Separation of Benzene-Cyclohexane Azeotropes Via Extractive Distillation Using Deep Eutectic Solvents as Entrainers
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of DES
2.3. Characterization of DES
2.4. VLE Measurements
3. Results and Discussion
3.1. Characterization of DES
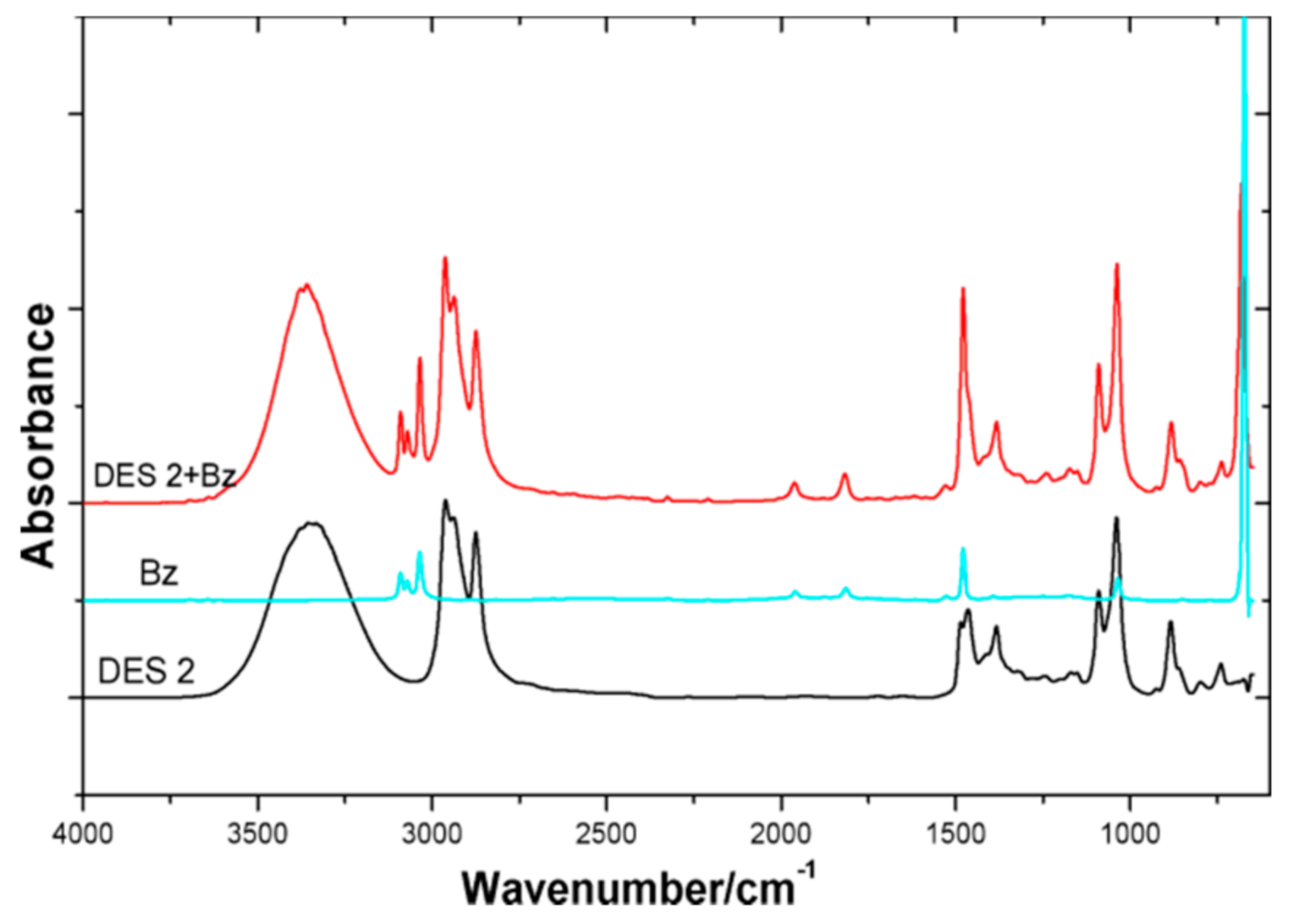
3.2. Physical Interactions between the DES
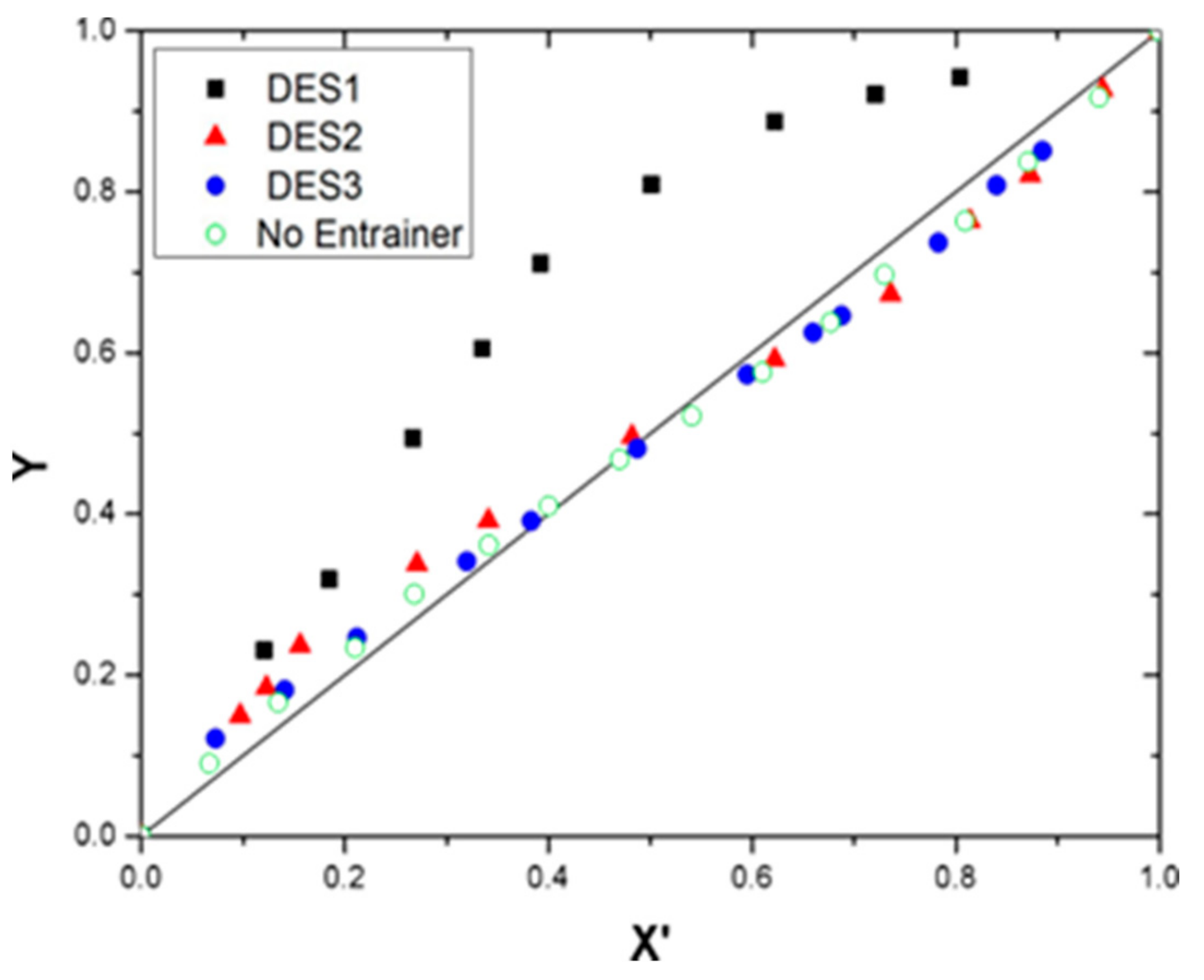
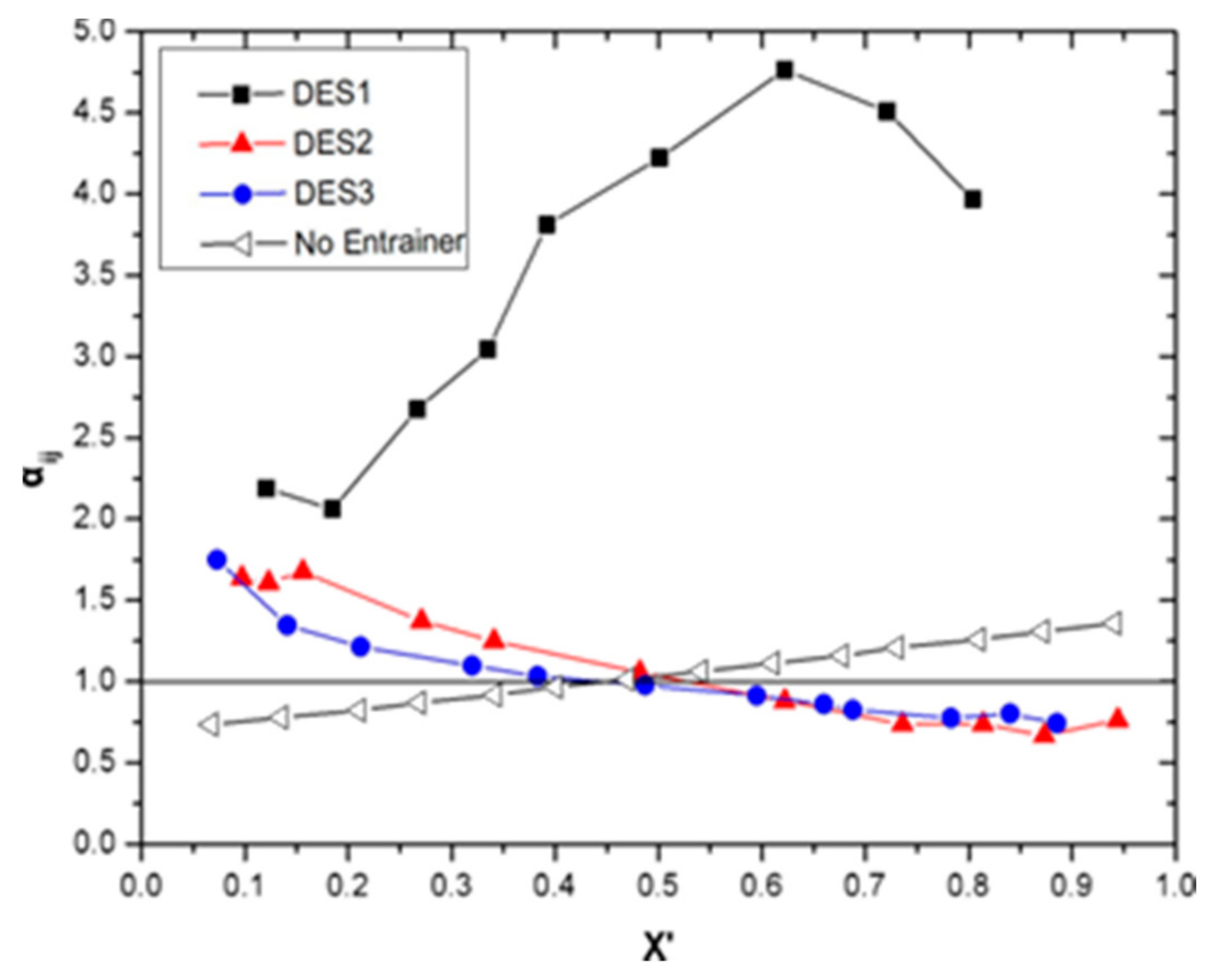
3.3. Vapor-Liquid Equilibrium and Selection Profile of the DES
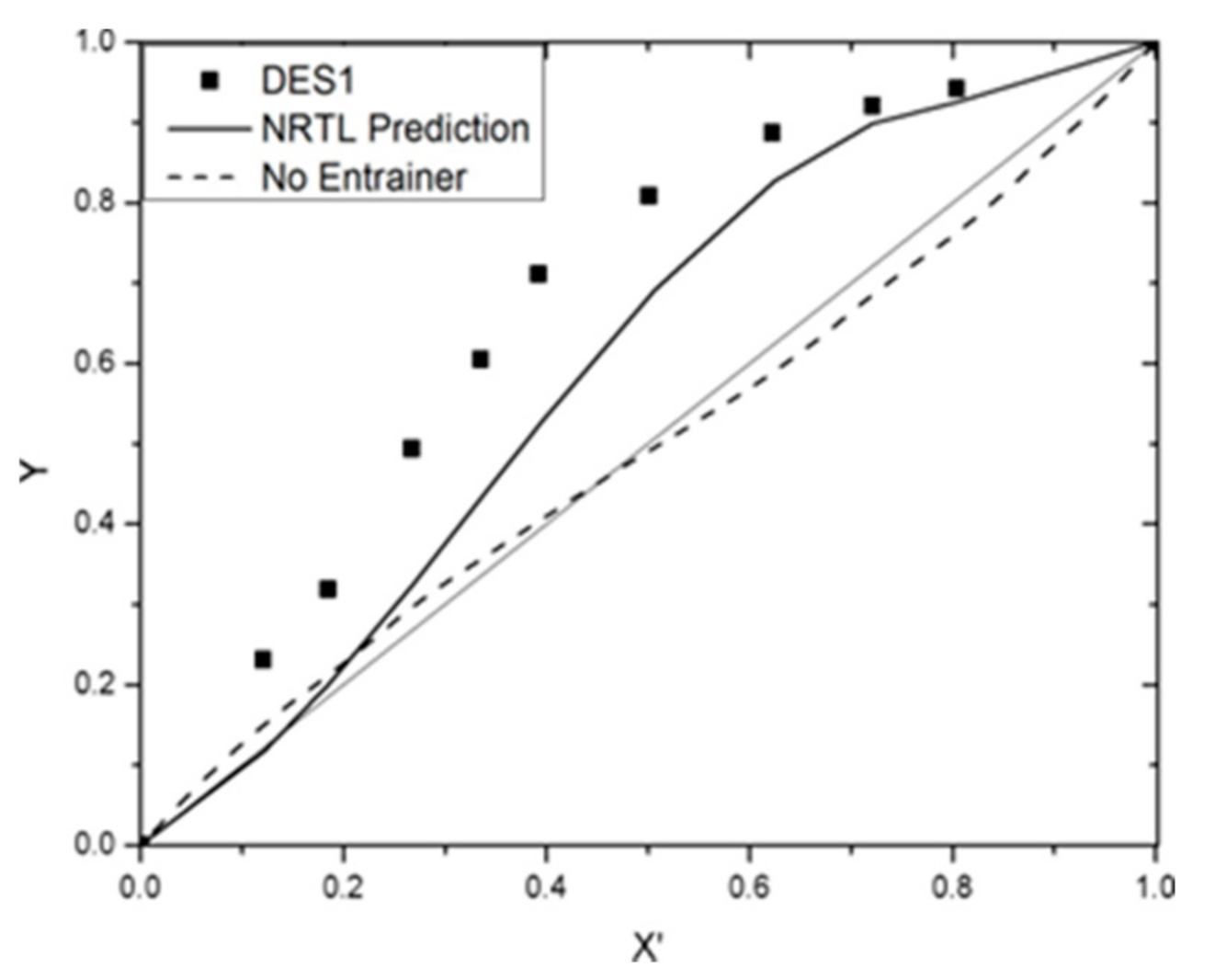
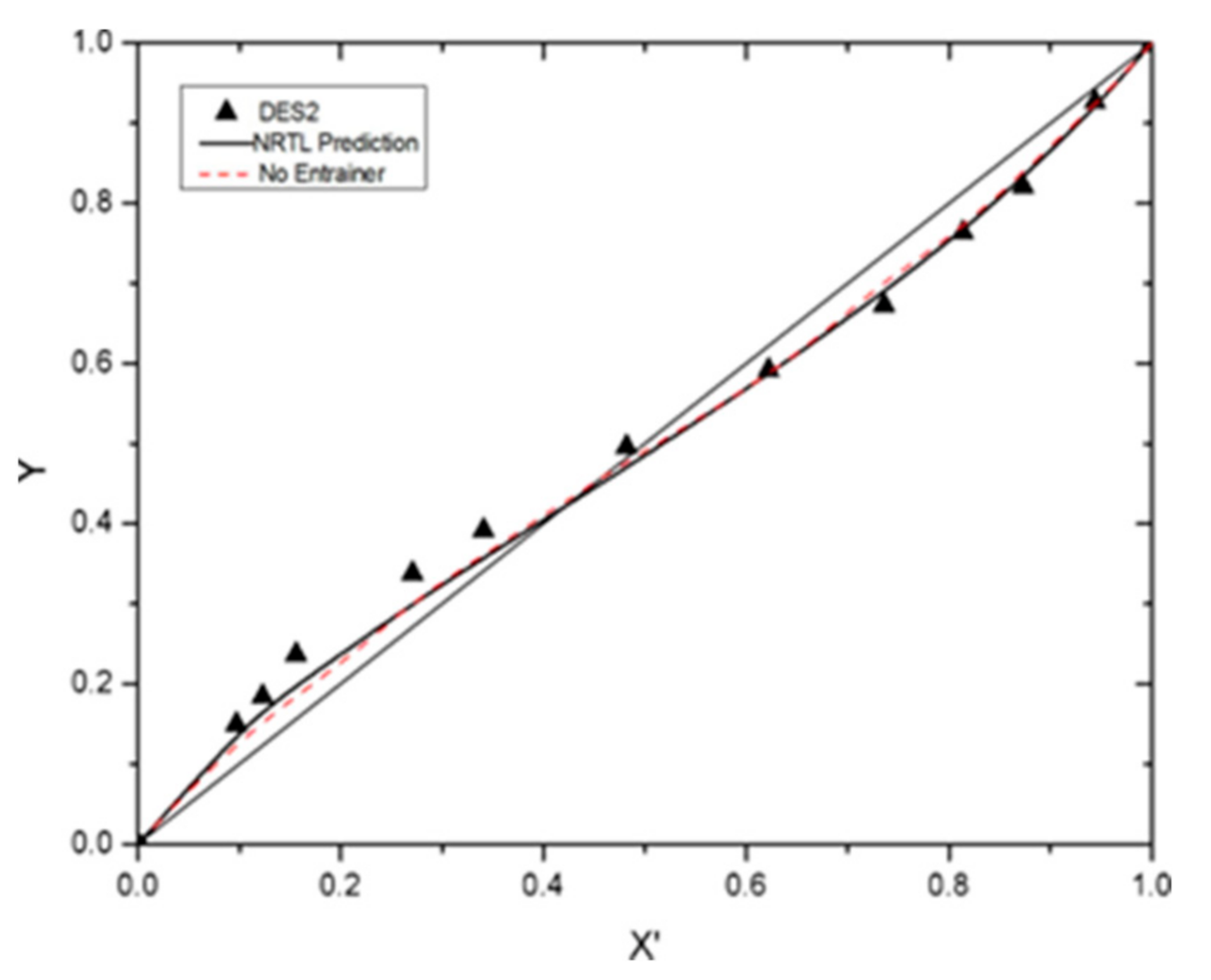
3.4. VLE Data Correlation
3.5. Mechanism Analysis of Extractive Distillation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Francisco, M.; González, A.S.B.; De Dios, S.L.G.; Weggemans, W.; Kroon, M.C. Comparison of a low transition temperature mixture (LTTM) formed by lactic acid and choline chloride with choline lactate ionic liquid and the choline chloride salt: Physical properties and vapour–liquid equilibria of mixtures containing water and ethanol. RSC Adv. 2013, 3, 23553–23561. [Google Scholar] [CrossRef]
- Durand, E.; LeComte, J.; Villeneuve, P. From green chemistry to nature: The versatile role of low transition temperature mixtures. Biochimie 2016, 120, 119–123. [Google Scholar] [CrossRef]
- Zeng, Q.; Wang, Y.; Huang, Y.; Ding, X.; Chen, J.; Xu, K. Deep eutectic solvents as novel extraction media for protein partitioning. Analyst 2014, 139, 2565–2573. [Google Scholar] [CrossRef] [PubMed]
- Hadi, N.A.; Ng, M.H.; Choo, Y.M.; Hashim, M.A.; Jayakumar, N.S. Performance of choline-based deep eutectic solvents in the extraction of tocols from crude palm oil. J. Am. Oil Chem. Soc. 2015, 92, 1709–1716. [Google Scholar] [CrossRef]
- Zhang, K.; Hou, Y.; Wang, Y.; Wang, K.; Ren, S.; Wu, W. Efficient and reversible absorption of CO2 by functional deep eutectic solvents. Energy Fuels 2018, 32, 7727–7733. [Google Scholar] [CrossRef]
- Li, N.; Wang, Y.; Xu, K.; Huang, Y.; Wen, Q.; Ding, X. Development of green Betaine-based deep eutectic solvent aqueous two-phase system for the extraction of protein. Talanta 2016, 152, 23–32. [Google Scholar] [CrossRef]
- Gu, Y.; Hou, Y.; Ren, S.; Sun, Y.; Wu, W. Hydrophobic functional deep eutectic solvents used for efficient and reversible capture of CO2. ACS Omega 2020, 5, 6809–6816. [Google Scholar] [CrossRef] [PubMed]
- Paradiso, V.M.; Clemente, A.; Summo, C.; Pasqualone, A.; Caponio, F. Towards green analysis of virgin olive oil phenolic compounds: Extraction by a natural deep eutectic solvent and direct spectrophotometric detection. Food Chem. 2016, 212, 43–47. [Google Scholar] [CrossRef]
- Qi, X.-L.; Peng, X.; Huang, Y.-Y.; Li, L.; Wei, Z.-F.; Zu, Y.; Fu, Y.-J. Green and efficient extraction of bioactive flavonoids from Equisetum palustre L. by deep eutectic solvents-based negative pressure cavitation method combined with macroporous resin enrichment. Ind. Crops Prod. 2015, 70, 142–148. [Google Scholar] [CrossRef]
- Wei, Z.-F.; Wang, X.-Q.; Peng, X.; Wang, W.; Zhao, C.; Zu, Y.; Fu, Y.-J. Fast and green extraction and separation of main bioactive flavonoids from Radix Scutellariae. Ind. Crops Prod. 2015, 63, 175–181. [Google Scholar] [CrossRef]
- Gonzalez, A.S.B.; Francisco, M.; de Dios, S.L.G.; Kroon, M.C. Liquid-liquid equilibrium data for the systems {LTTM plus benzene plus hexane} and {LTTM plus ethyl acetate plus hexane} at different temperatures and atmospheric pressure. Fluid Phase Equilib. 2013, 360, 54–62. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, B.; Row, K. Extraction of catechin compounds from green tea with a new green solvent. Chem Res. Chin. Univ. 2014, 30, 37–41. [Google Scholar] [CrossRef]
- Hou, Y.; Li, Z.; Ren, S.; Wu, W. Separation of toluene from toluene/alkane mixtures with phosphonium salt based deep eutectic solvents. Fuel Process. Technol. 2015, 135, 99–104. [Google Scholar] [CrossRef]
- Jiao, T.; Zhuang, X.; He, H.; Li, C.; Chen, H.; Zhang, S. Separation of phenolic compounds from coal tar via liquid–liquid extraction using amide compounds. Ind. Eng. Chem. Res. 2015, 54, 2573–2579. [Google Scholar] [CrossRef]
- Yao, C.F.; Hou, Y.C.; Sun, Y.; Wu, W.Z.; Ren, S.H.; Liu, H. Extraction of aromatics from aliphatics using a hydrophobic dica-tionic ionic liquid adjusted with small-content water. Sep. Purif. Technol. 2020, 236, 116287. [Google Scholar] [CrossRef]
- Kareem, M.A.; Mjalli, F.S.; Hashim, M.A.; Hadj-Kali, M.K.; Bagh, F.S.G.; Alnashef, I.M. Phase equilibria of toluene/heptane with tetrabutylphosphonium bromide based deep eutectic solvents for the potential use in the separation of aromatics from naphtha. Fluid Phase Equilib. 2012, 333, 47–54. [Google Scholar] [CrossRef]
- Maugeri, Z.; Leitner, W.; De Maria, P.D. Practical separation of alcohol–ester mixtures using Deep-Eutectic-Solvents. Tetrahedron Lett. 2012, 53, 6968–6971. [Google Scholar] [CrossRef]
- Oliveira, F.S.; Pereiro, A.B.; Rebelo, L.P.N.; Marrucho, I.M. Deep eutectic solvents as extraction media for azeotropic mixtures. Green Chem. 2013, 15, 1326–1330. [Google Scholar] [CrossRef]
- Hizaddin, H.F.; Hadj-Kali, M.K.; Ramalingam, A.; Hashim, M.A. Extraction of nitrogen compounds from diesel fuel using imidazolium- and pyridinium-based ionic liquids: Experiments, COSMO-RS prediction and NRTL correlation. Fluid Phase Equilib. 2015, 405, 55–67. [Google Scholar] [CrossRef]
- Peng, Y.; Lu, X.; Liu, B.; Zhu, J. Separation of azeotropic mixtures (ethanol and water) enhanced by deep eutectic solvents. Fluid Phase Equilib. 2017, 448, 128–134. [Google Scholar] [CrossRef]
- Pan, Q.; Shang, X.; Li, J.; Ma, S.; Li, L.; Sun, L. Energy-efficient separation process and control scheme for extractive distillation of ethanol-water using deep eutectic solvent. Sep. Purif. Technol. 2019, 219, 113–126. [Google Scholar] [CrossRef]
- Sharma, B.; Singh, N.; Kushwaha, J.P. Ammonium-based deep eutectic solvent as entrainer for separation of acetonitrile–water mixture by extractive distillation. J. Mol. Liq. 2019, 285, 185–193. [Google Scholar] [CrossRef]
- Shang, X.; Ma, S.; Pan, Q.; Li, J.; Sun, Y.; Ji, K.; Sun, L. Process analysis of extractive distillation for the separation of ethanol–water using deep eutectic solvent as entrainer. Chem. Eng. Res. Des. 2019, 148, 298–311. [Google Scholar] [CrossRef]
- Toikka, M.; Smirnov, M.A.; Toikka, A.M.; Prikhodko, I.V. Study of deep eutectic solvent on the base choline chloride as entrainer for the separation alcohol–ester systems. J. Chem. Eng. Data 2018, 63, 1877–1884. [Google Scholar] [CrossRef]
- Li, C.; Zhang, J.; Li, Z.; Yin, J.; Cui, Y.; Liu, Y.; Yang, G. Extraction desulfurization of fuels with ‘metal ions’ based deep eutectic solvents (MDESs). Green Chem. 2016, 18, 3789–3795. [Google Scholar] [CrossRef]
- Rodriguez, N.R.; Kroon, M.C. Isopropanol dehydration via extractive distillation using low transition temperature mixtures as entrainers. J. Chem. Thermodyn. 2015, 85, 216–221. [Google Scholar] [CrossRef]
- Gjineci, N.; Boli, E.; Tzani, A.; Detsi, A.; Voutsas, E. Separation of the ethanol/water azeotropic mixture using ionic liquids and deep eutectic solvents. Fluid Phase Equilib. 2016, 424, 1–7. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep eutectic solvents formed between choline chloride and carboxylic acids: Versatile alternatives to ionic liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef]
- Mi, W.; Tong, R.; Hua, C.; Yue, K.; Jia, D.; Lu, P.; Bai, F. Vapor–liquid equilibrium data for binary systems of n,n-dimethylacetamide with cyclohexene, cyclohexane, and benzene separately at atmospheric pressure. J. Chem. Eng. Data 2015, 60, 3063–3068. [Google Scholar] [CrossRef]
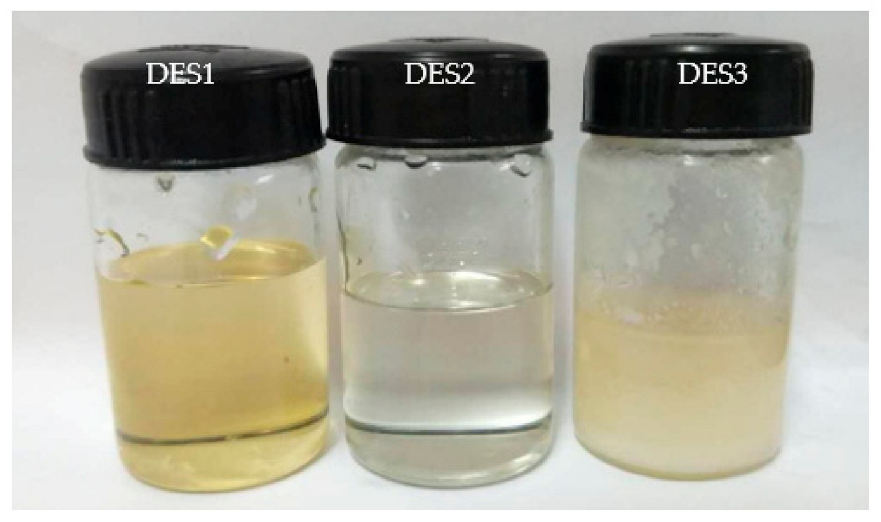


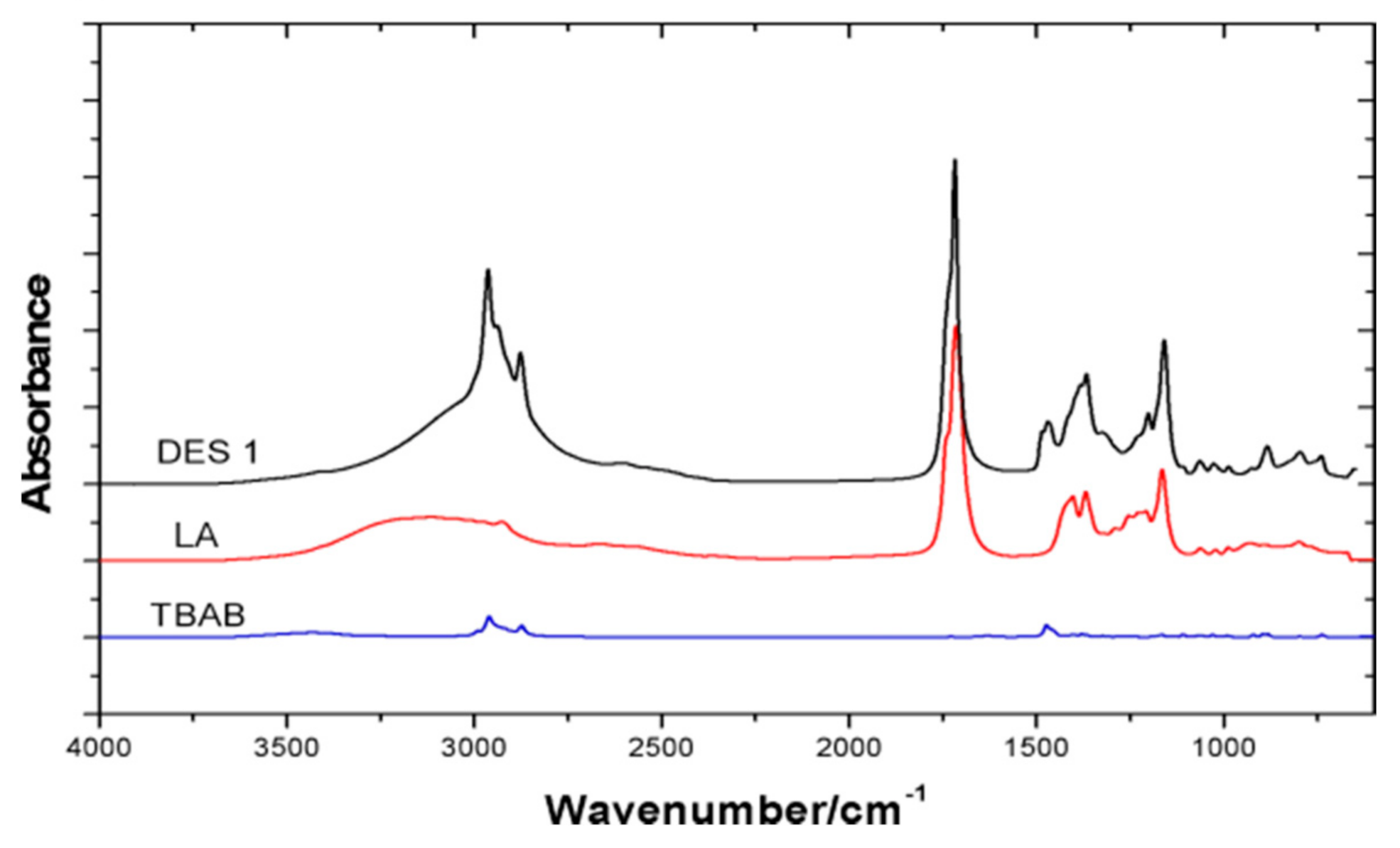
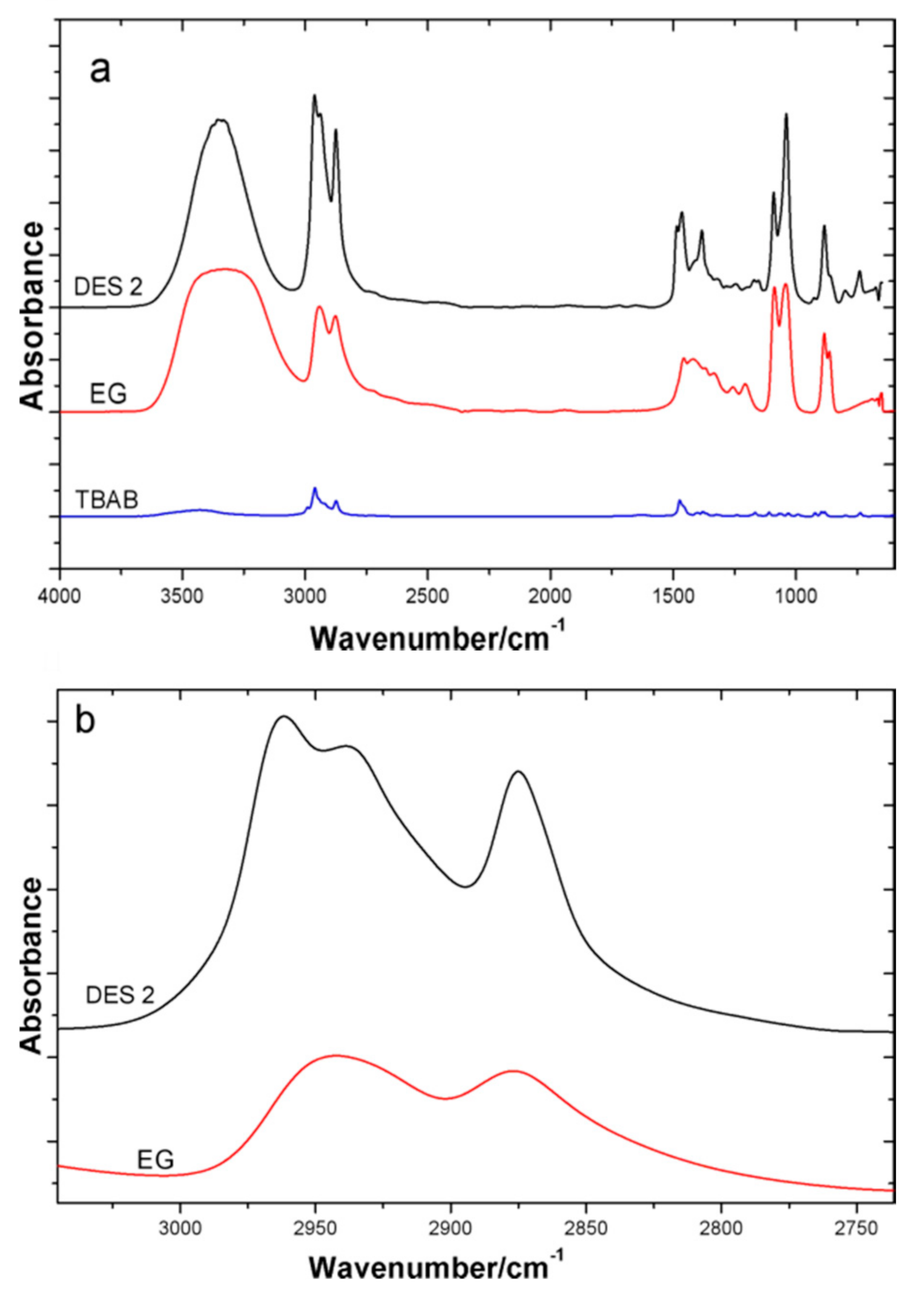
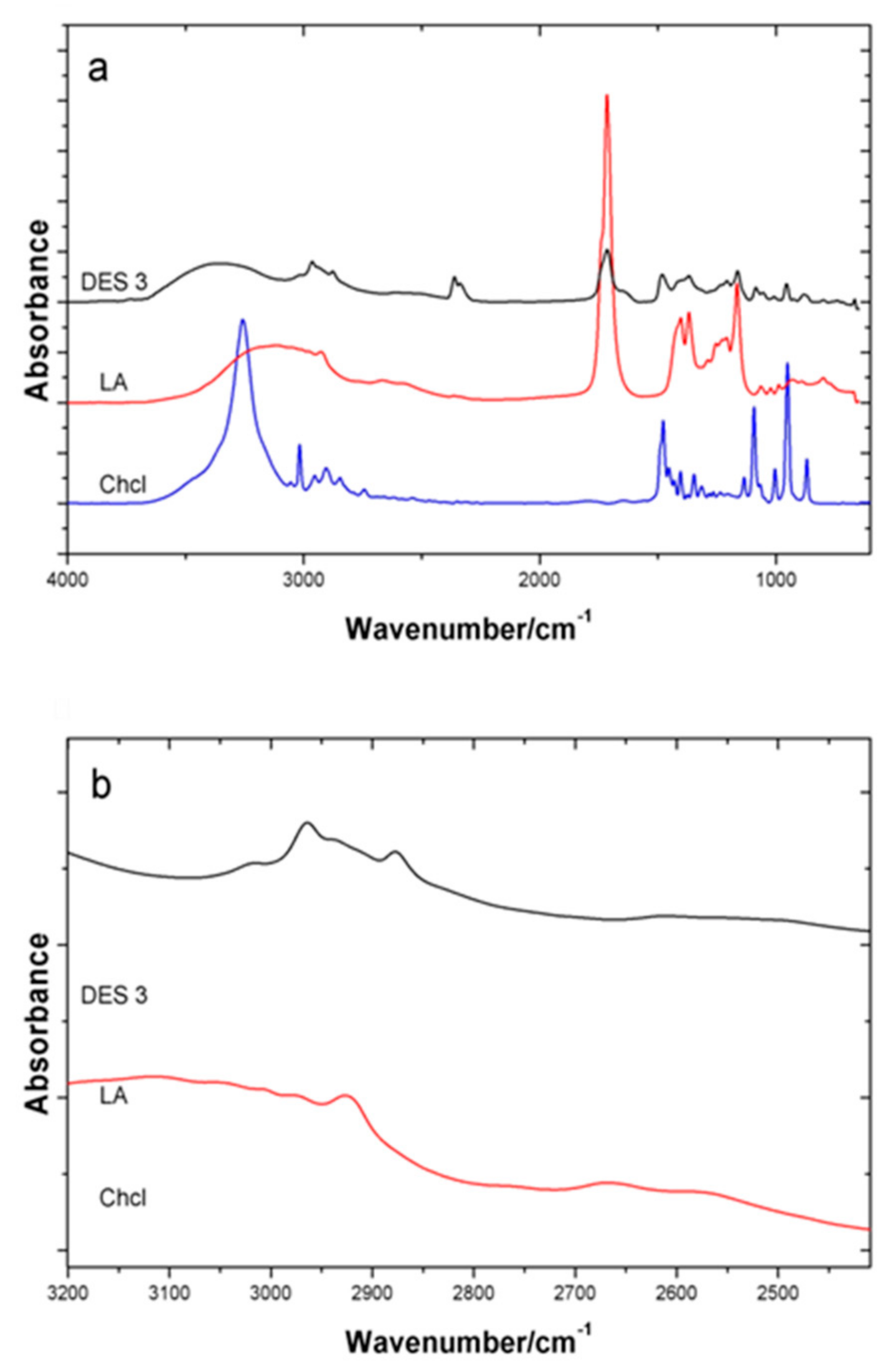


| Sample | Glass Transition Temperature (°C) | Melting Point (°C) | Melting Points of HBA Component (°C) | Melting Point of HBD Component (°C) |
|---|---|---|---|---|
| DES1 | −70~−65 | - | 102~106 | 37.2 |
| DES2 | −52~−54 | - | 102~106 | 12.9 |
| DES3 | −72~−65 | 63~66 | 302~305 | 37.2 |
| Benzene + Cyclohexane + DES1 at 101.3 kPa | ||||||
|---|---|---|---|---|---|---|
| X3 | X1 | X’1 | Y1 | T/K | αij | S |
| 0.1 | 0.000 | 0.000 | 0.000 | 353.25 | - | - |
| 0.1 | 0.109 | 0.121 | 0.231 | 351.96 | 2.188 | 1.7398 |
| 0.1 | 0.167 | 0.185 | 0.319 | 351.20 | 2.060 | 1.7050 |
| 0.1 | 0.241 | 0.267 | 0.494 | 350.64 | 2.675 | 2.4106 |
| 0.1 | 0.302 | 0.335 | 0.605 | 351.28 | 3.044 | 3.6987 |
| 0.1 | 0.353 | 0.392 | 0.711 | 351.74 | 3.809 | 4.8978 |
| 0.1 | 0.451 | 0.501 | 0.809 | 352.34 | 4.223 | 5.7581 |
| 0.1 | 0.560 | 0.622 | 0.887 | 352.67 | 4.763 | 6.4944 |
| 0.1 | 0.649 | 0.721 | 0.921 | 352.88 | 4.509 | 6.1481 |
| 0.1 | 0.723 | 0.804 | 0.942 | 353.17 | 3.968 | 5.4104 |
| 0.1 | 0.900 | 1.000 | 1.000 | 353.85 | - | - |
| Benzene + Cyclohexane + DES2 at 101.3 kPa | ||||||
| X3 | X1 | X’1 | Y1 | T/K | αij | S |
| 0.1 | 0.000 | 0.000 | 0.000 | 353.25 | - | - |
| 0.1 | 0.087 | 0.097 | 0.150 | 352.17 | 1.637 | 1.2526 |
| 0.1 | 0.111 | 0.123 | 0.184 | 351.91 | 1.607 | 1.2778 |
| 0.1 | 0.141 | 0.156 | 0.237 | 351.73 | 1.675 | 1.3319 |
| 0.1 | 0.244 | 0.271 | 0.338 | 351.39 | 1.371 | 1.1347 |
| 0.1 | 0.306 | 0.341 | 0.392 | 351.23 | 1.250 | 1.0346 |
| 0.1 | 0.434 | 0.482 | 0.496 | 351.03 | 1.056 | 0.9112 |
| 0.1 | 0.560 | 0.622 | 0.592 | 351.15 | 0.880 | 1.0693 |
| 0.1 | 0.786 | 0.873 | 0.821 | 352.16 | 0.666 | 0.9081 |
| 0.1 | 0.849 | 0.944 | 0.927 | 352.96 | 0.764 | 1.0417 |
| 0.1 | 0.900 | 1.000 | 1.000 | 353.85 | - | - |
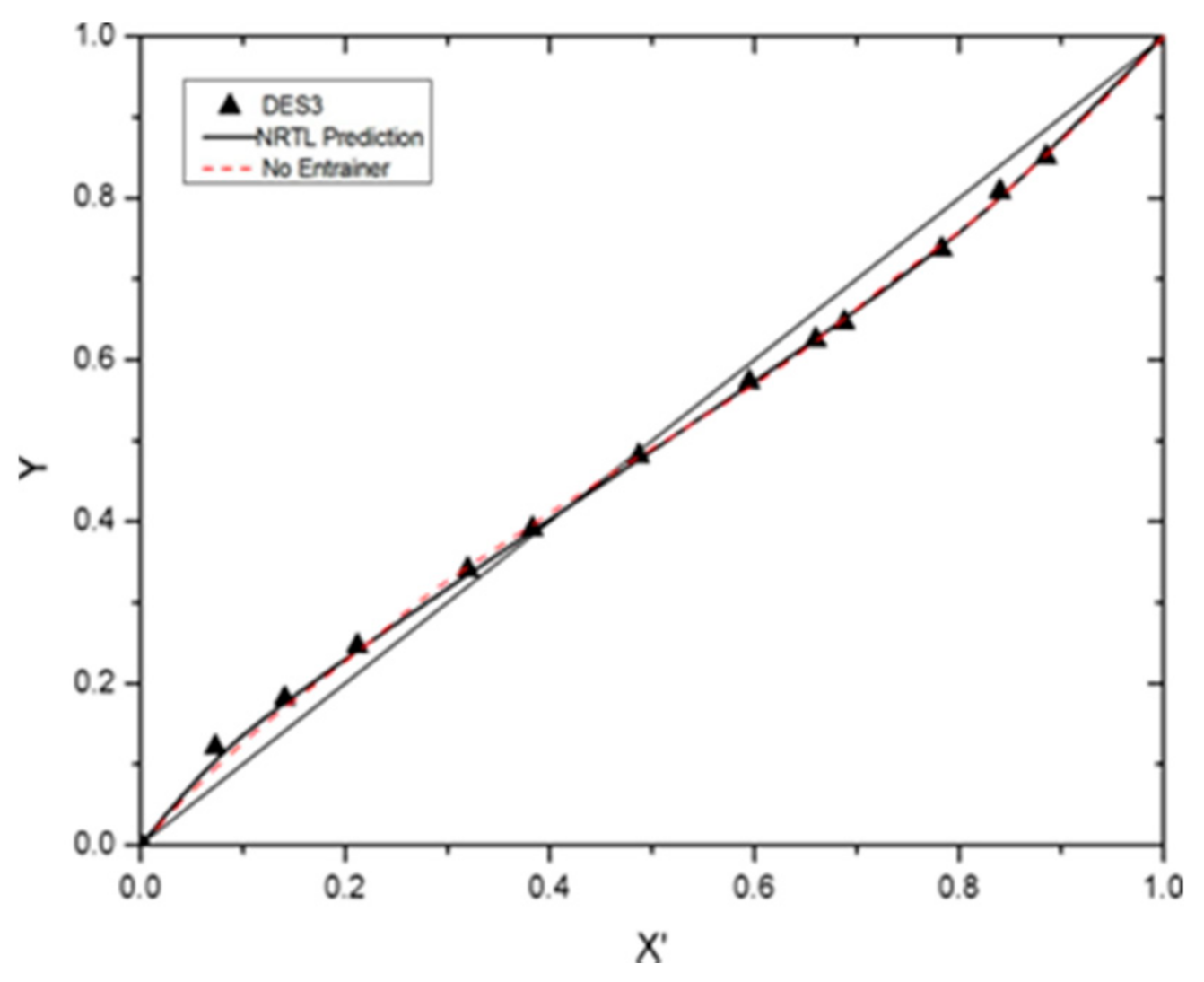
| Benzene + Cyclohexane + DES3 at 101.3 kPa | ||||||
| X3 | X1 | X’1 | Y1 | T/K | αij | S |
| 0.1 | 0.000 | 0.000 | 0.000 | 353.25 | - | - |
| 0.1 | 0.066 | 0.073 | 0.121 | 352.65 | 1.750 | 1.3390 |
| 0.1 | 0.127 | 0.141 | 0.181 | 352.15 | 1.344 | 1.0284 |
| 0.1 | 0.191 | 0.212 | 0.246 | 351.67 | 1.213 | 0.9645 |
| 0.1 | 0.288 | 0.320 | 0.341 | 351.27 | 1.096 | 0.9071 |
| 0.1 | 0.345 | 0.383 | 0.391 | 351.10 | 1.033 | 0.8914 |
| 0.1 | 0.438 | 0.487 | 0.481 | 351.17 | 0.977 | 1.1871 |
| 0.1 | 0.536 | 0.595 | 0.573 | 351.25 | 0.913 | 1.1094 |
| 0.1 | 0.594 | 0.660 | 0.625 | 351.30 | 0.861 | 1.0462 |
| 0.1 | 0.619 | 0.688 | 0.646 | 351.43 | 0.825 | 1.0608 |
| 0.1 | 0.705 | 0.783 | 0.737 | 351.95 | 0.775 | 0.9965 |
| 0.1 | 0.756 | 0.840 | 0.808 | 352.45 | 0.802 | 1.0935 |
| 0.1 | 0.797 | 0.885 | 0.851 | 352.87 | 0.742 | 1.0117 |
| 0.1 | 0.900 | 1.000 | 1.000 | 353.85 | - | - |
| System | R2 | ||
|---|---|---|---|
| UNIQUAC | WILSON | NRTL | |
| Benzene + cyclohexane + DES1 | 0.8232 | 0.6153 | 0.8761 |
| Benzene + cyclohexane + DES2 | 0.9932 | 0.9932 | 0.9999 |
| Benzene + cyclohexane + DES3 | 0.9992 | 0.9979 | 0.9995 |
| Extractant | Comp.i | Comp.j | Aij | Aji | Bij | Bji | Cij |
|---|---|---|---|---|---|---|---|
| DES1 | −17.8945 | 26.1684 | 10,000 | −10,000 | 0.1717 | ||
| DES2 | Cyclohexane | Benzene | 2.7875 | 13.7115 | −3150.39 | −2220.07 | 0.0170 |
| DES3 | −22.3132 | 181.9706 | 7823.07 | −63,763.92 | 0.9320 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, F.; Hua, C.; Li, J. Separation of Benzene-Cyclohexane Azeotropes Via Extractive Distillation Using Deep Eutectic Solvents as Entrainers. Processes 2021, 9, 336. https://doi.org/10.3390/pr9020336
Bai F, Hua C, Li J. Separation of Benzene-Cyclohexane Azeotropes Via Extractive Distillation Using Deep Eutectic Solvents as Entrainers. Processes. 2021; 9(2):336. https://doi.org/10.3390/pr9020336
Chicago/Turabian StyleBai, Fang, Chao Hua, and Jing Li. 2021. "Separation of Benzene-Cyclohexane Azeotropes Via Extractive Distillation Using Deep Eutectic Solvents as Entrainers" Processes 9, no. 2: 336. https://doi.org/10.3390/pr9020336
APA StyleBai, F., Hua, C., & Li, J. (2021). Separation of Benzene-Cyclohexane Azeotropes Via Extractive Distillation Using Deep Eutectic Solvents as Entrainers. Processes, 9(2), 336. https://doi.org/10.3390/pr9020336





