Chitosan-Coating Effect on the Characteristics of Liposomes: A Focus on Bioactive Compounds and Essential Oils: A Review
Abstract
:1. Introduction
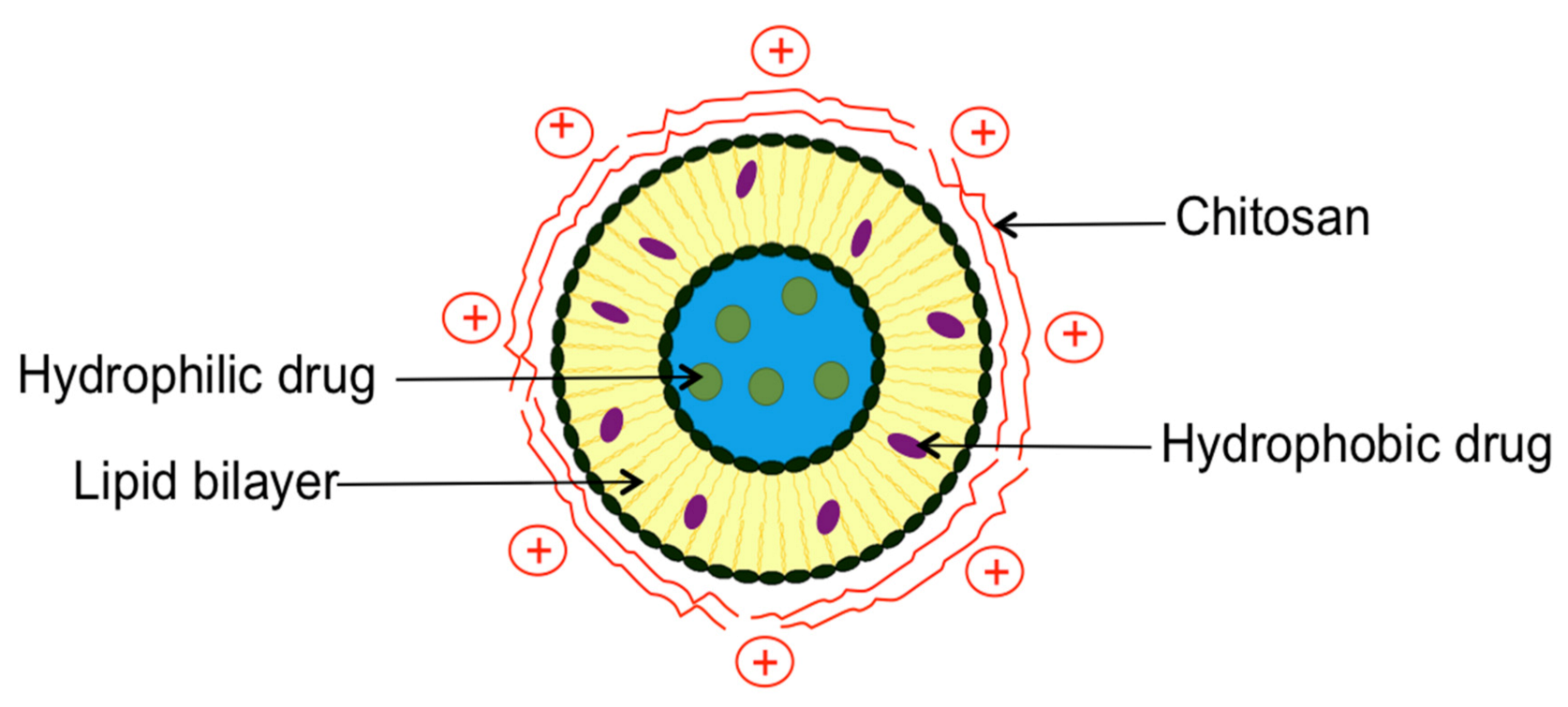
2. Chitosomes Preparation Method
3. Comparison between Conventional Liposomes and Chitosomes
3.1. Particles Size
3.2. Zeta Potential
3.3. Homogeneity
3.4. Morphology
3.5. Encapsulation Efficiency
| Natural Molecules | Liposomes a. Composition b. Preparation Method | Chitosan or Chitosan Derivative a. Concentration (% w/v) b. MW (kDa) c. DD (%) d. Chitosan Type | Size (nm) | Zeta Potential (mV) | pdI | EE | Ref | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CH-LP | UN-LP | CH-LP | UN-LP | CH-LP | UN-LP | CH-LP | UN-LP | ||||
| Artemisia afra | a. DSPC:DSPE 94:6 b. REV+sonication | a. 0.15 b. LMW c. NR d. Chitosan | 1079 | 8269 | 22.8 | 9.0 | 0.798 | 0.429 | NR | 18.7 | [36] |
| Berberine hydrochloride | a. EPC:CHO:DHP 1:0.242:0.036 * b. TFH+sonication | a. 0.1; 0.3 b. NR c. >90 d. Chitosan | 194–264 | 142 | 24.1–29.3 | −26.8 | 0.34–0.53 | 0.27 | 78.4–81.6 | 83.2 | [5] |
| Black mulberry extract | a. Lecithin (2% w/v) b. Homogenization | a. 0.4 b. NR c. 80 d. Chitosan | 473 | 173 | 41.8 | −32.4 | NR | NR | NR | NR | [65] |
| Carotenoids: lutein; β-carotene; lycopene; canthaxanthin | a. EPC:Tween-80 NR b. TFH+ sonication | a. 0.05; 0.1; 0.15 b. 200 c. 85 d. Chitosan | 78–83 76–78 72–75 125–130 | 77 75 70 120 | 9.3–20 9.3–20 9.3–20 9.3–20 | −5.3 −5.3 −5.3 −5.3 | 0.17–0.22 0.2–0.25 0.23–0.25 0.3–0.32 | 0.15 0.18 0.22 0.32 | 87–92 86–88 76–85 59–65 | 87 86 75 58 | [44] |
| Coenzyme Q10 | a. SPC:CHO 83:17 * b. EIM+ sonication | a. 0.1; 0.2; 0.5; 1 b. 100; 450 c. >85 d. N-trimethyl chitosan | 193–331 245–354 | 136 | 4.1–24.1 8.1–25.1 | −8.7 | NR | NR | 98 | 98 | [28] |
| Curcumin | a. EPC:CHO 2:1 b. EIM | a. 0.1–0.5 b. 30 c. 88 d. Chitosan | 123–204 | 101.4 | 30.1–32.1 | −14.1 | 0.185–0.216 | 0.247 | 64.93 | 54.7 | [43] |
| a. EPC:CHO 2:1 b. TFH+ sonication | a. 0.1–0.5 b. 30 c. 88 d. Chitosan | 104–192 | 115.7 | 30.4–43 | −20.1 | 0.218–0.281 | 0.388 | NR | 42.6 | ||
| a. PC:CHO 5:1 * b. EIM | a. 1 b. 28 c. 89 d. Chitosan | 332.7 | 93.2 | 67.1 | −24.3 | NR | NR | 52.08 | 41.42 | [66] | |
| a. EPC:DHP:CHO NR b. NR | a. 0.0025 b. NR c. 80 d. N-dodecyl chitosan | 140.3 | 51.7 | 31.6 | −39 | NR | NR | NR | NR | [26] | |
| a. 0.0025 b. NR c. 80 d. HPTMA-chitosan | 76.3 | 51.7 | 32 | −39 | NR | NR | NR | NR | |||
| a. 0.0025 b. NR c. 80 d. N-dodecyl chitosan-HPTMA | 73.1 | 51.7 | 32.3 | −39 | NR | NR | NR | NR | |||
| a. EPC: Phosphatidic acid 6.5:3.5 b. REV | a. 0.1 b. NR c. NR d. Chitosan | 300 | 129 | 33 | −49 | 0.1 | 0.095 | NR | NR | [67] | |
| a. SPC:CHO 20:2 * b. TFH+ extrusion | a. 0.5 b. 200 c. >85 d. N-trimethyl chitosan | 657.7 | 221.4 | 15.6 | −9.6 | 0.37 | 0.198 | 92.6 | 86.6 | [27] | |
| Eucalyptus globulus | a. DSPC:DSPE 94:6 b. REV+sonication | a. 0.15 b. LMW c. NR d. Chitosan | 885 | 9914 | 13.0 | −14.9 | 0.678 | 0.585 | NR | 69.2 | [36] |
| Grape seed extract | a. Lipoid S75 1% * b. High-pressure homogenizer | a. 0.1 b. NR c. 79 d. Chitosan | 173 | 84 | 63 | −49 | 0.4 | 0.3 | 86.6 | 85.4 | [68] |
| a. 1 b. NR c. 79 d. Chitosan | 160.3 | 86.5 | 64.9 | −42.5 | NR | NR | 99.5 | 88.2 | [62] | ||
| Melaleuca alternifolia | a. DSPC:DSPE 94:6 b. REV+sonication | a. 0.15 b. LMW c. NR d. Chitosan | 5781 | 9280 | 30.0 | 1.4 | 0.845 | 0.491 | NR | 41.7 | [36] |
| Resveratrol | a. EPC 2%(w/v) b. TFH+ sonication | a. 0.1; 0.3; 0.5 b. NR c. NR d. Chitosan | 279.8–558.3 | 212.8 | 26.3–39.2 | −9.4 | NR | NR | 81.3 | 83.9 | [42] |
| Rosmarinic acid esters | a. Lecithin 1%(w/v) b. Homogenization | a. 0.2 b. 205 c. 91.8 d. Chitosan | 205.1 | 87.8 | 66.3 | −37.8 | NR | NR | NR | NR | [69] |
| Vitamin E | a. PC:CHO 20:80; 40:60; 60:40; 80:20 b. Sonication | a. 0.1 b. 4 c. > 90 d. Chitosan | 144–531 | 133–357 | 53.5 | −29.5 | NR | NR | 55.4–99.8 | NR | [70] |
| Antimicrobials | Liposomes a. Composition b. Preparation Method | Chitosan or Chitosan Derivative a. Concentration (% w/v) b. MW (kDa) c. DD (%) d. Chitosan Type | Size (nm) | Zeta Potential (mV) | pdI | EE | Ref | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CH-LP | UN-LP | CH-LP | UN-LP | CH-LP | UN-LP | CH-LP | UN-LP | ||||
| Clotrimazole | a. Lipoid S100 200 mg b. TFH+ sonication | a. 0.1; 0.3; 0.6 b. NR c. 92 d. Chitosan | 135–190 | 107 | 25.9–43.8 | −1.6 | 0.27–0.29 | 0.34 | NR | 16.5 | [17] |
| Dicloxacillin | a. Lipoid S100: CHO:Tween-80 0.9:0.3:0.1 b. TFH+sonication | a. 1 b. NR c. NR d. Chitosan | 263.4 | 178.5 | 15.7 | −12.7 | 0.411 | 0.247 | 62 | 38 | [60] |
| Nisin Nisin silica | a. Lecithin:CHO 20:4 * b. TFH+ Homogenization | a. 0.1 b. NR c. NR d. Chitosan | 134 149 | NR | −42 −44 | NR | 0.27 0.283 | NR | 60 72 | NR | [71] |
| Triazavirin | a. SPC:CHO 85:15 * b. TFH+ extrusion | a. 0.275 b. 190 c. 95 d. Pelargonic chitosan | 188 | 147 | 20.4 | 30.2 | 0.16 | 0.13 | NR | 77.9 | [29] |
| a. 0.275 b. 190 c. 95 d. Lauric chitosan | 192 | 147 | 18.9 | 30.2 | 0.18 | 0.13 | NR | 77.9 | |||
| Vancomycin hydrochloride | a. Lecithin:CHO 32.5:5 * b. REV | a. 0.4 b. NR c. NR d. Chitosan | 220.4 | NR | 25.7 | NR | 0.21 | NR | 32.6 | 40 | [56] |
| Drugs | Liposomes a. Composition b. Preparation Method | Chitosan or Chitosan Derivative a. Concentration (% w/v) b. MW (kDa) c. DD (%) d. Chitosan Type | Size (nm) | Zeta Potential (mV) | pdI | EE | Ref | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CH-LP | UN-LP | CH-LP | UN-LP | CH-LP | UN-LP | CH-LP | UN-LP | ||||
| Atenolol | a. Lipoid S100 20 mg b. EIM | a. 0.1; 0.6 b. NR c. NR d. Chitosan | 240 –250 | 89 | 27 | −20 | NR | 0.223 | 24.6–25.7 | 21.6 | [63] |
| Butyric acid | a. PC:CHO 20:5 * b. TFH+ sonication | a. 0.1 b. NR c. NR d. Chitosan | 132.2 | 92.1 | 15.3 | −9.3 | 0.22 | 0.18 | NR | NR | [72] |
| Cyclosporin A | a. EPC:CHO: Pluronic F 127 28:5:11 b. TFH+ extrusion | a. 0.4 b. 200 c. 90 d. Chitosan | 207.8 | 165.2 | 41.7 | −7.6 | 0.187 | 0.132 | 82 | 85.1 | [59] |
| Diclofenac sodium | a. HSPC:PS:CHO 3:0.1:1 b. EIM | a. 0.1; 0.25; 0.5 b. 540 c. 97 d. Chitosan | 82.4–392.3 | 69 | −0.7–10.1 | −26.1 | NR | NR | 99.6–100 | 99.6 | [12] |
| Docetaxel | a. Lipoid S100: CHO:Tween-80: SDC:DCP 0.9:0.3:0.1:0.1:0.1 b. TFH+sonication | a. 1 b. NR c. NR d. Chitosan | 328.6 | 238 | 9.6 | −5.5 | 0.581 | 0.413 | 76.5 | 58.7 | [55] |
| Doxorubicin | a. HSPC:CHO NR b. TFH+ extrusion | a. NR b. NR c. NR d. Glycol chitosan | 142.7 | 123.7 | −14.3–9.1 | −25 | 0.068 | 0.04 | 90 | 90 | [23] |
| Epirubicin | a. PC:CHO 50:15 * b. TFH+ sonication | a. 12.5; 33; 75; 200 mg b. 80 c. 92 d. Chitosan | 180–262 | 148 | 21.1–25.4 | −4.7 | NR | NR | NR | NR | [47] |
| Fexofenadine | a. DPPC:DPPG: CHO 8:1:2.25 b. TFH+ extrusion | a. 0.1 b. NR c. NR d. Chitosan | 716 | 359 | 11.8 | −110 | 0.1 | <0.1 | 66.1 | 65.9 | [73] |
| Flurbiprofen | a. EPC:solutol HS15 7.5:1 b. Modified EIM | a. 0.05; 0.1; 0.2 b. 50 c. 95 d. Chitosan | 123.3–213.9 | 107.7 | 8.4–28.6 | −22.9 | NR | NR | 90.2–92.5 | 85.5 | [61] |
| Furosemide | a. SPC:CHO 10:1 b. TFH+ sonication | a. 0.5 b. NR c. NR d. Chitosan | 115.4 | 49.8 | 32.4 | −13.5 | NR | NR | 71.1 | 42.6 | [4] |
| Lidocaine | a. Lecithin:SDC 15% * b. TFH+ sonication+ extrusion | a. 0.1–0.5 b. 150 c. 90 d. Chitosan | 202–468.6 | 178.6 | −12.2–46.6 | −30.3 | 0.19–0.94 | 0.26 | 42.7–80.2 | 82.3 | [48] |
| Mitoxantrone | a. SPC:CHO 5:1 b. TFH+ sonication | a. 0.1; 0.3; 0.6; 1.2 b. 70 c. 92 d. Chitosan | 120–154 | 115 | 20–35 | −30.4 | NR | NR | 93.5–96.5 | 97.4 | [35] |
| Paclitaxel | a. Lecithin:CHO: SA:polyacrylic acid 1.225:0.575:0.1 * b. TFH+sonication | a. 0.1 b. 50 c. NR d. Chitosan | 215 | 152 | 27.9 | −37.6 | NR | NR | 70.9 | 77.1 | [74] |
| Prednisolone | a. SPC:CHO 6:3 * b. TFH+ sonication | a. 2 b. NR c. NR d. Chitosan | 235.8 | 99.9 | 35.3 | −33.1 | NR | NR | 92.8 | 94.2 | [58] |
| Macromolecules/Active Peptides | Liposomes a. Composition b. Preparation Method | Chitosan or Chitosan Derivative a. Concentration (% w/v) b. MW (kDa) c. DD (%) d. Chitosan Type | Size (nm) | Zeta Potential (mV) | pdI | EE | Ref | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CH-LP | UN-LP | CH-LP | UN-LP | CH-LP | UN-LP | CH-LP | UN-LP | ||||
| Anti-sense oligodeoxynucleotides | a. SPC:CHO 20:5 * b. TFH+sonication | a. 0.05–1 b. 100 c. 90 d. Chitosan | 65.6–95.2 | 55.7 | 3.8–17.2 | −11.7 | 0.181–0.701 | 0.426 | NR | NR | [53] |
| Bovine serum albumin (BSA) | a. EPC:sodium oleate 10:2 b. TFH+ sonication | a. 1.25–20 mM b. 276 c. 94 d. TMBz-chitosan | 108.2 –128 | 107.6 | −23.3–5.3 | −27.1 | 0.29–0.47 | 0.32 | NR | 50.1 | [25] |
| Calcitonin | a. DPPC:DPPE-MCC 3:0.3 b. TFH+ extrusion | a. 0.2 b. 150 c. NR d. TGA-chitosan | 709.2 | 174.8 | 43.5 | −39.8 | 0.34 | 0.19 | NR | NR | [30] |
| a. 0.2 b. 150 c. NR d. TGA-MNA-chitosan | 604.8 | 174.8 | 27.9 | −39.8 | 0.91 | 0.19 | NR | NR | |||
| DNA | a. Phospholipon 80:DCP:CHO 5:1:4 b. REV | a. 0.1 b. 300 c. 87 d. Chitosan | 346–554 | 441 | 22 | −52 | 0.19–0.37 | 0.47 | 84–87 | NR | [39] |
| Extracellular proteins | a. Lecithin 100 mg b. EIM | a. 0.3 b. NR c. NR d. Chitosan | 1200 | 500 | NR | NR | NR | NR | 70 | 80 | [57] |
| Insulin | a. Lecithin:CHO 4:1 b. REV | a. 0.1–0.5 b. 65; 140; 680; 1000 c. 90 d. Chitosan | 199.7–206.2 | 168.6 | 4.9–6.7 | −2.9 | 0.132–0.246 | 0.143 | 69.7–75.9 | 81.6 | [38] |
| Leuprolide | a. Epikuron 170: CHO 6:1 * Epikuron 200: CHO 6:1 * a. TFH+sonication | a. 0.1; 0.2; 0.5; 1 b. 1000 c. >90 d. Chitosan | 60–140 75–120 | 15 54 | 10–40 5–20 | −29.6 5 | NR | NR | 62.4 49.1 | 73.1 58.5 | [40] |
| Salmon protein hydrolysates | a. MFGM Phosphlac 700 3;5;10%(w/v) b. Heating+ Homogenization | a. 0.2–0.6 b. NR c. NR d. Chitosan | 200 | 100 | 40 | −55 | 0.2–0.7 | <0.19 | 40–70 | NR | [49] |
| siRNA-H1F1-α siRNA-VEGF | a. HSPC:DCP: CHO 1:0.1:1 b. TFH+ sonication | a.1 b. 75 c. 75–85 d. Chitosan | 641.7 609.4 | 167 159.3 | 24.1 27 | −23.1 −24.1 | NR | NR | NR | NR | [75] |
| Substance P | a. Lecithin:CHO 20:3.3 * b. TFH+ sonication | a. 0.1 b. NR c. 88 d. Chitosan | 243 | 151 | 32 | −49 | 0.3 | 0.2 | 66 | 81.3 | [34] |
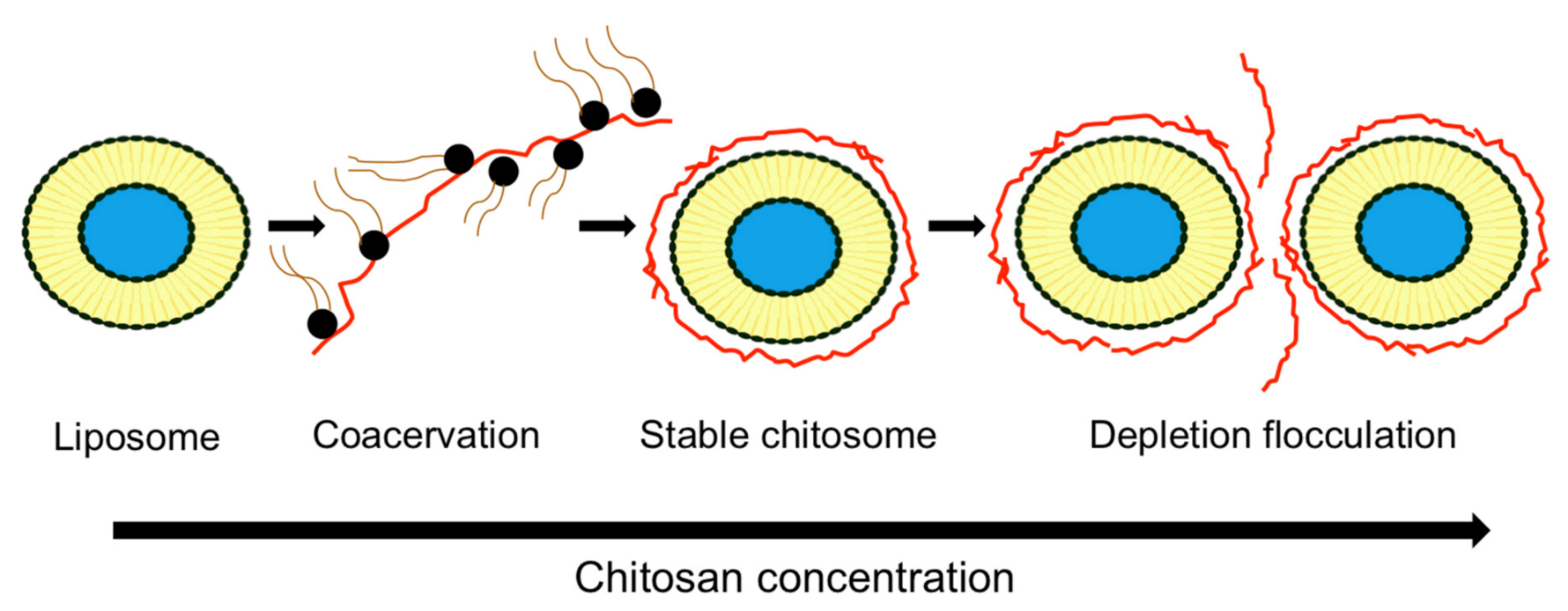
3.6. Chitosomes Stability
3.6.1. Physical Stability: Mechanism Controlled By Chitosan Concentration
3.6.2. Mechanism Controlled by Medium Composition
| Drug/EO | Liposomes a. Composition b. Preparation Method | Chitosan or Chitosan Derivative a. Concentration (% w/v) b. MW (kDa) c. DD (%) d. Chitosan Type | Storage Conditions a. Temperature b. Time c. Medium | Stability: Chitosomes and Liposomes | Ref |
|---|---|---|---|---|---|
| Berberine hydrochloride | a. EPC:CHO:DHP 1:0.242:0.036 * b. TFH + sonication | a. 0.1; 0.3 b. NR c. >90 d. Chitosan | a. 4; 25 °C b. 30 days c. Aqueous suspension | - Chitosomes displayed better stability at 4, and 25 °C since the changes in size, zeta potential and drug EE were less than those in uncoated ones - The changes in size, Zeta potential and leakage ratio at 25 °C for both systems were higher than that at 4 °C - 0.3% chitosomes were more stable than 0.1% chitosomes due to the thicker coating layer. | [5] |
| a. 37 °C b. 24 h c. SGF (pH 1.2); SIF (pH 7.4) | - The changes in size and zeta potential of coated liposomes were less than those for uncoated ones in SGF - In SIF, uncoated liposome size increased by 1.6-fold, while that of chitosomes increased by 6.2- and 4.2-fold for 0.1 and 0.3% chitosan, respectively | ||||
| Black mulberry extract | a. Lecithin 2% (w/v) b. Homogenization | a. 0.4 b. NR c. 80. d. Chitosan | a. 37 °C b. 2 h c. SGF (pH 2) | - The percentage recovery of anthocyanins in chitosomes (3.7%) was higher than that in liposomes (2.1%) after incubation in SGF | [65] |
| BSA | a. EPC:sodium oleate 10:2 b. TFH + sonication | a. 20 mM b. 276 c. 94 d. TMBz-chitosan | a. 37 °C b. 60 min c. SIF (pH 7.5) | - TMBz chitosan-coated liposomes protected BSA from pancreatin degradation in SIF more than conventional liposomes due to the interaction between positively charged derivative and negatively charged BSA | [25] |
| Calcitonin | a. DSPC:CHO: DCP 8:1:2 b. TFH + extrusion | a. 0.6 b. 150; 22 c. >85; 80 d. Chitosan | a. 37 °C b. 60 min c. Tris-HCl buffer (pH 8) | - LMW chitosomes had more efficiency to protect calcitonin from trypsin degradation than HMW chitosomes | [54] |
| Carotenoids: lutein; β-carotene; lycopene; canthaxanthin | a. EPC:Tween-80 NR b. TFH + sonication | a. 0.05; 0.1; 0.15 b. 200 c. 85 d. Chitosan | a. 37; 65; 90 °C b. 390 min c. Aqueous suspension | - Chitosan coating increased carotenoid retention rates in liposomes by 8–15% after coating - When heating at 37 and 65 °C, the retaining capacity of liposomes showed chitosan concentration dependency. The higher the chitosan concentration was, the stronger the thermal resistance of chitosomes - Whatever the heating conditions were, liposomes exhibited the strongest retaining ability to lutein, followed by β-carotene, lycopene and canthaxanthin | [44] |
| Chrysanthemum sp. | a. Lecithin:CHO 5:1 b. TFH + extrusion | a. 0.025; 0.05; 0.075; 0.1 mg/mL b. NR c. NR d. Chitosan | a. 4; 12; 25; 37 °C b. 15 days c. NR | - MDA content was lower in case of EO chitosomes compared to EO loaded liposomes | [78] |
| Curcumin | a. EPC:CHO 2:1 b. EIM and TFH + sonication | a. 0.1 b. 30 c. 88 d. Chitosan | a. 4; 25 °C b. 40 days c. Aqueous suspension | - Both systems prepared either by EIM or TFH were stable at both temperatures since no changes in mean size and pdI values were observed after 40 days | [43] |
| a. PC:CHO 5:1 * b. EIM | a. 1 b. 28 c. 89 d. Chitosan | a. 4; 25; 30; 40; 50; 60; 70; 80; 90 °C b. 40 days c. Aqueous suspension | - 90.68% of curcumin remained encapsulated in chitosomes compared to 26.03% in liposomes after 40 days at 4 °C - The remaining percentage of curcumin decreased to 75.77% when chitosomes were stored at 25 °C - Chitosomes showed the highest remaining percentage of curcumin at various temperatures tested up to 90 °C compared to liposomes and free curcumin | [66] | |
| a. EPC:DHP:CHO NR b. NR | a. 0.0025 b. NR c. 80 d. Chitosan; N-dodecyl chitosan; HPTMA-chitosan; N-dodecyl chitosan-HPTMA | a. NR b. NR c. Triton X100 pH 7.4 | - Alkyl anchors (N-dodecyl chitosan; N-dodecyl chitosan-HPTMA chloride) showed better stability compared to native chitosan and uncoated liposomes since the disruption process by triton X100 was slowed down considerably in the presence of these polymers on liposomes | [26] | |
| Diclofenac sodium | a. HSPC:PS:CHO 3:0.1:1 b. EIM | a. 0.25; 0.5 b. 540 c. 97 d. Chitosan | a. 4; 25 °C b. 30 days c. Aqueous suspension | - Both chitosomes and liposomes were stable at 4 °C without significant changes in their size, Zeta potential and EE - At 25 °C, chitosomes with both concentrations used showed better stability than liposomes in terms of size and EE | [12] |
| DNA | a. Phospholipon 80:DCP:CHO 5:1:4 b. REV | a. 0.1 b. 300 c. 87 d. Chitosan | a. 37 °C b. 1, 2 h c. SGF (pH 1.2); SIF (pH 6.8) | - Chitosomes protected the DNA from the endonuclease digestion after incubation in both SGF and SIF. However, conventional liposomes were less protective in SIF | [39] |
| Epirubicin | a. PC:CHO 50:15 * b. TFH + sonication | a. 12.5; 33; 75; 200 mg b. 80 c. 92 d. Chitosan | a. 4; 25; 37 °C b. 30 days c. Aqueous suspension | - Chitosomes showed better stability after 30 days of storage at 4 and 25 °C with no significant changes in size, contrary to conventional liposomes - Both systems were unstable at 37 °C with a significant increase in vesicle size | [47] |
| Extracellular proteins | a. Lecithin 100 mg b. EIM | a. 0.3 b. NR c. NR d. Chitosan | a. 4 °C b. 60 days c. Aqueous suspension | - Chitosomes improved liposome stability since more than 70% and 50% of extracellular proteins remained encapsulated after 2 months at 4 °C in coated and noncoated liposomes, respectively | [57] |
| Fexofenadine | a. DPPC:DPPG: CHO 8:1:2.25 b. TFH+ extrusion | a. 0.1 b. NR c. NR d. Chitosan | a. 4; 25 °C b. 180 days c. Freeze-dried powder | - Under different tested conditions, drug leakage was lower than 10%, and the size change was minimal for both systems | [73] |
| Glucose | a. DPPC 0.27 M b. ISCRPE | a. 0.005 b. NR c. 70–90 d. Chitosan | a. 25 °C b. 30 days c. Aqueous suspension | - Both systems were stable since the loss in glucose EE from chitosomes over time was almost similar to that in liposomes | [76] |
| Grape seed extract | a. Lipoid S75 1% (w/w) b. High-pressure homogenizer | a. 0.1 b. NR c. 79 d. Chitosan | a. 25 °C b. 98 days c. Aqueous suspension | - Both systems were stable since no significant changes in size and zeta potential were observed | [68] |
| a. 1 b. NR c. 79 d. Chitosan | a. 25 °C b. 15 days c. Aqueous suspension | - Both systems were stable since no significant changes in size and zeta potential were observed | [62] | ||
| Insulin | a. Lecithin:CHO 4:1 b. REV | a. 0.1–0.5 b. 65; 140; 680; 1000 c. 90 d. Chitosan | a. 37 °C b. NR c. Tris-HCl buffered saline (pH 2 and 7.4) | - Chitosan coating reduced peptic and tryptic digestion of insulin compared to uncoated liposomes - This protective action in chitosomes was enhanced by the increase in chitosan MW and concentration | [38] |
| Lidocaine | a. Lecithin:SDC15% * b. TFH + sonication+ extrusion | a. 0.3; 0.5 b. 150 c. 90 d. Chitosan | a. 4; 25 °C b. 90 days c. Aqueous suspension | - Elastic chitosomes with both chitosan concentrations used were more stable than uncoated ones for 3 months at 4 and 25 °C, where a slow increase in size and drug leakage ratio were observed - No significant difference in size and drug leakage ratio between elastic liposomes coated with 0.3 and 0.5% chitosan after 3 months; - A better stability was obtained at 4 °C since the changes in size and leakage ratio were less than those obtained at 25 °C | [48] |
| Mitoxantrone | a. SPC:CHO 5:1 b. TFH + sonication | a. 0.1; 0.3; 0.6; 1.2 b. 70 c. 92 d. Chitosan | a.–70 °C b. NR c. FD-RH | - Uncoated liposomes showed higher size after FD-RH compared to chitosomes, indicating the protective effect of chitosan-coating during FD-RH - As chitosan concentration increased from 0 to 0.3%, liposomes showed fewer changes in their size after FD-RH | [35] |
| Paclitaxel | a. Lecithin:CHO:SA 1.225:0.575:0.1 * b. TFH + sonication | a. 0.1 b. 50 c. NR d. Chitosan | a. 4; 25 °C b. 180 days c. Aqueous suspension | - Both systems were stable at 4 and 25 °C since no significant changes were observed in size, zeta potential and EE after storage | [74] |
| a. 37 °C b. 2 h, 6 h c. SGF (pH 1.2); SIF (pH 6.8) | - Chitosomes were more stable than liposomes in both SGF and SIF since no changes in size, zeta potential and EE were obtained | ||||
| Resveratrol | a. EPC 2% (w/v) b. TFH + sonication | a. 0.1; 0.3; 0.5 b. NR c. NR d. Chitosan | a. 25 °C b. 7 days c. Aqueous suspension | - Chitosan improved liposomes stability since the size increase after storage was lower than that of uncoated liposomes - 0.1% chitosan coating displayed very little change in size compared to high chitosan concentrations (0.3 and 0.5%) | [42] |
| Rosmarinic acid esters | a. Lecithin 1% (w/v) b. Homogenization | a. 0.2 b. 205 c. 91.8 | a. 55 °C b. 14 days c. pH 3 | - Chitosomes were more stable than liposomes, where chitosomes size increased by 1-fold after storage compared to 1.5-fold for uncoated ones | [69] |
| Salmon protein hydrolysates | a. MFGM Phosphlac 700 3; 5; 10% (w/v) b. Heating+ Homogenization | a. 0.4; 0.6 b. NR c. NR d. Chitosan | a. 4; 20 °C b. 30 days c. Aqueous suspension; and FD-RH | - Both systems were stable at 4 °C without significant changes in their size - A better stability at 4 °C for both systems. - The excess of chitosan (0.6%) resulted in more drug loss after 1 month at 20 °C compared to 0.4% chitosan - Conventional liposomes exhibited larger size and higher drug loss compared to chitosomes after FD-RH - No significant difference in drug loss between liposomes coated with either 0.4 or 0.6% chitosan after FD-RH | [49] |
| Substance P | a. Lecithin:CHO 20:3.3 * b. TFH+ sonication | a. 0.1 b. NR c. 88 d. Chitosan | a. 37 °C b. 24 h c. PBS (pH 7.4) | - Both systems were stable since the mean size and pdI did not increase over time | [34] |
| Triazavirin | a. SPC:CHO 85:15 * b. TFH+ extrusion | a. 0.275 b. 190 c. 95 d. Pelargonic chitosan | a. 4 °C b. 90 days c. Aqueous suspension | - Unmodified liposomes were proved to be unstable after one month of storage - Coating of liposomes with pelargonic chitosan extended the shelf life of liposomes up to 3 months at 4 °C compared to uncoated ones since the size and pdI was almost unchanged | [29] |
| Vitamin E | a. PC:CHO 60:40 b. Sonication | a. 0.1 b. 4 c. >90 d. Chitosan | a. 4; 25 °C b. 84 days c. Aqueous suspension | - After 12 weeks of storage at 4 °C, 97% of vitamin E remained encapsulated in chitosomes compared to 60.76% in liposomes - When chitosomes were stored at 25 °C, the stability of vitamin E decreased to 31.2% | [70] |
3.7. Drug Release
3.8. Pharmacokinetic Studies: Conventional Liposomes and Chitosomes
3.9. Pharmacodynamics: Conventional Liposomes and Chitosomes
3.9.1. Antimicrobial Property
| Drug/EO | Liposomes a. Composition b. Preparation Method | Chitosan a. Concentration (% w/v) b. MW (kDa ) c. DD (%) | Antibacterial Activity | Ref |
|---|---|---|---|---|
| Artemisia afra, Eucalyptus globulus, Melaleuca alternifolia | a. DSPC:DSPE 94:6 b. REV+sonication | a. 0.15 b. NR c. NR | - A. afra chitosomes displayed lower MIC values compared to liposomes and unencapsulated EO against Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, and Candida albicans, indicating that polymer coating overcomes the increased EO volatility - E. globulus chitosomes displayed lower MIC values compared to liposomes and unencapsulated EO against C. albicans - M. alternifolia chitosomes showed similar MIC compared to liposomes against S. aureus, E. coli, P. aeruginosa, and C. albicans | [36] |
| Chrysanthemumsp. | a. Lecithin:CHO 5:1 b. TFH+extrusion | a. 0.0025–0.01 b. NR c. NR | - EO chitosomes reduced Campylobacter jejuni viability in chicken compared to liposomes due to the antibacterial properties of the chitosan coating | [78] |
| Dicloxacillin | a. Lipoid S100:CHO:Tween-80 0.9:0.3:0.1 b. TFH+sonication | a. 1 b. NR c. NR | - Dicloxacillin-loaded liposomes exhibited a significantly wider zone of S. aureus inhibition compared to dicloxacillin or dicloxacillin-loaded chitosomes, probably due to liposome flexibility and relatively small size compared to chitosomes | [60] |
| Metronidazole | a. SPC b. TFH | a. 0.17 b. NR c. 77 | - Metronidazole loading chitosan-coated liposomes exhibited growth inhibition against C. albicans (MIC 0.11–0.22 mg/mL), whereas control carbopol-loaded liposomes and plain liposomes, as well as the metronidazole control solution, showed no inhibition | [84] |
| Nisin | a. Lecithin NR + tripolyphosphate as crosslinker (0.1% w/v) b. Stirring+sonication | a. 0.3–0.9 b. NR c. NR | Chitosomes controlled S. aureus, E. faecalis and L. monocytogenes growth better than free or liposomal-nisin | [85] |
| Nisin silica | a. Lecithin:CHO 20:4 * b. TFH+homogenization | a. 0.1 b. NR c. NR | - Chitosan-coated nisin-silica liposomes displayed better antibacterial activity against L. monocytogenes in cheese models compared to chitosan-coated nisin liposomes and uncoated ones | [71] |
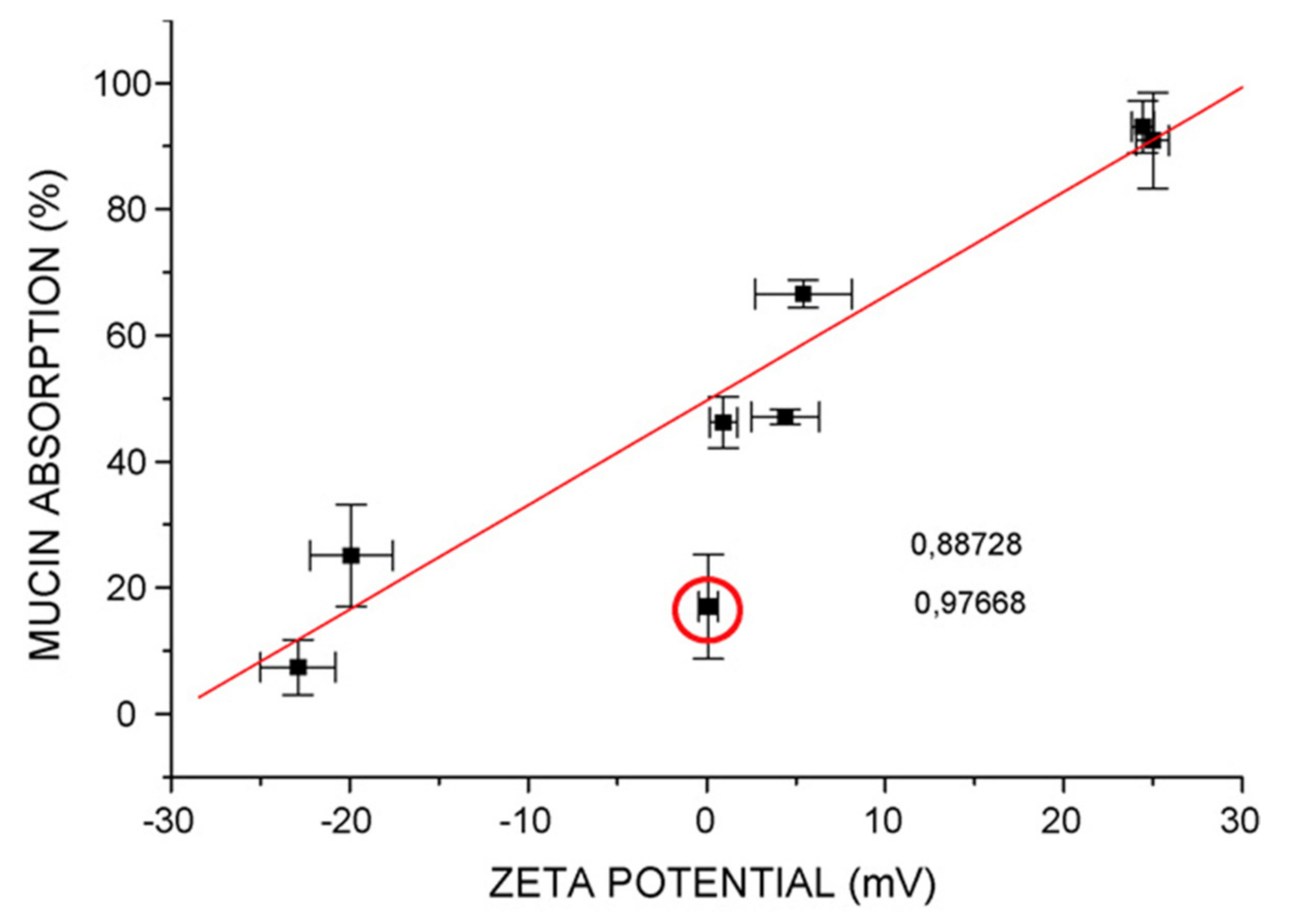
3.9.2. Mucoadhesive Property
| Drug | Liposomes a. Composition b. Preparation Method | Chitosan or Chitosan Derivative a. Concentration (% w/v) b. MW (kDa) c. DD (%) d. Chitosan Type | Mucoadhesive Property | Ref |
|---|---|---|---|---|
| Atenolol | a. Lipoid S100 20 mg b. EIM | a. 0.6 b. NR c. NR d. Chitosan | - Chitosomes have higher mucoadhesive strength performed on pork intestines (≈35%) compared to uncoated liposomes (10%) | [63] |
| Calcitonin | a. DPPC:DCP 4:1 DPPC:SA 40:1 b. TFH | a. 1.5 b. 150 c. 85 d. Chitosan | - The mucoadhesive percentage was as follows: chitosomes> noncoated positively charged liposomes> noncoated negatively charged liposomes | [81] |
| Calcitonin | a. DSPC:DCP:CHO 8:2:1 b. TFH+ sonication | a. 0.3 b. 150 c. 85 d. Chitosan | - Small-sized liposomes (200 nm) and chitosomes (400 nm) showed high penetration into intestinal mucosa, while such behavior was not observed for large vesicles (3810 nm) even when coated with chitosan (4130–4640 nm) | [82] |
| Clotrimazole | a. Lipoid S100 200 mg b. TFH+ sonication | a. 0.1; 0.3; 0.6 b. NR c. 92 d. Chitosan | - Mucin studies revealed that coating with low chitosan concentration (0.1%) increased the system’s mucoadhesive potential compared to coating with high chitosan concentrations (0.3 and 0.6%) | [17] |
| Curcumin | a. EPC:CHO 2:1 b. EIM and TFH+ sonication | a. 0.1 b. 30 c. 88 d. Chitosan | - Mucin adsorption was improved after chitosan coating with a value of 33.60 and 56.47%, respectively, for curcumin-loaded liposomes and curcumin-loaded chitosomes | [43] |
| Cyclosporin A | a. EPC:CHO:Pluronic F 127 28:5:11 b. TFH+ extrusion | a. 0.4 b. 200 c. 90 d. Chitosan | - After oral administration to rats, chitosomes were trapped by mucus and remained in the upper portion of the intestinal tract with limited penetration ability. However, deformable liposomes were found throughout the intestinal tract and were able to penetrate the mucus layers to reach the epithelial surface | [59] |
| DiI | a. DSPC:DCP:CHO 8:2:1 b. TFH+ sonication | a. 0.6 b. 150 c. 85 d. Chitosan | - Both liposomes and chitosomes were retained in the stomach at 40% in fed rats after 1 h oral administration, and intestinal transition was reduced compared to fasted rats | [88] |
| Fexofenadine | a. DPPC:DPPG:CHO 8:1:2.25 b. TFH+ extrusion | a. 0.1 b. NR c. NR d. Chitosan | - Mucoadhesive property was improved after chitosan coating with 30 and 90% for fexofenadine-loaded liposomes chitosomes, respectively | [73] |
| Rifampicin | a. EPC:CHO 2:1 EPC:PG:CHO 9:1:5 DSPC:CHO 2:1 DSPC:PG:CHO 9:1:5 b. TFH+ sonication | a. 0.001–0.66 b. NR c. 87 d. Chitosan | - The mucoadhesive percentage was in the following order: chitosomes> noncoated uncharged liposomes> noncoated negatively charged liposomes | [32] |
| Triazavirin | a. SPC:CHO 85:15 * b. TFH+ extrusion | a. 0.275 b. 190 c. 95 d. Pelargonic chitosan; lauric chitosan | - Unmodified chitosomes showed 1.3 and 1.6 times higher mucoadhesive properties than pelargonic- and lauric chitosan-coated liposomes, respectively | [29] |
3.9.3. Permeability and Drug Penetration Effect
| Drug | Liposomes a. Composition b. Preparation Method | Chitosan or Chitosan Derivative a. Concentration (% w/v) b. MW (kDa) c. DD (%) d. Chitosan Type | Permeability and drug Penetration Effect | Ref |
|---|---|---|---|---|
| Anti-sense oligodeoxynucleotides | a. SPC:CHO 20:5 * b. TFH+ sonication | a. 0.05–1 b. 100 c. 90 d. Chitosan | - Chitosomes significantly enhanced COS7 cells uptake of anti-sense oligodeoxynucleotides compared to the nucleotide alone or nucleotide-loaded liposomes | [53] |
| BSA | a. EPC:sodium oleate 10:2 b. TFH+ sonication | a. 20 mM b. 276 c. 94 d. TMBz-chitosan | - Compared to BSA-loaded liposomes, TMBz chitosan-coated liposomes enhanced BSA permeability across Caco-2 cell monolayers | [25] |
| Calcein | a. PC:CHO 3:1 * PC:Folate:PEG:CHO 1:1:1:1 * b. TFH+ sonication | a. 0.5 b. 50 c. NR d. Octadecyl-quaternized lysine modified chitosan | - Octadecyl-quaternized lysine-modified chitosan-coated deformable liposomes showed higher calcein delivery to MCF-7 cells compared to traditional liposomes | [22] |
| Calcitonin | a. DPPC:DPPE-MCC 3:s0.3 b. TFH+ extrusion | a. 0.2 b. 150 c. NR d. TGA chitosan; TGA-MNA-chitosan | - Calcitonin permeation enhancing effect through intestinal mucus was more pronounced for modified chitosomes than uncoated liposomes with 1.8-, 2.7- and a 3.8-fold increase for uncoated liposomes, TGA chitosan-coated liposomes and TGA-MNA-chitosan-coated liposomes, respectively, compared to a calcitonin buffer solution | [30] |
| Ciprofloxacin hydrochloride | a. PC:SA 10:0.5 PC:DCP 10:1 b. TFH | a. 0.3 b. NR c. 85 d. Chitosan | - Chitosomes exhibited high drug levels in the external eye of albino rabbits compared to uncoated liposomes and the commercially available eye drop, with no ocular irritation | [37] |
| Coenzyme Q10 | a. SPC:CHO 83:17 * b. EIM+ sonication | a. 0.5 b. 100; 450 c. >85 d. Trimethyl chitosan | - Trimethyl chitosan with HMW (450 kDa) led to higher precorneal retention times than that of LMW (100 kDa) and liposomes | [28] |
| Cyclosporin A | a. HSPC:PS:CHO 4:0.1:1 b. EIM | a. 0.25 b. 540 c. 94 d. Chitosan | - After topical instillation in rabbits, cyclosporin A concentrations in cornea, conjunctiva and sclera were higher in chitosomes than in liposomes | [79] |
| Diclofenac sodium | a. HSPC:PS:CHO 3:0.1:1 b. EIM | a. 0.25; 0.5 b. 540 c. 97 d. Chitosan | - Diclofenac sodium-loaded chitosomes improved the transcorneal drug penetration rate compared to uncoated liposomes or commercially available eye drops with no ocular irritation | [12] |
| Flurbiprofen | a. EPC:solutol HS15 7.5:1 b. Modified EIM | a. 0.1 b. 50 c. 95 d. Chitosan | - The apparent permeability coefficient of flurbiprofen-loaded deformable chitosomes evaluated using isolated rabbit corneas was 1.29-fold greater than that of uncoated flurbiprofen-loaded deformable liposomes | [61] |
| Furosemide | a. SPC:CHO 10:1 b. TFH+ sonication | a. 0.5 b. NR c. NR d. Chitosan | - Chitosomes increased the apical to basolateral permeability of furosemide by 8-fold through Caco-2 cells compared to furosemide loaded liposomes and furosemide solution | [4] |
| Lidocaine | a. Lecithin:SDC 15% * b. TFH+ sonication+ extrusion | a. 0.3 b. 150 c. 90 d. Chitosan | - Chitosan-coated elastic liposomes significantly improved lidocaine hydrochloride skin permeation compared to elastic liposome and chitosan solution | [48] |
| Resveratrol | a. EPC 2% * b. TFH+ sonication | a. 0.1 b. NR c. NR d. Chitosan | -Resveratrol permeated skin animal with 40.42 and 30.84% for chitosomes and liposomes, respectively | [42] |
3.9.4. Cytotoxicity
| Drug | Liposomes a. Composition b. Preparation Method | Chitosan or Chitosan Derivative a. Concentration (% w/v) b. MW (kDa) c. DD (%) d. Chitosan Type | Cytotoxicity and Anticancer Effects of Drugs | Ref |
|---|---|---|---|---|
| Butyric acid | a. PC:CHO 20:5 * b. TFH+ sonication | a. 0.1 b. NR c. NR d. Chitosan | - Chitosomes displayed higher cytotoxicity against human hepatoblastoma HepG2 cells with an IC50 value of 1.6 mM after 72 h of incubation than uncoated liposomes (2.7 mM) and free butyric acid (4.5 mM) | [72] |
| Cyclosporin A | a. HSPC:PS:CHO 4:0.1:1 b. EIM | a. 0.25; 0.5; 1; 2 b. 540 c. 94 d. Chitosan | - Chitosomes and liposomes loading cyclosporin A demonstrated low toxicity to rabbit conjunctival epithelium cells | [79] |
| Docetaxel | a. Lipoid S100:CHO: Tween-80:SDC:DCP 0.9:0.3:0.1:0.1:0.1 b. TFH+ sonication | a. 1 b. NR c. NR d. Chitosan | - Uncoated deformable liposomes displayed 52% of human colon cancer HT-29 cell growth, and cell viability was greatly reduced by 80% in deformable chitosomes, indicating enhanced cytotoxic activity for deformable chitosomes | [55] |
| Doxorubicin | a. HSPC:CHO NR b. TFH+ extrusion | a. NR b. NR c. NR d. Glycol chitosan | - Glycol chitosan-coated doxorubicin-loaded liposomes resulted in a 64% reduction in HT1080 cells viability at pH 6.5 and less than 15% reduction at pH 7.4 compared to uncoated liposomes exhibiting less than 20% reduction in viability regardless of pH | [23] |
| Doxorubicin | a. HSPC:CHO NR b. TFH+ extrusion | a. NR b. NR c. NR d. Glycol chitosan | - Hematoxylin and eosin-stained tumor sections excised from tumor-bearing mice following intravenous injection of free doxorubicin and doxorubicin-loaded liposomes and glycol chitosan-coated doxorubicin-loaded liposomes showed the strongest antitumor effect for glycol chitosan-coated doxorubicin-loaded liposomes | [23] |
| Furosemide | a. SPC:CHO 10:1 b. TFH+ sonication | a. 0.5 b. NR c. NR d. Chitosan | - Chitosomes showed less cytotoxicity toward Caco-2 cells than uncoated ones | [4] |
| Paclitaxel | a. Lecithin:CHO:SA 1.225:0.575:0.1 b. TFH+ sonication | a. 0.1 b. 50 c. NR d. Chitosan | - Chitosomes enhanced paclitaxel-induced cytotoxicity in human cervical cancer cells compared to paclitaxel loaded-liposomes | [74] |
| Rifampicin | a. EPC:CHO 2:1 EPC:PG:CHO 9:1:5 DSPC:CHO 2:1 DSPC:PG:CHO 9:1:5 b. TFH+ sonication | a. 0.001–0.66 b. NR c. 87 d. Chitosan | -The toxicity of rifampicin-loaded liposomes towards A549 epithelial cells was lower compared to the free drug for all the vesicles types (negatively charged and non-charged ones), especially chitosan-coated ones | [32] |
| si RNA-VEGF si RNA-H1F1-α | a. HSPC:CHO 1:1 HSPC:DCP:CHO1:0.1:1 HSPC:SA:CHO1:0.1:1 b. TFH+ sonication | a.1 b. 75 c. 75–85 d. Chitosan | - Chitosomes showed 96% of MCF7 cancer cell viability. However, anionic and cationic liposomes showed reduced cell viability of 76.27 and 67.79%, respectively | [75] |
| Substance P | a. Lecithin:CHO 20:3.3 * b. TFH+ sonication | a. 0.1 b. NR c. 88 d. Chitosan | -Both chitosomes and liposomes loading substance P showed no toxic effect on keratinocytes at different tested concentrations | [34] |
3.9.5. Other Biological Effects
| Drug | Liposomes a. Composition b. Preparation Method | Chitosan or Chitosan Derivative a. Concentration (% w/v) b. MW (kDa) c. DD (%) d. Chitosan Type | Other Biological Effects | Ref |
|---|---|---|---|---|
| Butyric acid | a. PC:CHO 20:5 * b. TFH+ sonication | a. 0.1 b. NR c. NR d. Chitosan | - Chitosomes showed higher anti-inflammatory effects by reducing IL-8, IL-6, TNF-α and TGF-β expression in HepG2 cells compared to free butyric acid and butyric acid-loaded liposomes at different tested concentrations | [72] |
| Extracellular proteins | a. Lecithin 100 mg b. EIM | a. 0.3 b. NR c. NR d. Chitosan | Nonspecific immune parameters myeloperoxidase, respiratory burst, hemagglutination, hemolytic, antiprotease activity and bacterial agglutination activity were tested after parenteral immunization in fish and rabbits. The specific antibody level was also measured. Chitosomes showed significantly higher specific and nonspecific immune responses than the liposomes | [57] |
| Lidocaine | a. Lecithin:SDC 15% * b. TFH+ sonication+ extrusion | a. 0.3 b. 150 c. 90 d. Chitosan | - Chitosan-coated elastic lidocaine loaded liposomes revealed greater suppression of formalin-induced nociceptive behavior in mice transdermally treated, thus a better analgesic effect compared to elastic liposome and chitosan solution | [48] |
| si RNA-VEGF si RNA-H1F1-α | a. HSPC:CHO 1:1 HSPC:DCP:CHO 1:0.1:1 HSPC:SA:CHO 1:0.1:1 b. TFH+ sonication | a.1 b. 75 c. 75–85 d. Chitosan | - VEGF and HIF1-α protein levels in cells treated with anionic liposomes were significantly lower than those treated with cationic and chitosan-coated ones. - In vitro codelivery of siVEGF and siHIF1-α in breast cancer cells using chitosomes significantly inhibited VEGF (89%) and HIF1-α (62%) protein expression compared to other liposome formulations. | [75] |
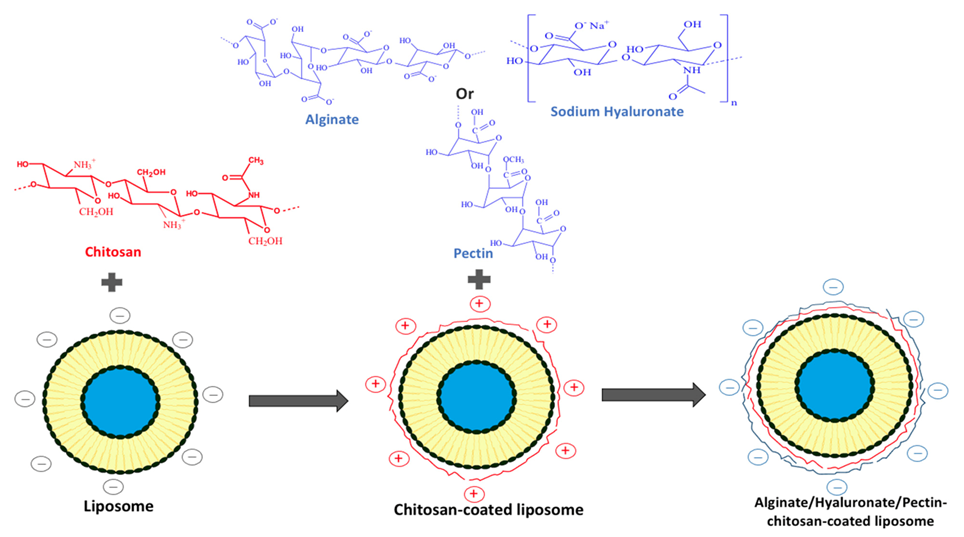
4. Multilayer Coating of Polyelectrolytes on the Liposomes
5. Encapsulation of Essential Oils: Chitosomes vs. Liposomes. Novel Formulation Strategies for Old Antimicrobials
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | area under curve |
| BSA | bovine serum albumin |
| Caco-2 | human colorectal adenocarcinoma |
| CH-LP | chitosan-coated liposomes |
| CHO | cholesterol |
| Cmax | maximum plasma concentration |
| DCP | dicetylphosphate |
| DD | degree of deacetylation |
| DDAB | dimethyldioctadecyl-ammonium bromide |
| DHP | dihexadecylphosphate |
| DiI | 1,1’-dioctadecyl-3,3,3’,3’-tetramethylindo carbocyanine perchlorate |
| DPPC | L-α-dipalmitoylphosphatidylcholine |
| DPPE-MCC | 1,2-dipalmitoylsn-glycero-3-phosphoethanolamine-N-[4-(p maleimidomethyl) cyclohexane-carboxamide]; |
| DSPC | distearoylphosphatidylcholine |
| EO | essential oil |
| EPC | egg phosphatidylcholine |
| FD-RH | freeze-drying followed by rehydration |
| GI | gastro-intestinal |
| H1F1-α; hypoxia inducible factor 1 | |
| HMW | high molecular weight |
| DSPE | distearoylphosphatidylethanolamine |
| EE | encapsulation efficiency |
| EIM | ethanol injection method |
| EO | essential oil |
| EPC | egg phosphatidylcholine |
| FD-RH | freeze-drying followed by rehydration |
| GI | gastro-intestinal |
| H1F1-α; hypoxia inducible factor 1 | |
| HCl | hydrogen chloride |
| HMW | high molecular weight |
| HPTMA | N-[(2-hydroxy-3-trimethylamine) propyl] chloride |
| HSPC | hydrogenated soy phosphatidylcholine |
| IC50 | half inhibitory concentration |
| ISCRPE | improved supercritical reverse-phase evaporation method |
| LMW | low molecular weight |
| MDA | malonaldehyde |
| MFGM | milk fat globule membrane |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide |
| MW | molecular weight |
| NR | not reported |
| PBS | phosphate-buffered saline |
| PC | phosphatidylcholine |
| pdI | polydispersity index |
| PDS | polyelectrolyte delivery system |
| PEG | polyethylene glycol |
| PG | phosphatidylglycerol |
| PS | phosphatidylserine |
| Ref | references |
| REV | reverse phase evaporation |
| SA | stearylamine |
| SDC | sodium deoxycholate |
| SGF | simulated gastric fluid |
| SIF | simulated intestinal fluid |
| SPC | soy phosphatidylcholine |
| TFH | thin film hydration |
| UN-LP | uncoated liposomes |
| VEGF | vascular endothelial growth factor |
References
- Anwekar, H.; Patel, S.; Singhai, A.K. Liposome as drug carriers. Int. J. Pharm. Life. Sci. 2011, 2, 945–951. [Google Scholar]
- Barba, A.A.; Bochicchio, S.; Bertoncin, P.; Lamberti, G.; Dalmoro, A. Coating of Nanolipid Structures by a Novel Simil-Microfluidic Technique: Experimental and Theoretical Approaches. Coatings 2019, 9, 491. [Google Scholar] [CrossRef] [Green Version]
- Laye, C.; McClements, D.J.; Weiss, J. Formation of Biopolymer-Coated Liposomes by Electrostatic Deposition of Chitosan. J. Food Sci. 2008, 73, N7–N15. [Google Scholar] [CrossRef]
- Vural, I.; Sarisozen, C.; Olmez, S.S. Chitosan coated furosemide liposomes for improved bioavailability. J. Biomed. Nanotechnol. 2011, 7, 426–430. [Google Scholar] [CrossRef]
- Nguyen, T.X.; Huang, L.; Liu, L.; Abdalla, A.M.E.; Gauthier, M.; Yang, G. Chitosan-coated nano-liposomes for the oral delivery of berberine hydrochloride. J. Mater. Chem. B 2014, 2, 7149–7159. [Google Scholar] [CrossRef] [PubMed]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [Green Version]
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomed. 2006, 1, 297–315. [Google Scholar]
- Trucillo, P.; Reverchon, E. Production of PEG-coated liposomes using a continuous supercritical assisted process. J. Supercrit. Fluids 2021, 167, 105048. [Google Scholar] [CrossRef]
- Patel, J. Liposomal doxorubicin: Doxil®. J. Oncol. Pharm. Pr. 1996, 2, 201–210. [Google Scholar] [CrossRef]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Wang, Q.Z.; Chen, X.G.; Liu, N.; Wang, S.X.; Liu, C.S.; Meng, X.H.; Liu, C.G. Protonation constants of chitosan with different molecular weight and degree of deacetylation. Carbohydr. Polym. 2006, 65, 194–201. [Google Scholar] [CrossRef]
- Li, N.; Zhuang, C.; Wang, M.; Sun, X.; Nie, S.; Pan, W. Liposome coated with low molecular weight chitosan and its potential use in ocular drug delivery. Int. J. Pharm. 2009, 379, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Progr. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Roy, J.; Salaün, F.; Giraud, S.; Ferri, A. Solubility of chitin: Solvents, solution behaviors and their related mechanisms. In Solubility of Polysaccharides; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef] [Green Version]
- Filipović-Grcić, J.; Skalko-Basnet, N.; Jalsenjak, I. Mucoadhesive chitosan-coated liposomes: Characteristics and stability. J. Microencapsul. 2001, 18, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R.A. Human enzymatic activities related to the therapeutic administration of chitin derivatives. Cell. Mol. Life Sci. 1997, 53, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Joraholmen, M.W.; Vanic, Z.; Tho, I.; Skalko-Basnet, N. Chitosan-coated liposomes for topical vaginal therapy: Assuring 2 localized drug effect. Int. J. Pharm. 2014, 472, 94–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngo, D.H.; Kim, S.K. Antioxidant effects of chitin, chitosan, and their derivatives. Adv. Food. Nutr. Res. 2014, 73, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Dai, T.; Tanaka, M.; Huang, Y.-Y.; Hamblin, M.R. Chitosan preparations for wounds and burns: Antimicrobial and wound-healing effects. Expert Rev. Anti Infect. Ther. 2011, 9, 857–879. [Google Scholar] [CrossRef]
- Raftery, R.; O’Brien, F.J.; Cryan, S.-A. Chitosan for Gene Delivery and Orthopedic Tissue Engineering Applications. Molecules 2013, 18, 5611–5647. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhao, P.; Liang, X.; Gong, X.; Song, T.; Niu, R.; Chang, J. Folate-PEG coated cationic modified chitosan—Cholesterol liposomes for tumor-targeted drug delivery. Biomaterials 2010, 31, 4129–4138. [Google Scholar] [CrossRef]
- Yan, L.; Crayton, S.H.; Thawani, J.P.; Amirshaghaghi, A.; Tsourkas, A.; Cheng, Z. A pH-Responsive Drug-Delivery Platform Based on Glycol Chitosan-Coated Liposomes. Small 2015, 11, 4870–4874. [Google Scholar] [CrossRef] [Green Version]
- Kowapradit, J.; Opanasopit, P.; Ngawhiranpat, T.; Apirakaramwong, A.; Rojanarata, T.; Ruktanonchai, U.; Sajomsang, W. Methylated N-(4-N,N-Dimethylaminobenzyl) Chitosan, a Novel Chitosan Derivative, Enhances Paracellular Permeability Across Intestinal Epithelial Cells (Caco-2). AAPS PharmSciTech 2008, 9, 1143–1152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowapradit, J.; Apirakaramwong, A.; Ngawhirunpat, T.; Rojanarata, T.; Sajomsang, W.; Opanasopit, P. Methylated N-(4-N,N-dimethylaminobenzyl) chitosan coated liposomes for oral protein drug delivery. Eur. J. Pharm. Sci. 2012, 47, 359–366. [Google Scholar] [CrossRef]
- Karewicz, A.; Bielska, D.; Loboda, A.; Gzyl-Malcher, B.; Bednar, J.; Jozkowicz, A.; Dulak, J.; Nowakowska, M. Curcumin-containing liposomes stabilized by thin layers of chitosan derivatives. Colloids Surf. B Biointerfaces 2013, 109, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wu, J.; Sun, M.; Guo, C.; Yu, A.; Cao, F.; Zhao, L.; Tan, Q.; Zhai, G. N-trimethyl chitosan chloride-coated liposomes for the oral delivery of curcumin. J. Liposome Res. 2012, 22, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, S. Topical use of Coenzyme Q10-loaded liposomes coated with trimethyl chitosan: Tolerance, precorneal retention and anti-cataract effect. Int. J. Pharm. 2009, 372, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Kozhikhova, K.V.; Ivantsova, M.N.; Tokareva, M.I.; Shulepov, I.D.; Tretiyakov, A.V.; Shaidarov, L.V.; Rusinov, V.L.; Mironov, M.A. Preparation of chitosan-coated liposomes as a novel carrier system for the antiviral drug Triazavirin. Pharm. Dev. Technol. 2018, 23, 334–342. [Google Scholar] [CrossRef]
- Gradauer, K.; Barthelmes, J.; Vonach, C.; Almer, G.; Mangge, H.; Teubl, B.; Roblegg, E.; Dünnhaupt, S.; Fröhlich, E.; Bernkop-Schnürch, A.; et al. Liposomes coated with thiolated chitosan enhance oral peptide delivery to rats. J. Control. Release 2013, 172, 872–878. [Google Scholar] [CrossRef] [Green Version]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [Green Version]
- Zaru, M.; Manca, M.L.; Fadda, A.M.; Antimisiaris, S.G. Chitosan-coated liposomes for delivery to lungs by nebulization. Colloids Surf. B. Biointerfaces 2009, 71, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Kim, D.W.; Lee, K.Y. Effects of chitosan coating for liposomes as an oral carrier. J. Biomed. Sci. 2011, 17, 211–216. [Google Scholar]
- Mengoni, T.; Adrian, M.; Pereira, S.; Santos-Carballal, B.; Kaiser, M.; Goycoolea, F.M. A Chitosan—Based Liposome Formulation Enhances the In Vitro Wound Healing Efficacy of Substance P Neuropeptide. Pharmaceutics 2017, 9, 56. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, J.; Ping, Q.; Song, Y.; Qi, J.; Cui, Z. Effects of chitosan coating on physical properties and pharmacokinetic behavior of mitoxantrone liposomes. Int. J. Nanomed. 2010, 5, 407–416. [Google Scholar] [CrossRef] [Green Version]
- Van Vuuren, S.F.; Du Toit, L.C.; Parry, A.; Pillay, V.; Choonara, Y.E. Encapsulation of Essential Oils within a Polymeric Liposomal Formulation for Enhancement of Antimicrobial Efficacy. Nat. Prod. Commun. 2010, 5, 1401–1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelbary, G. Ocular ciprofloxacin hydrochloride mucoadhesive chitosan-coated liposomes. Pharm. Dev. Technol. 2009, 16, 44–56. [Google Scholar] [CrossRef]
- Wu, Z.-H.; Ping, Q.-N.; Wei, Y.; Lai, J.-M. Hypoglycemic efficacy of chitosan-coated insulin liposomes after oral administration in mice. Acta Pharmacol. Sin. 2004, 25, 966–972. [Google Scholar]
- Channarong, S.; Chaicumpa, W.; Sinchaipanid, N.; Mitrevej, A. Development and Evaluation of Chitosan-Coated Liposomes for Oral DNA Vaccine: The Improvement of Peyer’s Patch Targeting Using a Polyplex-Loaded Liposomes. AAPS PharmSciTec. 2010, 12, 192–200. [Google Scholar] [CrossRef]
- Guo, J.; Ping, Q.; Jiang, G.; Huang, L.; Tong, Y. Chitosan-coated liposomes: Characterization and interaction with leuprolide. Int. J. Pharm. 2003, 260, 167–173. [Google Scholar] [CrossRef]
- Liu, N.; Park, H.-J. Factors effect on the loading efficiency of Vitamin C loaded chitosan-coated nanoliposomes. Colloids Surf. B Biointerfaces 2010, 76, 16–19. [Google Scholar] [CrossRef]
- Park, S.N.; Jo, N.R.; Jeon, S.H. Chitosan-coated liposomes for enhanced skin permeation of resveratrol. J. Ind. Eng. Chem. 2014, 20, 1481–1485. [Google Scholar] [CrossRef]
- Shin, G.H.; Chung, S.K.; Kim, J.T.; Joung, H.J.; Park, H.J. Preparation of Chitosan-Coated Nanoliposomes for Improving the Mucoadhesive Property of Curcumin Using the Ethanol Injection Method. J. Agric. Food Chem. 2013, 61, 11119–11126. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Feng, B.; Zhang, X.; Xia, W.; Xia, S. Biopolymer-coated liposomes by electrostatic adsorption of chitosan (chitosomes) as novel delivery systems for carotenoids. Food Hydrocoll. 2016, 52, 774–784. [Google Scholar] [CrossRef]
- Tan, H.W.; Misran, M. Characterization of fatty acid liposome coated with low-molecular-weight chitosan. J. Liposome Res. 2012, 22, 329–335. [Google Scholar] [CrossRef]
- Venturini, M.; Mazzitelli, S.; Mičetić, I.; Benini, C.; Fabri, J.; Mucelli, S.P.; Benetti, F.; Nastruzzi, C. Analysis of Operating Conditions Influencing the Morphology and In vitro Behaviour of Chitosan Coated Liposomes. J. Nanomed. Nanotechnol. 2014, 5, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Tu, S.; Li, R.; Yang, X.; Liu, L.; Zhang, Q. Cholesterol succinyl chitosan anchored liposomes: Preparation, characterization, physical stability, and drug release behavior. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 471–477. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Han, S.; Qu, Z.; Zhao, J.; Chen, Y.; Chen, Z.; Duan, J.; Pan, Y.; Tang, X. Penetration enhancement of lidocaine hydrochlorid by a novel chitosan coated elastic liposome for transdermal drug delivery. J. Biomed. Nanotechnol. 2011, 7, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Paulson, A.T.; Gill, T.A. Encapsulation of bioactive salmon protein hydrolysates with chitosan-coated liposomes. J. Funct. Foods 2015, 19, 733–743. [Google Scholar] [CrossRef]
- Diebold, Y.; Jarrín, M.; Sáez, V.; Carvalho, E.L.S.; Orea, M.; Calonge, M.; Seijo, B.; Alonso, M.J. Ocular drug delivery by liposome–chitosan nanoparticle complexes (LCS-NP). Biomaterials 2007, 28, 1553–1564. [Google Scholar] [CrossRef] [PubMed]
- Mady, M.M.; Darwish, M.M.; Khalil, S.; Khalil, W.M. Biophysical studies on chitosan-coated liposomes. Eur. Biophys. J. 2009, 38, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Mady, M.M.; Darwish, M.M. Effect of chitosan coating on the characteristics of DPPC liposomes. J. Adv. Res. 2010, 1, 187–191. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.-X.; Gao, J.-Q.; Liang, W.-Q. Chitosan-coated liposomes for intracellular oligonucleotides delivery: Characteristics and cell uptake behavior. Drug Deliv. 2010, 18, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Thongborisute, J.; Tsuruta, A.; Kawabata, Y.; Takeuchi, H. The effect of particle structure of chitosan-coated liposomes and type of chitosan on oral delivery of calcitonin. J. Drug Target. 2006, 14, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Alshraim, M.O.; Sangi, S.; Harisa, G.I.; Alomrani, A.H.; Yusuf, O.; Badran, M.M. Chitosan-Coated Flexible Liposomes Magnify the Anticancer Activity and Bioavailability of Docetaxel: Impact on Composition. Molecules 2019, 24, 250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Liu, J.; Gao, J.; Chen, S.; Huang, G. Chitosan coated vancomycin hydrochloride liposomes: Characterizations and evaluation. Int. J. Pharm. 2015, 495, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Behera, T.; Swain, P.; Sahoo, S. Antigen in chitosan coated liposomes enhances immune responses through parenteral immunization. Int. Immunopharmacol. 2011, 11, 907–914. [Google Scholar] [CrossRef]
- Ben, M.S.; Marina, K.; Mukund, G.S. Eudragit S-100 Encapsulated Chitosan Coated Liposomes Containing Prednisolone for Colon Targeting: In vitro, Ex vivo and In vivo Evaluation. J. Young Pharm. 2019, 11, 7–11. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Xia, D.; Li, X.; Zhu, Q.; Yu, H.; Zhu, C.; Gan, Y. Comparative study of Pluronic® F127-modified liposomes and chitosan-modified liposomes for mucus penetration and oral absorption of cyclosporine A in rats. Int. J. Pharm. 2013, 449, 1–9. [Google Scholar] [CrossRef]
- Alshamsan, A.; Aleanizy, F.S.; Badran, M.; Alqahtani, F.Y.; Alfassam, H.; Almalik, A.; Alosaimy, S. Exploring anti-MRSA activity of chitosan-coated liposomal dicloxacillin. J. Microbiol. Methods 2019, 156, 23–28. [Google Scholar] [CrossRef]
- Chen, H.; Pan, H.; Li, P.; Wang, H.; Wang, X.; Pan, W.; Yuan, Y. The potential use of novel chitosan-coated deformable liposomes in an ocular drug delivery system. Colloids Surf. B Biointerfaces 2016, 143, 455–462. [Google Scholar] [CrossRef]
- Gibis, M.; Ruedt, C.; Weiss, J. In vitro release of grape-seed polyphenols encapsulated from uncoated and chitosan-coated liposomes. Food Res. Int. 2016, 88, 105–113. [Google Scholar] [CrossRef]
- Karn, P.R.; Vanić, Z.; Pepić, I.; Škalko-Basnet, N. Mucoadhesive liposomal delivery systems: The choice of coating material. Drug Dev. Ind. Pharm. 2010, 37, 482–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Liu, W.; Ye, A.; Peng, S.; Wei, F.; Liu, C.; Han, J. Environmental stress stability of microencapsules based on liposomes decorated with chitosan and sodium alginate. Food Chem. 2016, 196, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Gültekin-Özgüven, M.; Karadağ, A.; Duman, S.; Özkal, B.; Özçelik, B. Fortification of dark chocolate with spray dried black mulberry (Morus nigra) waste extract encapsulated in chitosan-coated liposomes and bioaccessability studies. Food Chem. 2016, 201, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, D.; Zhu, L.; Gan, Q.; Le, X. Temperature-dependent structure stability and in vitro release of chitosan-coated curcumin liposome. Food Res. Int. 2015, 74, 97–105. [Google Scholar] [CrossRef]
- Cuomo, F.; Cofelice, M.; Venditti, F.; Ceglie, A.; Miguel, M.; Lindman, B.; Lopez, F. In-vitro digestion of curcumin loaded chitosan-coated liposomes. Colloids Surf. B Biointerfaces 2018, 168, 29–34. [Google Scholar] [CrossRef]
- Gibis, M.; Rahn, N.; Weiss, J. Physical and Oxidative Stability of Uncoated and Chitosan-Coated Liposomes Containing Grape Seed Extract. Pharmaceutics 2013, 5, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Panya, A.; Laguerre, M.; LeComte, J.; Villeneuve, P.; Weiss, J.; McClements, D.J.; Decker, E.A. Effects of Chitosan and Rosmarinate Esters on the Physical and Oxidative Stability of Liposomes. J. Agric. Food Chem. 2010, 58, 5679–5684. [Google Scholar] [CrossRef]
- Liu, N.; Park, H.-J. Chitosan-coated nanoliposome as vitamin E carrier. J. Microencapsul. 2009, 26, 235–242. [Google Scholar] [CrossRef]
- Cui, H.Y.; Wu, J.; Li, C.Z.; Lin, L. Anti-listeria effects of chitosan-coated nisin-silica liposome on Cheddar cheese. J. Dairy Sci. 2016, 99, 8598–8606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quagliariello, V.; Masarone, M.; Armenia, E.; Giudice, A.; Barbarisi, M.; Caraglia, M.; Barbarisi, A.; Persico, M. Chitosan-coated liposomes loaded with butyric acid demonstrate anticancer and anti-inflammatory activity in human hepatoma HepG2 cells. Oncol. Rep. 2019, 41, 1476–1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiang, F.; Shin, H.-J.; Lee, B.-J.; Han, H.-K. Enhanced systemic exposure of fexofenadine via the intranasal administration of chitosan-coated liposome. Int. J. Pharm. 2012, 430, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-X.; Li, B.-K.; Yin, D.-K.; Liang, J.; Li, S.-S.; Peng, D.-Y. Layer-by-layer assembly of chitosan stabilized multilayered liposomes for paclitaxel delivery. Carbohydr. Polym. 2014, 111, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Salva, E.; Turan, S.O.; Eren, F.; Akbuğa, J. The enhancement of gene silencing efficiency with chitosan-coated liposome formulations of siRNAs targeting HIF-1α and VEGF. Int. J. Pharm. 2015, 478, 147–154. [Google Scholar] [CrossRef]
- Otake, K.; Shimomura, T.; Goto, T.; Imura, T.; Furuya, T.; Yoda, S.; Takebayashi, Y.; Sakai, H.; Abe, M. One-Step Preparation of Chitosan-Coated Cationic Liposomes by an Improved Supercritical Reverse-Phase Evaporation Method. Langmuir 2006, 22, 4054–4059. [Google Scholar] [CrossRef]
- Mozuraityte, R.; Rustad, T.; Storrø, I. The Role of Iron in Peroxidation of Polyunsaturated Fatty Acids in Liposomes. J. Agric. Food Chem. 2008, 56, 537–543. [Google Scholar] [CrossRef]
- Lin, L.; Gu, Y.; Sun, Y.; Cui, H. Characterization of chrysanthemum essential oil triple-layer liposomes and its application against Campylobacter jejuni on chicken. LWT 2019, 107, 16–24. [Google Scholar] [CrossRef]
- Li, N.; Zhuang, C.-Y.; Wang, M.; Sui, C.-G.; Pan, W.-S. Low molecular weight chitosan-coated liposomes for ocular drug delivery: In vitro and in vivo studies. Drug Deliv. 2012, 19, 28–35. [Google Scholar] [CrossRef]
- Jeon, S.; Yoo, C.Y.; Park, S.N. Improved stability and skin permeability of sodium hyaluronate-chitosan multilayered liposomes by Layer-by-Layer electrostatic deposition for quercetin delivery. Colloids Surf. B Biointerfaces 2015, 129, 7–14. [Google Scholar] [CrossRef]
- Takeuchi, H.; Matsui, Y.; Yamamoto, H.; Kawashima, Y. Mucoadhesive properties of carbopol or chitosan-coated liposomes and their effectiveness in the oral administration of calcitonin to rats. J. Control. Release 2003, 86, 235–242. [Google Scholar] [CrossRef]
- Takeuchi, H.; Matsui, Y.; Sugihara, H.; Yamamoto, H.; Kawashima, Y. Effectiveness of submicron-sized, chitosan-coated liposomes in oral administration of peptide drugs. Int. J. Pharm. 2005, 303, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Jøraholmen, M.W.; Bhargava, A.; Julin, K.; Johannessen, M.; Škalko-Basnet, N. The Antimicrobial Properties of Chitosan Can Be Tailored by Formulation. Mar. Drugs 2020, 18, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, T.; Mishchenko, E.; Flaten, G.E.; Sollid, J.U.E.; Mattsson, S.; Tho, I.; Škalko-Basnet, N. Chitosan-Based Nanomedicine to Fight Genital Candida Infections: Chitosomes. Mar. Drugs 2017, 15, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niaz, T.; Shabbir, S.; Noor, T.; Rahman, A.; Bokhari, H.; Imran, M. Potential of polymer stabilized nano-liposomes to enhance antimicrobial activity of nisin Z against foodborne pathogens. LWT 2018, 96, 98–110. [Google Scholar] [CrossRef]
- Serra, L.; Doménech, J.; Peppas, N. Engineering design and molecular dynamics of mucoadhesive drug delivery systems as targeting agents. Eur. J. Pharm. Biopharm. 2009, 71, 519–528. [Google Scholar] [CrossRef] [Green Version]
- Dünnhaupt, S.; Barthelmes, J.; Thurner, C.C.; Waldner, C.; Sakloetsakun, D.; Bernkop-Schnürch, A. S-protected thiolated chitosan: Synthesis and in vitro characterization. Carbohydr. Polym. 2012, 90, 765–772. [Google Scholar] [CrossRef] [Green Version]
- Sugihara, H.; Yamamoto, H.; Kawashima, Y.; Takeuchi, H. Effects of food intake on the mucoadhesive and gastroretentive properties of submicron-sized chitosan-coated liposomes. Chem. Pharm. Bull. 2012, 60, 1320–1323. [Google Scholar] [CrossRef] [Green Version]
- Haidar, Z.S.; Hamdy, R.C.; Tabrizian, M. Protein release kinetics for core-shell hybrid nanoparticles based on the layer-by-layer assembly of alginate and chitosan on liposomes. Biomaterials 2008, 29, 1207–1215. [Google Scholar] [CrossRef]
- Liu, W.; Liu, J.; Liu, W.; Li, T.; Liu, C. Improved Physical and in Vitro Digestion Stability of a Polyelectrolyte Delivery System Based on Layer-by-Layer Self-Assembly Alginate–Chitosan-Coated Nanoliposomes. J. Agric. Food Chem. 2013, 61, 4133–4144. [Google Scholar] [CrossRef]
- Gibis, M.; Zeeb, B.; Weiss, J. Formation, characterization, and stability of encapsulated hibiscus extract in multilayered liposomes. Food Hydrocoll. 2014, 38, 28–39. [Google Scholar] [CrossRef]
- Dajic Stevanovic, Z.; Sieniawska, E.; Glowniak, K.; Obradovic, N.; Pajic-Lijakovic, I. Natural Macromolecules as Carriers for Essential Oils: From Extraction to Biomedical Application. Front. Bioeng. Biotechnol. 2020, 8, 563. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial Properties of Plant Essential Oils against Human Pathogens and Their Mode of Action: An Updated Review. Evid. Based Complement. Altern. Med. 2016, 2016, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Dilworth, L.L.; Riley, C.K.; Stennett, D.K. Plant Constituents: Carbohydrates, Oils, Resins, Balsams, and Plant Hormones. In Pharmacognosy. Fundamentals, Applications and Strategy; Badal, S., Delgoda, R., Eds.; Academic Press: London, UK, 2017; pp. 61–80. [Google Scholar]
- Schmidt, E. Production of Essential Oils. In Handbook of Essential Oils: Science, Technology, and Applications; Can Baser, K.H., Buchbauer, G., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 83–120. [Google Scholar]
- Bucar, F.; Wube, A.; Schmid, M. Natural product isolation: How to get from biological material to pure compounds. Nat. Prod. Rep. 2013, 30, 525–545. [Google Scholar] [CrossRef] [Green Version]
- Simona, J.; Dani, D.; Petr, S.; Marcela, N.; Jakub, T.; Bohuslava, T. Edible Films from Carrageenan/Orange Essential Oil/Trehalose—Structure, Optical Properties, and Antimicrobial Activity. Polymers 2021, 13, 332. [Google Scholar] [CrossRef] [PubMed]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential Oils in Food Preservation: Mode of Action, Synergies, and Interactions with Food Matrix Components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crop. Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Sarkic, A.; Stappen, I. Essential Oils and Their Single Compounds in Cosmetics—A Critical Review. Cosmetics 2018, 5, 11. [Google Scholar] [CrossRef] [Green Version]
- Hammer, K.A.; Carson, C.F. Antibacterial and antifungal activities of essential oils. In Lipids and Essential Oils as Antimicrobial Agents; Thormar, H., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2011; pp. 255–295. [Google Scholar]
- Bassolé, I.H.N.; Juliani, H.R. Essential Oils in Combination and Their Antimicrobial Properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- Langeveld, W.T.; Veldhuizen, E.J.A.; Burt, S.A. Synergy between essential oil components and antibiotics: A review. Crit. Rev. Microbiol. 2014, 40, 76–94. [Google Scholar] [CrossRef] [PubMed]
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Bhat, M.A.; Prabhakar, A.; Shalla, A.H.; Rather, M.A. A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [CrossRef]
- Trifan, A.; Luca, S.V.; Greige-Gerges, H.; Miron, A.; Gille, E.; Aprotosoaie, A.C. Recent advances in tackling microbial multidrug resistance with essential oils: Combinatorial and nano-based strategies. Crit. Rev. Microbiol. 2020, 46, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Kon, K.V.; Rai, M.K. Plant essential oils and their constituents in coping with multidrug-resistant bacteria. Expert Rev. Anti Infect. Ther. 2012, 10, 775–790. [Google Scholar] [CrossRef] [PubMed]
- Subramani, R.; Narayanasamy, M.; Feussner, K.-D. Plant-derived antimicrobials to fight against multi-drug-resistant human pathogens. 3 Biotech 2017, 7, 172. [Google Scholar] [CrossRef]
- Kfoury, M.; Auezova, L.; Greige-Gerges, H.; Fourmentin, S. Promising applications of cyclodextrins in food: Improvement of essential oils retention, controlled release and antiradical activity. Carbohydr. Polym. 2015, 131, 264–272. [Google Scholar] [CrossRef]
- Doost, A.S.; Nasrabadi, M.N.; Kassozi, V.; Nakisozi, H.; Van Der Meeren, P. Recent advances in food colloidal delivery systems for essential oils and their main components. Trends Food Sci. Technol. 2020, 99, 474–486. [Google Scholar] [CrossRef] [Green Version]
- Sherry, M.; Charcosset, C.; Fessi, H.; Greige-Gerges, H. Essential oils encapsulated in liposomes: A review. J. Liposome Res. 2013, 23, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Majeed, H.; Bian, Y.-Y.; Ali, B.; Jamil, A.; Majeed, U.; Khan, Q.F.; Iqbal, K.J.; Shoemaker, C.F.; Fang, Z. Essential oil encapsulations: Uses, procedures, and trends. RSC Adv. 2015, 5, 58449–58463. [Google Scholar] [CrossRef]
- Ahmad, R.; Srivastava, S.; Ghosh, S.; Khare, S.K. Phytochemical delivery through nanocarriers: A review. Colloids Surf. B Biointerfaces 2021, 197, 111389. [Google Scholar] [CrossRef]
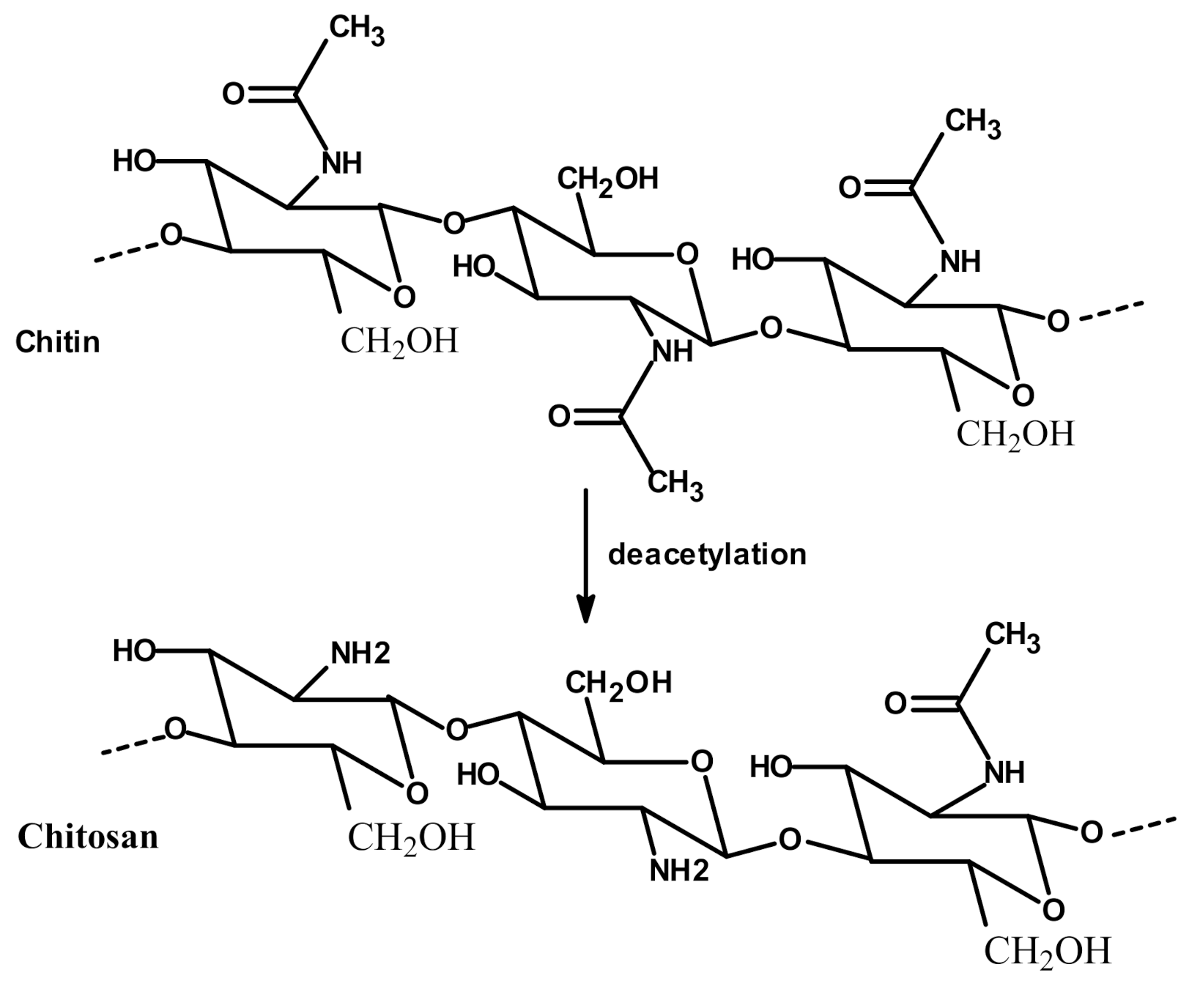
| Chitosan Derivatives | Chemical Structure | Aim of Chitosan Derivatization | Ref |
|---|---|---|---|
| Chitosan | 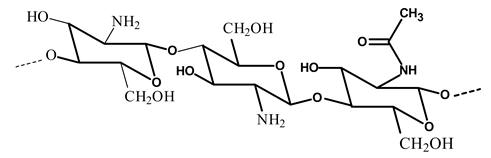 | - Improve chitosan properties | [22] |
| Glycol chitosan | 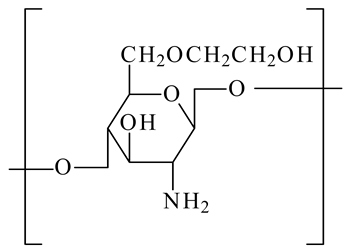 | - Target acidic tumor microenvironment - Improve anticancer drug efficacy | [23] |
| Methylated N-(4-N,N-dimethylaminobenzyl) chitosan (TMBz-chitosan) | 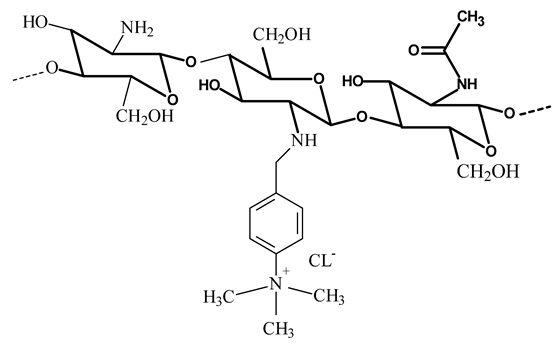 The methylation could also occur at the primary amino group of chitosan The methylation could also occur at the primary amino group of chitosan | - Provide the hydrophobic moiety to improve the hydrophobic interaction with the cell membrane | [24,25] |
| N-dodecyl chitosan | 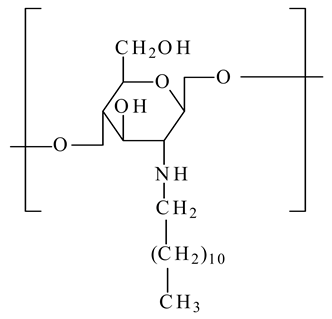 | - Improve the liposome stability by anchoring the liposome bilayer by the long alkyl chain | [26] |
| N-[(2-hydroxy-3-trimethylamine) propyl] chitosan chloride (HPTMA-chitosan) |  | - Improve the stability of coated liposomes - Increase chitosan solubility | [26] |
| N-dodecyl-chitosan N-[(2-hydroxy-3-trimethylamine) propyl]chloride (N-dodecyl-chitosan-HPTMA) |  | ||
| N-trimethyl chitosan |  | - Improve chitosan solubility over a wide pH range - Improve liposomal stability | [27,28] |
| PEGylated octadecyl-quaternized lysine modified chitosan | 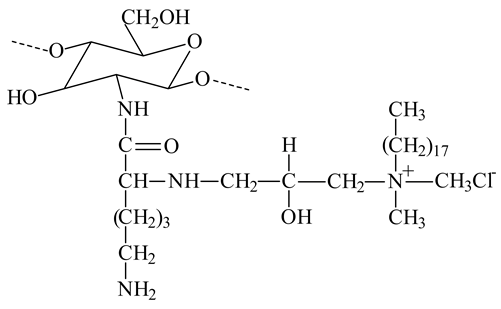 | - Provide amphiphilic character and steric stabilizations | [22] |
| Pelargonic chitosan (n=7) Lauric chitosan (n=10) | 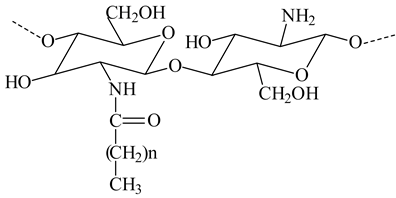 | - Coat positively charged liposomes with mucoadhesive properties. - The side groups introduced into the polysaccharide chains provide additional steric stabilization for liposomes in solutions | [29] |
| Thioglycolic acid chitosan (TGA-chitosan) | 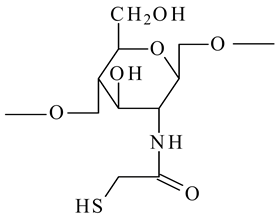 | - Improve the mucoadhesive property and enhance oral bioavailability of peptides | [30] |
| Thioglycolic acid 6-mercaptonicotinamide-conjugate chitosan (TGA-MNA-chitosan) | 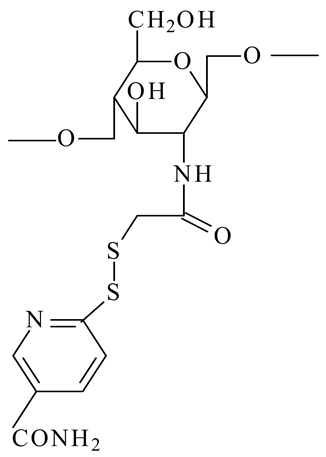 | - Thioglycolic acid chitosan was S-protected via conjugation with 6,6′-dithionicotinamide resulting in a derivative being less prone to oxidation and exhibiting higher mucoadhesive properties | [30] |
| Drug | Liposomes A. Composition B. Preparation Method | Chitosan or Chitosan Derivative a. Concentration (% w/v) b. MW (kDa) c. DD (%) d. Chitosan Type | Pharmacokinetic Behavior | Ref |
|---|---|---|---|---|
| Berberine Hydrochloride | a. EPC:CHO:DHP 1:0.242:0.036 b. TFH+ sonication | a. 0.1; 0.3 b. NR c. >90 d. Chitosan | - Pharmacokinetics parameters (AUC, Tmax, Cmax) for berberine hydrochloride-loaded chitosomes after oral administration to rabbits were higher than those obtained with uncoated liposomes | [5] |
| Calcitonin | a. DPPC:DCP 4:1 DPPC:SA 4:1 b. TFH | a. 1.5 b. 150 c. 85 d. Chitosan | - The area under the plasma calcium concentration curve was 2.4 and 2.8 times higher for chitosomes than for positively and negatively charged uncoated liposomes, respectively, after oral administration to rats | [81] |
| a. DSPC:DCP:CHO 8:2:1 b. TFH+ sonication | a. 0.3 b. 150 c. 85 d. Chitosan | - The area under plasma calcium concentration obtained after oral administration to rats increased with decreasing liposomes size | [82] | |
| a. DSPC:DCP:CHO 8:2:1 b. TFH+ extrusion | a. 0.6 b. 150; 22 c. >85; 80 d. Chitosan | - After oral administration to rats, calcitonin-loaded chitosomes prepared with LMW chitosan showed more pharmacological effectiveness in decreasing blood calcium concentration than did HMW chitosomes | [54] | |
| a. DPPC:DPPE-MCC 3:0.3 b. TFH+ extrusion | a. 0.2 b. 150 c. NR d. TGA chitosan; TGA-MNA-chitosan | - The highest reduction in blood calcium level after oral administration to rats was achieved for TGA-MNA-chitosan-coated liposomes | [30] | |
| Curcumin | a. SPC:CHO 20:2 b. TFH+ extrusion | a. 0.5 b. 200 c. >85 d. N-trimethyl chitosan chloride | - After oral administration to rats, curcumin incorporated into N-trimethyl chitosan-coated liposomes exhibited different pharmacokinetic parameters (Cmax, T1/2, AUC), the greatest absorption, the slowest elimination and enhanced bioavailability compared to curcumin-loaded liposomes | [27] |
| Cyclosporin A | a. EPC:CHO:Pluronic F 127 28:5:11 b. TFH+ extrusion | a. 0.4 b. 200 c. 90 d. Chitosan | - Pharmacokinetic analysis after oral administration to rats showed that Cmax and AUC of deformable liposomes were 1.73- and 1.84-fold higher than those of chitosomes, respectively | [59] |
| Docetaxel | a. Lipoid S100:CHO: Tween-80:SDC:DCP 0.9:0.3:0.1:0.1:0.1 b. TFH+ sonication | a. 1 b. NR c. NR d. Chitosan | - After intraperitoneal administration to rats, the pharmacokinetic parameters (AUC, Cmax, mean residence time) were higher in deformable chitosomes than in deformable liposomes | [55] |
| Fexofenadine | a. DPPC:DPPG:CHO 8:1:2.25 b. TFH+ extrusion | a. 0.1 b. NR c. NR d. Chitosan | - Bioavailability of fexofenadine was increased up to 34.7% via intranasal administration of chitosomes in rats compared to uncoated liposomes (24.5%) | [73] |
| Vancomycin hydrochloride | a. Lecithin:CHO 32.5:5 * b. REV | a. 0.4 b. NR c. NR d. Chitosan | - After intravenous injection to mice, chitosomes loading vancomycin hydrochloride showed a longer retention time and higher AUC values compared to vancomycin hydrochloride injection and vancomycin hydrochloride-loaded liposomes | [56] |
| Liposome a. Composition b. Preparation Method c. Drug/EO | Chitosan a. Concentration (% w/v) b. MW (kDa) c. DD (%) | a. Anionic Polysaccharide b. Concentration (% w/v) c. MW (kDa) | Size (nm) a. PDS b. CH-LP c. UN-LP | Zeta Potential (mv) a. PDS b. CH-LP c. UN-LP | Drug Release | Ref |
|---|---|---|---|---|---|---|
| a. DPPC:CHO: DDAB NR b. TFH+ extrusion c. BSA | a. 0.1 b. 91.11 c. 85 | a. Alginate b. 0.1 c. 12 | a. 345 b. NR c. 180 | a. 36.6 b. NR c. 35.4 | - BSA released from uncoated liposomes is faster than from PDS - The increase in coating thickness by increasing polyelectrolyte layers number decreased protein release rate | [89] |
| a. Lecithin:CHO 5:1 b. TFH+extrusion c. Chrysanthemum sp. | a. 0.0025–0.01 b. NR c. NR | a. Pectin b. 0.0025–0.01 c. NR | a. 642–3235 b. 530–793 c. 98–231 | a. -19.3– -13.5 b. 34.2–45.4 c. -37.6– -27.7 | - PDS strongly reduced the leakage of EO from the liposomes - Liposomes exhibited the highest release rate (88.2%), followed by chitosomes (60.2%), and lastly, by triple layer liposomes (25.2%) | [78] |
| a. Lipoid S75 1, 2, 5% * b. High shear disperser + microfluidization c. Hibiscus extract | a. 1 b. NR c. 79 | a. Pectin b. 0.005–0.1 c. 55 | a. 98–343 b. 60–150 c. 32–46 | a.-25 b. 78 c. -29 | NR | [91] |
| a. SPC:CHO: Tween-80:6:1:1.8 * b. TFH+ high-pressure microfluidization c. Medium-chain fatty acids | a. 0.05–2 b. 50 c. NR | a. Alginate b. 0.1–2 c. 12 | a. 170–3229 b. 124–255 c. 89 | a. 1.5– -16.1 b. -1.1– 3.1 c. -6.3 | - In SGF, all liposomes formulations showed low medium-chain fatty acids release rates (29.8 and 20.4% for the liposomes and PDS, respectively) - In SIF, liposomes exhibited the highest medium-chain fatty acids (79.8%) release rate compared to PDS (56.9%) | [90] |
| a. EPC:CHO: DHP 10:2.5:1 b. TFH+ sonication c. Quercetin | a. 0.1 b. 50–190 c. NR | a. Sodium hyaluronate b. 0.1 c. 490 | a. 528 b. NR c. 121 | a. -50 b. NR c. -38.2 | - Uncoated liposomes exhibited the highest quercetin released amount after 24 h (62% at pH 5.5 and 50% at pH 7.4) - Quercetin exhibited a sustained release as the number of polyelectrolyte layers increased up to 5 (below 20% at both pH) | [80] |
| a. SPC:CHO: Tween-80 6:1:1.8 * b. TFH+ high-pressure microfluidization c. Vitamin C | a. 0.6 b. 50 c. NR | a. Alginate b. 0.5 c. 12 | a. 1809 b. 1098 c. 601 | a. -25.2 b. 24.5 c. -12.5 | In SGF, all liposome formulations showed low vitamin C release rates (25, 27 and 14% for the liposomes, chitosomes and PDS, respectively). In SIF, PDS exhibited the slowest vitamin release rate (10%) compared to chitosomes (30%) and liposomes (82%) | [64] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sebaaly, C.; Trifan, A.; Sieniawska, E.; Greige-Gerges, H. Chitosan-Coating Effect on the Characteristics of Liposomes: A Focus on Bioactive Compounds and Essential Oils: A Review. Processes 2021, 9, 445. https://doi.org/10.3390/pr9030445
Sebaaly C, Trifan A, Sieniawska E, Greige-Gerges H. Chitosan-Coating Effect on the Characteristics of Liposomes: A Focus on Bioactive Compounds and Essential Oils: A Review. Processes. 2021; 9(3):445. https://doi.org/10.3390/pr9030445
Chicago/Turabian StyleSebaaly, Carine, Adriana Trifan, Elwira Sieniawska, and Hélène Greige-Gerges. 2021. "Chitosan-Coating Effect on the Characteristics of Liposomes: A Focus on Bioactive Compounds and Essential Oils: A Review" Processes 9, no. 3: 445. https://doi.org/10.3390/pr9030445
APA StyleSebaaly, C., Trifan, A., Sieniawska, E., & Greige-Gerges, H. (2021). Chitosan-Coating Effect on the Characteristics of Liposomes: A Focus on Bioactive Compounds and Essential Oils: A Review. Processes, 9(3), 445. https://doi.org/10.3390/pr9030445








