Evolution of Nitrogen-Based Alkylating Anticancer Agents
Abstract
:1. Introduction
2. Nitrogen-Based Alkylating Agents
2.1. First-Generation Nitrogen-Based Alkylators
2.2. Second-Generation Nitrogen-Based Alkylators
2.3. Third-Generation Nitrogen Mustards
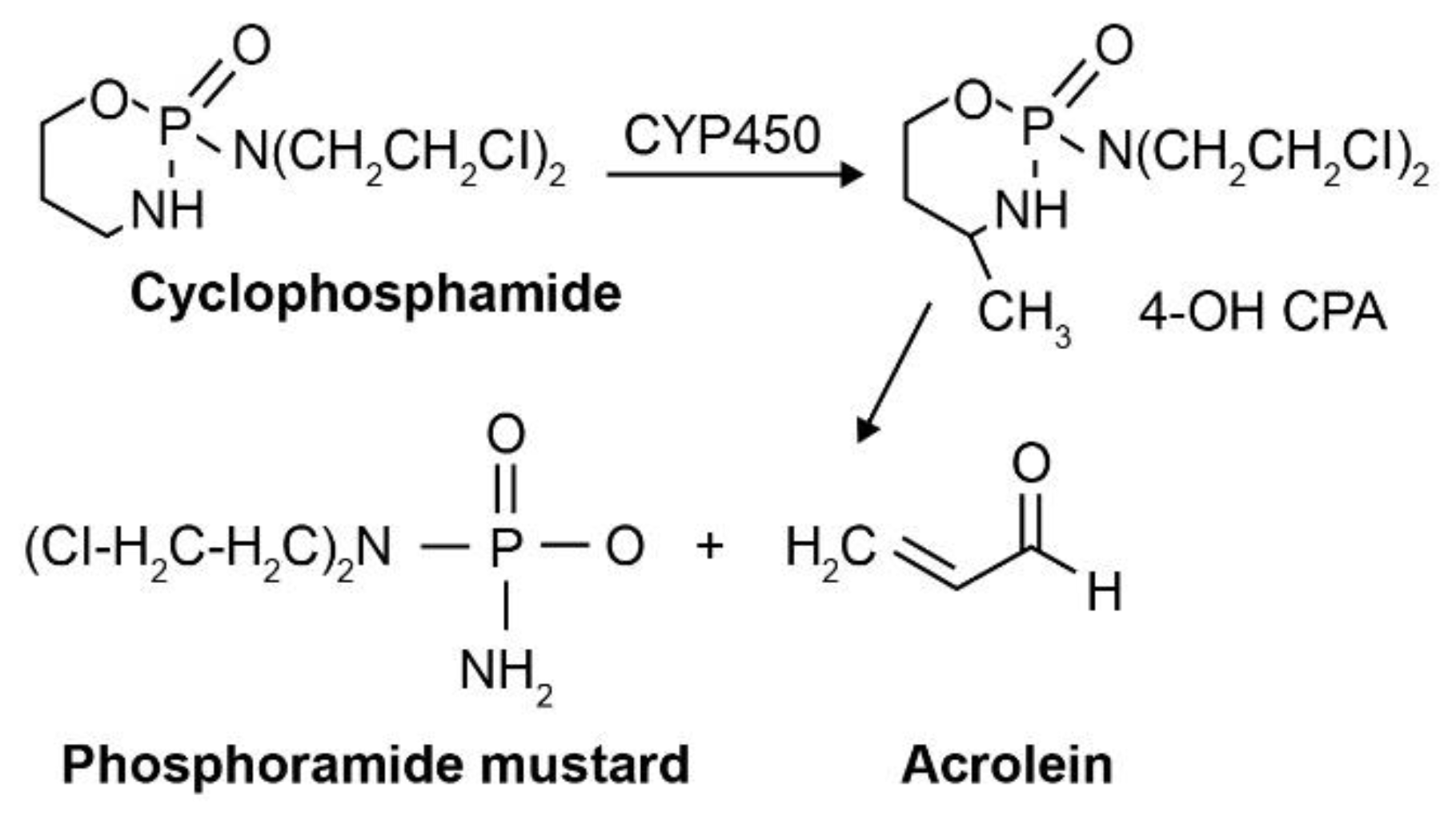
2.4. Phosphoramide Mustards
2.5. Steroid-Coupled Nitrogen Mustards
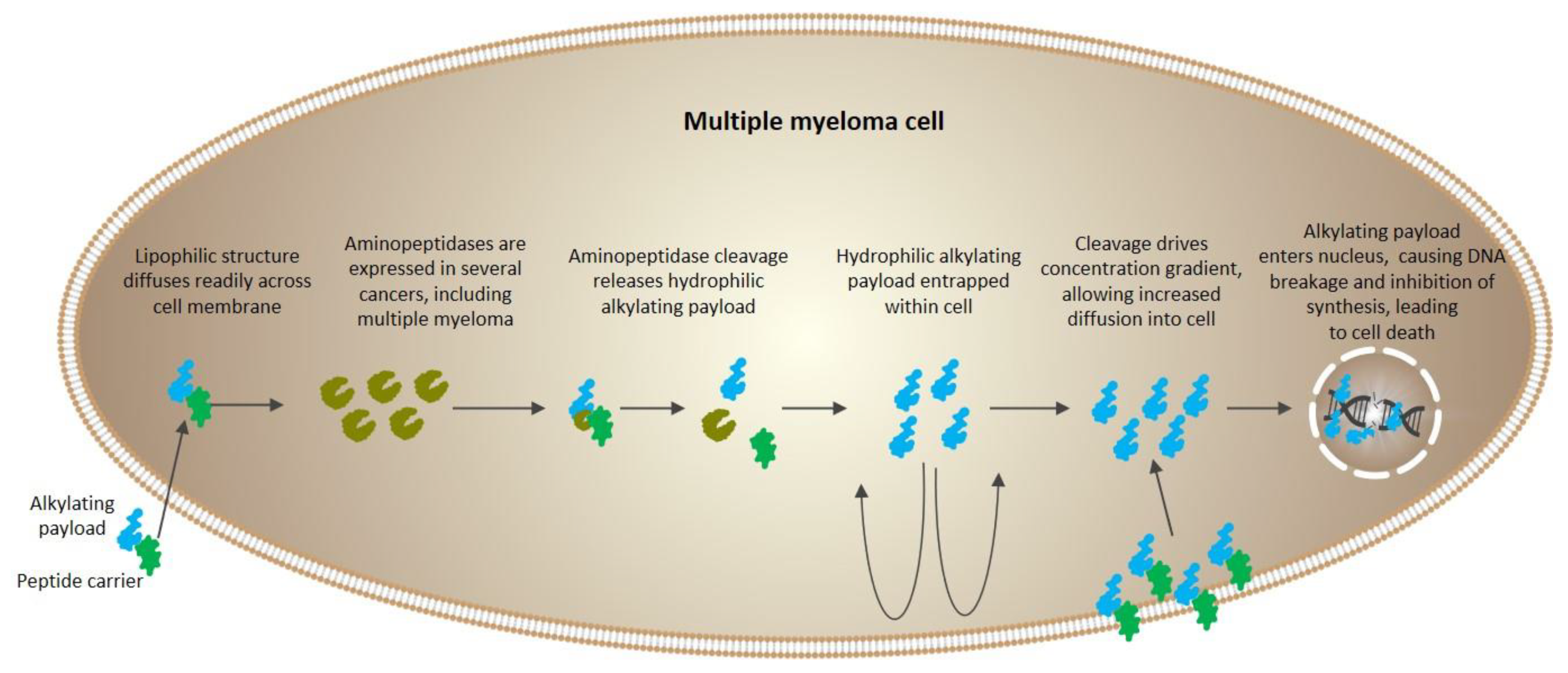
2.6. Peptide Conjugation
3. Alkylating Anticancer Agents: An Evolving Landscape
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Cancer. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 22 December 2020).
- Chen, Y.; Jia, Y.; Song, W.; Zhang, L. Therapeutic Potential of Nitrogen Mustard Based Hybrid Molecules. Front. Pharmacol. 2018, 9, 1453. [Google Scholar] [CrossRef]
- Ralhan, R.; Kaur, J. Alkylating agents and cancer therapy. Expert Opin. Ther. Patents 2007, 17, 1061–1075. [Google Scholar] [CrossRef]
- Weber, G.F. DNA Damaging Drugs. Mol. Ther. Cancer 2015, 9–112. [Google Scholar] [CrossRef]
- Wickström, M.; Dyberg, C.; Milosevic, J.; Einvik, C.; Calero, R.; Sveinbjörnsson, B.; Sandén, E.; Darabi, A.; Siesjö, P.; Kool, M.; et al. Wnt/β-catenin pathway regulates MGMT gene expression in cancer and inhibition of Wnt signalling prevents chemoresistance. Nat. Commun. 2015, 6, 8904. [Google Scholar] [CrossRef]
- Zheng, H.-C. The molecular mechanisms of chemoresistance in cancers. Oncotarget 2017, 8, 59950–59964. [Google Scholar] [CrossRef] [Green Version]
- Sarkaria, J.N.; Kitange, G.J.; James, C.D.; Plummer, R.; Calvert, H.; Weller, M.; Wick, W. Mechanisms of Chemoresistance to Alkylating Agents in Malignant Glioma. Clin. Cancer Res. 2008, 14, 2900–2908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R.K.; Kumar, S.; Prasad, D.N.; Bhardwaj, T.R. Therapeutic journery of nitrogen mustard as alkylating anticancer agents: Historic to future perspectives. Eur. J. Med. Chem. 2018, 151, 401–433. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, F.; Wennerberg, J. Melflufen: A journey from discovery to multi-kilogram production. In Complete Accounts of Integrated Drug Discovery and Development: Recent Examples from the Pharmaceutical Industry Volume 3; Pesti, J.A., Abdel-Magid, A.F., Vaidyanathan, R., Eds.; ACS Symposium Series; American Chemical Society: Washington DC, USA, 2020; Volume 1369, Chapter 5; pp. 157–177. [Google Scholar] [CrossRef]
- Gilman, A.; Philips, F.S. The Biological Actions and Therapeutic Applications of the B-Chloroethyl Amines and Sulfides. Science 1946, 103, 409–436. [Google Scholar] [CrossRef]
- Gilman, A. The initial clinical trial of nitrogen mustard. Am. J. Surg. 1963, 105, 574–578. [Google Scholar] [CrossRef]
- Colvin, M. Alkylating Agents. In Holland-Frei Cancer Medicine, 6th ed.; Kufe, D.W., Pollock, R.E., Weichselbaum, R.R., Bast, R.C., Gansler, T.S., Holland, J.F., Frei, E., Eds.; BC Decker: Hamilton, ON, Canada; Toronto, ON, Canada, 2003. Available online: https://www.ncbi.nlm.nih.gov/books/NBK12772/ (accessed on 22 December 2020).
- Diethelm-Varela, B.; Ai, Y.; Liang, D.; Xue, F. Nitrogen Mustards as Anticancer Chemotherapies: Historic Perspective, Current Developments and Future Trends. Curr. Top. Med. Chem. 2019, 19, 691–712. [Google Scholar] [CrossRef]
- Pass, G.J.; Carrie, D.; Lorimore, S.; Wright, E.; Houston, B.; Henderson, C.J.; Boylan, M.; Wolf, C.R. Role of Hepatic Cytochrome P450s in the Pharmacokinetics and Toxicity of Cyclophosphamide: Studies with the Hepatic Cytochrome P450 Reductase Null Mouse. Cancer Res. 2005, 65, 4211–4217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- A Fleming, R. An overview of cyclophosphamide and ifosfamide pharmacology. Pharmacother. J. Hum. Pharmacol. Drug Ther. 1997, 17, 146–154. [Google Scholar]
- Wickström, M.; Nygren, P.; Larsson, R.; Harmenberg, J.; Lindberg, J.; Sjöberg, P.; Jerling, M.; Lehmann, F.; Richardson, P.; Anderson, K.; et al. Melflufen - a peptidase-potentiated alkylating agent in clinical trials. Oncotarget 2017, 8, 66641–66655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gullbo, J.; Tullberg, M.; Våbenø, J.; Ehrsson, H.; Lewensohn, R.; Nygren, P.; Larsson, R.; Luthman, K. Structure–Activity Relationship for Alkylating Dipeptide Nitrogen Mustard Derivatives. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2003, 14, 113–132. [Google Scholar] [CrossRef]
- Wickström, M.; Viktorsson, K.; Lundholm, L.; Aesoy, R.; Nygren, H.; Sooman, L.; Fryknäs, M.; Vogel, L.K.; Lewensohn, R.; Larsson, R.; et al. The alkylating prodrug J1 can be activated by aminopeptidase N, leading to a possible target directed release of melphalan. Biochem. Pharmacol. 2010, 79, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, D.; Ray, A.; Viktorsson, K.; Spira, J.; Paba-Prada, C.; Munshi, N.; Richardson, P.; Lewensohn, R.; Anderson, K.C. In Vitro and In Vivo Antitumor Activity of a Novel Alkylating Agent, Melphalan-Flufenamide, against Multiple Myeloma Cells. Clin. Cancer Res. 2013, 19, 3019–3031. [Google Scholar] [CrossRef] [Green Version]
- Ray, A.; Ravillah, D.; Das, D.S.; Song, Y.; Nordström, E.; Gullbo, J.; Richardson, P.G.; Chauhan, D.; Anderson, K.C. A novel alkylating agent Melflufen induces irreversible DNA damage and cytotoxicity in multiple myeloma cells. Br. J. Haematol. 2016, 174, 397–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wickström, M.; Haglund, C.; Lindman, H.; Nygren, P.; Larsson, R.; Gullbo, J. The novel alkylating prodrug J1: Diagnosis directed activity profile ex vivo and combination analyses in vitro. Investig. New Drugs 2007, 26, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Oncopeptides Submits a New Drug Application to the FDA for Accelerated Approval of Melflufen in Triple-Class Refractory Multiple Myeloma Patients. Available online: https://www.prnewswire.com/news-releases/oncopeptides-submits-a-new-drug-application-to-the-fda-for-accelerated-approval-of-melflufen-in-triple-class-refractory-multiple-myeloma-patients-301085607.html (accessed on 22 December 2020).
- FDA Grants Priority Review of Melflufen for Patients with Triple-Class Refractory Multiple Myeloma. Available online: https://www.prnewswire.com/news-releases/fda-grants-priority-review-of-melflufen-for-patients-with-triple-class-refractory-multiple-myeloma-301120645.html (accessed on 22 December 2020).
- Gram, H.F.; Mosher, C.W.; Baker, B.R. Potential Anticancer Agents.1XVII. Alkylating Agents to Phenylalanine Mustard. I. J. Am. Chem. Soc. 1959, 81, 3103–3108. [Google Scholar] [CrossRef]
- Bartzatt, R.L. Synthesis and Alkylation Activity of a Nitrogen Mustard Agent to Penetrate the Blood-Brain Barrier. Drug Deliv. 2004, 11, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Lin, M.; Guo, W.; Zhang, Z.; Zhou, Y.; Chen, J.; Wang, T.; Zhong, X.; Lu, Y.; Yang, Q.; Wei, Q.; et al. Reduced Toxicity of Liposomal Nitrogen Mustard Prodrug Formulation Activated by an Intracellular ROS Feedback Mechanism in Hematological Neoplasm Models. Mol. Pharm. 2019, 17, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Denny, W.A. DNA minor groove alkylating agents. Curr. Med. Chem. 2001, 8, 533–544. [Google Scholar] [CrossRef] [PubMed]


| Generation | Compound (Chemical Formula) | First FDA Approval (yr) | Molecular Mass | Partition Coefficient (log P) | Dosage |
|---|---|---|---|---|---|
| First-generation | Mechlorethamine (C5H11Cl2N) | 1949 | 192.5 | −1.24 | 0.4 mg/kg |
| Second-generation | Chlorambucil (C14H19Cl2NO2) | 1957 | 304.2 | 3.21 | 0.1–0.2 mg/kg/day |
| Bendamustine (C16H21Cl2N3O2) | 2008 | 358.3 | 3.09 | 100–120 mg/m2 | |
| Melphalan (C13H18Cl2N2O2) | 1964 | 305.2 | 1.79 | 6–10 mg/day | |
| Third-generation | Uramustine (C8H11Cl2N3O2) | 1962 | 252.1 | 1.13 | 150 μg/kg |
| Melflufen (Melphalan flufenamide; C24H30Cl2FN3O3) | 2020 a | 498.5 | 4.04 | 40 mg (fixed dose) | |
| Steroid-coupled mustards | Estramustine (C23H32Cl2NO6P) | 1981 | 520.4 | 4.97 | 14 mg/kg |
| Phosphoramide mustards | Cyclophosphamide (C7H15Cl2N2O2P) | 1959 | 261.1 | 0.63 | 200 mg/day |
| Ifosfamide (C7H15Cl2N2O2P) | 1987 | 261.1 | 0.86 | 1.2 g/m2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lehmann, F.; Wennerberg, J. Evolution of Nitrogen-Based Alkylating Anticancer Agents. Processes 2021, 9, 377. https://doi.org/10.3390/pr9020377
Lehmann F, Wennerberg J. Evolution of Nitrogen-Based Alkylating Anticancer Agents. Processes. 2021; 9(2):377. https://doi.org/10.3390/pr9020377
Chicago/Turabian StyleLehmann, Fredrik, and Johan Wennerberg. 2021. "Evolution of Nitrogen-Based Alkylating Anticancer Agents" Processes 9, no. 2: 377. https://doi.org/10.3390/pr9020377
APA StyleLehmann, F., & Wennerberg, J. (2021). Evolution of Nitrogen-Based Alkylating Anticancer Agents. Processes, 9(2), 377. https://doi.org/10.3390/pr9020377






