Pretreatment of Switchgrass for Production of Glucose via Sulfonic Acid-Impregnated Activated Carbon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Switchgrass Preparation
2.2. Catalyst Preparation
2.3. Catalyst Characterization
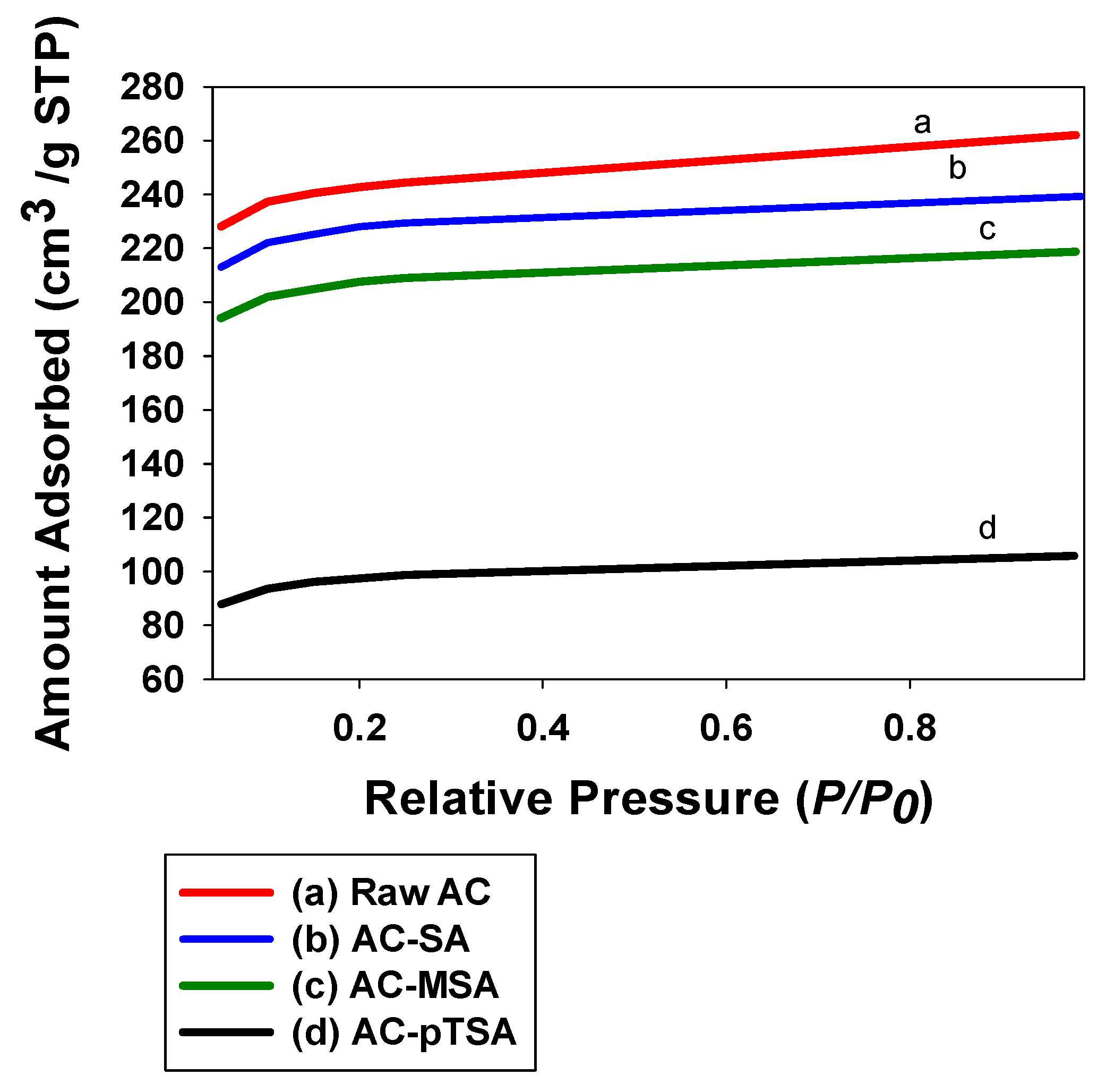
2.3.1. BET Surface Area Analysis
2.3.2. Determination of Surface Functional Groups
2.3.3. Thermogravimetric Analysis
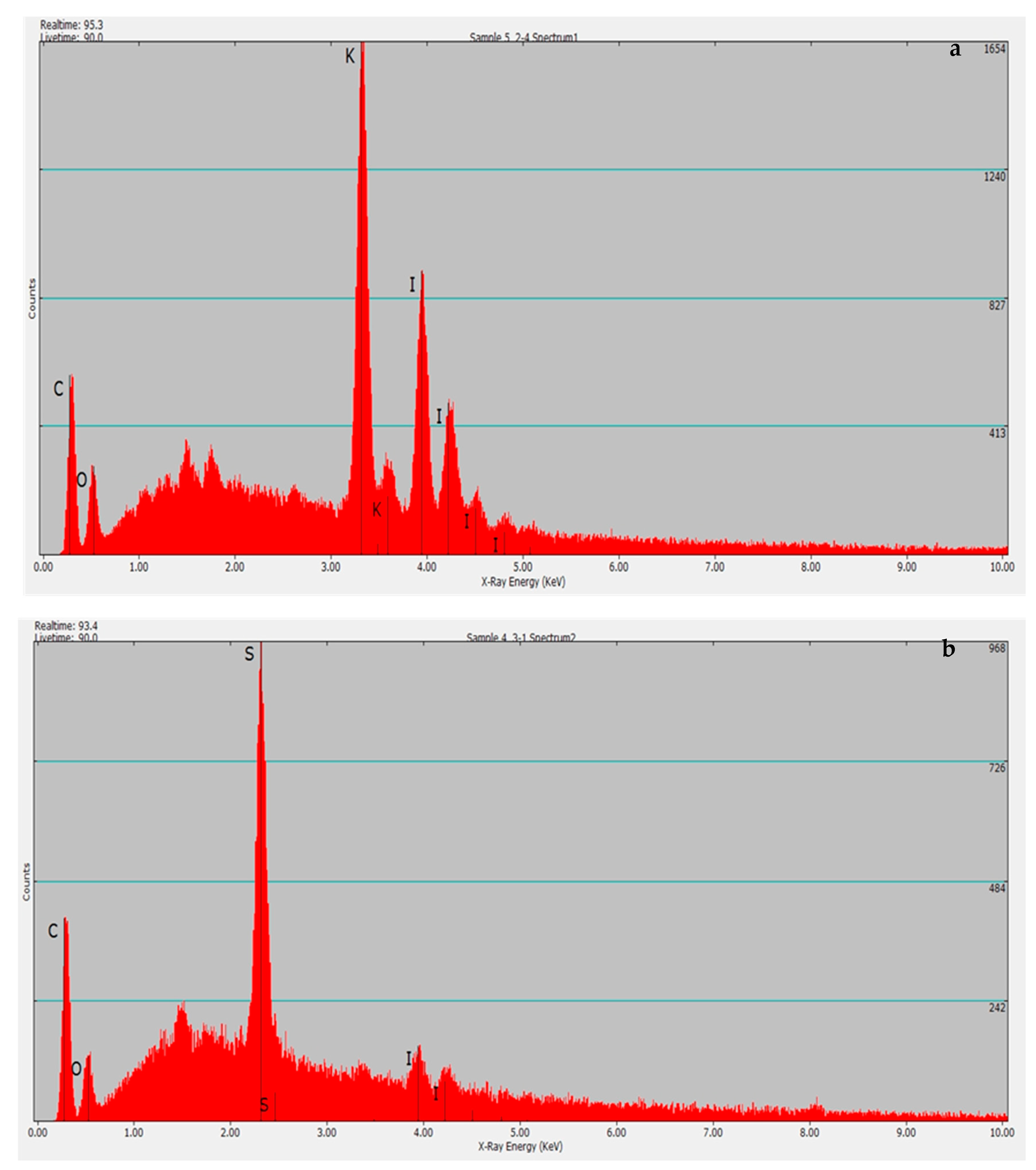
2.3.4. Energy-Dispersive Spectroscopy
2.3.5. X-ray Photoelectron Spectroscopy
2.4. Catalytic Pretreatment of Switchgrass
2.5. Reusability of the Catalyst
2.6. Enzymatic Hydrolysis
2.7. Composition Analysis
2.8. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. Catalyst Characterization
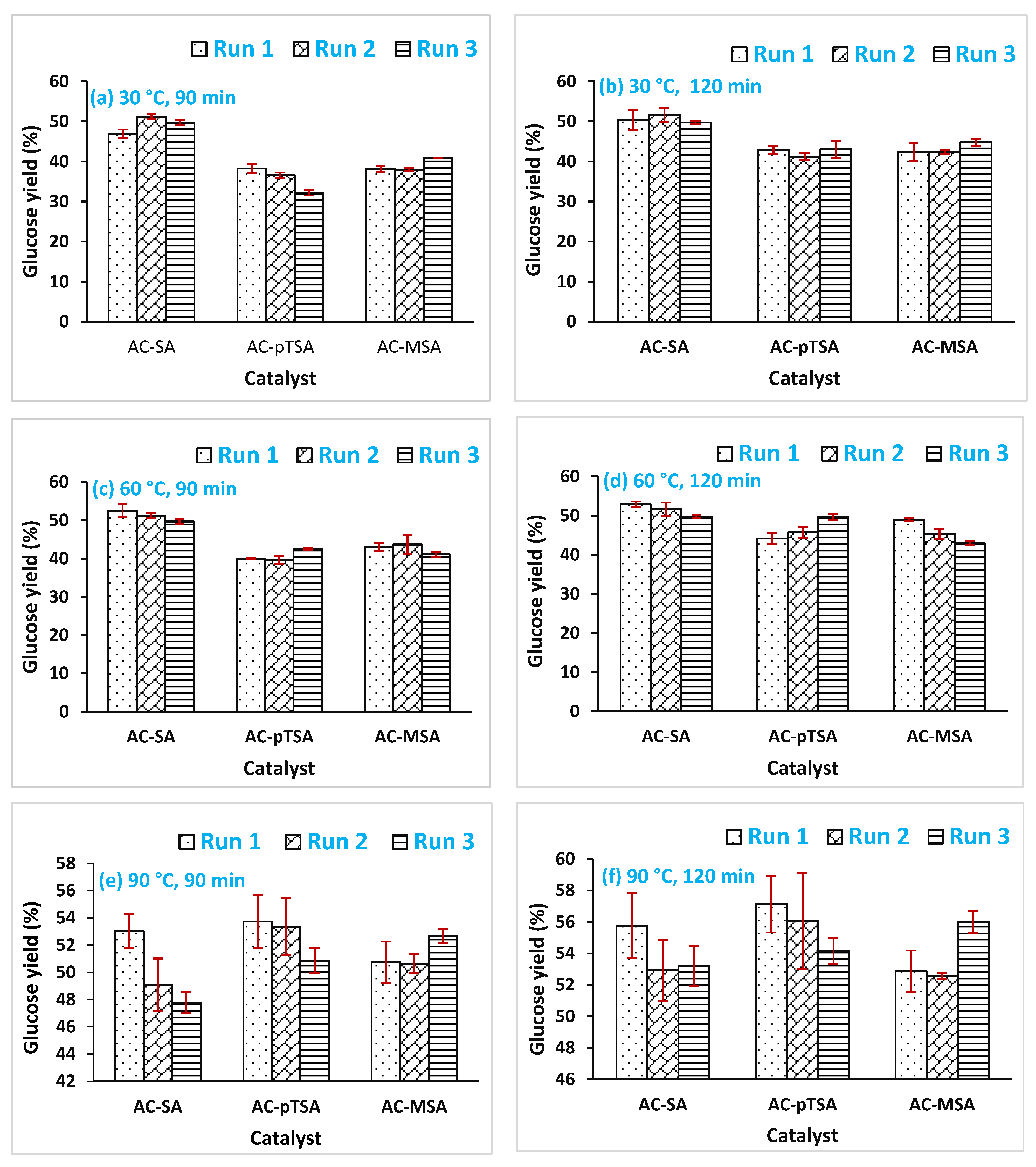
3.2. Effect of Pretreatment Temperature on Glucose Production
3.3. Effect of Pretreatment Time on Glucose Production
3.4. Effect of the Reusability of Catalyst on Glucose Production
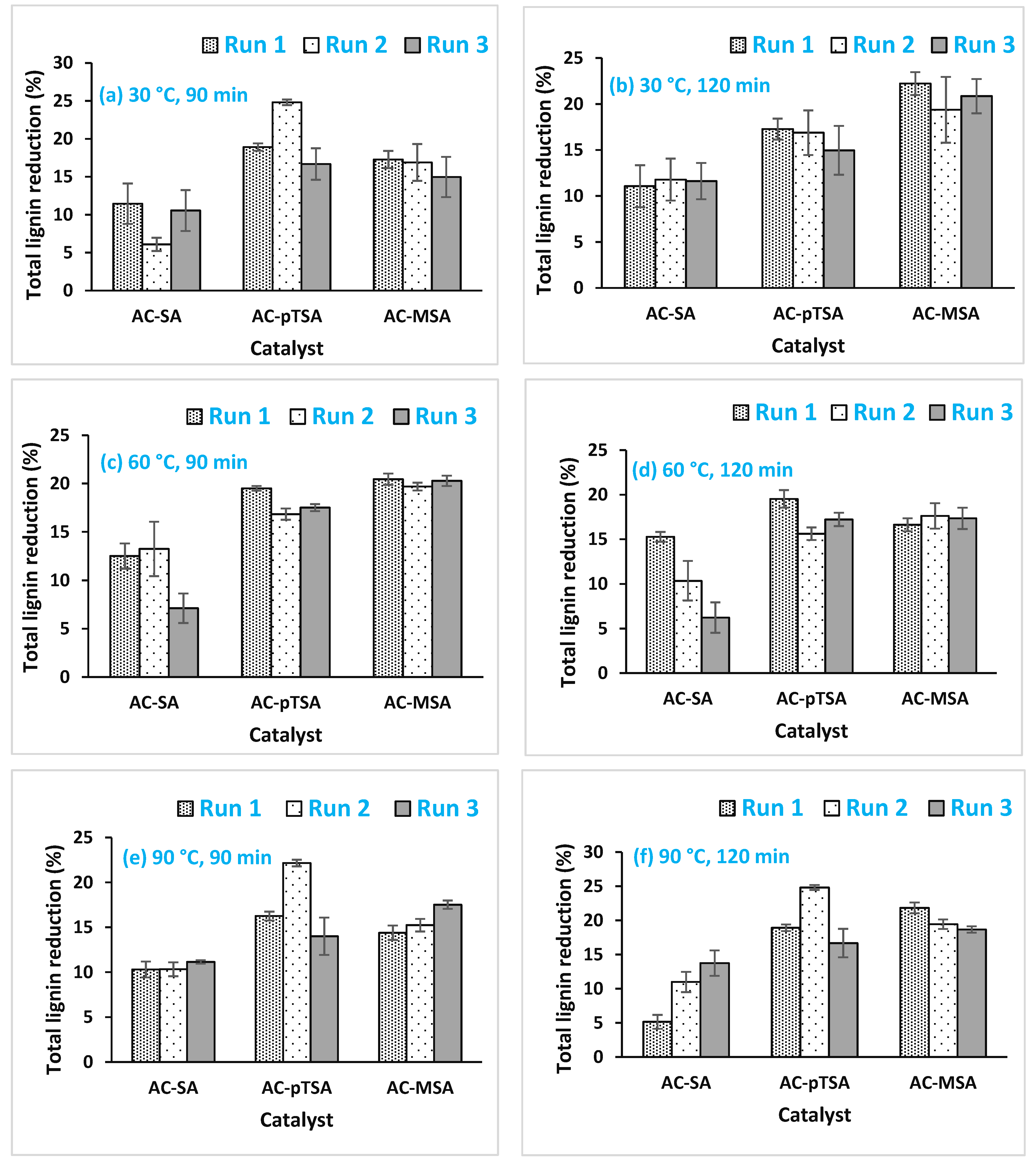
3.5. Effect of Sulfonic Solid Acid Pretreatment on Delignification
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresource Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Shirkavand, E.; Baroutian, S.; Gapes, D.J.; Young, B.R. Combination of fungal and physicochemical processes for lignocellulosic biomass pretreatment—A review. Renew. Sustain. Energ. Rev. 2016, 54, 217–234. [Google Scholar] [CrossRef]
- Su, T.; Zhao, D.; Khodadadi, M.; Len, C. Lignocellulosic biomass for bioethanol: Recent advances, technology trends, and barriers to industrial development. Curr. Opin. Green Sustain. Chem. 2020, 24, 56–60. [Google Scholar] [CrossRef]
- Wright, L.; Turhollow, A. Switchgrass selection as a “model” bioenergy crop: A history of the process. Biomass Bioenergy 2010, 34, 851–868. [Google Scholar] [CrossRef]
- Somerville, C.; Youngs, H.; Taylor, C.; Davis, S.; Long, S. Feedstocks for lignocellulosic biofuels. Science 2010, 329, 790–792. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Rondon, V.; Weeks, K.; Pullammanappallil, P.; Ingram, L.O.; Shanmugam, K.T. Phosphoric acid based pretreatment of switchgrass and fermentation of entire slurry to ethanol using a simplified process. Bioresour. Technol. 2018, 251, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Shi, D.; Han, J.; Zhang, G.; Jiang, X.; Yang, M.; Wu, Z.; Fu, C.; Li, Z.; Xian, M.; et al. Comparative study on pretreatment processes for different utilization purposes of switchgrass. ACS Omega 2020, 5, 21999–22007. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.; Clark, C.D.; Ellis, P.; English, B.; Menard, J.; Walsh, M.; Ugarte, D.L.T. Farmer willingness to grow switchgrass for energy production. Biomass Bioenergy 2007, 31, 773–781. [Google Scholar] [CrossRef] [Green Version]
- Schmer, M.R.; Vogel, K.P.; Mitchell, R.B.; Perrin, R.K. Net energy of cellulosic ethanol from switchgrass. Proc. Natl. Acad.Sci. USA 2008, 105, 464–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, Z.; Yang, G.; Song, J.; Zheng, P.; Liu, J.; Zhu, W.; Huang, L.; Chen, L.; Luo, X.; Shuai, L. Understanding the promoting effect of non-catalytic protein on enzymatic hydrolysis effciency of lignocelluloses. Bioresour. Bioprocess. 2021, 8. [Google Scholar] [CrossRef]
- Kothari, N.; Bhagia, S.; Pu, Y.; Yoo, C.G.; Mi, L.; Venketachalam, S.; Pattathil, S.; Kumar, R.; Cai, C.M.; Hahn, M.G.; et al. The effect of switchgrass plant cell wall properties on its deconstruction by thermochemical pretreatments coupled with fungal enzymatic hydrolysis or Clostridium thermocellum consolidated bioprocessing. Green Chem. 2020, 22, 7924. [Google Scholar] [CrossRef]
- Karp, E.M.; Resch, M.G.; Donohoe, B.S.; Ciesielski, P.N.; O’Brien, M.H.; Nill, J.E.; Mittal, A.; Biddy, M.J.; Beckham, G.T. Alkaline pretreatment of switchgrass. Sustain. Chem. Eng. 2015, 3, 1479–1491. [Google Scholar] [CrossRef]
- Kumar, S.; Kothari, U.; Kong, L.; Lee, Y.; Gupta, R. Hydrothermal pretreatment of switchgrass and corn stover for production of ethanol and carbon microspheres. Biomass Bioenergy 2011, 35, 956–968. [Google Scholar] [CrossRef]
- Yang, Y.; Sharma-Shivappa, R.; Burns, J.; Cheng, J. Dilute acid pretreatment of oven-dried switchgrass germplasms for bioethanol production. Energy Fuels Energy Fuels 2009, 759–3766. [Google Scholar] [CrossRef]
- Chen, H.; Liu, J.; Chang, X.; Chen, D.; Xue, Y.; Liu, P.; Lin, H.; Han, S. A review on the pretreatment of lignocellulose for high-value chemicals. Fuel Process. Technol. 2017, 160, 196–206. [Google Scholar] [CrossRef]
- Kumar, P.; Barrett, D.; Delwiche, M.; Stroeve, P. Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind. Eng. Chem. Res. 2009, 3713–3729. [Google Scholar] [CrossRef]
- Huang, Y.; Fu, Y. Hydrolysis of cellulose to glucose by solid acid catalysts. Green Chem. 2013, 15, 1095–1111. [Google Scholar] [CrossRef]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresource. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef]
- Wyman, C.E.; Dale, B.E.; Elander, R.T.; Holtzapple, M.; Ladisch, M.R.; Lee, Y.Y. Coordinated development of leading biomass pretreatment technologies. Bioresour. Technol. 2005, 96, 1959–1966. [Google Scholar] [CrossRef]
- Dien, B.S.; Jung, H.J.G.; Vogel, K.P.; Casler, M.D.; Lamb, J.F.S.; Iten, L.; Mitchell, R.B.; Sarath, G. Chemical composition and response to dilute-acid pretreatment and enzymatic saccharification of alfalfa, reed canarygrass, and switchgrass. Biomass Bioenergy 2006, 30, 880–891. [Google Scholar] [CrossRef] [Green Version]
- Wyman, C.E.; Balan, V.; Dale, B.E.; Elander, R.T.; Falls, M.; Hames, B.; Holtzapple, M.T.; Ladisch, M.R.; Lee, Y.Y.; Mosier, N.; et al. Comparative data on effects of leading pretreatments and enzyme loadings and formulations on sugar yields from different switchgrass sources. Bioresour. Technol. 2011, 102, 11052–11062. [Google Scholar] [CrossRef] [PubMed]
- Chang, V.S.; Burr, B.; Holtzapple, M.T. Lime pretreatment of switchgrass. Appl. Biochem. Biotechnol. 1997, 63, 3–19. [Google Scholar] [CrossRef]
- Alkaline Pretreatment of Coastal Bermudagrass for Bioethanol Production. Available online: https://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1035&context=biosysengpres (accessed on 9 March 2021).
- Alizadeh, H.; Teymouri, F.; Gilbert, T.I.; Dale, B.E. Pretreatment of switchgrass by ammonia fiber explosion (AFEX). Appl. Biochem. Biotechnol. 2005, 124, 1133–1141. [Google Scholar] [CrossRef]
- Kurakake, M.; Kisaka, W.; Ouchi, K.; Komaki, T. Pretreatment with ammonia water for enzymatic hydrolysis of corn husk, bagasse, and switchgrass. Appl. Biochem. Biotechnol. 2001, 90, 251–259. [Google Scholar] [CrossRef]
- Vidal, P.F.; Molinier, J. Ozonolysis of lignin improvement of in vitro digestibility of poplar sawdust. Biomass 1988, 16, 1–17. [Google Scholar] [CrossRef]
- Panneerselvam, A.; Sharma-Shivappa, R.R.; Kolar, P.; Ranney, T.; Peretti, S. Potential of ozonolysis as a pretreatment for energy grasses. Bioresour. Technol. 2013, 148, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Qian, E. Pretreatment and saccharification of lignocellulosic biomass. In Research Approaches to Sustainable Biomass System; Academic Press: Cambridge, MA, USA, 2013; pp. 181–204. ISBN 9780124046832. [Google Scholar]
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D.B. Biomass pretreatment: Fundamentals toward application. Biotechnol. Adv. 2011, 29, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Fang, Z. Solid- and Nano-Catalysts pretreatment and hydrolysis techniques. In Pretreatment Techniques for Biofuels and Biorefineries; Springer: Heidelberg, Germany; Dordrecht, The Netherlands; London, UK; New York, NY, USA, 2013; pp. 339–366. [Google Scholar] [CrossRef]
- Ansanay, Y.; Kolar, P.; Sharma-Shivappa, R.R.; Cheng, J.J. Niobium oxide catalyst for delignification of switchgrass for fermentable sugar production. Ind. Crops. Prod. 2014, 52, 790–795. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Xia, X.; Lin, C.; Tong, D.; Beltramini, J. Catalytic conversion of lignocellulosic biomass to fine chemicals and fuels. Chem. Soc. Rev. 2011, 40, 5588–5617. [Google Scholar] [CrossRef]
- Guo, F.; Fang, Z.; Xu, C.; Smith, R. Solid acid mediated hydrolysis of biomass for producing biofuels. Prog. Energy Combust. Sci. 2012, 38, 672–690. [Google Scholar] [CrossRef]
- Qi, B.; Vu, A.; Wickramasinghe, S.R.; Qian, X. Glucose production from lignocellulosic biomass using a membrane-based polymeric solid acid catalyst. Biomass Bioenergy 2018, 117, 137–145. [Google Scholar] [CrossRef]
- Peña, L.; Xu, F.; Hohn, K.; Li, J.; Wang, D. Propyl-sulfonic acid functionalized nanoparticles as catalyst for pretreatment of corn stover. J. Biomater. Nanobiotechnol. 2014, 5, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Tan, I.S.; Lee, K.T. Solid acid catalysts pretreatment and enzymatic hydrolysis of macroalgae cellulosic residue for the production of bioethanol. Carbohyd Polym. 2015, 124, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; He, C.; Wang, Q.; Liu, S.; Yu, Q.; Wang, W.; Leksawasdi, N.; Wang, C.; Yuan, Z. Carbon-based solid acid pretreatment in corncob saccharification: Specific xylose production and efficient enzymatic hydrolysis. ACS Sustain. Chem. Eng. 2018, 6, 3640–3648. [Google Scholar] [CrossRef]
- Hara, M. Biomass conversion by a solid acid catalyst. Energy Environ. Sci. 2010, 3, 601–607. [Google Scholar] [CrossRef]
- Li, Z.; Su, K.; Ren, J.; Yang, D.; Cheng, B.; Kim, C.K.; Yao, X. Direct catalytic conversion of glucose and cellulose. Green Chem. 2018, 20, 863–872. [Google Scholar] [CrossRef]
- Huang, F.; Li, W.; Zhang, T.; Li, D.; Liu, Q.; Zhu, X.; Ma, L. Conversion of biomass-derived carbohydrates into 5-hydroxymethylfurfural catalyzed by sulfonic acid-functionalized carbon material with high strong-acid density in γ-valerolactone. Res. Chem. Intermed. 2018, 44, 5439–5453. [Google Scholar] [CrossRef]
- Alam, M.I.; De, S.; Khan, T.S.; Haider, M.A.; Saha, B. Acid functionalized ionic liquid catalyzed transformation of non-food biomass into platform chemical and fuel additive. Ind. Crops. Prod. 2018, 123, 629–637. [Google Scholar] [CrossRef]
- Wu, Y.; Fu, Z.; Yin, D.; Xu, Q.; Liu, F.; Lu, C.; Mao, L. Microwave-assisted hydrolysis of crystalline cellulose catalyzed by biomass char sulfonic acids. Green Chem. 2010, 12, 696–700. [Google Scholar] [CrossRef]
- Wang, J.; Xu, W.; Ren, J.; Liu, X.; Lu, G.; Wang, Y. Efficient catalytic conversion of fructose into hydroxymethylfurfural by a novel carbon-based solid acid. Green Chem. 2011, 13, 2678. [Google Scholar] [CrossRef]
- Rackemann, D.W.; Bartley, J.P.; Doherty, W.O.S. Methanesulfonic acid-catalyzed conversion of glucose and xylose mixtures to levulinic acid and furfural. Ind. Crop. Prod. 2014, 52, 46–57. [Google Scholar] [CrossRef]
- Rackemann, D.W.; Bartley, J.P.; Harrison, M.D.; Doherty, W.O.S. The effect of pretreatment on methanesulfonic acid-catalyzed hydrolysis of bagasse to levulinic acid, formic acid, and furfural. RSC Adv. 2016, 6, 74525. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Ren, J.; Liu, X.; Guo, Y.; Guo, Y.; Lu, G.; Wang, Y. Novel sulfonated carbonaceous materials from p-toluenesulfonic acid/glucose as a high-performance solid-acid catalyst. Catal. Commun. 2010, 11, 629–632. [Google Scholar] [CrossRef]
- Evangelin, C.; Naren, S.; Dharmendirakumar, M. Comparison of the Surface features of the three chemically modified silk cotton hull activated carbons. Orient. J. Chem. 2012, 28, 1761–1768, ISSN 0970-020 X. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Tao, H.; Zhang, B.; Ren, J.; Lu, G.; Wang, Y. One-pot synthesis of carbonaceous monolith with surface sulfonic groups and its carbonization/activation. Carbon 2011, 49, 1811–1820. [Google Scholar] [CrossRef]
- Reye, J.T.; Lu, J.; Maxwell, K.E.; Banerjee, S. Enhancement of cellulase catalysis of wood pulp fiber by cationic polyelectrolytes. Biomass Bioenergy 2011, 35, 4887–4891. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.D.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of structural carbohydrates and lignin in biomass. In Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Liu, X.; Huang, M.; Ma, H.; Zhang, Z.; Gao, J.; Zhu, Y.; Han, X.; Guo, X. Preparation of a carbon-based solid acid catalyst by sulfonating activated carbon in a chemical reduction process. Molecules 2010, 7188–7196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shu, Q.; Zhang, Q.; Xu, G.; Nawaz, Z.; Wang, D.; Wang, J. Synthesis of biodiesel from cottonseed oil and methanol using a carbon-based solid acid catalyst. Fuel Process Technol. 2009, 90, 1002–1008. [Google Scholar] [CrossRef]
- Liang, X.; Zeng, M.; Qi, C. One-step synthesis of carbon functionalized with sulfonic acid groups using hydrothermal carbonization. Carbon 2010, 48, 1844–1848. [Google Scholar] [CrossRef]
- Kitano, M.; Yamaguchi, D.; Suganuma, S.; Nakajima, K.; Kato, H.; Hayashi, S.; Hara, M. Adsorption-enhanced hydrolysis of β-1,4-glucan on graphene-based amorphous carbon bearing SO3H, COOH, and OH Groups. Langmuir 2009, 25, 5068–5075. [Google Scholar] [CrossRef]
- Nasef, M.M.; Saidi, H. Surface studies of radiation grafted sulfonic acid membranes: XPS and SEM analysis. Appl. Surf. Sci. 2006, 252, 3073–3084. [Google Scholar] [CrossRef]
- Mo, X.; Lotero, E.; Lu, C.; Liu, Y.; Goodwin, J.G. A novel sulfonated carbon composite solid acid catalyst for biodiesel synthesis. Catal Lett. 2008, 123, 1–6. [Google Scholar] [CrossRef]
- Alvear-Daza, J.J.; Pasquale, G.A.; Rengifo-Herrera, J.A.; Romanelli, G.P.; Pizzio, L.R. Mesoporous activated carbon from sunflower shells modified with sulfonic acid groups as solid acid catalyst for itaconic acid esterification. Catal. Today 2021, in press. [Google Scholar] [CrossRef]
- Suganuma, S.; Nakajima, K.; Kitano, M.; Yamaguchi, D.; Kato, H.; Hayashi, S.; Hara, M. Hydrolysis of cellulose by amorphous carbon bearing SO3H, COOH, and OH groups. J. Am. Chem. Soc. 2008, 130, 12787–12793. [Google Scholar] [CrossRef]
- Li, X.; Luo, X.; Li, K.; Zhu, J.; Fougere, J.; Clarke, K. Effects of SPORL and dilute acid pretreatment on substrate morphology, cell physical and chemical wall structures, and subsequent enzymatic hydrolysis of lodgepole. Appl. Biochem. Biotechnol. 2012, 168, 1556–1567. [Google Scholar] [CrossRef]


| No | Composition | Raw Switchgrass | SA-Treated Switchgrass | MSA-Treated Switchgrass | pTSA-Treated Switchgrass |
|---|---|---|---|---|---|
| Weight Percentage (%) | Weight Percentage (%) | Weight Percentage (%) | Weight Percentage (%) | ||
| 1 | Glucan | 33.36 ± 1.02 | 29.15 ± 2.03 | 26.35 ± 1.08 | 26.22 ± 0.34 |
| 2 | Xylan | 20.84 ± 1.88 | 18.58 ± 1.28 | 15.78 ± 0.75 | 16. 54 ± 1.02 |
| 3 | Arabinan | 3.78 ± 1.1 | 2.88 ± 0.9 | 3.02 ± 0.34 | 2. 95 ± 0.8 |
| 4 | Ash | 2.65 ± 0.9 | 2.04 ± 0.04 | 1.95 ± 0.1 | 1.88 ± 0.43 |
| 5 | Acid-Soluble Lignin | 3.46 ± 0.75 | 2.86 ± 0.09 | 2.58 ± 0.07 | 2.46 ± 0.09 |
| 6 | Acid-Insoluble Lignin | 22.78 ± 0.4 | 20.62 ± 0.96 | 18. 84 ± 0.81 | 18.98 ± 0.93 |
| 7 | Others | 13.13 ± 0.3 | Some parts lost during treatment. Solid Recovery less than 100% | ||
| Total | 100% | ||||
| Characteristic | Raw AC | AC-SA | AC-MSA | AC-pTSA |
|---|---|---|---|---|
| Surface Area (m2/g) | 781 ± 31 | 734.5 ± 29 | 668.9 ± 26 | 317.8 ± 7 |
| Pore Volume (cm3/g) | 0.4 ± 0.02 | 0.37 ± 0.03 | 0.33 ± 0.02 | 0.14 ± 0.00 |
| Pore Size (Å) | 20.75 ± 0.8 | 20.24 ± 0.8 | 20.23 ± 0.8 | 20.6 ± 0.4 |
| Total Acidity (mmol g−1) | 0.1 | 0.365 | 0.3 | 0.425 |
| Carboxylic and Sulfonic (mmol g−1) | 0.025 | 0.2 | 0.2 | 0.175 |
| Lactone (mmol g−1) | 0.025 | 0.09 | 0.05 | 0.125 |
| Phenolic (mmol g−1) | 0.05 | 0.075 | 0.05 | 0.125 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ansanay, Y.; Kolar, P.; Sharma-Shivappa, R.; Cheng, J.; Arellano, C. Pretreatment of Switchgrass for Production of Glucose via Sulfonic Acid-Impregnated Activated Carbon. Processes 2021, 9, 504. https://doi.org/10.3390/pr9030504
Ansanay Y, Kolar P, Sharma-Shivappa R, Cheng J, Arellano C. Pretreatment of Switchgrass for Production of Glucose via Sulfonic Acid-Impregnated Activated Carbon. Processes. 2021; 9(3):504. https://doi.org/10.3390/pr9030504
Chicago/Turabian StyleAnsanay, Yane, Praveen Kolar, Ratna Sharma-Shivappa, Jay Cheng, and Consuelo Arellano. 2021. "Pretreatment of Switchgrass for Production of Glucose via Sulfonic Acid-Impregnated Activated Carbon" Processes 9, no. 3: 504. https://doi.org/10.3390/pr9030504
APA StyleAnsanay, Y., Kolar, P., Sharma-Shivappa, R., Cheng, J., & Arellano, C. (2021). Pretreatment of Switchgrass for Production of Glucose via Sulfonic Acid-Impregnated Activated Carbon. Processes, 9(3), 504. https://doi.org/10.3390/pr9030504








