Chemical Profiling of Pistacia lentiscus var. Chia Resin and Essential Oil: Ageing Markers and Antimicrobial Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Standards
2.2. Starting Material and Production of Essential Oil
2.3. Profiling of the Resin and Essential Oil by HPTLC
2.4. Determination of Essential Oil Constituents by GC-MS
2.5. Media and Growth Conditions
2.6. Epifluorescence Microscopy
3. Results and Discussion
3.1. Hydrodistillation Yield
3.2. HPTLC Profiling
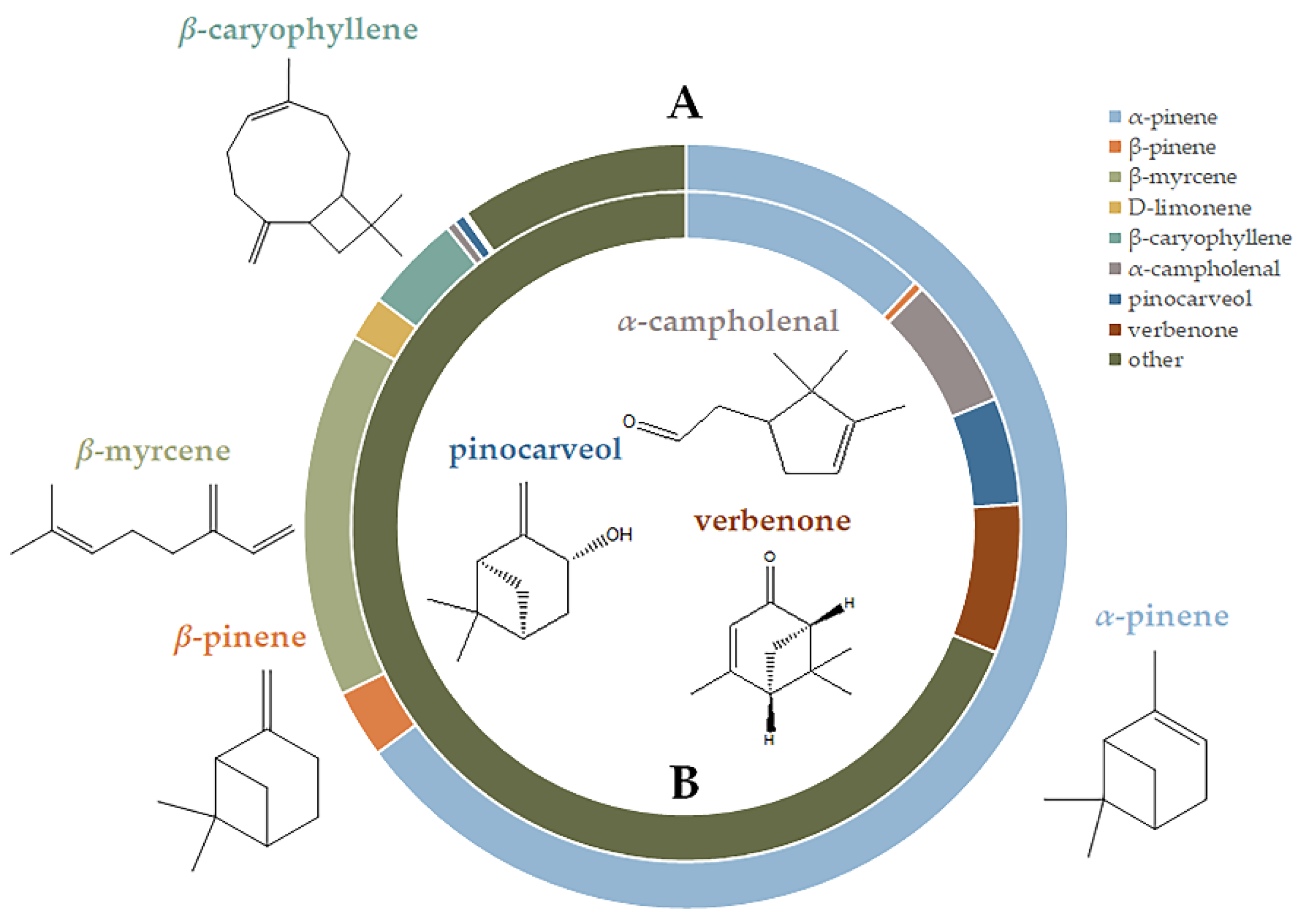
3.3. GC-MS Analysis
3.4. Essential Oil Ageing
3.5. Antifungal and Antibacterial Εffects of Resins and Essential Oils
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pachi, V.K.; Mikropoulou, E.V.; Gkiouvetidis, P.; Siafakas, K.; Argyropoulou, A.; Angelis, A.; Mitakou, S.; Halabalaki, M. Traditional Uses, Phytochemistry and Pharmacology of Chios Mastic Gum (Pistacia lentiscus Var. Chia, Anacardiaceae): A Review. J. Ethnopharmacol. 2020, 254. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No 123/97 of 23 January 1997 Supplementing the Annex to Commission Regulation (EC) No 1107/96 on the Registration of Geographical Indications and Designations of Origin. Off. J. 1997, L 22, 19–20. [Google Scholar]
- United Nations Educational, Scientific and Cultural Organization. Decision of the Intergovernmental Committee: 9.COM 10.18, Inscribing the Know-How of Cultivating Mastic on the Island of Chios on the Representative List of the Intangible Cultural Heritage of Humanity. 2014. Available online: https://ich.unesco.org/en/Decisions/9.COM/10.18 (accessed on 1 November 2020).
- European Medicines Agency. Assessment Report on Pistacia lentiscus, L., Resin (Mastix). 2015. Available online: https://www.ema.europa.eu/en/documents/herbal-report/draft-assessment-report-pistacia-lentiscus-l-resin-mastic_en.pdf (accessed on 1 November 2020).
- Dioscorides, P. De Materia Medica, 1st ed.; Militos: Alimos, Greece, 1999. [Google Scholar]
- Galen. Pharmacy, 2nd ed.; Lindsay and Blakiston: Philadelphia, PA, UAS, 1846. [Google Scholar]
- Moussaieff, A.; Fride, E.; Amar, Z.; Lev, E.; Steinberg, D.; Gallily, R.; Mechoulam, R. The Jerusalem Balsam: From the Franciscan Monastery in the Old City of Jerusalem to Martindale 33. J. Ethnopharmacol. 2005, 101, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Assimopoulou, A.N.; Zlatanos, S.N.; Papageorgiou, V.P. Antioxidant Activity of Natural Resins and Bioactive Triterpenes in Oil Substrates. Food Chem. 2005, 92, 721–727. [Google Scholar] [CrossRef]
- Dedoussis, G.V.Z.; Kaliora, A.C.; Psarras, S.; Chiou, A.; Mylona, A.; Papadopoulos, N.G.; Andrikopoulos, N.K. Antiatherogenic Effect of Pistacia lentiscus via GSH Restoration and Downregulation of CD36 MRNA Expression. Atherosclerosis 2004, 174, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Kaliora, A.C.; Stathopoulou, M.G.; Triantafillidis, J.K.; Dedoussis, G.V.Z.; Andrikopoulos, N.K. Chios Mastic Treatment of Patients with Active Crohn’s Disease. World J. Gastroenterol. 2007, 13, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Tabanca, N.; Nalbantsoy, A.; Kendra, P.E.; Demirci, F.; Demirci, B. Chemical Characterization and Biological Activity of the Mastic Gum Essential Oils of Pistacia lentiscus Var. Chia from Turkey. Molecules 2020, 25, 2136. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, I.; Peroulis, N.; Pantazis, P.; Hadzopoulou-Cladaras, M. Camphene, a Plant-Derived Monoterpene, Reduces Plasma Cholesterol and Triglycerides in Hyperlipidemic Rats Independently of HMG-CoA Reductase Activity. PLoS ONE 2011, 6, e20516. [Google Scholar] [CrossRef] [PubMed]
- Andreadou, I.; Mitakou, S.; Paraschos, S.; Efentakis, P.; Magiatis, P.; Kaklamanis, L.; Halabalaki, M.; Skaltsounis, L.; Iliodromitis, E.K. “Pistacia lentiscus”, L. Reduces the Infarct Size in Normal Fed Anesthetized Rabbits and Possess Antiatheromatic and Hypolipidemic Activity in Cholesterol Fed Rabbits. Phytomedicine 2016, 23, 1220–1226. [Google Scholar] [CrossRef] [PubMed]
- Tzani, A.I.; Doulamis, I.P.; Konstantopoulos, P.S.; Pasiou, E.D.; Daskalopoulou, A.; Iliopoulos, D.C.; Georgiadis, I.V.; Kavantzas, N.; Kourkoulis, S.K.; Perrea, D.N. Chios Mastic Gum Decreases Renin Levels and Ameliorates Vascular Remodeling in Renovascular Hypertensive Rats. Biomed. Pharmacother. 2018, 105, 899–906. [Google Scholar] [CrossRef]
- Spyridopoulou, K.; Tiptiri-Kourpeti, A.; Lampri, E.; Fitsiou, E.; Vasileiadis, S.; Vamvakias, M.; Bardouki, H.; Goussia, A.; Malamou-Mitsi, V.; Panayiotidis, M.I.; et al. Dietary Mastic Oil Extracted from Pistacia lentiscus Var. Chia Suppresses Tumor Growth in Experimental Colon Cancer Models. Sci. Rep. 2017, 7, 3782–3796. [Google Scholar] [CrossRef] [PubMed]
- Magkouta, S.; Stathopoulos, G.T.; Psallidas, I.; Papapetropoulos, A.; Kolisis, F.N.; Roussos, C.; Loutrari, H. Protective Effects of Mastic Oil from Pistacia lentiscus Variation Chia against Experimental Growth of Lewis Lung Carcinoma. Nutri. Cancer 2009, 61, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Loutrari, H.; Magkouta, S.; Pyriochou, A.; Koika, V.; Kolisis, F.N.; Papapetropoulos, A.; Roussos, C. Mastic Oil from Pistacia lentiscus Var. Chia Inhibits Growth and Survival of Human K562 Leukemia Cells and Attenuates Angiogenesis. Nutr. Cancer 2006, 55, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Loutrari, H.; Magkouta, S.; Papapetropoulos, A.; Roussos, C. Mastic Oil Inhibits the Metastatic Phenotype of Mouse Lung Adenocarcinoma Cells. Cancers 2011, 3, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Buriani, A.; Fortinguerra, S.; Sorrenti, V.; Dall’Acqua, S.; Innocenti, G.; Montopoli, M.; Gabbia, D.; Carrara, M. Human Adenocarcinoma Cell Line Sensitivity to Essential Oil Phytocomplexes from Pistacia Species: A Multivariate Approach. Molecules 2017, 22, 1336. [Google Scholar] [CrossRef] [PubMed]
- Papada, E.; Amerikanou, C.; Torović, L.; Kalogeropoulos, N.; Tzavara, C.; Forbes, A.; Kaliora, A.C. Plasma Free Amino Acid Profile in Quiescent Inflammatory Bowel Disease Patients Orally Administered with Mastiha (Pistacia lentiscus); a Randomised Clinical Trial. Phytomedicine 2019, 56, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Triantafyllou, A.; Chaviaras, N.; Sergentanis, T.N.; Protopapa, E.; Tsaknis, J. Chios Mastic Gum Modulates Serum Biochemical Parameters in a Human Population. J. Ethnopharmacol. 2007, 111, 43–49. [Google Scholar] [CrossRef]
- Kartalis, A.; Didagelos, M.; Georgiadis, I.; Benetos, G.; Smyrnioudis, N.; Marmaras, H.; Voutas, P.; Zotika, C.; Garoufalis, S.; Andrikopoulos, G. Effects of Chios Mastic Gum on Cholesterol and Glucose Levels of Healthy Volunteers: A Prospective, Randomized, Placebo-Controlled, Pilot Study (CHIOS-MASTIHA). Eur. J. Prev. Cardiol. 2015, 23, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Kontogiannis, C.; Georgiopoulos, G.; Loukas, K.; Papanagnou, E.-D.; Pachi, V.K.; Bakogianni, I.; Laina, A.; Kouzoupis, A.; Karatzi, K.; Trougakos, I.P.; et al. Chios Mastic Improves Blood Pressure Haemodynamics in Patients with Arterial Hypertension: Implications for Regulation of Proteostatic Pathways. Eur. J. Prev. Cardiol. 2019, 26, 328–331. [Google Scholar] [CrossRef]
- Sterer, N. Antimicrobial Effect of Mastic Gum Methanolic Extract Against Porphyromonas gingivalis. J. Med. Food 2006, 9, 290–292. [Google Scholar] [CrossRef]
- Koychev, S.; Dommisch, H.; Chen, H.; Pischon, N. Antimicrobial Effects of Mastic Extract Against Oral and Periodontal Pathogens. J. Periodontol. 2017, 88, 511–517. [Google Scholar] [CrossRef]
- Miyamoto, T.; Okimoto, T.; Kuwano, M. Chemical Composition of the Essential Oil of Mastic Gum and Their Antibacterial Activity Against Drug-Resistant Helicobacter pylori. Nat. Prod. Bioprospecting 2014, 4, 227–231. [Google Scholar] [CrossRef]
- Karygianni, L.; Cecere, M.; Skaltsounis, A.L.; Argyropoulou, A.; Hellwig, E.; Aligiannis, N.; Wittmer, A.; Al-Ahmad, A. High-Level Antimicrobial Efficacy of Representative Mediterranean Natural Plant Extracts against Oral Microorganisms. BioMed Res. Int. 2014, 2014, 839019. [Google Scholar] [CrossRef] [PubMed]
- Tassou, C.C.; Nychas, G.J.E. Antimicrobial Activity of the Essential Oil of Mastic Gum (Pistacia lentiscus Var. Chia) on Gram Positive and Gram Negative Bacteria in Broth and in Model Food System. Int. Biodeterior. Biodegrad. 1995, 36, 411–420. [Google Scholar] [CrossRef]
- Vrouvaki, I.; Koutra, E.; Kornaros, M.; Avgoustakis, K.; Lamari, F.N.; Hatziantoniou, S. Polymeric Nanoparticles of Pistacia lentiscus Var. Chia Essential Oil for Cutaneous Applications. Pharmaceutics 2020, 12, 353. [Google Scholar] [CrossRef] [PubMed]
- Magiatis, P.; Melliou, E.; Skaltsounis, A.L.; Chinou, I.B.; Mitaku, S. Chemical Composition and Antimicrobial Activity of the Essential Oils of Pistacia lentiscus Var. Chia. Planta Med. 1999, 65, 749–752. [Google Scholar] [CrossRef]
- Sharifi, M.S.; Hazell, S.L. Fractionation of Mastic Gum in Relation to Antimicrobial Activity. Pharmaceuticals 2009, 2, 2–10. [Google Scholar] [CrossRef]
- Paraskevopoulou, A.; Kiosseoglou, V. Chios Mastic Gum and Its Food Applications. In Functional Properties of Traditional Foods; Springer: Berlin/Heidelberg, Germany, 2016; pp. 271–287. [Google Scholar]
- Fazeli-Nasab, B.; Fooladvand, Z. Classification and Evaluation of Medicinal Plant and Medicinal Properties of Mastic. Int. J. Adv. Biol. Biomed. Res. 2014, 2, 2155–2161. [Google Scholar]
- CMGA. Available online: https://www.gummastic.gr/en (accessed on 5 November 2020).
- Mavrakis, C.; Kiosseoglou, V. The Structural Characteristics and Mechanical Properties of Biopolymer/Mastic Gum Microsized Particles Composites. Food Hydrocoll. 2008, 22, 854–861. [Google Scholar] [CrossRef]
- Paraschos, S. Phytochemical and Pharmacological Study of Chios Mastic Gum; National and Kapodistrian University of Athens: Athens, Greece, 2010. [Google Scholar] [CrossRef]
- Xynos, N.; Termentzi, A.; Fokialakis, N.; Skaltsounis, L.A.; Aligiannis, N. Supercritical CO2 extraction of Mastic Gum and Chemical Characterization of Bioactive Fractions Using LC-HRMS/MS and GC–MS. J. Supercrit. Fluids 2018, 133, 349–356. [Google Scholar] [CrossRef]
- Papanicolaou, D.; Melanitou, M.; Katsaboxakis, K. Changes in Chemical Composition of the Essential Oil of Chios “Mastic Resin” from Pistacia lentiscus Var. Chia Tree during Solidification and Storage. Dev. Food Sci. 1995, 37, 303–310. [Google Scholar] [CrossRef]
- Daferera, D.; Pappas, C.; Tarantilis, P.A.; Polissiou, M. Quantitative Analysis of α-Pinene and β-Myrcene in Mastic Gum Oil Using FT-Raman Spectroscopy. Food Chem. 2002, 77, 511–515. [Google Scholar] [CrossRef]
- Koutsoudaki, C.; Krsek, M.; Rodger, A. Chemical Composition and Antibacterial Activity of the Essential Oil and the Gum of Pistacia lentiscus Var. Chia. J. Agric. Food Chem. 2005, 53, 7681–7685. [Google Scholar] [CrossRef]
- Hellenic Republic, Ministry of Rural Development and Food. Available online: www.minagric.gr (accessed on 25 November 2020).
- Ierapetritis, D.; Fotaki, M. Συνεταιριστική Κοινωνική Επιχειρηματικότητα: H ανάπτυξη δικτύου καταστημάτων λιανικής πώλησης της Ένωσης Μαστιχοπαραγωγών Χίου και οι επιπτώσεις της (text in Greek). In Kainotomo-Epicheiro; Lioukas, S., Ed.; Athens University of Economics and Business: Athens, Greece, 2013. [Google Scholar]
- Rigling, M.; Fraatz, M.A.; Trögel, S.; Sun, J.; Zorn, H.; Zhang, Y. Aroma Investigation of Chios Mastic Gum (Pistacia lentiscus Variety Chia) Using Headspace Gas Chromatography Combined with Olfactory Detection and Chiral Analysis. J. Agric. Food Chem. 2019, 67, 13420–13429. [Google Scholar] [CrossRef]
- European Pharmacopoeia 9.0; 2011; Volume 1, pp. 2277–2278. Available online: https://www.tsoshop.co.uk/?DI=647707 (accessed on 28 January 2021).
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- NIST. Available online: https://webbook.nist.gov/chemistry/ (accessed on 20 October 2020).
- Maryutina, T.A.; Savonina, E.Y.; Fedotov, P.S.; Smith, R.M.; Siren, H.; Hibbert, D.B. Terminology of Separation Methods (IUPAC Recommendations 2017). Pure Appl. Chem. 2018, 90, 181–213. [Google Scholar] [CrossRef]
- FGSC. Available online: http://www.fgsc.net. (accessed on 2 November 2020).
- Papanicolaou, D.; Melanitou, M.; Katsaboxakis, K. Effect of α-Tocopherol (Vitamin E) on the Retention of Essential Oil, Color and Texture of Chios Mastic Resin during Storage. Dev. Food Sci. 1998, 40, 689–694. [Google Scholar] [CrossRef]
- Papageorgiou, V.P.; Mellidis, A.S.; Argyriadou, N. The Chemical Composition of the Essential Oil of Mastic Gum. J. Essent. Oil Res. 1991, 3, 107–110. [Google Scholar] [CrossRef]
- Paraschos, S.; Magiatis, P.; Gikas, E.; Smyrnioudis, I.; Skaltsounis, A.-L. Quality Profile Determination of Chios Mastic Gum Essential Oil and Detection of Adulteration in Mastic Oil Products with the Application of Chiral and Non-Chiral GC–MS Analysis. Fitoterapia 2016, 114, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Van Den Berg, K.J.; Van Der Horst, J.; Boon, J.J.; Sudmeijer, O.O. Cis-1,4-Poly-β-Myrcene: The Structure of the Polymeric Fraction of Mastic Resin (Pistacia lentiscus, L.) Elucidated. Tetrahedron Lett. 1998, 39, 2645–2648. [Google Scholar] [CrossRef]
- Behr, A.; Johnen, L. Myrcene as a Natural Base Chemical in Sustainable Chemistry: A Critical Review. ChemSusChem 2009, 2, 1072–1095. [Google Scholar] [CrossRef] [PubMed]
- Mikropoulou, E.V.; Petrakis, E.A.; Argyropoulou, A.; Halabalaki, M.; Skaltsounis, L.A. Quantification of Bioactive Lignans in Sesame Seeds Using HPTLC Densitometry: Comparative Evaluation by HPLC-PDA. Food Chem. 2019, 288, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Meier, B.; Spriano, D. Modern HPTLC-A Perfect Tool for Quality Control of Herbals and Their Preparations. J. AOAC Int. 2010, 93, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Assimopoulou, A.N.; Papageorgiou, V.P. GC-MS Analysis of Penta- and Tetra-Cyclic Triterpenes from Resins of Pistacia Species. Part, I. Pistacia lentiscus Var. Chia. Biomed. Chromatogr. 2005, 19, 285–311. [Google Scholar] [CrossRef]
- Papageorgiou, V.P.; Bakola-Christianopoulou, M.N.; Apazidou, K.K.; Psarros, E.E. Gas Chromatographic-Mass Spectroscopic Analysis of the Acidic Triterpenic Fraction of Mastic Gum. J. Chromatography A 1997, 769, 263–273. [Google Scholar] [CrossRef]
- Serifi, I.; Tzima, E.; Bardouki, H.; Lampri, E.; Papamarcaki, T. Effects of the Essential Oil from Pistacia lentiscus Var. Chia on the Lateral Line System and the Gene Expression Profile of Zebrafish (Danio rerio). Molecules 2019, 24, 3919. [Google Scholar] [CrossRef]
- Papageorgiou, V.P.; Sagredos, A.N.; Mose, R. GLC-MS Computer Analysis of the Essential Oil of Mastic Gum. Chim. Chronica New Ser. 1981, 10, 119–124. [Google Scholar]
- Boelens, M.H.; Jimenez, R. Chemical Composition of the Essential Oils from the Gum and from Various Parts of Pistacia lentiscus L. (Mastic Gum Tree). Flavour Fragr. J. 1991, 6, 271–275. [Google Scholar] [CrossRef]
- Duru, M.E.; Cakir, A.; Kordali, S.; Zengin, H.; Harmandar, M.; Izumi, S.; Hirata, T. Chemical Composition and Antifungal Properties of Essential Oils of Three Pistacia Species. Fitoterapia 2003, 74, 170–176. [Google Scholar] [CrossRef]
- Burham, B.O.; EL-Kamali, H.H.; EL-Egami, A.A. Volatile Components of the Resin of Pistacia lentiscus “Mistica” Used in Sudanese Traditional Medicine. J. Chem. Pharm. Res. 2011, 3, 478–482. [Google Scholar]
- Neuenschwander, U.; Guignard, F.; Hermans, I. Mechanism of the Aerobic Oxidation of α-Pinene. ChemSusChem 2010, 3, 75–84. [Google Scholar] [CrossRef]
- Neuenschwander, U.; Meier, E.; Hermans, I. Peculiarities of β-Pinene Autoxidation. ChemSusChem 2011, 4, 1613–1621. [Google Scholar] [CrossRef] [PubMed]
- Slaghenaufi, D.; Ugliano, M. Norisoprenoids, Sesquiterpenes and Terpenoids Content of Valpolicella Wines during Aging: Investigating Aroma Potential in Relationship to Evolution of Tobacco and Balsamic Aroma in Aged Wine. Front. Chem. 2018, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Karlberg, A.; Magnusson, K.; Nilsson, U. Air Oxidation of D-limonene (the Citrus Solvent) Creates Potent Allergens. Contact Dermat. 1992, 26, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Scazzocchio, C. Aspergillus: A Multifaceted Genus. In Encyclopedia of Microbiology; Academic Press: Cambridge, MA, USA, 2009; ISBN 9780123739445. [Google Scholar]
- Jerez Puebla, L.E. Fungal Infections in Immunosuppressed Patients. In Immunodeficiency; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Latgé, J.P.; Chamilos, G. Aspergillus Fumigatus and Aspergillosis in 2019. Clin. Microbiol. Rev. 2020, 33. [Google Scholar] [CrossRef]
- Ghuman, H.; Voelz, K. Innate and Adaptive Immunity to Mucorales. J. Fungi 2017, 3, 48. [Google Scholar] [CrossRef]
- Chamilos, G.; Lewis, R.E.; Kontoyiannis, D.P. Lovastatin Has Significant Activity against Zygomycetes and Interacts Synergistically with Voriconazole. Antimicrob. Agents Chemother. 2006, 50, 96–103. [Google Scholar] [CrossRef]
- Wiederhold, N.P. Antifungal Resistance: Current Trends and Future Strategies to Combat. Infect. Drug Resist. 2017, 10, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Pantazopoulou, A.; Lemuh, N.D.; Hatzinikolaou, D.G.; Drevet, C.; Cecchetto, G.; Scazzocchio, C.; Diallinas, G. Differential Physiological and Developmental Expression of the UapA and AzgA Purine Transporters in Aspergillus nidulans. Fungal Genet. Biol. 2007, 44, 627–640. [Google Scholar] [CrossRef] [PubMed]
- Toews, M.W.; Warmbold, J.; Konzack, S.; Rischitor, P.; Veith, D.; Vienken, K.; Vinuesa, C.; Wei, H.; Fischer, R. Establishment of MRPF1 as a Fluorescent Marker in Aspergillus nidulans and Construction of Expression Vectors for High-Throughput Protein Tagging Using Recombination in Vitro (GATEWAY). Curr. Genet. 2004, 45, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Sant, D.G.; Tupe, S.G.; Ramana, C.V.; Deshpande, M.V. Fungal Cell Membrane—Promising Drug Target for Antifungal Therapy. J. Appl. Microbiol. 2016, 121, 1498–1510. [Google Scholar] [CrossRef] [PubMed]
- Gkogka, E.; Hazeleger, W.C.; Posthumus, M.A.; Beumer, R.R. The Antimicrobial Activity of the Essential Oil of Pistacia lentiscus Var. Chia. J. Essent. Oil Bear. Plants 2013, 16, 714–729. [Google Scholar] [CrossRef]



| Collection Time (month/year) | Resin | Essential Oil | Yield (mL/kg) |
|---|---|---|---|
| 01/2020 | CMG_1 | CMO_1 | 11.9 |
| 01/2020 | CMG_2 | CMO_2 | 10.3 |
| 02/2020 | CMG_3 | CMO_3 | 23.4 |
| 02/2020 | CMG_4 | CMO_4 | 19.5 |
| 02/2020 | CMG_5 | CMO_5 | 24.8 |
| 06/2020 | CMG_6 | CMO_6 | 9.9 |
| 06/2020 | CMG_7 | CMO_7 | 14.3 |
| 11/2018 | CMG_8 | CMO_8 | 3.5 |
| Minimum | 3.5 | ||
| Maximum | 24.8 | ||
| Average ± SD 1 | 14.7 ± 7.3 | ||
| 2010 | CMG_9 | CMO_9 | traces |
| 2010 | CMG_10 | CMO_10 | - |
| 2010 | CMG_11 | CMO_11 | - |
| A/A | Rt (min) 1 | Compound | Molecular Formula | MW (g/mol) 2 | Main Fragments (Descending Intensity) | RI 3 | IM 4 |
|---|---|---|---|---|---|---|---|
| 1 | 9.75 | N/I_C01 | C10H16 | 136.23 | 93, 67, 49, 41, 84, 79, 108, 121, 136 | - | MS |
| 2 | 10.36 | α-Pinene | C10H16 | 136.23 | 93, 77, 105, 121, 67, 41, 53, 136 | - | MS |
| 3 | 12.10 | Sabinene | C10H16 | 136.23 | 93, 77, 41, 136, 121, 105 | - | MS |
| 4 | 12.31 | β-Pinene | C10H16 | 136.23 | 93, 69, 41, 79, 121, 136 | - | MS |
| 5 | 12.88 | β-Myrcene | C10H16 | 136.23 | 69, 93, 41, 79, 53, 121, 107, 136 | - | MS |
| 6 | 14.06 | o-Methyl-anisole | C8H10O | 122.16 | 122, 107, 77, 91, 51, 69, 41, 83 | - | MS |
| 7 | 14.76 | D-Limonene | C10H16 | 136.23 | 68, 93, 79, 107, 53, 121, 41, 136 | - | MS |
| 8 | 16.22 | N/I_C02 | C10H18O | 154.25 | 49, 84, 93, 41, 69, 77, 121, 107, 136 | - | MS |
| 9 | 16.88 | N/I_C03 | C10H18O | 154.25 | 49, 84, 93, 69, 41, 79, 107, 122, 137, 152 | - | MS |
| 10 | 17.64 | Terpinolene | C10H16 | 136.23 | 93, 121, 136, 69, 79, 41, 105, 152 | - | MS |
| 11 | 17.86 | Camphenol | C10H16O | 152.23 | 93, 108, 67, 41, 79, 121, 137, 152 | - | MS |
| 12 & 13 | 18.25 | Perillene & α-Linalool | C10H14O & C10H18O | 150.22 & 154.25 | 69, 71, 41, 93, 55, 81, 150, 121, 107, 135 | - | MS |
| 14 | 18.81 | N/I_C04 | C10H18O | 154.25 | 69, 71, 93, 41, 55, 84, 121, 150, 107, 136, 154 | - | MS |
| 15 | 19.67 | α-Campholenal | C10H16O | 152.23 | 108, 93, 67, 41, 81, 150, 119, 136 | - | MS |
| 16 | 20.30 | Pinocarveol | C10H16O | 152.23 | 55, 92, 69, 41, 83, 109, 119, 134, 150 | - | MS |
| 17 | 20.57 | Cis-Verbenol | C10H16O | 152.23 | 109, 41, 81, 91, 69, 119, 150, 137 | - | MS |
| 18 | 21.40 | N/I_C05 | C11H18 | 150.26 | 49, 84, 69, 41, 108, 93, 135, 122, 150 | - | MS |
| 19 | 22.33 | Verbenol | C10H16O | 152.23 | 59, 94, 79, 69, 83, 41, 109, 119, 136, 150 | - | MS |
| 20 | 23.07 | Myrtenal | C10H14O | 150.22 | 79, 107, 91, 41, 67, 119, 135, 150 | 1203.0 | RI, MS |
| 21 | 23.81 | Verbenone | C10H14O | 150.22 | 107, 91, 79, 135, 67, 41, 150, 122 | 1220.5 | RI, MS |
| 22 | 26.27 | N/I_C06 | C10H16O | 152.23 | 93, 69, 49, 41, 84, 79, 136, 164, 121, 109, 150 | - | MS |
| 23 | 26.89 | Bornyl acetate | C12H20O2 | 196.29 | 95, 43, 121, 136, 108, 67, 55, 80, 154, 196 | 1291.6 | RI, MS |
| 24 | 29.46 | N/I_C07 | C15H26O | 222.37 | 105, 161, 119, 49, 91, 69, 41, 84, 58, 204, 148, 133 | - | MS |
| 25 | 29.64 | α-Longipinene | C15H24 | 204.35 | 119, 105, 93, 133, 69, 41, 55, 79, 204, 161, 152, 189 | 1354.4 | RI, MS |
| 26 | 30.46 | α-Ylangene | C15H24 | 204.35 | 105, 119, 93, 161, 41, 69, 79, 55, 133, 204, 189, 148 | 1373.0 | RI, MS |
| 27 | 30.76 | α-Copaene | C15H24 | 204.35 | 105, 119, 161, 93, 69, 81, 41, 148, 133, 204, 189 | 1379.6 | RI, MS |
| 28 | 31.10 | β-Bourbonene | C15H24 | 204.35 | 81, 123, 91, 105, 41, 69, 161, 148, 133, 204 | 1387.3 | RI, MS |
| 29 | 31.33 | β-Elemene | C15H24 | 204.35 | 93, 81, 69, 148, 41, 105, 121, 133, 161, 189, 176, 204 | 1392.7 | RI, MS |
| 30 | 31.98 | Isocaryophyllene | C15H24 | 204.35 | 93, 69, 133, 105, 41, 79, 55, 148, 119, 161, 189, 175, 204 | 1407.6 | RI, MS |
| 31 | 32.65 | β-Caryophyllene | C15H24 | 204.35 | 93, 133, 105, 69, 79, 41, 55, 120, 147, 161, 189, 175, 204 | 1423.9 | RI, MS |
| 32 | 34.21 | α-Humulene | C15H24 | 204.35 | 93, 121, 80, 107, 147, 67, 41, 53, 204, 136, 189, 161, 175 | 1461.1 | RI, MS |
| 33 | 35.11 | α-Muurolene | C15H24 | 204.35 | 105, 161, 91, 119, 79, 133, 69, 41, 204, 55, 148, 189, 178 | 1482.3 | RI, MS |
| 34 | 35.39 | D-Germacrene | C15H24 | 204.35 | 161, 105, 91, 79, 119, 41, 148, 133, 69, 204, 178 | 1489.0 | RI, MS |
| 35 | 39.46 | Caryophyllene oxide | C15H24O | 220.35 | 79, 93, 41, 69, 107, 55, 121, 135, 147, 161, 178, 187, 205, 220 | 1591.2 | RI, MS |
| 36 | 40.68 | α-Humulene epoxide II | C15H24O | 220.35 | 109, 67, 96, 138, 43, 55, 81, 123, 178, 148, 164, 205, 191, 220 | - | MS |
| 37 | 50.40 | p-Camphorene/Dimyrcene | C20H32 | 272.47 | 69, 41, 93, 105, 55, 119, 79, 133, 229, 147, 187, 161, 272, 175, 202, 216, 243, 257 | - | MS |
| 38 | 51.15 | Dimyrcene | C20H32 | 272.47 | 69, 93, 41, 105, 55, 79, 121, 187, 147, 229, 133, 159, 203, 272, 175, 257, 215, 243 | - | MS |
| 39 | 52.26 | m-Camphorene/Dimyrcene | C20H32 | 272.47 | 69, 41, 91, 105, 119, 79, 133, 55, 147, 229, 203, 161, 187, 272, 257, 173, 216, 243 | - | MS |
| 40 | 53.58 | Dimyrcene | C20H32 | 272.47 | 69, 93, 41, 105, 79, 133, 119, 55, 229, 147, 203, 161, 187, 272, 257, 175 | - | MS |
| Compounds | (Min–Max) % in Fresh Samples (n = 7) | Average % ± SD in Fresh Samples (n = 7) | % in the 2-Year-Old Sample (n = 1) | % in the 10-Year-Old Sample (n = 1) |
|---|---|---|---|---|
| Monoterpene hydrocarbons | 79.40–91.15 | 86.01 ± 4.37 | 81.96 | 13.2 |
| Oxygenated monoterpenes1 & Benzenoids 2 | 3.75–6.25 | 4.60 ± 0.78 | 4.35 | 52.51 |
| Sesquiterpene hydrocarbons | 2.84–7.94 | 4.99 ± 1.96 | 5.91 | 0.08 |
| Oxygenated sesquiterpenes | 0.29–0.96 | 0.63 ± 0.26 | 0.90 | 1.91 |
| Diterpene hydrocarbons | 0.29–2.62 | 1.47 ± 0.89 | 3.23 | - |
| Ketones | - | - | 3.23 | |
| Total identified | 96.12–98.43 | 97.69 ± 0.79 | 96.35 | 70.85 |
| Total non-identified | 0.76–1.66 | 1.10 ± 0.31 | 1.98 | 29.00 |
| Compounds | (Min–Max) % in Fresh Samples (n = 7) | Average % ± SD in Fresh Samples (n = 7) | % in the 2-Year-Old Sample (n = 1) | % in the 10-Year-Old Sample (n = 1) |
| Monoterpene Hydrocarbons | ||||
| α-Pinene | 56.42–72.99 | 64.83 ± 5.78 | 65.65 | 12.00 |
| β-Pinene | 2.47–3.08 | 2.75 ± 0.25 | 3.81 | 0.45 |
| β-Myrcene | 12.6- 19.94 | 16.40 ± 3.17 | 8.90 | - |
| Sabinene | 0.18–0.3 | 0.23 ± 0.04 | 0.28 | - |
| D-Limonene | 1.24–1.98 | 1.66 ± 0.32 | 3.24 | - |
| Terpinolene | 0.11–0.2 | 0.15 ± 0.04 | 0.08 | - |
| Camphene | - | - | - | 0.75 |
| Oxygenated Monoterpenes 1 & Benzenoids 2 | ||||
| Perillene1 & α-Linalool 1 | 0.84–2.53 | 1.68 ± 0.64 | 1.73 | - |
| Camphenol 1 | 0.06–0.09 | 0.08 ± 0.01 | 0.07 | - |
| α-Campholenal 1 | 0.33–0.47 | 0.39 ± 0.05 | 0.42 | 6.32 |
| Pinocarveol 1 | 0.31–0.72 | 0.50 ± 0.14 | 0.29 | 5.16 |
| cis-Verbenol 1 | 0.3–1.05 | 0.61 ± 0.25 | 0.2 | - |
| Verbenol 1 | 0.15–0.31 | 0.21 ± 0.057 | 0.17 | - |
| Verbenone 1 | 0.07–0.22 | 0.13 ± 0.059 | 0.07 | 7.21 |
| Bornyl acetate 1 | 0.07–0.2 | 0.11 ± 0.046 | 0.06 | 0.98 |
| Campholene group 1 | - | - | - | 4.62 |
| Camphor 1 | - | - | - | 0.78 |
| 3,6,6-Trimethyl norpinan-2-one 1 & Pinocarvone1 | - | - | - | 4.99 |
| cis-3-Pinanone 1 | - | - | - | 0.27 |
| cis-Carveol 1 | - | - | - | 1.47 |
| 1-Ethenyl-2,4-dimethylbenzene or 1-Methyl-4-(2-propenyl)-benzene2 & o-Methyl-anisole 2 | 0.36–0.84 | 0.60 ± 0.16 | 1.14 | 1.93 |
| o-, p- & m-Cymene 2 | - | - | - | 5.30 |
| β-Methyl-cinnamaldehyde 2 | - | - | - | 0.15 |
| Myrtenal1 & p-Cymen-8-ol 2 | 0.22–0.39 | 0.28 ± 0.058 | 0.2 | 11.11 |
| Carvone1 & Trimethyl-hydroquinone 2 | - | - | - | 2.22 |
| Sesquiterpene Hydrocarbons | ||||
| β-Caryophyllene | 2.21–6.38 | 4.13 ± 1.53 | 4.74 | - |
| α-Humulene | 0.28–0.91 | 0.54 ± 0.22 | 0.69 | - |
| α-Longipinene | 0.01–0.07 | 0.03 ± 0.02 | 0.02 | - |
| α-Ylangene | 0.04–0.16 | 0.10 ± 0.04 | 0.08 | 0.08 |
| α-Copaene | 0.02–0.08 | 0.05 ± 0.02 | 0.06 | - |
| β-Bourbonene | 0.01–0.07 | 0.04 ± 0.02 | 0.07 | - |
| β-Elemene | 0.01–0.09 | 0.04 ± 0.03 | 0.07 | - |
| Isocaryophyllene | 0.02–0.11 | 0.06 ± 0.03 | 0.07 | - |
| a-Muurolene | 0.03–0.09 | 0.05 ± 0.02 | 0.08 | - |
| D-Germacrene | 0.05–0.1 | 0.06 ± 0.02 | 0.03 | - |
| Oxygenated Sesquiterpenes | ||||
| Caryophyllene oxide | 0.27–0.89 | 0.58 ± 0.24 | 0.82 | 1.63 |
| α-Humulene epoxide II | 0.02–0.07 | 0.05 ± 0.02 | 0.08 | - |
| 3,8,8-Trimethyl-1,2,3,4,5,6,7,8-octahydro-2-naphthalenyl methyl acetate | - | - | - | 0.28 |
| Ketones | ||||
| 4-Acetyl-1-methylcyclohexene | - | - | - | 1.53 |
| 2-Undecanone | - | - | - | 1.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pachi, V.K.; Mikropoulou, E.V.; Dimou, S.; Dionysopoulou, M.; Argyropoulou, A.; Diallinas, G.; Halabalaki, M. Chemical Profiling of Pistacia lentiscus var. Chia Resin and Essential Oil: Ageing Markers and Antimicrobial Activity. Processes 2021, 9, 418. https://doi.org/10.3390/pr9030418
Pachi VK, Mikropoulou EV, Dimou S, Dionysopoulou M, Argyropoulou A, Diallinas G, Halabalaki M. Chemical Profiling of Pistacia lentiscus var. Chia Resin and Essential Oil: Ageing Markers and Antimicrobial Activity. Processes. 2021; 9(3):418. https://doi.org/10.3390/pr9030418
Chicago/Turabian StylePachi, Vasiliki K., Eleni V. Mikropoulou, Sofia Dimou, Mariangela Dionysopoulou, Aikaterini Argyropoulou, George Diallinas, and Maria Halabalaki. 2021. "Chemical Profiling of Pistacia lentiscus var. Chia Resin and Essential Oil: Ageing Markers and Antimicrobial Activity" Processes 9, no. 3: 418. https://doi.org/10.3390/pr9030418
APA StylePachi, V. K., Mikropoulou, E. V., Dimou, S., Dionysopoulou, M., Argyropoulou, A., Diallinas, G., & Halabalaki, M. (2021). Chemical Profiling of Pistacia lentiscus var. Chia Resin and Essential Oil: Ageing Markers and Antimicrobial Activity. Processes, 9(3), 418. https://doi.org/10.3390/pr9030418