Thermodynamics and Machine Learning Based Approaches for Vapor–Liquid–Liquid Phase Equilibria in n-Octane/Water, as a Naphtha–Water Surrogate in Water Blends
Abstract
:1. Introduction
2. Approach Adopted in the Present Study
3. Specific Strategy
| n-Octane | Naphtha [21] | |
|---|---|---|
| Carbon number | 8 | 6–13 |
| Molecular weight (g/gmole) | 114.23 | 145 |
| Boiling point (°C) | 125.6 | 65–230 |
| Density (kg/m3) | 703 | 781 |
| Conditions | Ref. | |
|---|---|---|
| Temperature: 5–25 °C | Mutual solubilities. | [22] |
| Temperature: 0–430 °C | Mutual solubilities. Liquid–liquid equilibrium. | [23] |
| Temperature: 5–75 °C | Vapor–liquid equilibrium | [24] |
| Temperature: 0–568 °C | Vapor–liquid equilibrium | [25] |
| Temperature: 25 °C | Mutual solubilities | [26] |
| Temperature: 0–25 °C | Mutual solubilities | [27] |
| Temperature: 357–387 °C Pressure: 19–23 MPa | Liquid–liquid–vapor equilibrium | [28] |
3.1. Materials
3.2. CREC Vapor Liquid Equilibrium Cell
4. Mathematical Formulation
4.1. Vapor–Liquid–Liquid Equilibrium Using NRTL Model
4.2. Gibbs Energy Analysis from Activity Coefficient Model
5. Results and Discussion
5.1. Issues with Available Models while Evaluating VLLE
- Discrepancies between models when running with two different available software (e.g. HYSYS V9 and Aspen Plus V9).
- Inconsistency of the available thermodynamic model predictions (e.g. Aspen Plus V9) with available experimental data.
5.2. Theoretical Discussion of Model Discrepancy


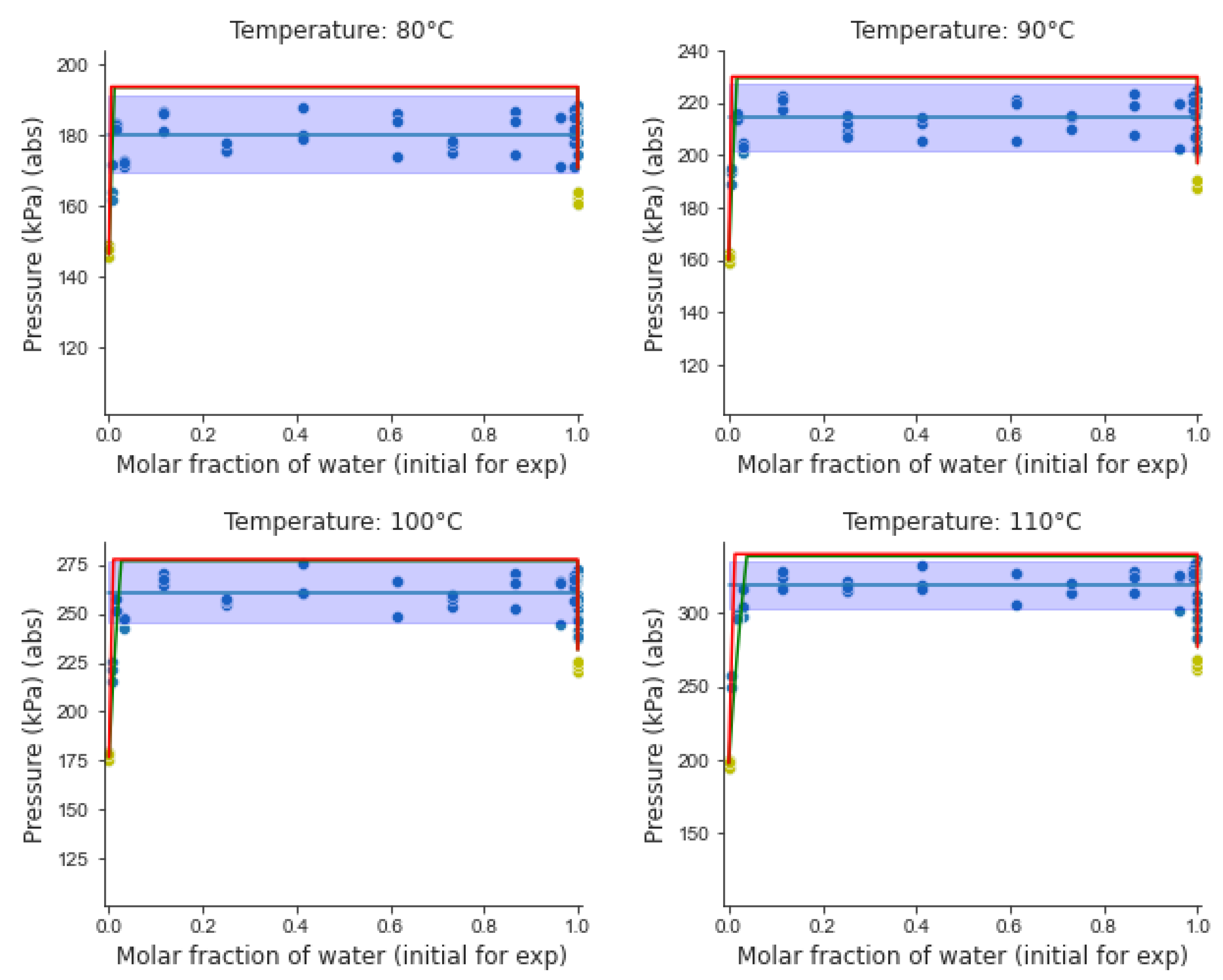
5.3. Analysis of Experimental Results
- (a)
- Three coexisting liquid–liquid–vapor (VLL) phases with the vapor pressure remaining unchanged, while the initial water composition is varied (horizontal broken line).
- (b)
- Two liquids phases at higher pressures, with every phase involving highly diluted blends,
- (c)
- Two phases, vapor and liquid, with the liquid phase encompassing completely solubilized species.
- (d)
- A mixed vapor phase at low pressures.

6. The Machine Learning Approach
6.1. Classification Methodology
6.2. Classification Models Results
7. Conclusions
- It is shown that reliable models, based on fundamentals principles, are still needed to represent the number of phases, in diluted hydrocarbon in water mixtures at phase equilibria.
- It is proven that a phase stability analysis involving the Gibbs energy of mixing, can be used to explain calculation result discrepancies, in water/n-octane mixtures when using available simulation software.
- It is demonstrated that runs in a CREC VL Cell employing a dynamic technique (1.22 °C/min temperature ramp), can provide the “big data sets” required to accurately determine the fully miscible, partially miscible, and fully immiscible octane/water blend states.
- It is proven that ML models based on the obtained “big data sets” can be proposed for the prediction of the number of phases under the studied conditions, with the KNN model and the weighted SVC model, identified as the ones with best performance.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Symbols with Latin letter | |
| F | Function |
| G | Gibbs Free Energy |
| P | Pressure |
| R | Universal Gas Constant |
| T | Temperature |
| x | Molar Fraction of Liquid Phase |
| y | Molar Fraction of Vapor Phase |
| z | Overall Molar Fraction |
| Parameter for accounting local composition variations (NRTL method) | |
| Activity coefficient | |
| Interaction energy (NRTL method) | |
| Dimensionless interaction parameters (NRTL method) | |
| Subindex and Superindex | |
| identifies component of the solution | |
| identifies component of the solution | |
| identifies a subgroup | |
| L | Liquid |
| mix | mixing |
| obj | objective |
| sat | Saturation |
| V | vapor |
| I | Phase I |
| II | Phase II |
| Acronyms | |
| ANN | Artificial Neural Networks |
| AUC | Area Under Curve |
| BIP | Binary Interaction Parameters |
| CREC | Chemical Reactors Engineering Center |
| EoS | Equation of State |
| FN | False Negative |
| FNN | Feedforward Neural Networks |
| FP | False Positive |
| FPR | False Positive Rate |
| KNN | K-Nearest Neighbors |
| LL | Liquid–Liquid |
| LLE | Liquid–Liquid Equilibrium |
| ML | Machine Learning |
| NRTL | Non-Random Two-Liquid Model |
| NRU | Naphtha Recovery Unit |
| PC | Personal Computer |
| PNN | Probabilistic Neural Networks |
| PR-EoS | Peng–Robinson-Equation of State |
| ROC | Receiver Operating Characteristic |
| RVM | Relevance Vector Machines |
| SVM | Support Vector Machine |
| SVC | Support Vector Classification |
| TN | True Negative |
| TP | True Positive |
| TPR | Three Phase Region |
| UNIQUAC | Universal Quasichemical model |
| USB | Universal Serial Bus |
| VL | Vapor–Liquid |
| VL Cell | Vapor–Liquid Cell |
| VLE | Vapor-Liquid Equilibrium |
| VLL | Vapor–Liquid–Liquid |
| VLLE | Vapor–Liquid–Liquid Equilibrium |
Appendix A
Appendix A.1. Brief Description of Classification Models
Appendix A.1.1. Logistic Regression
Appendix A.1.2. Decision Tree Classifier
Appendix A.1.3. K-Nearest Neighbors (KNN)
Appendix A.1.4. Support Vector Machine (SVM)
Appendix A.2. Additional Figures



Appendix A.3. The CREC VL Cell and Its Instrumentation
| Name | Volume |
|---|---|
| Thermofluid | 2.4 L |
| CREC-VL-Cell | 275 mL |
| Sample analyzed | 100 to 140 mL |
| Instrument | Parameter | Data Range | Uncertainty (±) [10,11] |
|---|---|---|---|
| OMEGATM Transducer PX409-50GUSBH Series | Pressure | 0–345 kPa5 Hz frequency | ±0.28 kPa |
| OMEGATM USB TC-08 Unit | Temperature data acquisition rate | 10 Hz frequency | 1% |
| OMEGATM PID Controller | Temperature increase rate in the Cell | 4 to 20 mA | ±0.5 °C |
| Thermocouple:OMEGATM type k | Temperature | −200 to 1250 °C | ±2.2 °C |
| VELP® DLS Digital Overhead Stirrer | Torque Maximum | 40 Ncm | N/A |
| Mixing speed | 50 to 2000 rpm |
| Operation | Set Value |
|---|---|
| Impeller Mixing Speed | 1080 rpm |
| Heating Rate | 1.22 °C/min |
| Temperature Range | 30 to 120 °C |
| Run Time | 90 min |
References
- De Tommaso, J.; Rossi, F.; Moradi, N.; Pirola, C.; Patience, G.S.; Galli, F. Experimental methods in chemical engineering: Process simulation. Can. J. Chem. Eng. 2020, 98, 2301–2320. [Google Scholar] [CrossRef]
- Banerjee, D.K. Oil Sands, Heavy Oil & Bitumen: From Recover to Refinery; PennWell Corporation: Tilsa, OK, USA, 2012. [Google Scholar]
- Pedersen, K.S.; Christensen, P.L.; Shaikh, J.A. Phase Behavior of Petroleum Reservoir Fluids, 2nd ed.; Taylor & Francis Group, LLC: Boca Raton, FL, USA, 2015. [Google Scholar]
- Du, J.; Cluett, W. Modelling of a Naphtha Recovery Unit (NRU) with Implications for Process Optimization. Processes 2018, 6, 74. [Google Scholar] [CrossRef] [Green Version]
- Matsoukas, T. Fundamentals of Chemical Engineering Thermodynamics: With Applications to Chemical Processes; Pearson Education, Inc: Ann Arbor, MI, USA, 2013. [Google Scholar]
- Carlson, E.C. Don’t Gamble With Physical Properties. Chem. Eng. Prog. 1996, 92, 35–46. [Google Scholar]
- Jia, H.; Wang, H.; Ma, K.; Yu, M.; Zhu, Z.; Wang, Y. Effect of thermodynamic parameters on prediction of phase behavior and process design of extractive distillation. Chinese J. Chem. Eng. 2018, 26, 993–1002. [Google Scholar] [CrossRef]
- Marcilla, A.; Reyes-Labarta, J.A.; Olaya, M.M. Should we trust all the published LLE correlation parameters in phase equilibria? Necessity of their assessment prior to publication. Fluid Phase Equilib. 2017, 433, 243–252. [Google Scholar] [CrossRef] [Green Version]
- Venkatasubramanian, V. The promise of artificial intelligence in chemical engineering: Is it here, finally? AIChE J. 2019, 65, 466–478. [Google Scholar] [CrossRef]
- Kong, J. Multiphase Equilibrium in A Novel Batch Dynamic VL-Cell Unit with High Mixing: Apparatus Design and Process Simulation. The University of Western Ontario, 2020. Available online: https://ir.lib.uwo.ca/etd/7283/ (accessed on 31 August 2020).
- Escobedo, S.; Kong, J.; Lopez-Zamora, S.; de Lasa, H. Synthetic Naphtha Recovery from Water Streams: Vapor-Liquid-Liquid Equilibrium (VLLE) Studies in a Dynamic VL-Cell Unit with High Intensity Mixing. Can. J. Chem. Eng. 2021. (In Press) [Google Scholar]
- He, Q.P.; Wang, J. Application of systems engineering principles and techniques in biological big data analytics: A review. Processes 2020, 8, 951. [Google Scholar] [CrossRef]
- Duever, T.A. Data science in the chemical engineering curriculum. Processes 2019, 7, 830. [Google Scholar] [CrossRef] [Green Version]
- Chiang, L.; Lu, B.; Castillo, I. Big data analytics in chemical engineering. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 63–85. [Google Scholar] [CrossRef]
- Trappenberg, T.P. Fundamentals of Machine Learning; Oxford University Press (OUP): Oxford, UK, 2019. [Google Scholar]
- Pereira, F.C.; Borysov, S.S. Machine Learning Fundamentals. In Mobility Patterns, Big Data and Transport Analytics: Tools and Applications for Modeling; Antoniou, C., Dimitriou, L., Pereira, F., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 9–29. [Google Scholar]
- Pecht, M.G. Prognostics and Health Management of Electronics; John Wiley & Sons Ltd: Hoboken, NJ, USA, 2018. [Google Scholar]
- Schmitz, J.E.; Zemp, R.J.; Mendes, M.J. Artificial neural networks for the solution of the phase stability problem. Fluid Phase Equilib. 2006, 245, 83–87. [Google Scholar] [CrossRef]
- Poort, J.P.; Ramdin, M.; van Kranendonk, J.; Vlugt, T.J.H. Solving vapor-liquid flash problems using artificial neural networks. Fluid Phase Equilib. 2019, 490, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Kashinath, A.; Szulczewski, M.L.; Dogru, A.H. A fast algorithm for calculating isothermal phase behavior using machine learning. Fluid Phase Equilib. 2018, 465, 73–82. [Google Scholar] [CrossRef]
- Naphtha (petroleum), hydrotreated heavy [MAK Value Documentation, 2010]. In The MAK-Collection for Occupational Health and Safety (eds and); Wiley-VCH: Weinheim, Germany, 2015. [CrossRef]
- Black, C.; Joris, G.G.; Taylor, H.S. The solubility of water in hydrocarbons. J. Chem. Phys. 1948, 16, 537–543. [Google Scholar] [CrossRef]
- Mączyński, A.; Wiśniewska-Gocłowska, B.; Góral, M. Recommended liquid-liquid equilibrium data. Part 1. Binary alkane-water systems. J. Phys. Chem. Ref. Data 2004, 33, 549–577. [Google Scholar] [CrossRef]
- Tu, M.; Fei, D.; Liu, Y.; Wang, J. Phase Equilibrium for Partially Miscible System of Octane-Water. J. Chem. Eng. Chinese Univ. 1998, 12, 325–330. [Google Scholar]
- Heidman, J.L.; Tsonopoulos, C.; Brady, C.J.; Wilson, G.M. High-temperature mutual solubilities of hydrocarbons and water. Part II: Ethylbenzene, ethylcyclohexane, and n-octane. AIChE J. 1985, 31, 376–384. [Google Scholar] [CrossRef]
- Tsonopoulos, C. Thermodynamic analysis of the mutual solubilities of hydrocarbons and water. Fluid Phase Equilib. 2001, 186, 185–206. [Google Scholar] [CrossRef]
- Polak, J.; Lu, B.C.-Y. Mutual Solubilities of Hydrocarbons and Water at 0 and 25 °C. Can. J. Chem. 1973, 51, 4018–4023. [Google Scholar] [CrossRef] [Green Version]
- Aktiengesellschaft, B.; Ludwigshqfen, D. Fluid mixtures at high pressures IX. Phase phenomena mixtures separation and critical + water). J. Chem. Thermodyn. 1990, 22, 335–353. [Google Scholar]
- Renon, H.; Prausnitz, J.M. Local compositions in thermodynamic excess functions for liquid mixtures. AIChE J. 1968, 14, 135–144. [Google Scholar] [CrossRef]
- Klauck, M.; Grenner, A.; Schmelzer, J. Liquid-liquid(-liquid) equilibria in ternary systems of water + cyclohexylamine + aromatic hydrocarbon (toluene or propylbenzene) or aliphatic hydrocarbon (heptane or octane). J. Chem. Eng. Data 2006, 51, 1043–1050. [Google Scholar] [CrossRef]
- Chien, H.H. Formulations for three-phase flash calculations. AIChE J. 1994, 40, 957–965. [Google Scholar] [CrossRef]
- Paarsch, H.J.; Golyaev, K. A Gentle Introduction to Effective Computing in Quantitative Research What Every Research Assistant Should Know; MIT Press: London, UK, 2016. [Google Scholar]
- Connolly, M. An isenthalpic-Based Compositional Framework for Nonlinear Thermal Simulation; Stanford University: Stanford, CA, USA, 2018. [Google Scholar]
- Privat, R.; Jaubert, J.N.; Berger, E.; Coniglio, L.; Lemaitre, C.; Meimaroglou, D.; Warth, V. Teaching the Concept of Gibbs Energy Minimization through Its Application to Phase-Equilibrium Calculation. J. Chem. Educ. 2016, 93, 1569–1577. [Google Scholar] [CrossRef]
- Olaya, M.D.M.; Reyes-Labarta, J.A.; Serrano, M.D.; Marcilla, A. Vapor-liquid equilibria: Using the gibbs energy and the common tangent plane criterion. Chem. Eng. Educ. 2010, 44, 236–244. [Google Scholar]
- Jaubert, J.N.; Privat, R. Application of the double-tangent construction of coexisting phases to Any Type of Phase Equilibrium For Binary Systems Modeled With the Gamma-Phi Approach. Chem. Eng. Educ. 2014, 48, 42–56. [Google Scholar]
- Liaw, H.J.; Chen, C.T.; Gerbaud, V. Flash-point prediction for binary partially miscible aqueous-organic mixtures. Chem. Eng. Sci. 2008, 63, 4543–4554. [Google Scholar] [CrossRef] [Green Version]
- Baker, L.E.; Pierce, A.C.; Luks, K.D. Gibbs Energy Analysis of Phase Equilibria. Soc. Pet. Eng. J. 1982, 22, 731–742. [Google Scholar] [CrossRef]
- Soares, M.E.; Medina, A.G.; McDermott, C.; Ashton, N. Three phase flash calculations using free energy minimisation. Chem. Engng. Sci. 1982, 37, 521–528. [Google Scholar] [CrossRef]
- Michelsen, M.L. The isothermal flash problem. Part II. Phase-split calculation. Fluid Phase Equilib. 1982, 9, 21–40. [Google Scholar] [CrossRef]
- Delen, D. Predictive Analytics: Data Mining, Machine Learning and Data Science for Practitioners, 2nd ed.; Pearson Education Inc.: Old Tappan, NJ, USA, 2020. [Google Scholar]
- Sahu, P.K.; Pal, S.R.; Das, A.K. Estimation and Inferential Statistics; Springer: New Delhi, India, 2015. [Google Scholar]
- Buitinck, L.; Louppe, G.; Blondel, M.; Pedregosa, F.; Andreas, C.M.; Grisel, O.; Niculae, V.; Prettenhofer, P.; Gramfort, A.; Grobler, J.; et al. API design for machine learning software: Experiences From the Scikit-Learn Project. arXiv 2013, arXiv:1309.0238. [Google Scholar]
- Géron, A. Hands-On Machine Learning with Scikit-Learn, Keras, and TensorFlow, 2nd ed.; O’Reilly Media, Inc.: Newton, MA, USA, 2019. [Google Scholar]
- Pozzolo, A.D.; Caelen, O.; Johnson, R.A.; Bontempi, G. Calibrating probability with undersampling for unbalanced classification. In Proceedings of the 2015 IEEE Symposium Series Computational Intelligence, Cape Town, South Africa, 7–10 December 2015. [Google Scholar] [CrossRef]
- Cranganu, C.; Breaban, M.E.; Luchian, H. Artificial Intelligent Approaches in Petroleum Geosciences; Springer International Publishing: Dordrecht, The Netherlands, 2015. [Google Scholar]
- Sinha, U.; Dindoruk, B.; Soliman, M. Prediction of CO Minimum Miscibility Pressure MMP using Machine Learning Techniques. In Proceedings of the Society of Petroleum Engineers-SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 18–22 April 2020. [Google Scholar] [CrossRef]
- Kubat, M. An Introduction to Machine Learning; Springer International Publishing: Dordrecht, The Nerthlands, 2015. [Google Scholar]
- Kazemi, P.; Steyer, J.P.; Bengoa, C.; Font, J.; Giralt, J. Robust data-driven soft sensors for online monitoring of volatile fatty acids in anaerobic digestion processes. Processes 2020, 8, 67. [Google Scholar] [CrossRef] [Green Version]
















| Model | Boiling Point Difference | Dew Point Difference |
|---|---|---|
| Peng Robinson | Min: 5.67% Mean: 7.98% Max: 11.58% | Min: 1.78% Mean: 3.91% Max: 6.83% |
| NRTL | Min: 92.88% Mean: 99.95% Max: 104.66% | Min: 34.12% Mean: 49.96% Max: 66.45% |
| UNIQUAC | Min: 83.20% Mean: 86.28% Max: 90.39% | Min: 37.86% Mean: 62.98% Max: 48.89% |
| 70 °C | 100 °C | |||
|---|---|---|---|---|
| BIP reference | Water in Hydrocarbon phase (x1I) | n-Octane in Aqueous phase (x2II) | Water in Hydrocarbon phase (x1I) | n-Octane in Aqueous phase (x2II) |
| Klauck et al. (2006) [6,29] | 3.32245 × 10−3 | 9.1571 × 10−7 | 9.56125 × 10−3 | 9.3652 × 10−7 |
| Aspen Plus (Python) | 7.50095 × 10−3 | 8.1543 × 10−6 | 2.644388 × 10−2 | 2.1395 × 10−5 |
| HYSYS (estimated BIP) | 8.9570 × 10−6 | 0.02069 | 1.3065 × 10−5 | 0.05936 |
| HYSYS (zero) (* one single phase) | −6.1590 × 10−10 | 1 | −6.1590 × 10−10 | 1 |
| Model # | Type | Hyper-Parameters | Class Weight Option |
|---|---|---|---|
| 1 | Logistic Regression | penalty: 12, tol: 0.0001, C: 1.0, fit_intercept: True, intercept_scaling: 1 | Yes |
| 2 | Decision Tree Classifier | criterion: entropy, splitter: best, max_depth: 3, min_samples_split: 2, min_samples_leaf: 1 | Yes |
| 3 | K-Neighbors Classifier | n_neighbors: 5, weights: uniform, algorithm: auto, leaf_size: 30, p: 2, metric: Minkowski | No |
| 4 | Support Vector Classifier (SVC) | C:1.0, kernel: rbf, degree: 3, gamma: scale, shrinking: True, probability: True, tol = 0.001 | Yes |
| Logistic Regression | |||
| Precision | Recall | F1 score | |
| 3-Phases | 0.92 | 0.93 | 0.94 |
| 2-Phases | 0.78 | 0.83 | 0.80 |
| Decision Tree Classifier | |||
| Precision | Recall | F1 score | |
| 3-Phases | 1.00 | 0.90 | 0.95 |
| 2-Phases | 0.75 | 0.99 | 0.85 |
| K-Neighbors Classifier (KNN) | |||
| Precision | Recall | F1 score | |
| 3-Phases | 1.00 | 0.97 | 0.98 |
| 2-Phases | 0.91 | 1.00 | 0.95 |
| SVC | |||
| Precision | Recall | F1 score | |
| 3-Phases | 1.00 | 0.93 | 0.96 |
| 2-Phases | 0.80 | 0.99 | 0.88 |
| Logistic Regression (penalized) | |||
| Precision | Recall | F1 score | |
| 3-Phases | 0.92 | 1 | 0.96 |
| 2-Phases | 1 | 0.72 | 0.84 |
| Decision Tree Classifier (penalized) | |||
| Precision | Recall | F1 score | |
| 3-Phases | 0.95 | 1.00 | 0.97 |
| 2-Phases | 1.00 | 0.82 | 0.90 |
| K-Neighbors Classifier (KNN) | |||
| Precision | Recall | F1 score | |
| 3-Phases | 1.00 | 0.99 | 0.99 |
| 2-Phases | 0.97 | 0.98 | 0.98 |
| SVC (penalized) | |||
| Precision | Recall | F1 score | |
| 3-Phases | 0.98 | 1 | 0.99 |
| 2-Phases | 1 | 0.94 | 0.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez-Zamora, S.; Kong, J.; Escobedo, S.; Lasa, H.d. Thermodynamics and Machine Learning Based Approaches for Vapor–Liquid–Liquid Phase Equilibria in n-Octane/Water, as a Naphtha–Water Surrogate in Water Blends. Processes 2021, 9, 413. https://doi.org/10.3390/pr9030413
Lopez-Zamora S, Kong J, Escobedo S, Lasa Hd. Thermodynamics and Machine Learning Based Approaches for Vapor–Liquid–Liquid Phase Equilibria in n-Octane/Water, as a Naphtha–Water Surrogate in Water Blends. Processes. 2021; 9(3):413. https://doi.org/10.3390/pr9030413
Chicago/Turabian StyleLopez-Zamora, Sandra, Jeonghoon Kong, Salvador Escobedo, and Hugo de Lasa. 2021. "Thermodynamics and Machine Learning Based Approaches for Vapor–Liquid–Liquid Phase Equilibria in n-Octane/Water, as a Naphtha–Water Surrogate in Water Blends" Processes 9, no. 3: 413. https://doi.org/10.3390/pr9030413








