Effect of Surface Textures and Wettability on Droplet Impact on a Heated Surface
Abstract
:1. Introduction
2. Materials and Experimental Methods
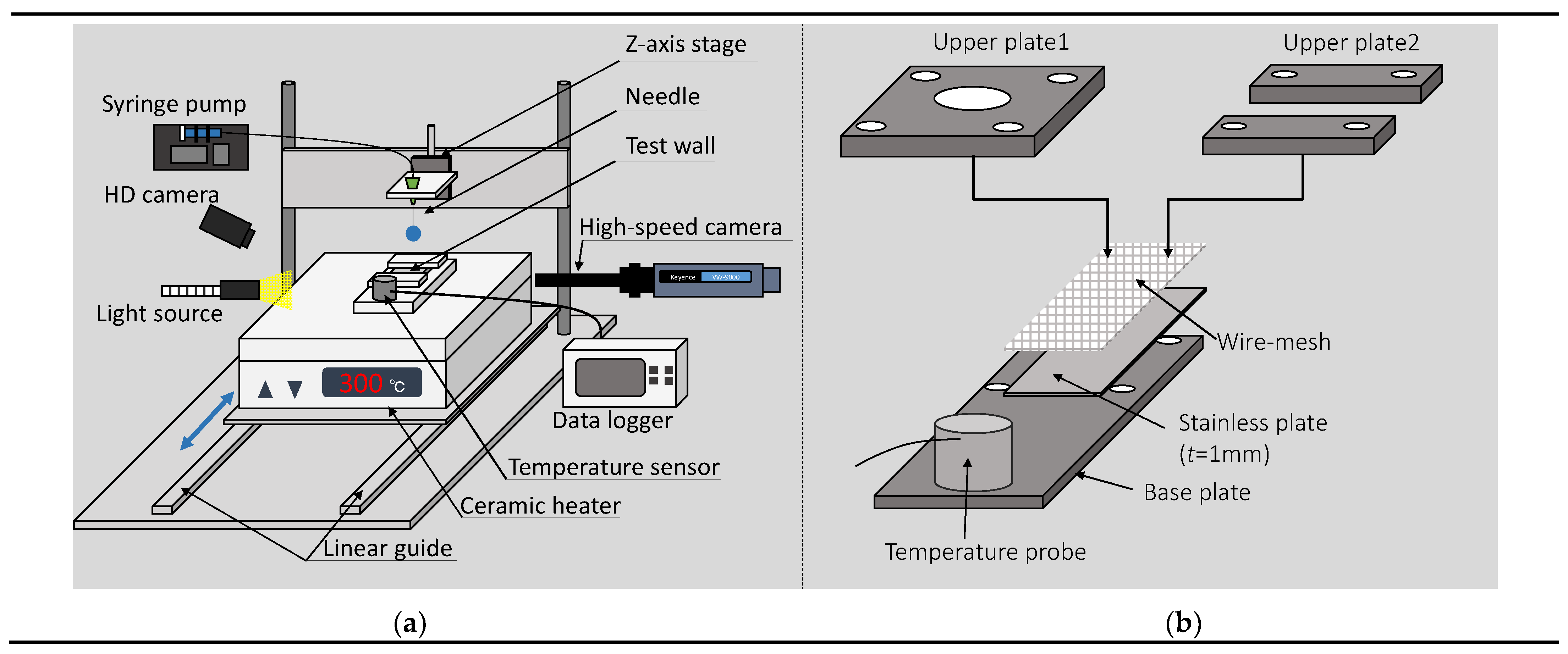
2.1. Experimental Setup
2.2. Hydrophilic Treatment
2.3. Test Wall and Jig
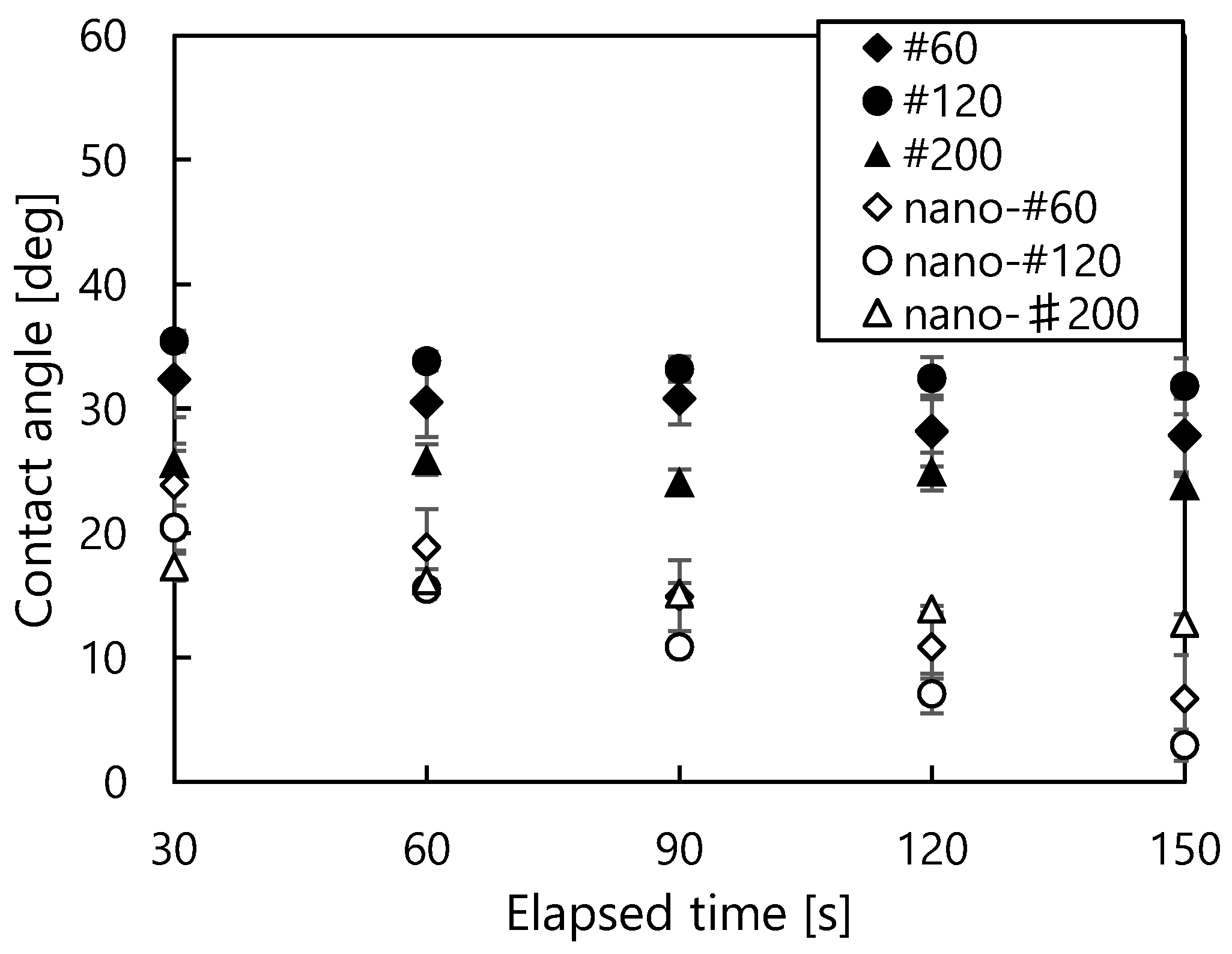
2.4. Contact Angle Measure
2.5. Measurement of LFP
2.6. Validation
3. Results and Discussion
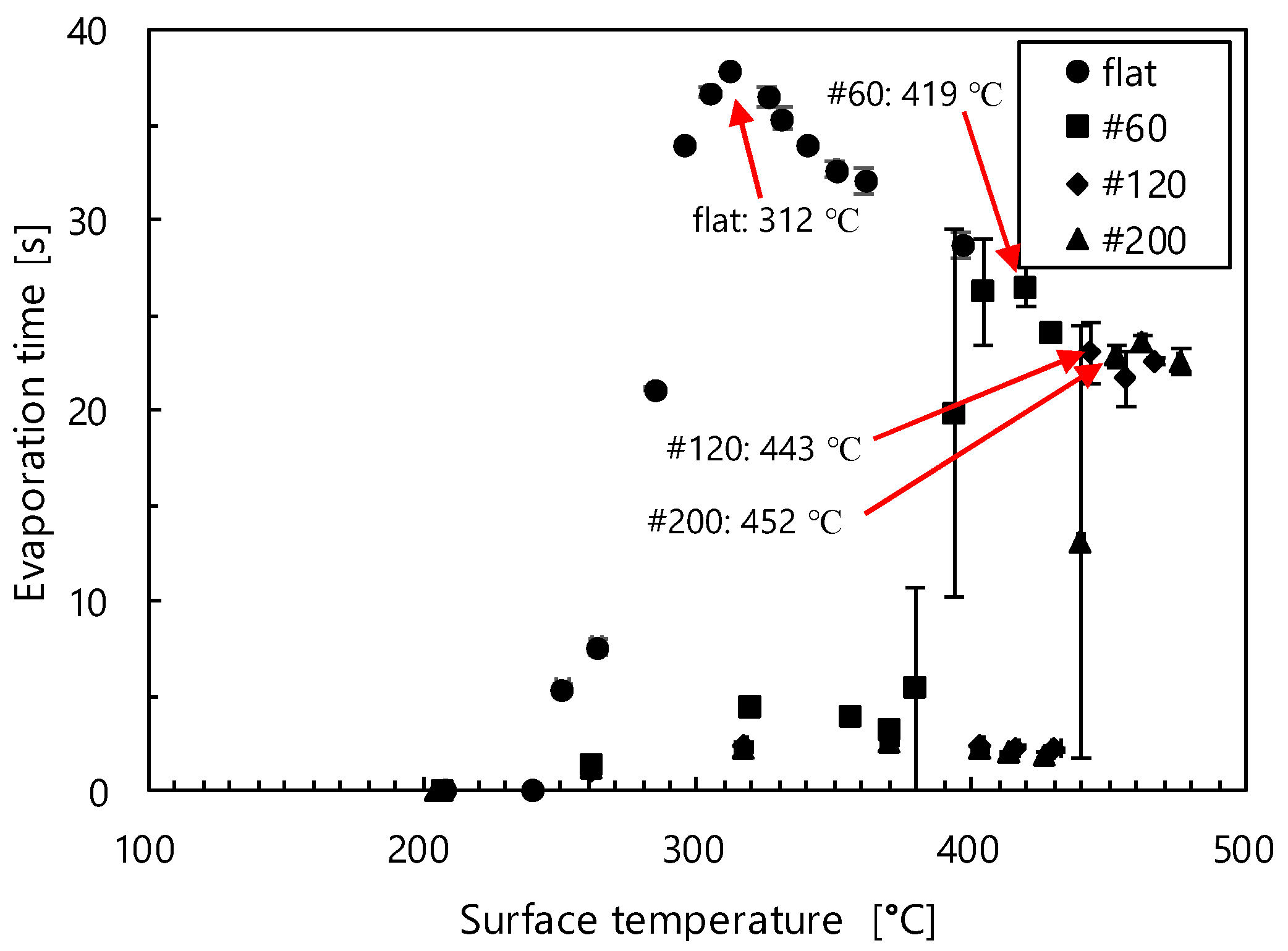
3.1. Droplet Evaporation Time and LFP
3.1.1. Stainless Surface/Nanocoated Stainless Surface
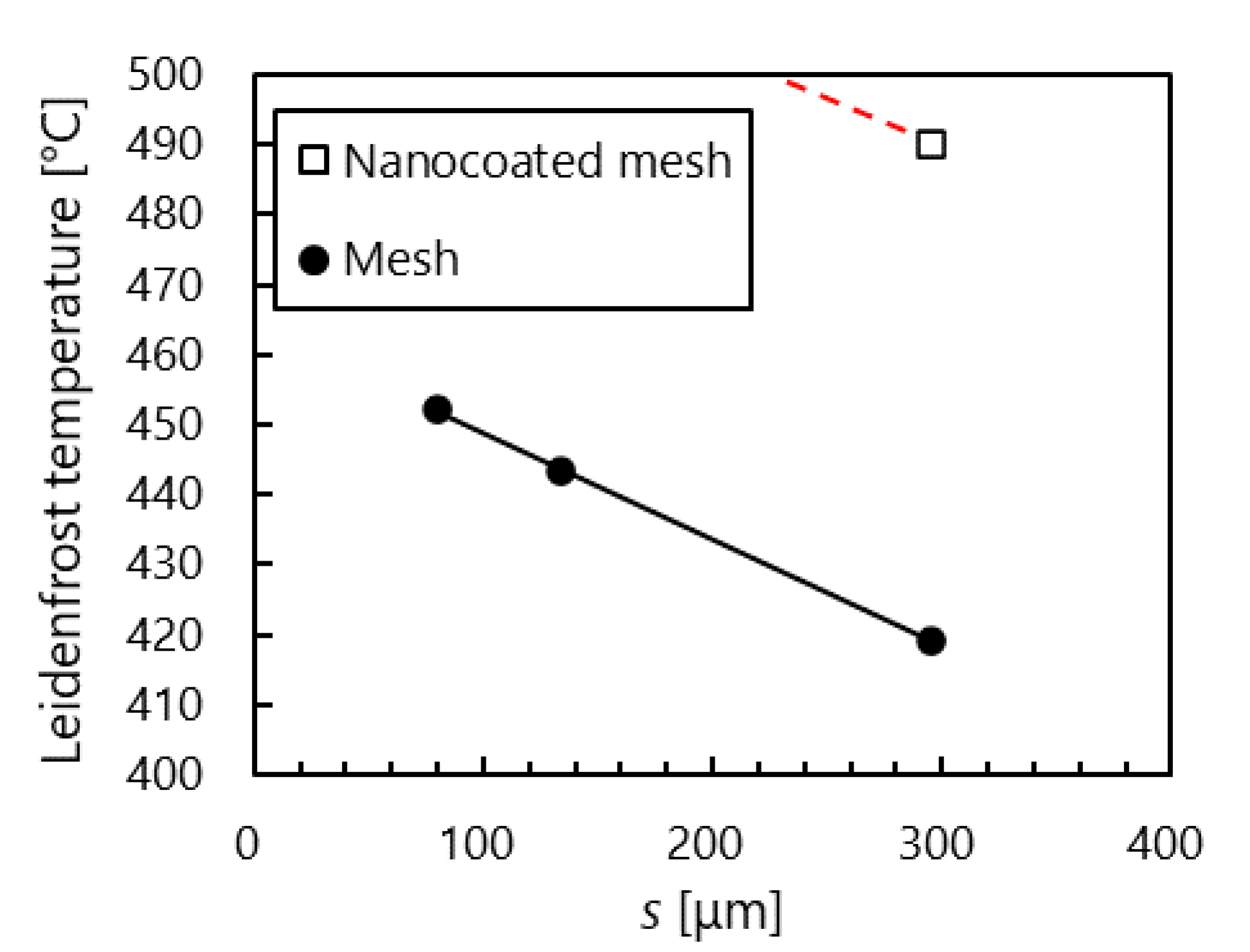
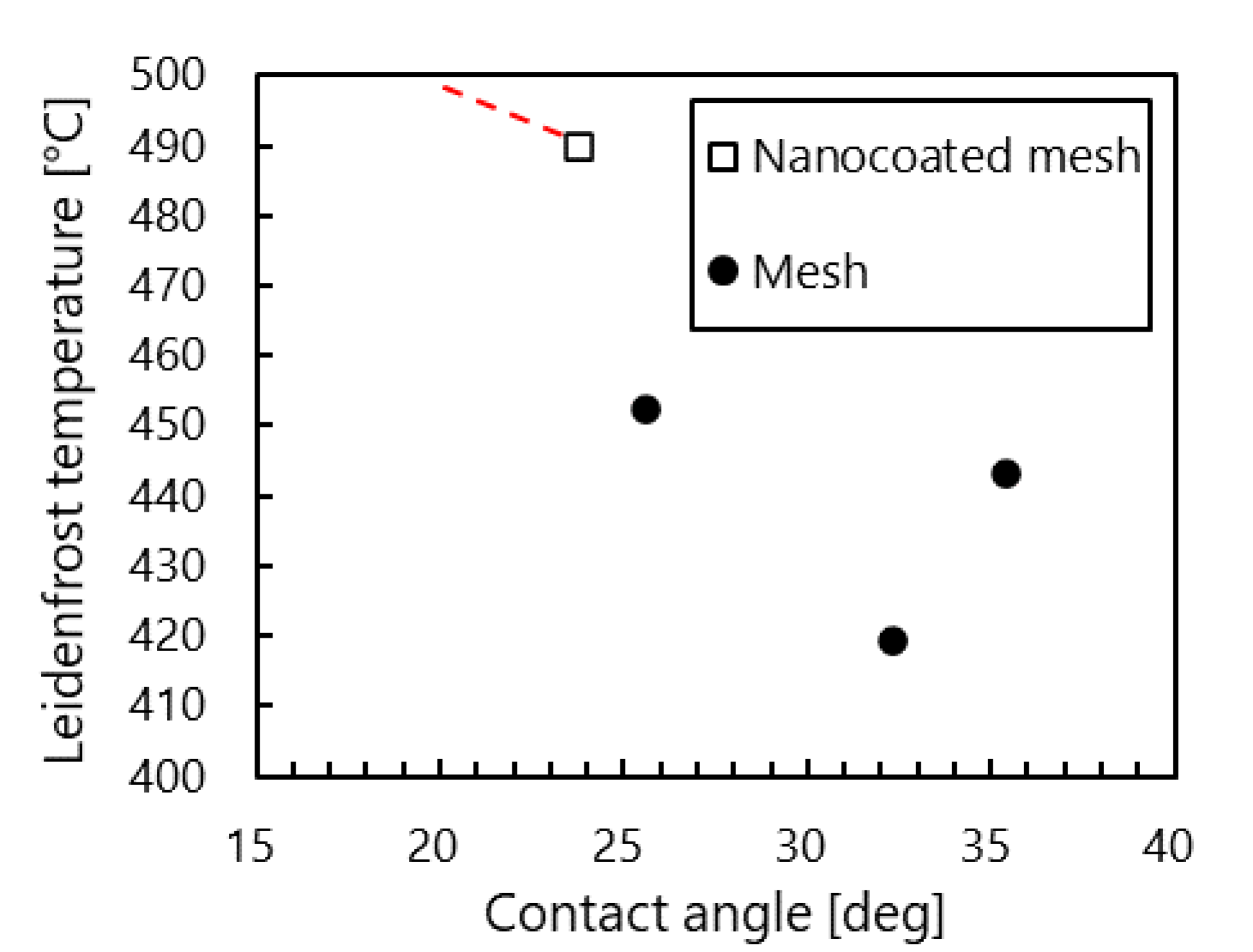
3.1.2. Mesh Surfaces
3.1.3. Nanocoated Mesh Surface
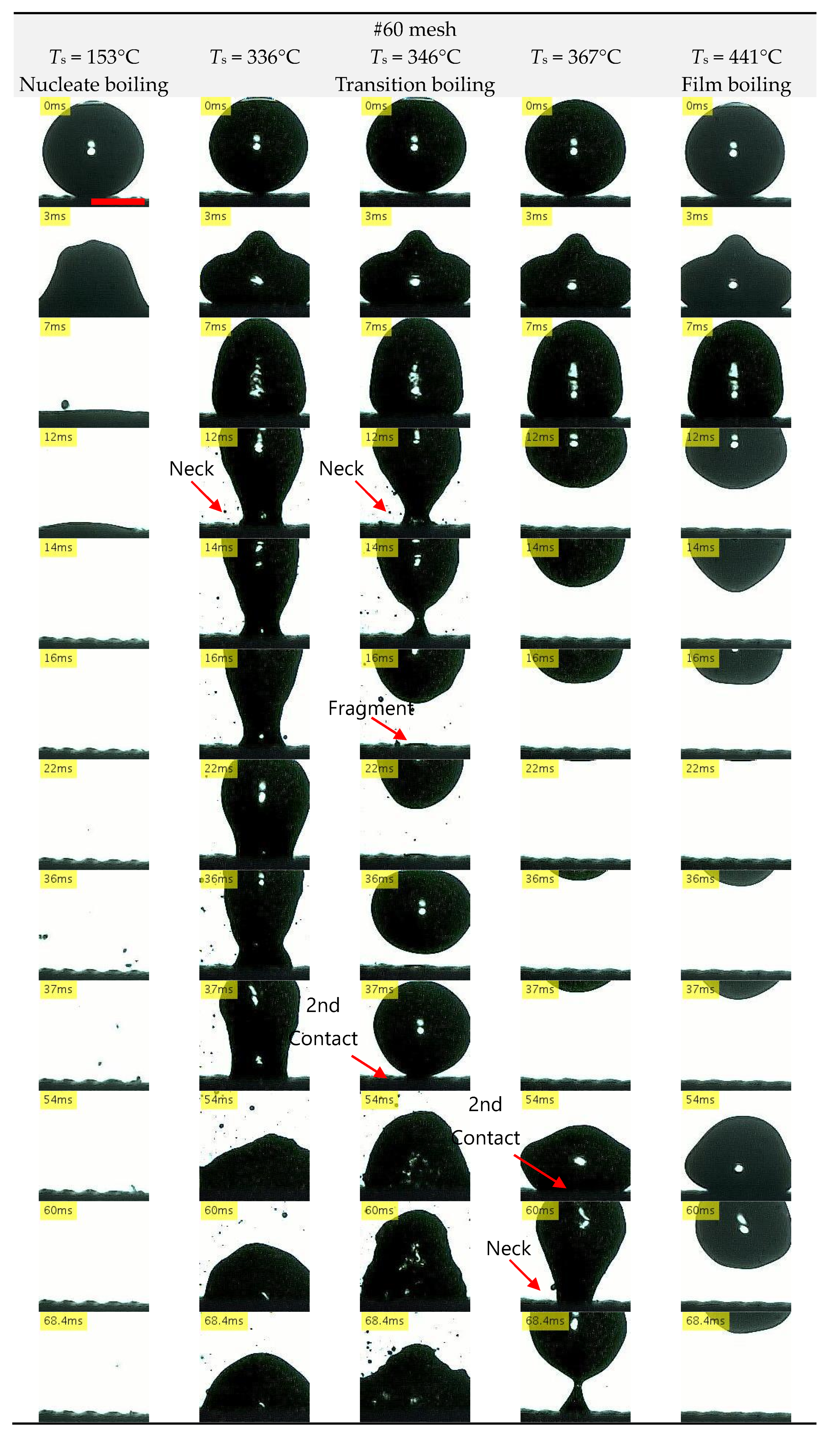
3.2. Droplet Impact Behavior
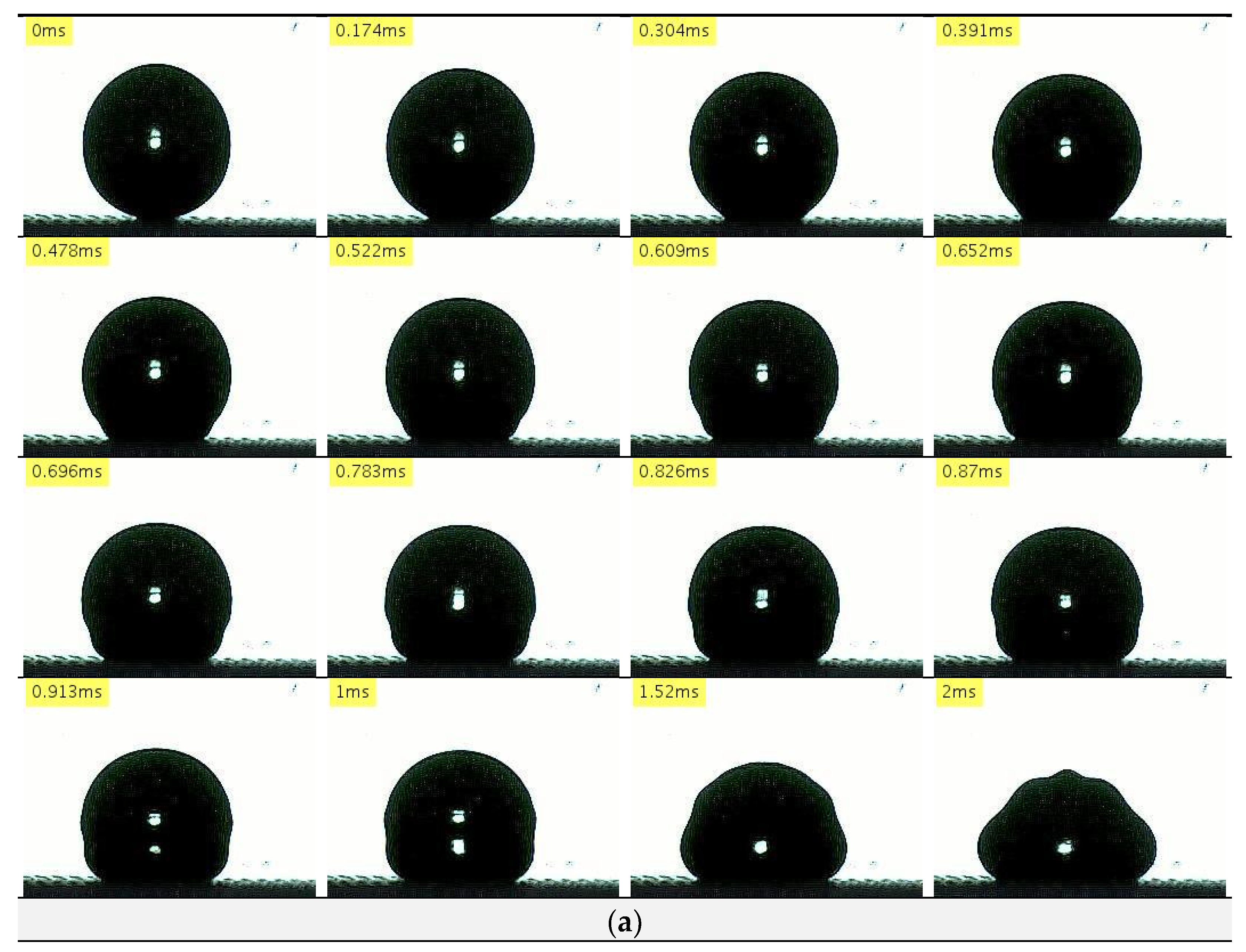
3.2.1. Stainless Surface/Nanocoated Stainless Surface
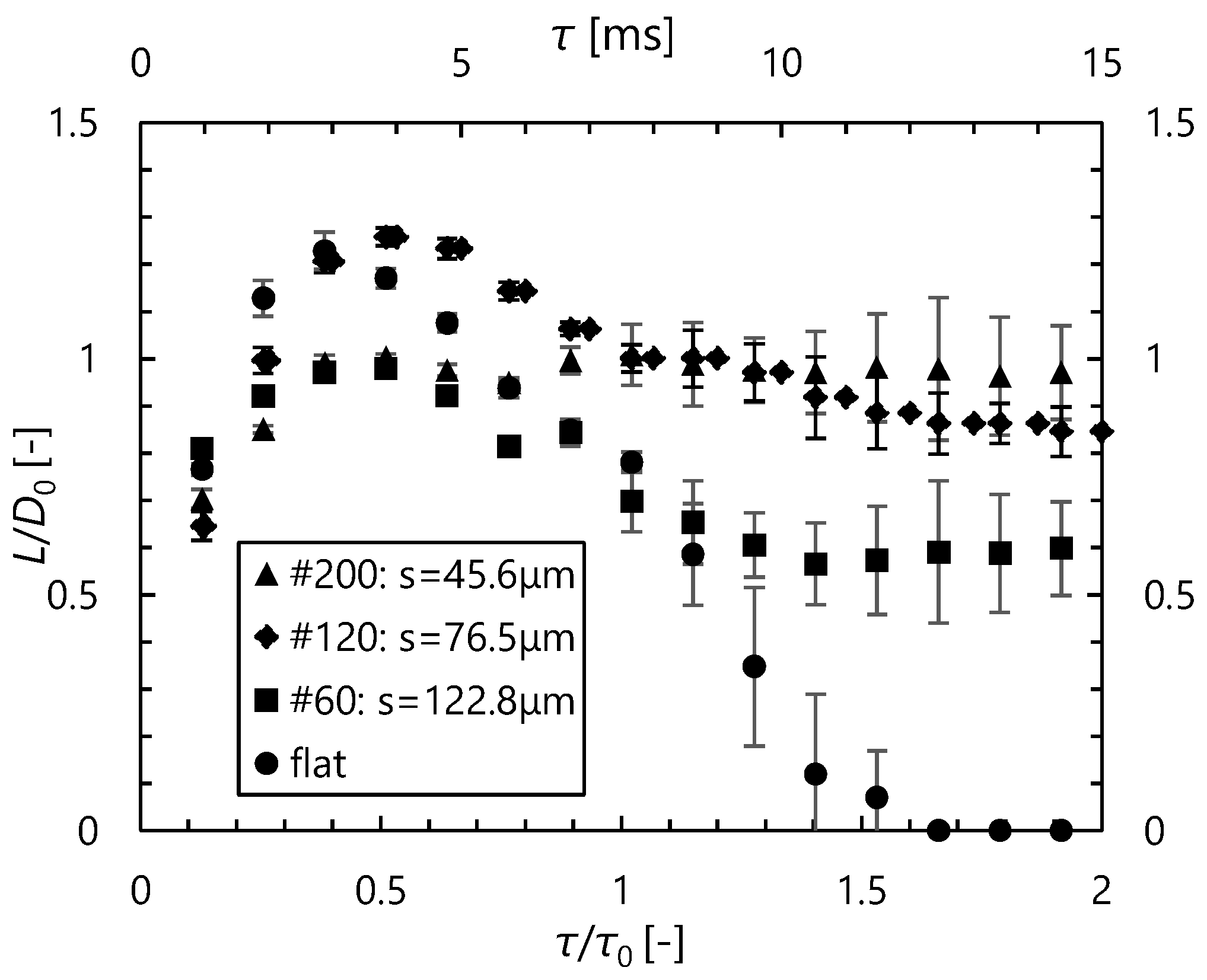
3.2.2. Mesh Surface
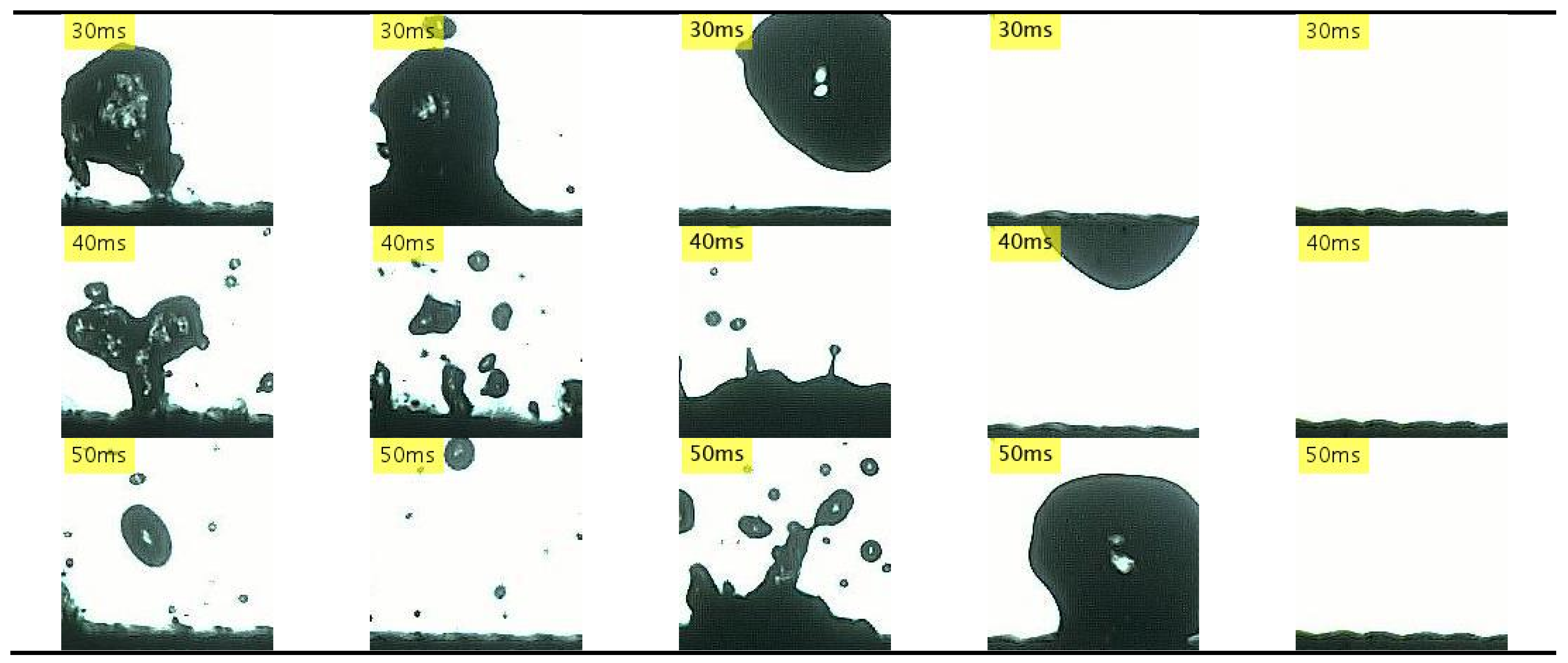
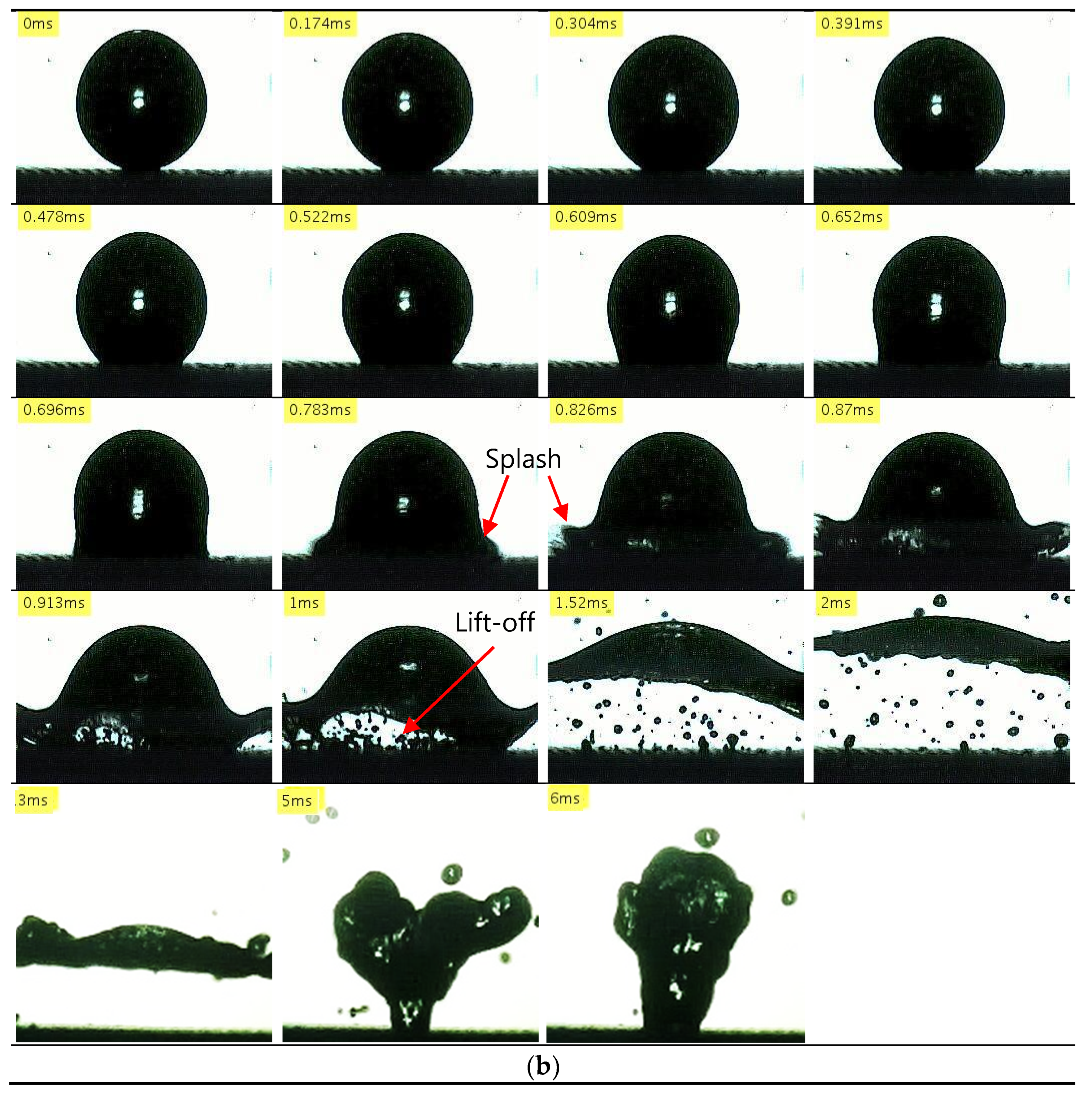
3.2.3. Nanocoated Mesh Surfaces
4. Conclusions
- SEM observations confirmed that nanocoated mesh surfaces have a micro/nanostructure that combines fine pores of micrometer order and roughness of nanometer order.
- Compared with stainless steel surfaces, the LFP on mesh surfaces was enhanced by 107 °C to 140 °C. Further, the LFP on mesh surfaces was negatively correlated with wire spacing. In addition, we captured images of a droplet hitting a mesh surface, which had not been done in previous studies. These images showed that the contact area between the mesh and the droplet decreased with the wire spacing. These findings suggest that the fine pores on the mesh surface produced capillarity that impeded the transition to film boiling and enhanced the LFP.
- With nanocoated mesh surfaces, a maximum evaporation time was not observed even when the temperature was increased to the limit of the experimental system. This resulted in an LFP of least 490 °C, which is higher than those achieved in previous studies. In addition, sequential images of the drop impact revealed that after a splash occurs at the bottom of the droplet, it deforms into a disk shape and bounces. This so-called explosive-like behavior occurred in a very short interval after the impact (t < 1 ms). Previous studies attributed this explosive-like behavior to steam bubbles produced by nanostructures.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, J. Spray cooling heat transfer: The state of the art. Int. J. Heat Fluid Flow 2007, 28, 753–767. [Google Scholar] [CrossRef]
- Murav’ev, V.P. Emergency cooling of nuclear power plant reactors with heat removal by a forced-draft cooling tower. Power Technol. Eng. 2016, 50, 176–179. [Google Scholar] [CrossRef]
- Yeh, H.C. An analysis of rewetting of a nuclear fuel rod in water reactor emergency core cooling. Nucl. Eng. Des. 1975, 34, 317–322. [Google Scholar] [CrossRef]
- Hall, D.D.; Mudawar, I.; Morgan, R.E.; Ehlers, S.L. Validation of a systematic approach to modeling spray quenching of aluminum alloy extrusions, composites, and continuous castings. J. Mater. Eng. Perform. 1997, 6, 77–92. [Google Scholar] [CrossRef]
- Liang, G.; Mudawar, I. Review of spray cooling—Part 2: High temperature boiling regimes and quenching applications. Int. J. Heat Mass Transf. 2017, 115, 1206–1222. [Google Scholar] [CrossRef]
- Kandlikar, S.G.; Bapat, A.V. Evaluation of jet impingement, spray and microchannel chip cooling options for high heat flux removal. Heat Transf. Eng. 2007, 28, 911–923. [Google Scholar] [CrossRef]
- Bernardin, J.D.; Mudawar, I. The Leidenfrost point: Experimental study and assessment of existing models. J. Heat Transf. 1999, 121, 894. [Google Scholar] [CrossRef]
- Kim, H.; Truong, B.; Buongiorno, J.; Hu, L.W. On the effect of surface roughness height, wettability, and nanoporosity on Leidenfrost phenomena. Appl. Phys. Lett. 2011, 98, 2011–2014. [Google Scholar] [CrossRef]
- Kwon, H.M.; Bird, J.C.; Varanasi, K.K. Increasing Leidenfrost point using micro-nano hierarchical surface structures. Appl. Phys. Lett. 2013, 103. [Google Scholar] [CrossRef] [Green Version]
- Feng, R.T.; Wu, X.D.; Xue, Q.J. Profile characterization and temperature dependence of droplet control on textured surfaces. Chin. Sci. Bull. 2011, 56, 1930–1934. [Google Scholar] [CrossRef] [Green Version]
- Geraldi, N.R.; McHale, G.; Xu, B.B.; Wells, G.G.; Dodd, L.E.; Wood, D.; Newton, M.I. Leidenfrost transition temperature for stainless steel meshes. Mater. Lett. 2016, 176, 205–208. [Google Scholar] [CrossRef] [Green Version]
- Park, I.W.; Fernandino, M.; Dorao, C.A. Effect of micropillar characteristics on Leidenfrost. ASME. Int. Conf. Nanochannels Microchannels Minichannels 2016, 1–8. [Google Scholar] [CrossRef]
- Tran, T.; Staat, H.J.J.; Susarrey-Arce, A.; Foertsch, T.C.; van Houselt, A.; Gardeniers, H.J.G.E.; Prosperetti, A.; Lohse, D.; Sun, C. Droplet impact on superheated microstructured surfaces. Soft Matter 2013, 9, 3272. [Google Scholar] [CrossRef]
- Takata, Y.; Hidaka, S.; Cao, J.M.; Nakamura, T.; Yamamoto, H.; Masuda, M.; Ito, T. Effect of surface wettability on boiling and evaporation. Energy 2005, 30, 209–220. [Google Scholar] [CrossRef]
- Nair, H.; Staat, H.J.; Tran, T.; van Houselt, A.; Prosperetti, A.; Lohse, D.; Sun, C. The Leidenfrost temperature increase for impacting droplets on carbon-nanofiber surfaces. Soft Matter 2014, 10, 2102–2109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.H.; Ahn, H.S.; Kim, J.; Kaviany, M.; Kim, M.H. Dynamics of water droplet on a heated nanotubes surface. Appl. Phys. Lett. 2013, 102. [Google Scholar] [CrossRef] [Green Version]
- Tong, W.; Qiu, L.; Jin, J.; Sun, L.; Duan, F. Unique lift-off of droplet impact on high temperature nanotube surfaces. Appl. Phys. Lett. 2017, 111. [Google Scholar] [CrossRef]
- Liu, L.; Cai, G.; Tsai, P.A. Drop impact on heated nanostructures. Langmuir 2020, 36, 10051. [Google Scholar] [CrossRef]
- Young, T. An essay on the cohesion of fluids. Philos. Trans. R. Soc. Lond. 1805, 95, 65–87. [Google Scholar]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, B.A.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Davarpanah, A. A feasible visual investigation for associative foam >\ polymer injectivity performances in the oil recovery enhancement. Eur. Polym. J. 2018, 105, 405–411. [Google Scholar] [CrossRef]
- Esfandyari, H.; Hoseini, A.H.; Shadizadeh, S.R.; Davarpanah, A. Simultaneous evaluation of capillary pressure and wettability alteration based on the USBM and imbibition tests on carbonate minerals. J. Petrol. Sci. Eng. 2020, 108285. [Google Scholar] [CrossRef]
- Pan, F.; Zhang, Z.; Zhang, X.; Davarpanah, A. Impact of anionic and cationic surfactants interfacial tension on the oil recovery enhancement. Powder Technol. 2020, 373, 93–98. [Google Scholar] [CrossRef]
- Esfandyari, H.; Shadizadeh, S.R.; Esmaeilzadeh, F.; Davarpanah, A. Implications of anionic and natural surfactants to measure wettability alteration in EOR. Fuel 2020, 278, 118392. [Google Scholar] [CrossRef]
- Farokhnia, N.; Sajadi, S.M.; Irajizad, P.; Ghasemi, H. Decoupled hierarchical structures for suppression of Leidenfrost phenomenon. Langmuir 2017, 33, 2541–2550. [Google Scholar] [CrossRef]
- Hassebrook, A.; Kruse, C.; Wilson, C.; Anderson, T.; Zuhlke, C.; Alexander, D.; Gogos, G.; Ndao, S. Effects of droplet diameter on the Leidenfrost temperature of laser processed multiscale structured surfaces. Thermomech. Phenom. Electron. Syst. Proc. Intersoc. Conf. 2014, 452–457. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Ahn, H.S.; Kim, J.; Kim, M.H. Dynamics of a Water Droplet on the Heated Surface of Nano- and Microstructures. In Proceedings of the ASME 2012 10th International Conference on Nanochannels, Microchannels, and Minichannels Collo-cated with the ASME 2012 Heat Transfer Summer Conference and the ASME 2012 Fluids Engineering Division Summer Meeting, Rio Grande, Puerto Rico, USA, 8–12 July 2012; pp. 1–10. [Google Scholar]
- Ng, B.T.; Hung, Y.M.; Tan, M.K. Acoustically-controlled Leidenfrost droplets. J. Colloid Interface Sci. 2016, 465, 26–32. [Google Scholar] [CrossRef] [PubMed]







| d [μm] | s [μm] | d [μm] | s [μm] |  | ||
| #60 | 122.8 ± 0.8 | 295.6 ± 0.8 | nano-#60 | 129.4 ± 2.8 | 293.8 ± 10.5 | |
| #120 | 76.5 ± 2.1 | 134.1 ± 2.8 | nano-#120 | 71.1 ± 0.7 | 132.5 ± 2.2 | |
| #200 | 45.6 ± 1.2 | 80.2 ± 6.2 | nano-#200 | 37.5 ± 2.5 | 87.2 ± 2.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogata, S.; Nakanishi, R. Effect of Surface Textures and Wettability on Droplet Impact on a Heated Surface. Processes 2021, 9, 350. https://doi.org/10.3390/pr9020350
Ogata S, Nakanishi R. Effect of Surface Textures and Wettability on Droplet Impact on a Heated Surface. Processes. 2021; 9(2):350. https://doi.org/10.3390/pr9020350
Chicago/Turabian StyleOgata, Satoshi, and Ryo Nakanishi. 2021. "Effect of Surface Textures and Wettability on Droplet Impact on a Heated Surface" Processes 9, no. 2: 350. https://doi.org/10.3390/pr9020350
APA StyleOgata, S., & Nakanishi, R. (2021). Effect of Surface Textures and Wettability on Droplet Impact on a Heated Surface. Processes, 9(2), 350. https://doi.org/10.3390/pr9020350







