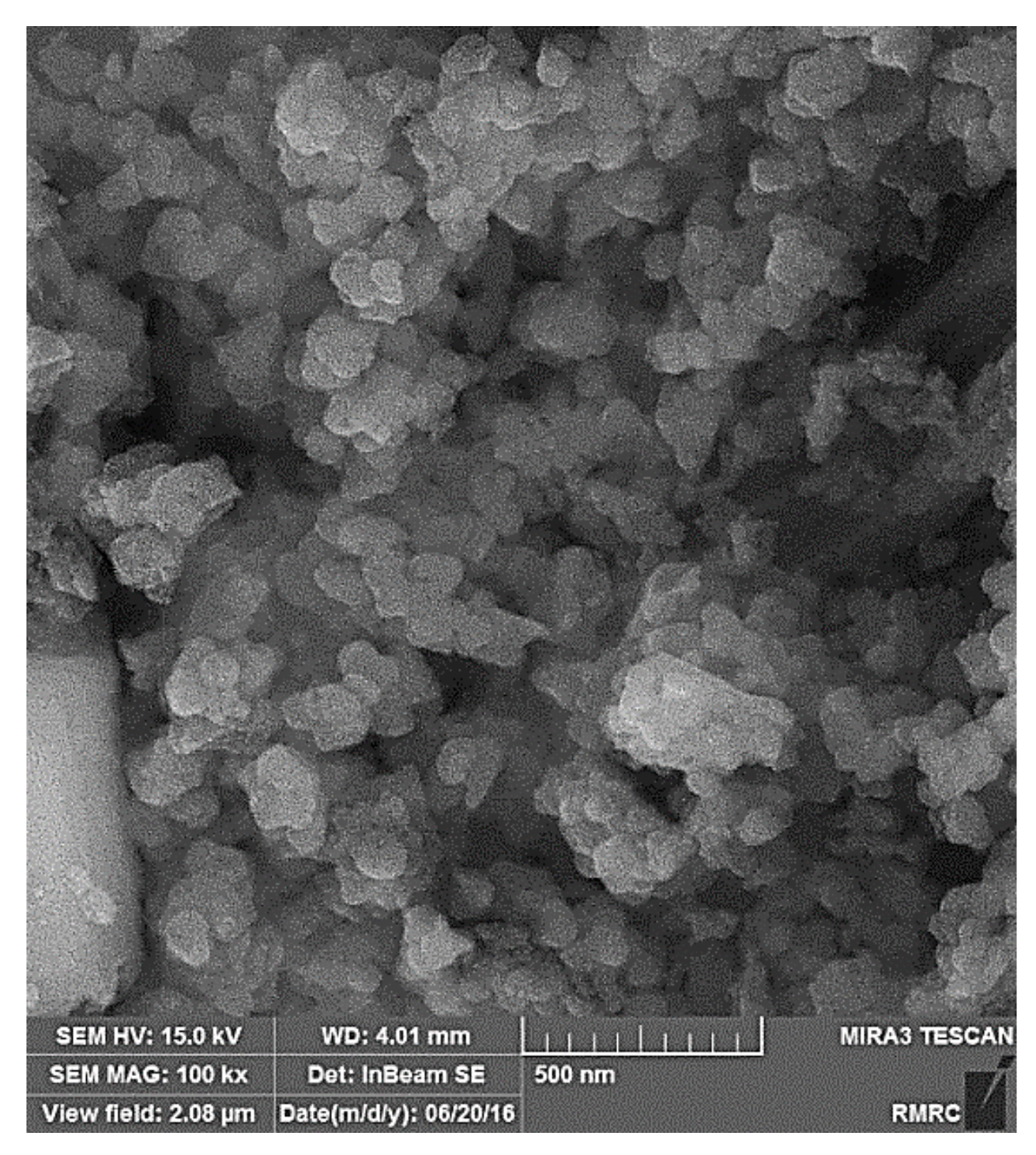
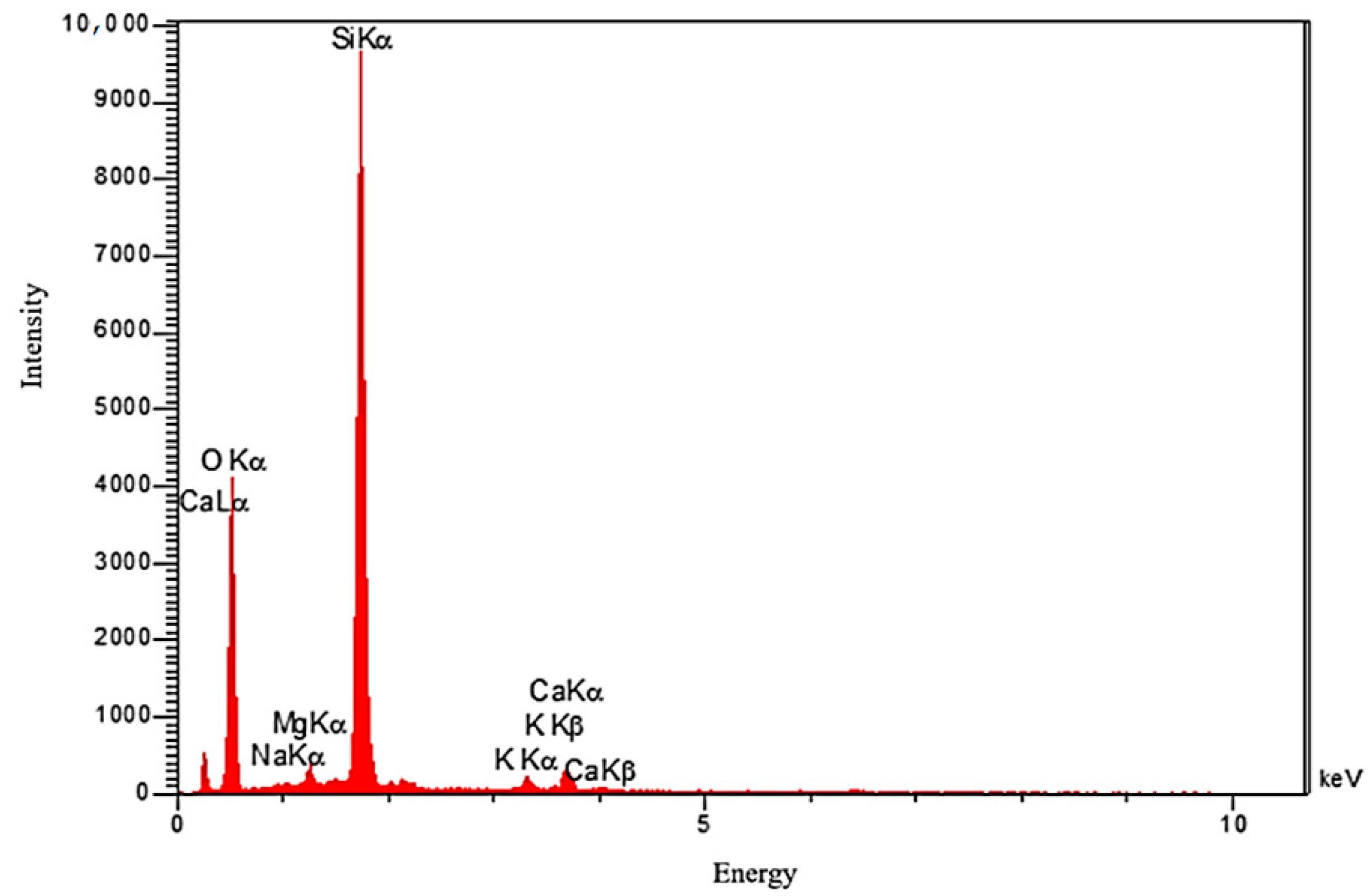


| One Time Duration | Two Time Duration | Three Time Duration | Four Time Duration | Five Time Duration | |
|---|---|---|---|---|---|
| One step (rpm) | 300 | 400 | 500 | 600 | 700 |
| Two step (rpm) | 400 | 500 | 600 | 700 | 700 |
| Three step (rpm) | 500 | 600 | 700 | 700 | 700 |
| Components | Si | O | K | Ca | Mg | Na |
|---|---|---|---|---|---|---|
| Weight % | 33.96 | 60.61 | 1.41 | 2.48 | 1.14 | 0.04 |
| Composition | SiO2 | P2O5 | Fe2O3 | K2O | CaO | Cl | Na2O | Al2O3 | TiO2 | L.O.I | La&Lu |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Weight % | 94.5 | 0.39 | 0. 36 | 0.66 | 1.1 | 0.17 | N.D | N.D | 0.01 | 2.05 | <1 |
| Elements | Average Weight % | |
|---|---|---|
| HCL acid leached | Citric acid leached | |
| Si | 45.6 | 61.9 |
| O | 54.4 | 38.1 |
| Total | 100 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarei, V.; Mirzaasadi, M.; Davarpanah, A.; Nasiri, A.; Valizadeh, M.; Hosseini, M.J.S. Environmental Method for Synthesizing Amorphous Silica Oxide Nanoparticles from a Natural Material. Processes 2021, 9, 334. https://doi.org/10.3390/pr9020334
Zarei V, Mirzaasadi M, Davarpanah A, Nasiri A, Valizadeh M, Hosseini MJS. Environmental Method for Synthesizing Amorphous Silica Oxide Nanoparticles from a Natural Material. Processes. 2021; 9(2):334. https://doi.org/10.3390/pr9020334
Chicago/Turabian StyleZarei, Vahid, Mojtaba Mirzaasadi, Afshin Davarpanah, Alireza Nasiri, Majid Valizadeh, and Mohammad Javad Sarbaz Hosseini. 2021. "Environmental Method for Synthesizing Amorphous Silica Oxide Nanoparticles from a Natural Material" Processes 9, no. 2: 334. https://doi.org/10.3390/pr9020334
APA StyleZarei, V., Mirzaasadi, M., Davarpanah, A., Nasiri, A., Valizadeh, M., & Hosseini, M. J. S. (2021). Environmental Method for Synthesizing Amorphous Silica Oxide Nanoparticles from a Natural Material. Processes, 9(2), 334. https://doi.org/10.3390/pr9020334







