Techno-Economic Analysis of a Process for the Aqueous Conversion of Corn Stover into Lactic and Levulinic Acid through Sn-Beta Catalysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reactive Extraction
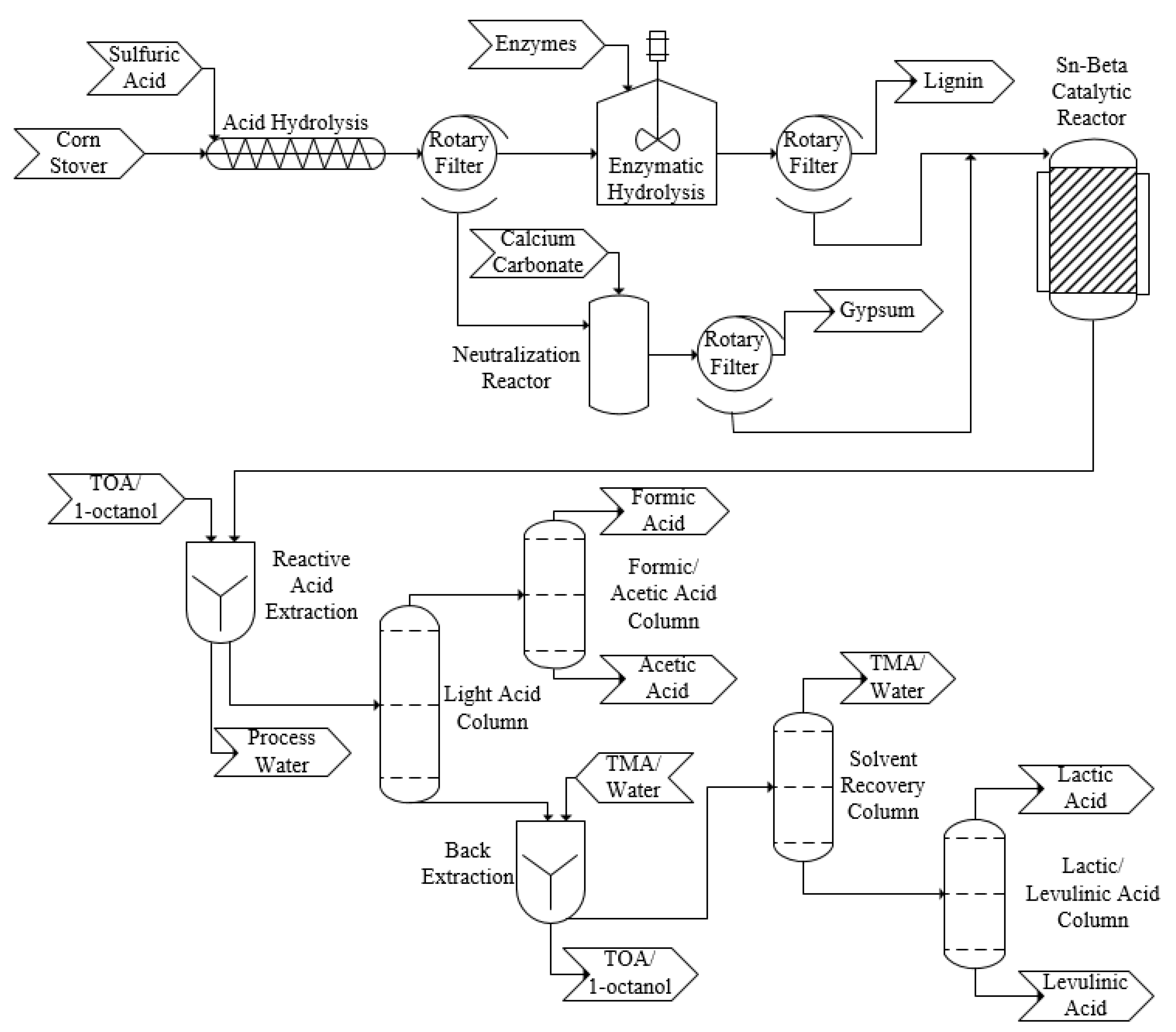
2.2. Design
2.3. Economic Analysis
2.4. Process Design Assumptions
- Processes were designed as grassroot plants with a 20 year lifespan.
- Designed processes have an operating factor of 95%.
- Corn stover is available pre-milled at a mean size 5 mm and a density of 560 kg/m3.
- Corn stover has an initial composition (dry wt%) of 35.1% glucan, 19.5% xylan, 15.8% lignin, 4.9% ash, 3.1% protein, 2.4% arabinan, 1.8% acetate, 1.4% galactan, 0.8% sucrose, 0.6% mannan, and 14.7% extractives [21]. The extractives component is a general term used to sum the initial composition of corn stover to 100% [21].
- Deacetylation removes 100% of water-soluble extractives, 88% of acetate, 75% of ash, 20% of lignin, and 2% of xylan from the initial corn stover [21].
- Solids leaving the deacetylation reactor are assumed to be 20 wt% liquid and all are removed from the screw and recycled back to the reactor [21].
- Acid hydrolysis requires a sulfuric acid loading of 9 mg/g of dry biomass. Sulfuric acid is diluted to 0.3 wt% in water [21].
- At 160 °C and 560 kPa for 5 min, the acid hydrolysis reactor has a xylan conversion of 90 wt% to xylose, 5 wt% to furfural, and 2.4 wt% to xylose oligomers. The rates of glucan conversion are 9.9 wt% glucose, 3 wt% glucose oligomers, and 3 wt% HMF. Meanwhile, 100 wt% of the remaining acetate is converted to acetic acid.
- Corn stover has a heat capacity of 1300 J/(kg-K) [24].
- Heats of formation used are −1271 kJ/mol for glucose, −973 kJ/mol xylose, −484.5 kJ/mol for acetic acid, −425 kJ/mol for formic acid, −590 kJ/mol for lactic acid, −581 kJ/mol for levulinic acid, and −286 kJ/mol for water [25].
- Enzymatic hydrolysis requires an enzyme loading of 10 mg/g of cellulose and operates at a residence time of 84 h [21].
- Enzymatic hydrolysis has glucan conversions of 91.2 wt% to glucose and 4 wt% to glucose oligomers [21].
- Since HMF is an intermediate of levulinic acid formation, it is assumed that all HMF produced from acid hydrolysis is converted to levulinic acid and formic acid with Sn-Beta.
- Glucose and xylose are assumed to have 100% conversion in the Sn-Beta reactor. Sugars that are not converted into organic acid products are assumed to be hydrochar [26].
- Hydrochar can be calcined off the catalyst using compressed air at 260 °C and 1800 kPa.
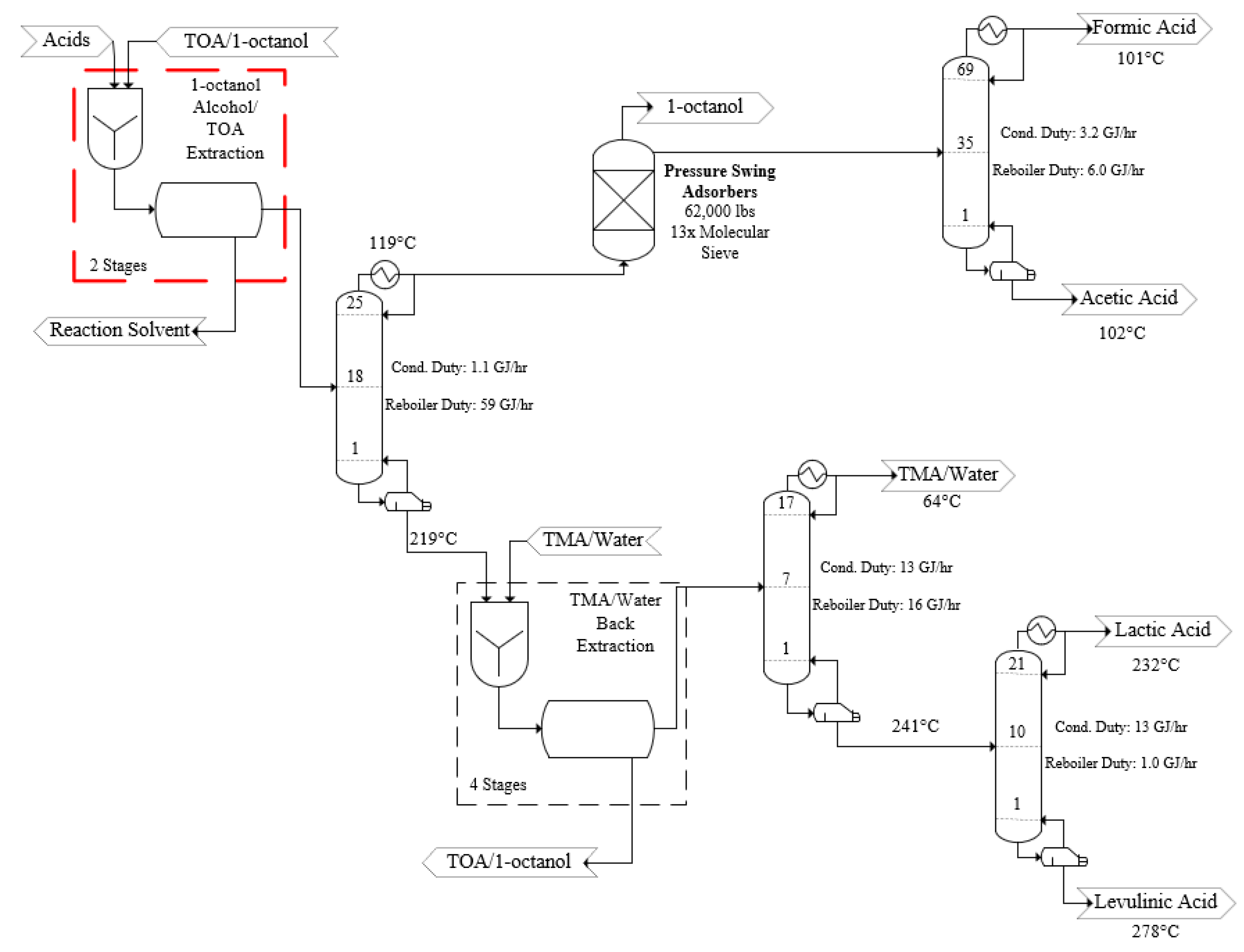
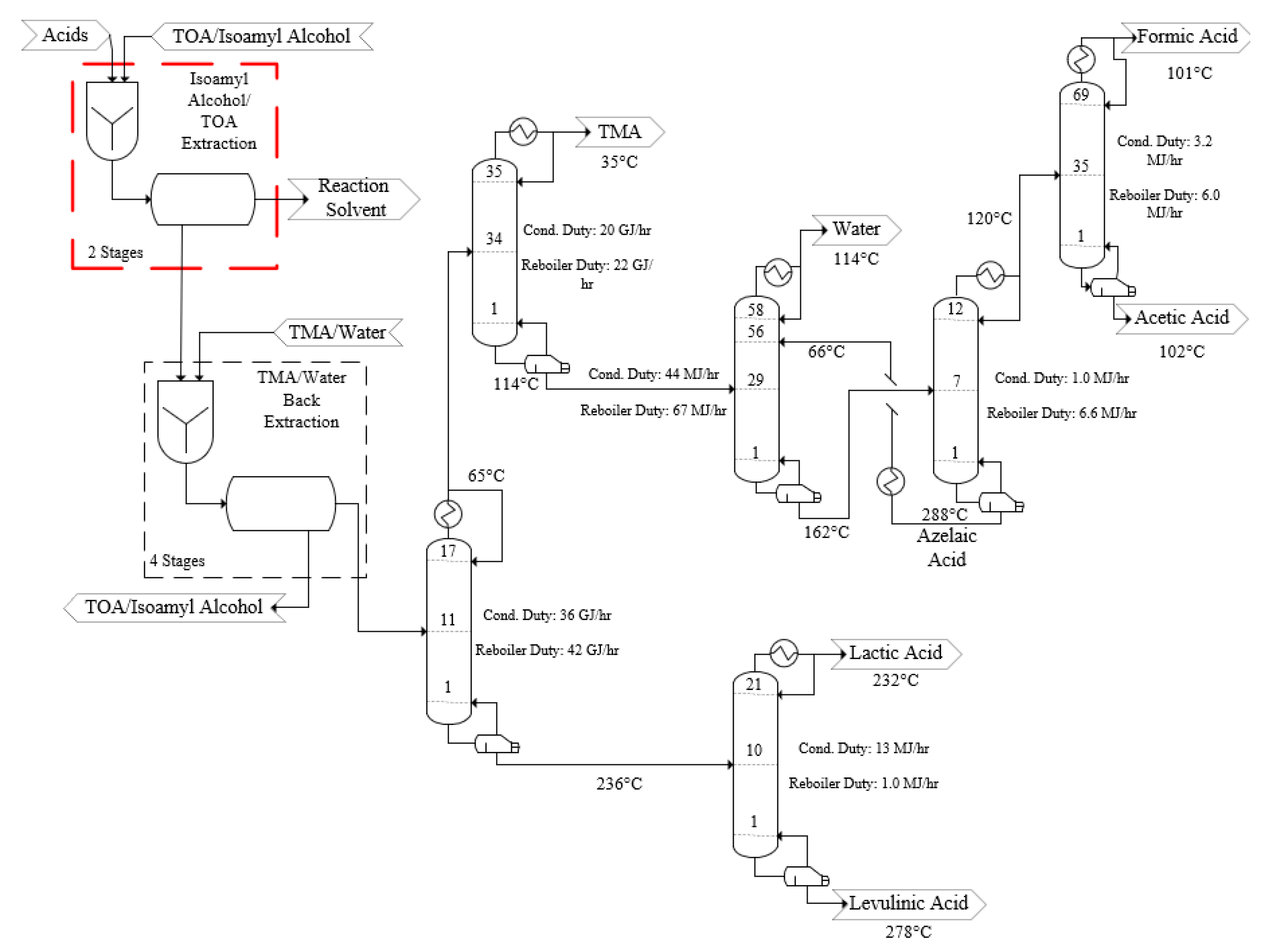
- The target efficiencies of reactive extraction for both TOA and trimethylamine (TMA) extractors were 99% for all product acids.
2.5. Equipment Design Assumptions
- Conveyors anticipated to stay within one process area were 15 m long. Conveyors anticipated to go between process areas were sized at 150 m in length. All conveyors were 0.61 m wide.
- Pumps operate at 65% efficiency.
- Compressors have a polytrophic efficiency of 75%.
- Hold up time in a reflux drum is 450 s.
- The height-to-diameter ratio used for reflux and hold up drums sizing was 2.
- The liquid residence time for hold up drums is 5 min and the total drum volume is double the liquid space.
- The liquid residence time for mixers is 5 min with a height to diameter ratio of 2.
- Distillation columns were sized with sieve trays at a spacing of 0.61 m.
- A 20 kPa pressure drop was assumed across all units with an additional 14 kPa for an anticipated control valve and 20 kPa for a mist eliminator.
- A 70 kPa pressure drop was assumed for every 4 m of catalyst packing.
- Trayed columns have pressure changes of 0.7 kPa per tray, with pressure decreasing with increased height. Other pressure drops for distillation columns include 100 kPa for feed above 9 m and 1 kPa for thermosyphon reboilers.
- Interstage cooling systems for multistage compressors have a 14 kPa pressure drop across a heat exchanger and 7 kPa across a knockout drum.
2.6. Utility Assumptions
- Steam grades produced in the auxiliary facilities include: low pressure steam at 148 °C and 450 kPa, medium-low pressure steam at 175 °C and 900 kPa, moderate pressure steam at 204 °C and 1700 kPa, medium-high pressure steam at 239 °C and 3300 kPa, and high pressure steam at 400 °C and 4500 kPa. All grades of steam are rated at their saturation pressures except for high pressure steam which is superheated.
- Cooling and boiler feed water are produced in the auxiliary facilities at 30 °C and 310 kPa.
2.7. Economic Assumptions
- Economic estimates were brought to a basis date of January of 2019 using CEPCI values of 400 and 616 for January 2004 and January 2019, respectively.
- Annual maintenance costs were estimated as 4% of the fixed capital investment.
- The fixed capital investment was depreciated over 17 years using the modified accelerated cost recovery system (MACRS).
- Income tax rates were applied as 21% for Federal and 4.31% for State (North Dakota) [23].
- A rough project completion schedule was estimated using a 30% design, 40% procurement, and 30% implementation rule of thumb. Procurement was based on the longest equipment lead time (14 months) with no overlap of project phases, yielding a total schedule of 36 months.
- No royalties or patent fees are required for the process
- Recycled solvents are replaced at a rate of 15% per year while solid catalysts and sorbents are replaced at a rate of 5% per year.
- Solid catalysts and sorbents are completely changed-out every 4 years.
- The loaded cost of an operator in a chemical plant in North Dakota is $30/h [27].
- A natural gas price of $4.4/GJ was used in utility cost correlations [28].
- The price of electricity was assumed to be the 2019 industrial rate in North Dakota, $0.0619/kWh [29]
- The project hurdle rate (minimum acceptable rate of return) is 20%.
3. Results and Discussion
3.1. Process Design—Sugar Recovery and Conversion
- Areas 01 and 02: Corn stover extraction, TRL 7—the technology has been demonstrated at the pilot scale level by the US DOE National Renewable Energy Laboratory (NREL).
- Area 03: Catalytic reactions to convert corn stover, TRL 2—this design is based on novel research results from a recent study on reaction conditions in a lab-scale batch reactor.
- Areas 04/05: Separation and purification, TRL 3—this design is based on new experimental data related to the solvent extraction steps.
3.2. Process Design—Acid Recovery and Purification
3.3. Capital Cost Estimates
3.4. Operating Cost Estimates
3.5. Revenues
3.6. Overall Profitability
3.7. Sensitivity Analysis
4. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Humbird, D.; Davis, R.; Tao, L.; Kinchin, C.; Hsu, D.; Aden, A.; Shoen, P.; Lukas, J.; Olthof, B.; Worley, M.; et al. Process Design and Economics for Biochemical Conversion of Lignocellulosic Biomass to Ethanol (Report No. TP-5100-62498); National Renewable Energy Laboratory and Harris Group Inc.: Golden, CO, USA, 2011. [Google Scholar] [CrossRef] [Green Version]
- Lloyd, T.A.; Wyman, C.E. Combined sugar yields for dilute sulfuric acid pretreatment of corn stover followed by enzymatic hydrolysis of the remaining solids. Bioresour. Technol. 2005, 96, 1967–1977. [Google Scholar] [CrossRef]
- Hammond, C.; Conrad, S.; Hermans, I. Simple and Scalable Preparation of Highly Active Lewis Acidic Sn-beta. Angew. Chem. Int. Ed. 2012, 51, 11736–11739. [Google Scholar] [CrossRef]
- Holm, M.S.; Saravanamurugan, S.; Taarning, E. Conversion of Sugars to Lactic Acid Derivatives Using Heterogeneous Zeotype Catalysts. Science 2010, 328, 602–605. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Shi, L.; Wang, H.; Miao, G.; Kong, L.; Li, S.; Sun, Y. Efficient production of lactic acid from sugars over Sn-Beta zeolite in water: Catalytic performance and mechanistic insights. Sustain. Energy Fuels 2019, 3, 1163–1171. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Zhou, L.; Gao, B.; Lu, T.; Su, Y.; Xu, J. Production of lactic acid derivatives from sugars over post-synthesized Sn-Beta zeolite promoted by WO3. Food Chem. 2019, 289, 285–291. [Google Scholar] [CrossRef]
- Taarning, E.; Saravanamurugan, S.; Holm, M.S.; Xiong, J.M.; West, R.M.; Christensen, C.H. Zeolite-Catalyzed Isomerization of Triose Sugars [Article]. ChemSusChem 2009, 2, 625–627. [Google Scholar] [CrossRef]
- Xia, M.; Dong, W.J.; Gu, M.Y.; Chang, C.; Shen, Z.; Zhang, Y.L. Synergetic effects of bimetals in modified beta zeolite for lactic acid synthesis from biomass-derived carbohydrates. RSC Adv. 2018, 8, 8965–8975. [Google Scholar] [CrossRef]
- Acharjee, T.C.; Lee, Y.Y. Production of Levulinic Acid from Glucose by Dual Solid-Acid Catalysts. Environ. Prog. Sustain. Energy 2018, 37, 471–480. [Google Scholar] [CrossRef]
- Conn, R.E.; Kolstad, J.J.; Borzelleca, J.F.; Dixler, D.S.; Filer, L.J.; Ladu, B.N.; Pariza, M.W. Safety assessment of polylactide (pla) for use as a food-contact polymer. Food Chem. Toxicol. 1995, 33, 273–283. [Google Scholar] [CrossRef]
- Datta, R.; Henry, M. Lactic acid: Recent advances in products, processes and technologies—A review. J. Chem. Technol. Biotechnol. 2006, 81, 1119–1129. [Google Scholar] [CrossRef]
- Hara, M.; Nakajima, K.; Kamata, K. Recent progress in the development of solid catalysts for biomass conversion into high value-added chemicals. Sci. Technol. Adv. Mater. 2015, 16, 034903. [Google Scholar] [CrossRef]
- Lim, L.T.; Cink, K.; Vanyo, T. Processing of Poly(Lactic Acid). In Poly(Lactic Acid): Synthesis, Structures, Properties, Processing, and Applications; Auras, R., Lim, L.T., Selke, S.E.M., Tsuji, H., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar] [CrossRef]
- Holm, M.S.; Pagan-Torres, Y.J.; Saravanamurugan, S.; Riisager, A.; Dumesic, J.A.; Taarning, E. Sn-Beta catalysed conversion of hemicellulosic sugars. Green Chem. 2012, 14, 702–706. [Google Scholar] [CrossRef]
- Tolborg, S.; Sadaba, I.; Osmundsen, C.M.; Fristrup, P.; Holm, M.S.; Taarning, E. Tin-containing Silicates: Alkali Salts Improve Methyl Lactate Yield from Sugars. Chemsuschem 2015, 8, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Kohler, A.; Seames, W.; Foerster, I.; Kadrmas, C. Catalytic Formation of Lactic and Levulinic Acids from Biomass Derived Monosaccarides through Sn-Beta Formed by Impregnation. Catalysts 2020, 10, 1219. [Google Scholar] [CrossRef]
- Horvarth, I.T.; Mehdi, H.; Fabos, V.; Boda, L.; Mika, L.T. Gamma-Valerolactone—A sustainable liquid for energy and carbon-based chemicals. Green Chem. 2008, 10, 238–242. [Google Scholar] [CrossRef]
- Fegyverneki, D.; Orha, L.; Lang, G.; Horvath, I.T. Gamma-valerolactone-based solvents. Tetrahedron 2010, 66, 1078–1081. [Google Scholar] [CrossRef]
- Gallezot, P. Conversion of biomass to selected chemical products. Chem. Soc. Rev. 2012, 41, 1538–1558. [Google Scholar] [CrossRef]
- Ulrich, G.D.; Vasudevan, P.T. Capital Cost Estimation. In Chemical Engineering—Process Design and Economics, A Practical Guide, 2nd ed.; Process Publishing: Durham, NH, USA, 2004. [Google Scholar]
- Davis, R.; Tao, L.; Scarlata, C.; Tan, E.C.D.; Ross, J.; Lukas, J.; Sexton, D. Process Design and Economics for the Conversion of Lignocellulosic Biomass to Hydrocarbons-Dilute-Acid and Enzymatic Deconstruction of Biomass to Sugars and Catalytic Conversion of Sugars to Hydrocarbons (Report No. TP-5100-62498); National Renewable Energy Laboratory and Harris Group Inc.: Golden, CO, USA, 2015. [Google Scholar] [CrossRef]
- AACE Intl. Cost Estimate Classification System—As Applied in Engineering, Procurement, and Construction for the Process Industries; AACE International Recommended Practice No. 18R-97; AACE Intl: Morgantown, WV, USA, 2005. [Google Scholar]
- North Dakota Office of the Tax Commissioner. Corporate Income Tax. 2019. Available online: https://www.nd.gov/tax/user/businesses/formspublications/corporate-income-tax (accessed on 27 March 2019).
- Popovic, M.; Woodfield, B.F.; Hansen, L.D. Thermodynamics of hydrolysis of cellulose to glucose from 0 to 100 degrees C: Cellulosic biofuel applications and climate change implications. J. Chem. Thermodyn. 2019, 128, 244–250. [Google Scholar] [CrossRef]
- Cheméo—Chemical & Physical Properties by Cheméo. 2016. Available online: www.chemeo.com (accessed on 12 February 2019).
- Sevilla, M.; Fuertes, A.B. Chemical and Structural Properties of Carbonaceous Products Obtained by Hydrothermal Carbonization of Saccharides. Chem. A Eur. J. 2009, 15, 4195–4203. [Google Scholar] [CrossRef]
- Southwest Information Office. Employer Costs for Employee Compensation for the Regions—December 2018. 2018. Available online: https://www.bls.gov/regions/southwest/news-release/employercostsforemployeecompensation_regions.htm (accessed on 20 March 2019).
- Energy Information Administration. North Dakota Natural Gas Industrial Price (Dollars per Thousand Cubic Feet). 2019. Available online: https://www.eia.gov/dnav/ng/hist/n3035nd3m.htm (accessed on 23 March 2019).
- Electricity Local. Wahpeton Electricity Rates. Available online: www.electricitylocal.com/states/minnesota/minnesota-city (accessed on 23 March 2019).
- Urbanchuk, J. Current State of the U.S. Ethanol Industry (Report No. 02-5025); U.S. Department of Energy: Washington, DC, USA, 2010. [CrossRef] [Green Version]
- Jansky, D. Ethanol Facilities Capacity by State and Plant. Nebraska Energy Office. 2018. Available online: https://neo.ne.gov/programs/stats/inf/122.htm (accessed on 31 January 2019).
- Lange, J.P. Renewable Feedstocks: The Problem of Catalyst Deactivation and its Mitigation. Angew. Chem.-Int. Ed. 2015, 54, 13186–13197. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, T.; Blahusiak, M.; Babic, K.; Schuur, B. Reactive extraction and recovery of levulinic acid, formic acid and furfural from aqueous solutions containing sulphuric acid. Sep. Purif. Technol. 2017, 185, 186–195. [Google Scholar] [CrossRef]
- Choudhury, B.; Basha, A.; Swaminathan, T. Study of lactic acid extraction with higher molecular weight aliphatic amines. J. Chem. Technol. Biotechnol. 1998, 72, 111–116. [Google Scholar] [CrossRef]
- Eda, S.; Borra, A.; Parthasarathy, R.; Bankupalli, S.; Bhargava, S.; Thella, P.K. Recovery of levulinic acid by reactive extraction using tri-n-octylamine in methyl isobutyl ketone: Equilibrium and thermodynamic studies and optimization using Taguchi multivariate approach. Sep. Purif. Technol. 2018, 197, 314–324. [Google Scholar] [CrossRef]
- Sprakel LM, J.; Schuur, B. Solvent developments for liquid-liquid extraction of carboxylic acids in perspective. Sep. Purif. Technol. 2019, 211, 935–957. [Google Scholar] [CrossRef]
- Komesu, A.; Maciel, M.R.W.; Maciel, R. Separation and Purification Technologies for Lactic Acid—A Brief Review. Bioresources 2017, 12, 6885–6901. [Google Scholar] [CrossRef]
- Qin, W.; Li, Z.Y.; Dai, Y.Y. Extraction of monocarboxylic acids with trioctylamine: Equilibria and correlation of apparent reactive equilibrium constant. Ind. Eng. Chem. Res. 2003, 42, 6196–6204. [Google Scholar] [CrossRef]
- Wasewar, K.L.; Heesink, A.B.M.; Versteeg, G.F.; Pangarkar, V.G. Reactive extraction of lactic acid using alamine 336 in MIBK: Equilibria and kinetics. J. Biotechnol. 2002, 97, 59–68. [Google Scholar] [CrossRef] [Green Version]
- Solutions, I. Caustic Soda Price History. 2019. Available online: www.intratec.us/chemical-markets/caustic-soda-prices (accessed on 23 March 2019).
- CCM: China’s Market Price of Furfural Bounces Back in April 05-18-2016 4648. CCM—Data and Business Intelligence. 2016. Available online: www.cnchemicals.com/Press/86385-CCM (accessed on 29 March 2019).
- China CN: Market Price: Monthly Avg: Organic Chemical Material: Formic acid 94%. CEIC. 2018. Available online: https://www.ceicdata.com/en/china/china-petroleum--chemical-industry-association-petrochemical-price-organic-chemical-material/cn-market-price-monthly-avg-organic-chemical-material-formic-acid-94> (accessed on 31 January 2019).
- Global Bio-Based Platform Chemical Market—Market Research Report, Cumulative Impact of COVID-19; 360iResearch: Pune, Maharashtra, India, 2020.
- Global Gypsum Market Set for 9.9% Growth. Smithers Apex. 2016. Available online: www.smithersapex.com/news/2016/february/global-gypsum-market-set-for-growth (accessed on 29 March 2019).
- Shao, Y.W.; Sun, K.; Li, Q.Y.; Liu, Q.H.; Zhang, S.; Liu QHu, G.Z.; Hu, X. Copper-based catalysts with tunable acidic and basic sites for the selective conversion of levulinic acid/ester to gamma-valerolactone or 1,4-pentanediol. Green Chem. 2019, 21, 4499–4511. [Google Scholar] [CrossRef]

| Alternative | Sugar | Lactic Acid | Levulinic Acid | Acetic Acid | Formic Acid |
|---|---|---|---|---|---|
| Sn-Beta | Glucose | 11% | 20% | 2.4% | 8.0% |
| Sn-Beta | Xylose | 19% | 1.8% | 2.4% | 7.4% |
| Sn-Beta + CaSO4 | Glucose | 68% | 2.0% | 3.6% | 3.2% |
| Sn-Beta + CaSO4 | Xylose | 50% | 2.5% | 5.2% | 5.4% |
| Diluent | ||
|---|---|---|
| Condition | Isoamyl Alcohol | 1-octanol |
| Organic/Aqueous Ratio | 0.47 | 0.65 |
| Extractant Concentration (wt%) | 13 | 22 |
| Lactic/Levulinic Acid Ratio | 1.3 | 0.69 |
| Lactic Acid KD | 58 | 35 |
| Levulinic Acid KD | 33 | 21 |
| Acetic Acid KD | 29 | 32 |
| Formic Acid KD | 97 | 102 |
| Measure | 1-octanol | Isoamyl |
|---|---|---|
| Average Annual Operating Costs | $10.1 million/year | $10.3 million/year |
| Total Capital Investment | $13.2 million | $18.1 million |
| Net Present Value @ 20% | ($51.5 million) | ($57.6 million) |
| Sn-Beta | Sn-Beta + CaSO4 | |
|---|---|---|
| Lactic Acid KD | 35 | 1.9 |
| Levulinic Acid KD | 21 | 6.4 |
| Acetic Acid KD | 32 | 2.3 |
| Formic Acid KD | 102 | 3.4 |
| Equipment Type | # of Units | PEC ($2019) A | Total BMC ($) B | |
|---|---|---|---|---|
| Process Vessels | 221 | 3,300,000 | 19,000,000 | |
| Heat Exchangers | 51 | 3,100,000 | 11,000,000 | |
| Tanks | 2 | 74,000 | 260,000 | |
| Compressors | 7 | 2,500,000 | 3,800,000 | |
| Filters | 11 | 2,600,000 | 11,000,000 | |
| Conveyors | 36 | 1,200,000 | 2,600,000 | |
| Pumps | 74 | 860,000 | 3,400,000 | |
| Mixers | 23 | 4,900,000 | 10,000,000 | |
| Reactors | 36 | 3,300,000 | 19,000,000 | |
| Total Bare Modular Cost | CTBM C » | 81,000,000 | ||
| Contingency and Fees | CTBM A * 0.18 = | 15,000,000 | ||
| Total Module Cost | CTM D » | 95,000,000 | ||
| Auxiliary Facilities | CAUX E | CTM B * 0.30 = | 29,000,000 | |
| Fixed Capital Investment | FCI F » | 120,000,000 | ||
| Working Capital | CWC G » | FCI D * 0.15 = | 19,000,000 | |
| Initial Charge of Chemicals and Catalysts | 2,100,000 | |||
| Total Capital Investment | TCI H » | 140,000,000 |
| Equipment Type | # of Units | PEC ($2019) | Total BMC ($) | |
|---|---|---|---|---|
| Process Vessels | 227 | 3,900,000 | 23,000,000 | |
| Heat Exchangers | 51 | 3,300,000 | 13,000,000 | |
| Tanks | 2 | 74,000 | 260,000 | |
| Compressors | 7 | 1,400,000 | 2,200,000 | |
| Filters | 11 | 2,600,000 | 11,000,000 | |
| Conveyors | 36 | 1,200,000 | 2,600,000 | |
| Pumps | 74 | 930,000 | 3,700,000 | |
| Mixers | 23 | 5,200,000 | 11,000,000 | |
| Reactors | 36 | 3,300,000 | 19,000,000 | |
| Total Bare Modular Cost | CTBM » | 86,000,000 | ||
| Contingency and Fees | CTBM A * 0.18 = | 15,000,000 | ||
| Total Module Cost | CTM » | 100,000,000 | ||
| Auxiliary Facilities | CAUX | CTM B * 0.30 = | 30,000,000 | |
| Fixed Capital Investment | FCI » | 130,000,000 | ||
| Working Capital | CWC » | FCI D * 0.15 = | 20,000,000 | |
| Initial Charge of Chemicals and Catalysts | 2,400,000 | |||
| Total Capital Investment | TCI » | 150,000,000 |
| Process Alternative | Year | Raw Materials | Chemicals and Catalysts | Operating Labor | Maintenance | Utilities | Waste Disposal | Other Expenses | Total |
|---|---|---|---|---|---|---|---|---|---|
| Base Sn-Beta | 1–20 | 21,000,000 | 4,600,000 | 7,200,000 | 5,100,000 | 29,000,000 | 1,300,000 | 12,000,000 | 79,000,000 |
| Sn-Beta + CaSO4 | 1–20 | 21,000,000 | 4,900,000 | 7,200,000 | 5,200,000 | 29,000,000 | 1,200,000 | 12,000,000 | 80,000,000 |
| Base Sn-Beta Alternative | Sn-Beta +CaSO4 Alternative | |||
|---|---|---|---|---|
| Utility Description | Annual Cost ($/year) | Requirement | Annual Cost ($/year) | Requirement |
| Electricity | 5,600,000 | Amount: 90 million kWh/year | 3,600,000 | Amount: 59 million kWh/year |
| Cooling Water | 660,000 | Amount: 3.4 billion kg/year Temperature: 30 °C Pressure: 300 kPa | 710,000 | Amount: 4.4 billion kg/year Temperature: 30 °C Pressure: 300 kPa |
| Boiler Feed Water | 6,700,000 | Amount: 1.8 billion kg/year Temperature: 30 °C Pressure: 450 kPa | 7,600,000 | Amount: 2.0 billion kg/year Temperature: 30 °C Pressure: 450 kPa |
| Medium-High Pressure Steam | 9,100,000 | Amount: 480 million kg/year Temperature: 239 °C Pressure: 3300 kPa | 3,400,000 | Amount: 160 million kg/year Temperature: 239 °C Pressure: 3300 kPa |
| High Pressure Steam | 6,400,000 | Amount: 320 million kg/year Temperature: 400 °C Pressure: 4500 kPa | 14,000,000 | Amount: 730 million kg/year Temperature: 400 °C Pressure: 4500 kPa |
| Total | 29 million/year | 29 million/year | ||
| Base Sn-Beta Alternative | Sn-Beta + CaSO4 Alternative | ||||
|---|---|---|---|---|---|
| Products | $/kg | Amount (kg/year) | Revenue ($/year) | Amount (kg/year) | Revenue ($/year) |
| Lactic Acid | 1.48 | 18,000,000 | 26,000,000 | 78,000,000 | 150,000,000 |
| Levulinic Acid | 5.00 | 15,000,000 | 75,000,000 | 3,400,000 | 17,000,000 |
| Acetic Acid | 0.67 | 3,000,000 | 2,100,000 | 9,600,000 | 6,600,000 |
| Formic Acid | 0.68 | 15,000,000 | 10,000,000 | 9,800,000 | 6,700,000 |
| Furfural | 1.65 | 1,600,000 | 2,600,000 | 1,600,000 | 2,600,000 |
| Gypsum | 0.04 | 2,100,000 | USD 75,000 | -- | -- |
| Saturated Water Credit | -- | -- | 3,100,000 | -- | 3,500,000 |
| Lignin Energy Credit | -- | -- | 4,200,000 | 4,200,000 | |
| TOTALS | USD 123,000,000 | 191,000,000 | |||
| Year | Revenues | Operating Costs | Gross Profit | Depreciation | Federal Taxable Profit | Federal Income Tax | State Taxable Profit | State Income Tax | Nontaxable Charges | Net Profit | Present Value @ 20% |
|---|---|---|---|---|---|---|---|---|---|---|---|
| −2 | (41) | (41) | (59) | ||||||||
| −1 | (41) | (41) | (50) | ||||||||
| 0 | (62) | (62) | (62) | ||||||||
| 1 | 120 | (79) | 44 | 15 | 29 | (6.1) | 23 | (1.0) | 37 | 31 | |
| 2 | 120 | (79) | 44 | 13 | 32 | (6.6) | 25 | (1.1) | 37 | 25 | |
| 3 | 120 | (79) | 44 | 11 | 33 | (6.9) | 26 | (1.1) | 36 | 21 | |
| 4 | 120 | (79) | 44 | 10 | 34 | (7.2) | 27 | (1.2) | 36 | 17 | |
| 5 | 120 | (80) | 43 | 8.8 | 34 | (7.1) | 27 | (1.2) | 35 | 14 | |
| 6 | 120 | (79) | 44 | 7.7 | 37 | (7.7) | 29 | (1.2) | 35 | 12 | |
| 7 | 120 | (79) | 44 | 6.8 | 37 | (7.9) | 30 | (1.3) | 35 | 10 | |
| 8 | 120 | (79) | 44 | 6.0 | 38 | (8.0) | 30 | (1.3) | 35 | 8.0 | |
| 9 | 120 | (80) | 43 | 5.3 | 37 | (7.9) | 30 | (1.3) | 34 | 7.0 | |
| 10 | 120 | (79) | 44 | 5.0 | 39 | (8.2) | 31 | (1.3) | 35 | 5.6 | |
| 11 | 120 | (79) | 44 | 5.0 | 39 | (8.2) | 31 | (1.3) | 35 | 4.7 | |
| 12 | 120 | (79) | 44 | 5.0 | 39 | (8.2) | 31 | (1.3) | 35 | 3.9 | |
| 13 | 120 | (80) | 43 | 5.0 | 38 | (7.9) | 30 | (1.3) | 34 | 3.1 | |
| 14 | 120 | (79) | 44 | 5.0 | 39 | (8.2) | 31 | (1.3) | 35 | 2.7 | |
| 15 | 120 | (79) | 44 | 5.0 | 39 | (8.2) | 31 | (1.3) | 35 | 2.3 | |
| 16 | 120 | (79) | 44 | 5.0 | 39 | (8.2) | 31 | (1.3) | 35 | 1.9 | |
| 17 | 120 | (80) | 43 | 5.0 | 38 | (7.9) | 30 | (1.3) | 34 | 1.5 | |
| 18 | 120 | (79) | 44 | 44 | (9.3) | 35 | (1.5) | 34 | 1.3 | ||
| 19 | 120 | (79) | 44 | 44 | (9.3) | 35 | (1.5) | 34 | 1.0 | ||
| 20 | 120 | (79) | 44 | 44 | (9.3) | 35 | (1.5) | 19 | 52 | 1.4 | |
| Note: Numbers in parentheses represent negative values | NPV@20% | 3.3 | |||||||||
| DCFROR | 20% | ||||||||||
| Year | Revenues | Operating Costs | Gross Profit | Depreciation | Federal Taxable Profit | Federal Income Tax | State Taxable Profit | State Income Tax | Nontaxable Charges | Net Profit | Present Value @ 20% |
|---|---|---|---|---|---|---|---|---|---|---|---|
| −2 | (44) | (44) | (63) | ||||||||
| −1 | (44) | (44) | (53) | ||||||||
| 0 | (66) | (66) | (66) | ||||||||
| 1 | 160 | (80) | 75 | 16 | 59 | (12) | 46 | (2.0) | 61 | 51 | |
| 2 | 160 | (80) | 75 | 14 | 61 | (13) | 49 | (2.1) | 60 | 42 | |
| 3 | 160 | (80) | 75 | 12 | 63 | (13) | 50 | (2.1) | 60 | 34 | |
| 4 | 160 | (80) | 75 | 11 | 64 | (14) | 51 | (2.2) | 59 | 29 | |
| 5 | 160 | (82) | 74 | 9.3 | 64 | (13) | 51 | (2.2) | 58 | 23 | |
| 6 | 160 | (80) | 75 | 8.2 | 67 | (14) | 53 | (2.3) | 59 | 20 | |
| 7 | 160 | (80) | 75 | 7.2 | 68 | (14) | 54 | (2.3) | 58 | 16 | |
| 8 | 160 | (80) | 75 | 6.4 | 69 | (14) | 54 | (2.3) | 58 | 14 | |
| 9 | 160 | (82) | 74 | 5.6 | 68 | (14) | 54 | (2.3) | 57 | 11 | |
| 10 | 160 | (80) | 75 | 5.3 | 70 | (15) | 55 | (2.4) | 58 | 9.4 | |
| 11 | 160 | (80) | 75 | 5.3 | 70 | (15) | 55 | (2.4) | 58 | 7.8 | |
| 12 | 160 | (80) | 75 | 5.3 | 70 | (15) | 55 | (2.4) | 58 | 6.5 | |
| 13 | 160 | (82) | 74 | 5.3 | 68 | (14) | 54 | (2.3) | 57 | 5.3 | |
| 14 | 160 | (80) | 75 | 5.3 | 70 | (15) | 55 | (2.4) | 58 | 4.5 | |
| 15 | 160 | (80) | 75 | 5.3 | 70 | (15) | 55 | (2.4) | 58 | 3.8 | |
| 16 | 160 | (80) | 75 | 5.3 | 70 | (15) | 55 | (2.4) | 58 | 3.1 | |
| 17 | 160 | (82) | 74 | 5.3 | 68 | (14) | 54 | (2.3) | 57 | 2.6 | |
| 18 | 160 | (80) | 75 | 75 | (16) | 59 | (2.6) | 57 | 2.1 | ||
| 19 | 160 | (80) | 75 | 75 | (16) | 59 | (2.6) | 57 | 1.8 | ||
| 20 | 160 | (80) | 75 | 75 | (16) | 59 | (2.6) | 20 | 76 | 2.0 | |
| Note: Numbers in parentheses represent negative values | NPV@20% | 110 | |||||||||
| DCFROR | 30% | ||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kohler, A.; Seames, W.; Shaeffer, C.; Bjerke, C.; Dahl, J. Techno-Economic Analysis of a Process for the Aqueous Conversion of Corn Stover into Lactic and Levulinic Acid through Sn-Beta Catalysis. Processes 2021, 9, 436. https://doi.org/10.3390/pr9030436
Kohler A, Seames W, Shaeffer C, Bjerke C, Dahl J. Techno-Economic Analysis of a Process for the Aqueous Conversion of Corn Stover into Lactic and Levulinic Acid through Sn-Beta Catalysis. Processes. 2021; 9(3):436. https://doi.org/10.3390/pr9030436
Chicago/Turabian StyleKohler, Andrew, Wayne Seames, Cassandra Shaeffer, Cara Bjerke, and Jacob Dahl. 2021. "Techno-Economic Analysis of a Process for the Aqueous Conversion of Corn Stover into Lactic and Levulinic Acid through Sn-Beta Catalysis" Processes 9, no. 3: 436. https://doi.org/10.3390/pr9030436








