Hydroponic Farm Wastewater Treatment Using an Indigenous Consortium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains, Medium, and Effluent
2.2. Culture Conditions
2.3. Analytical Techniques
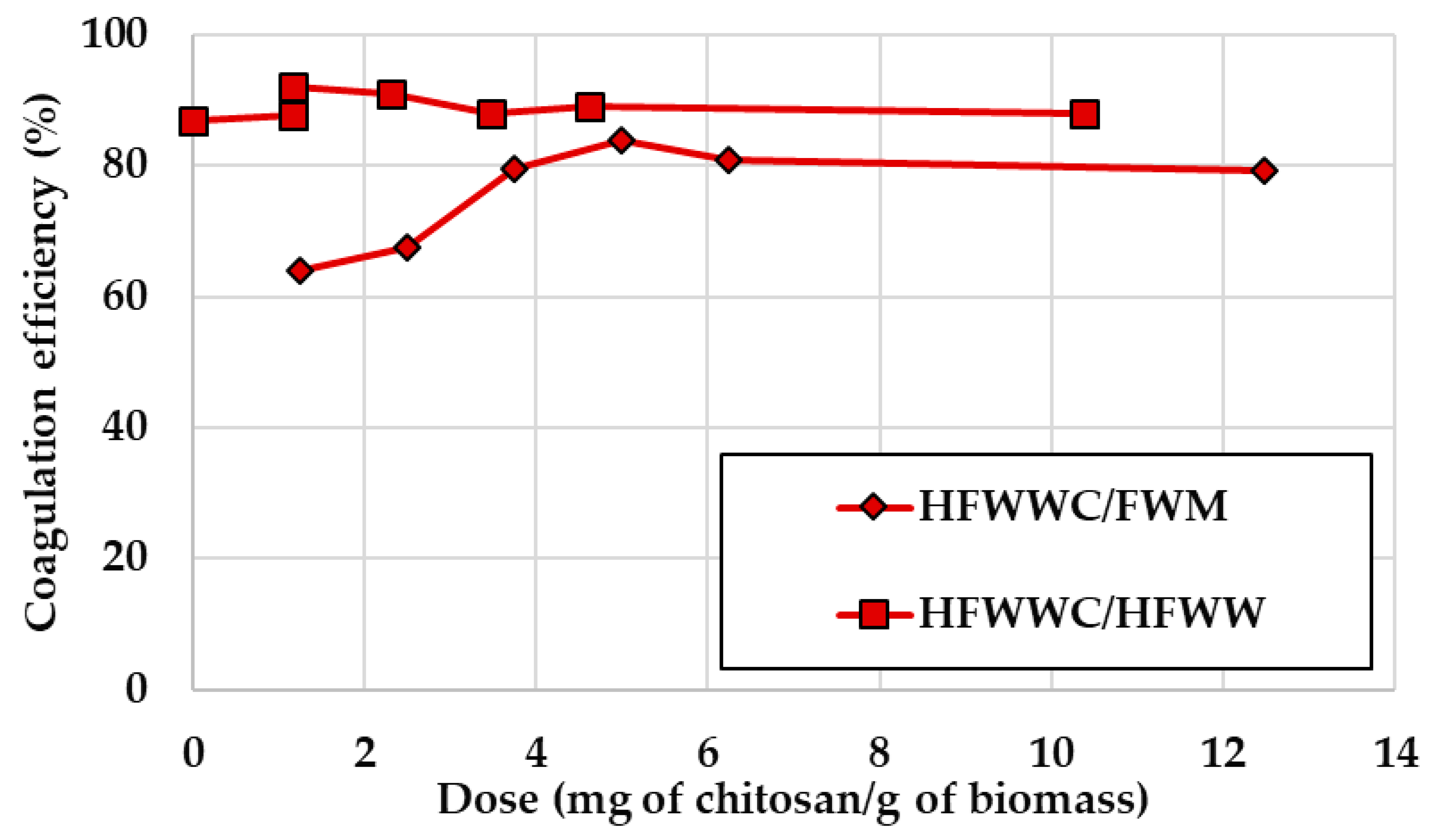
2.4. Chitosan Coagulation Experiments
3. Results
3.1. Effluent Characterization
3.2. Three-Step Screening Process
3.2.1. Ability to Grow with HFWW in Microplates
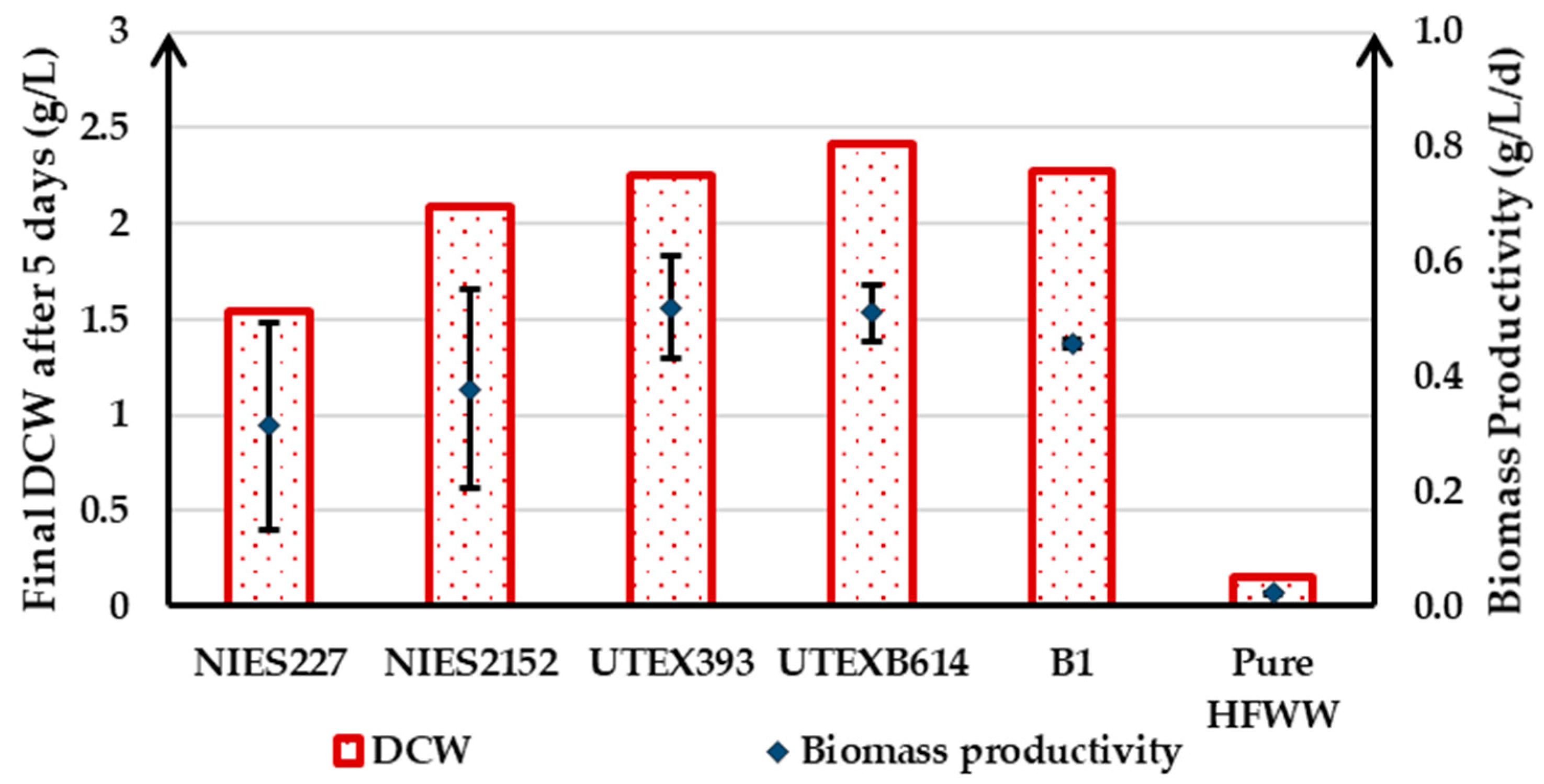
3.2.2. Microalgae Bioremediation Potential of HFWW in Flasks
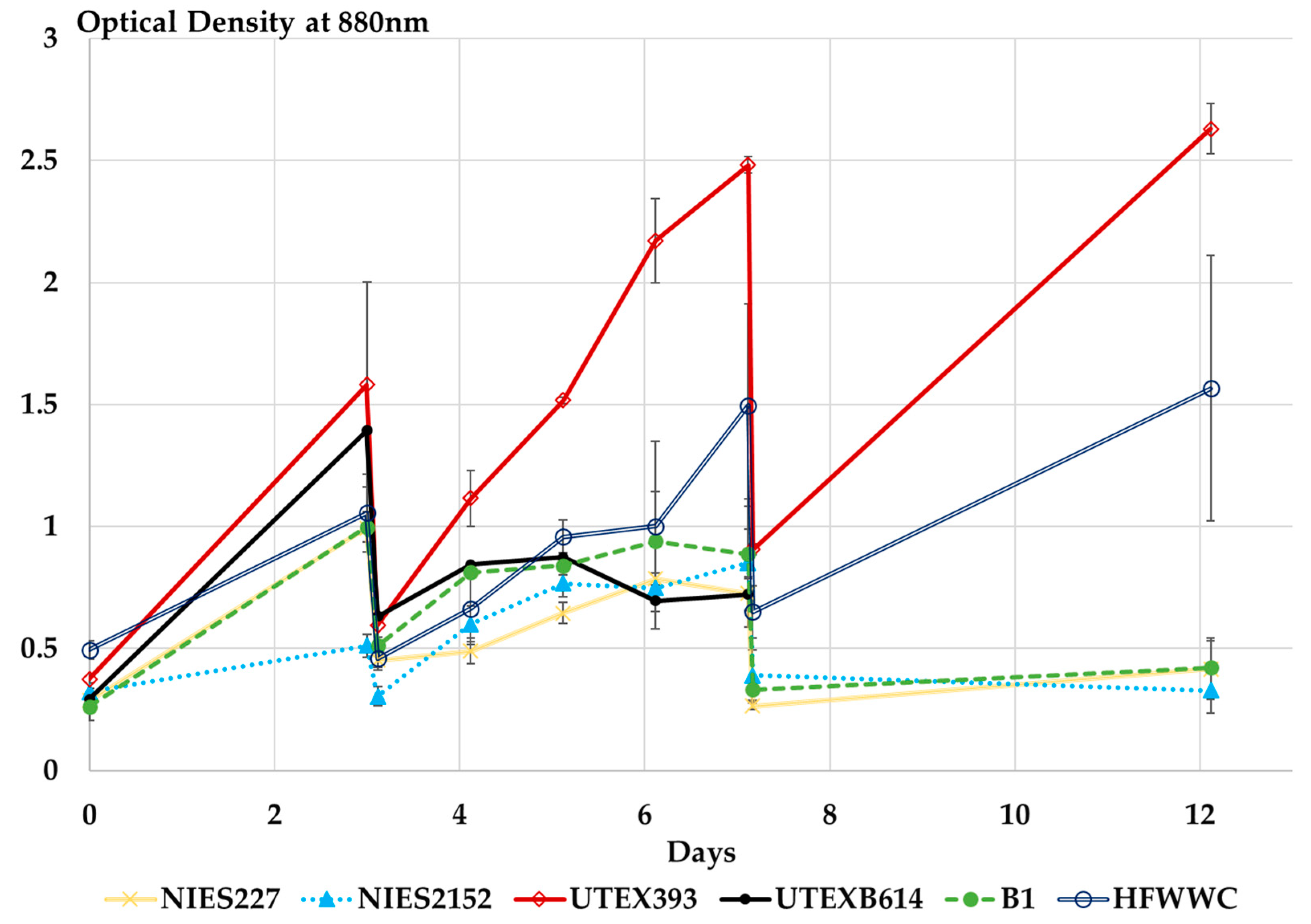
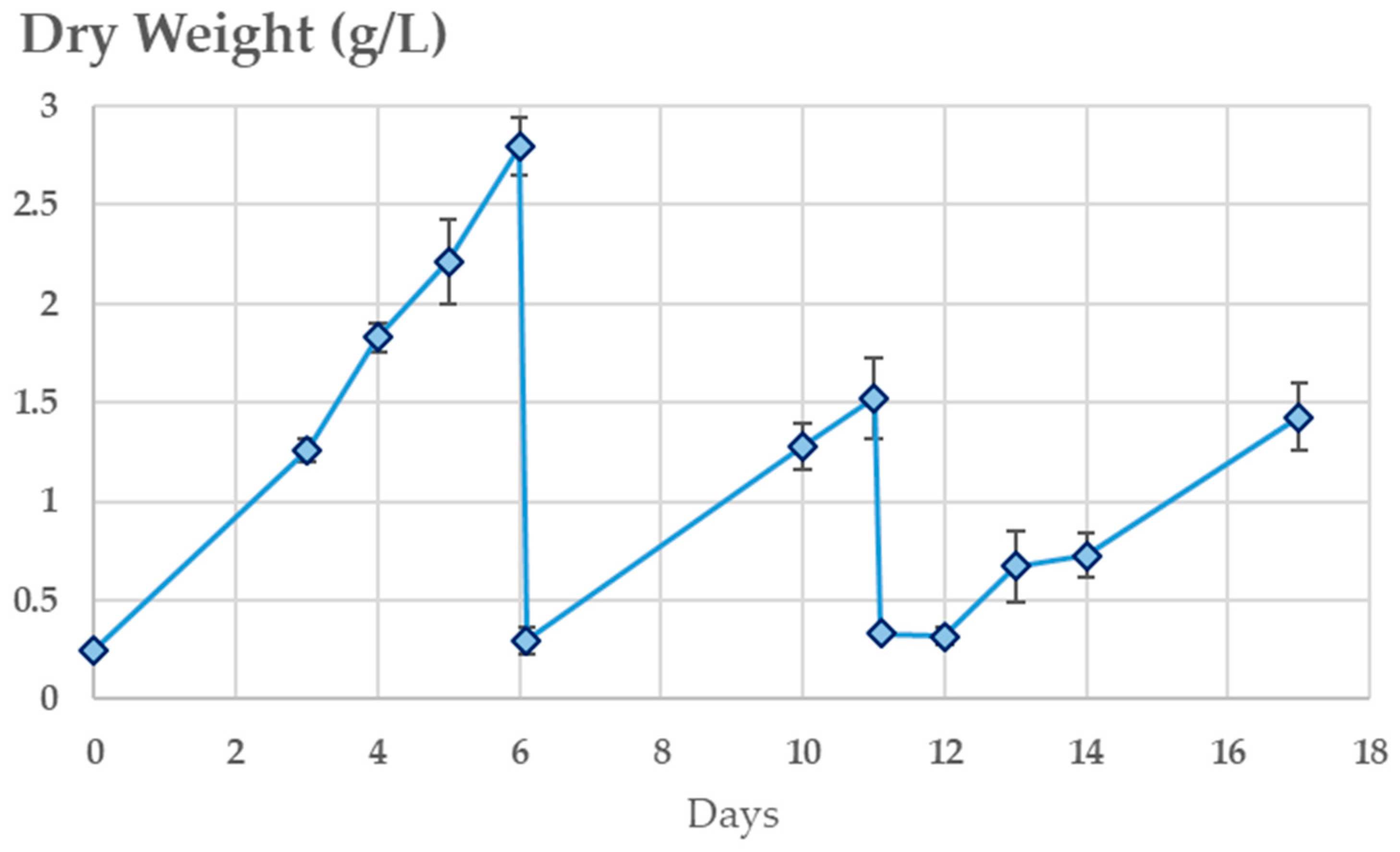
3.2.3. Microalgae Bioremediation Potential of HFWW in Semi-Continuous Operation
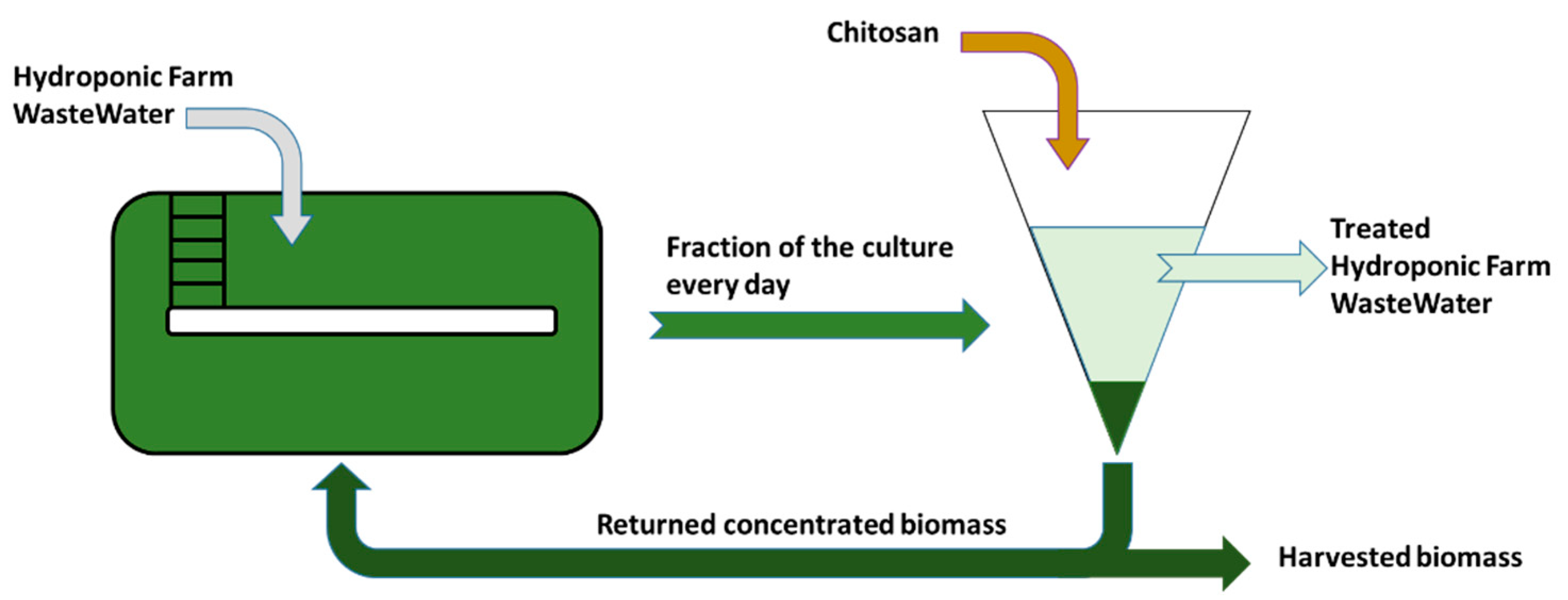
3.3. Design of an Integrated Process
3.3.1. Preliminary Test
3.3.2. Continuous Integrated Process
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Micrographs of Consortium B1 and HFWWC

Appendix B. Evolution of the Nitrate, Phosphate, and Sulfate Concentrations during the Semi-Continuous Operation

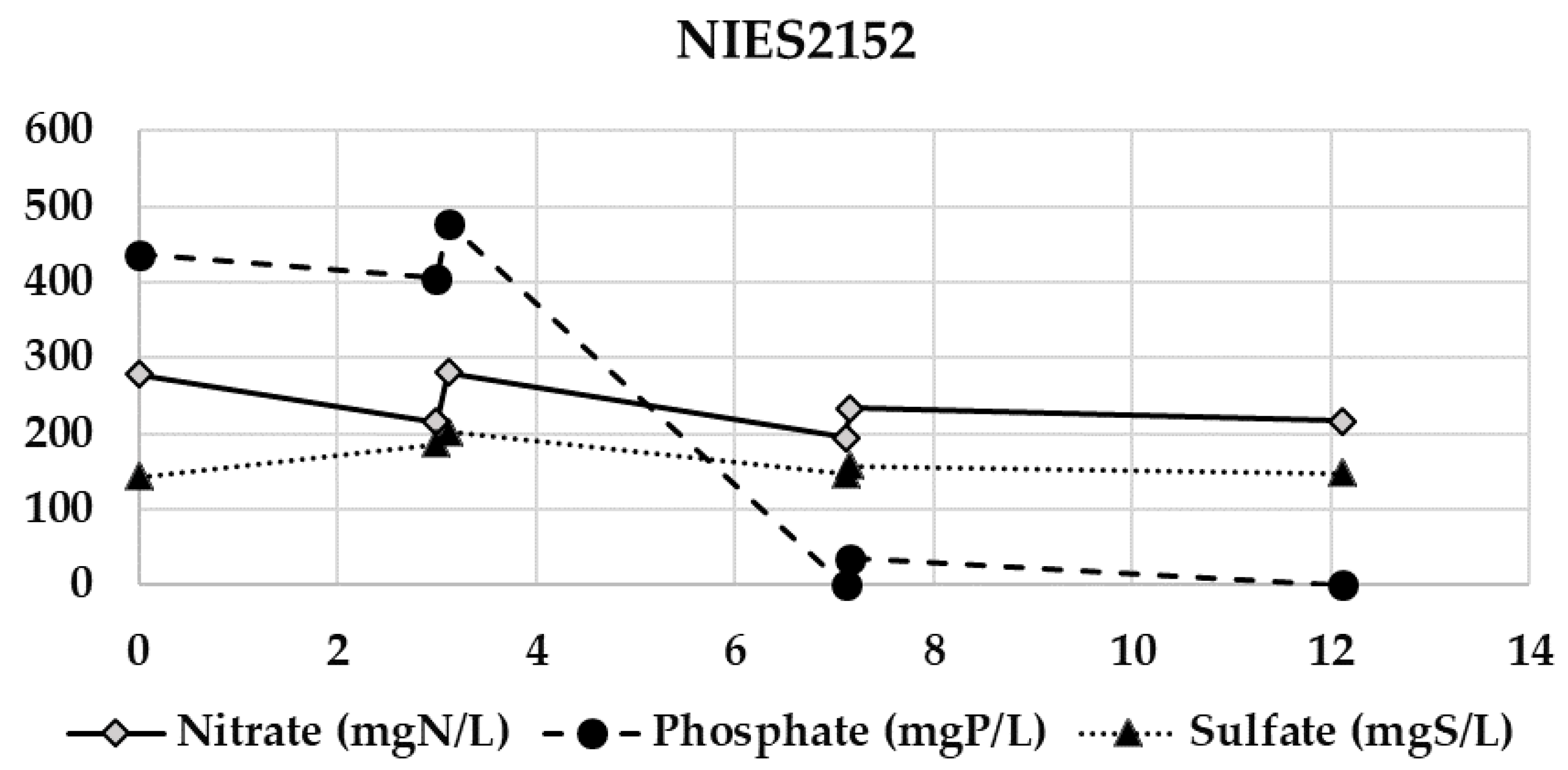
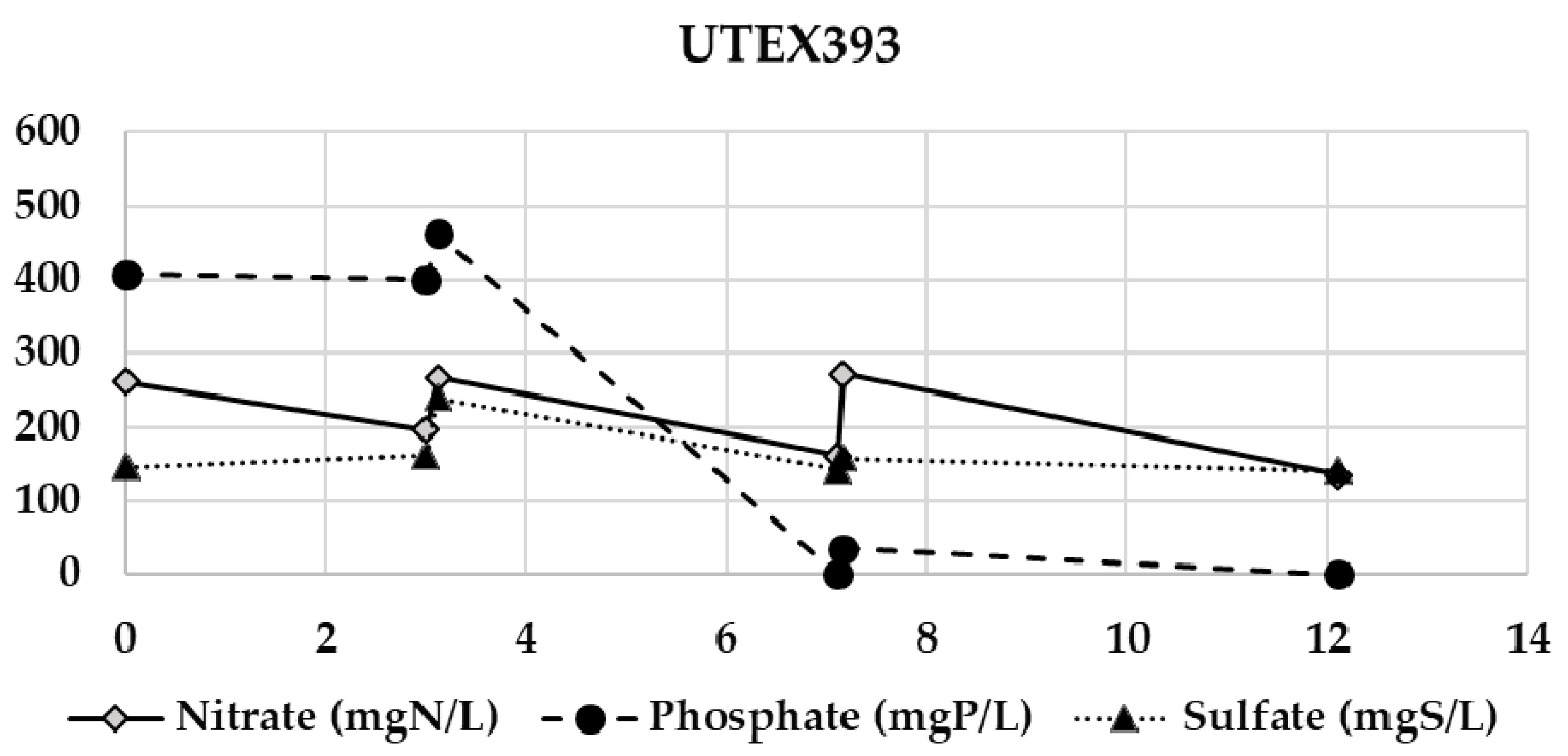

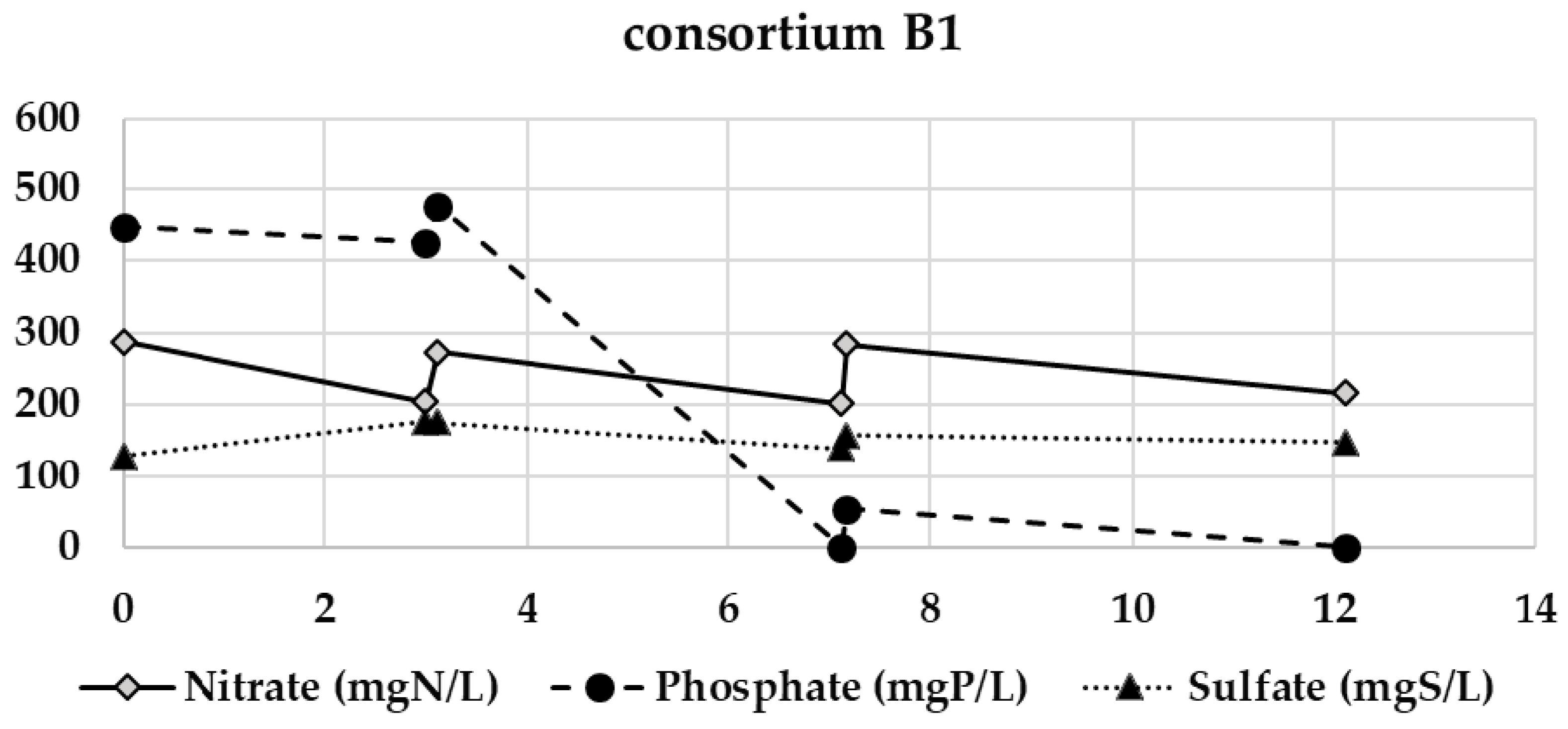
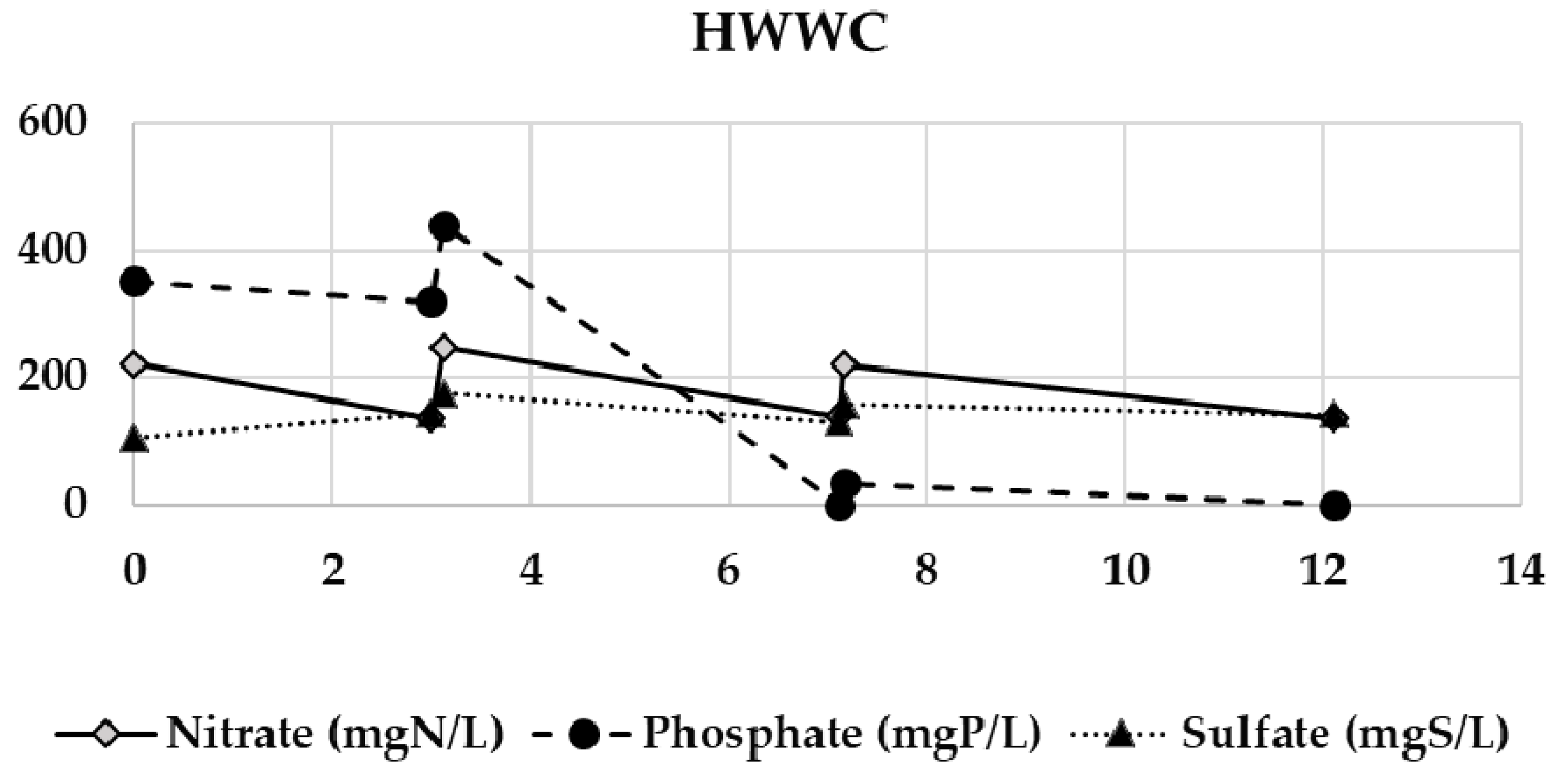
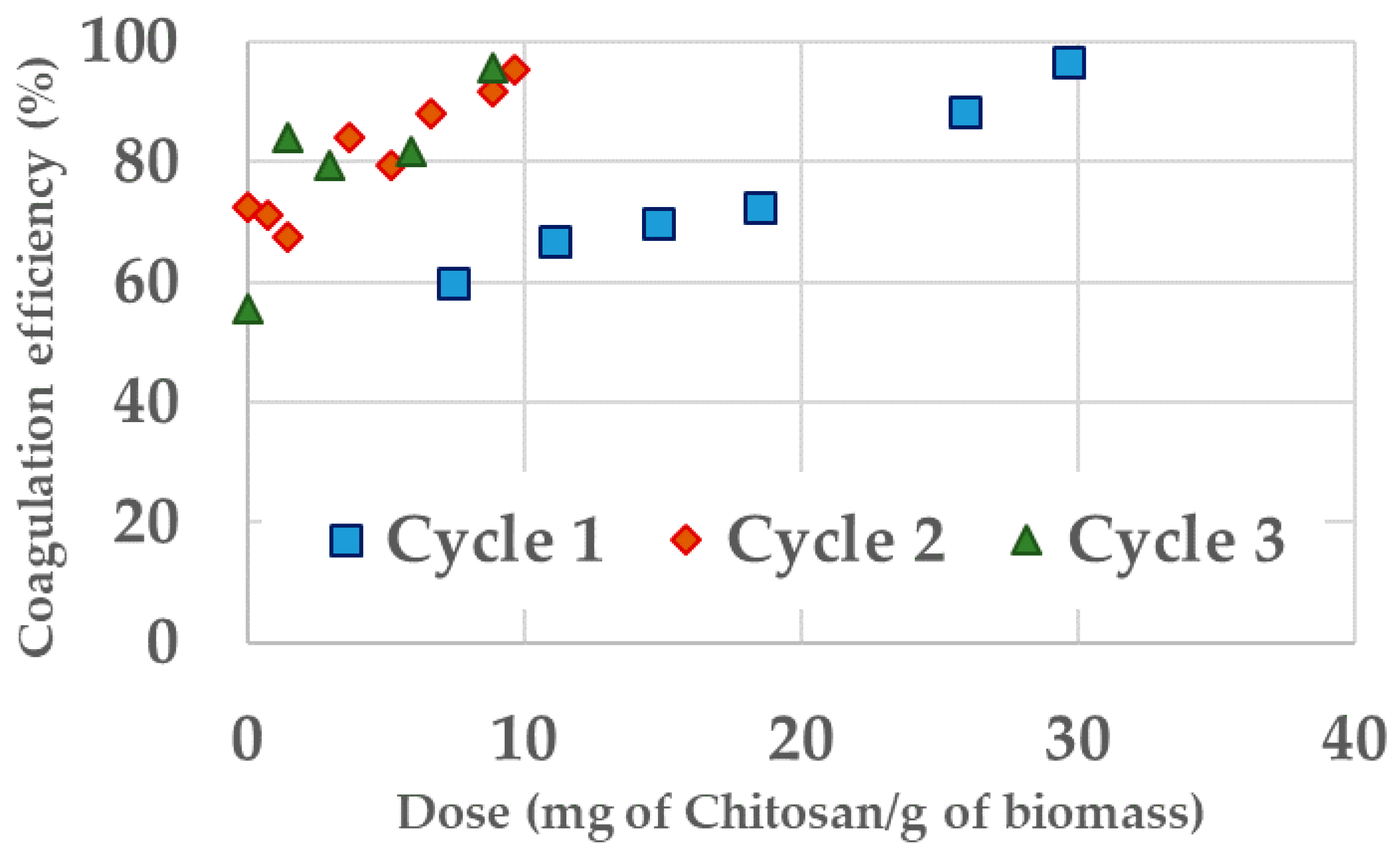
Appendix C. Chitosan Coagulation Efficiency during the Continuous Integrated Process Experience
References
- Wang, M.; Zhang, D.; Dong, J.; Tan, S.K. Application of constructed wetlands for treating agricultural runoff and agro-industrial wastewater: A review. Hydrobiologia 2018, 805, 1–31. [Google Scholar] [CrossRef]
- Kronvang, B.; Wendland, F.; Kovar, K.; Fraters, D. Land Use and Water Quality. Water 2020, 12, 2412. [Google Scholar] [CrossRef]
- Putra, A.; Yuliando, H. Soilless Culture System to Support Water Use Efficiency and Product Quality: A Review. Agric. Agric. Sci. Procedia 2015, 3, 283–288. [Google Scholar] [CrossRef] [Green Version]
- Hultberg, M.; Carlsson, A.S.; Gustafsson, S. Treatment of drainage solution from hydroponic greenhouse production with microalgae. Bioresour. Technol. 2013, 136, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Saxena, P.; Bassi, A. Removal of nutrients from hydroponic greenhouse effluent by alkali precipitation and algae cultivation method. J. Chem. Technol. Biotechnol. 2013, 88, 858–863. [Google Scholar] [CrossRef]
- Delrue, F.; Alvarez-Diaz, P.D.; Fon-Sing, S.; Fleury, G.; Sassi, J.-F. The Environmental Biorefinery: Using Microalgae to Remediate Wastewater, a Win-Win Paradigm. Energies 2016, 9, 132. [Google Scholar] [CrossRef]
- Delrue, F.; Setier, P.A.; Sahut, C.; Cournac, L.; Roubaud, A.; Peltier, G.; Froment, A.-K. An economic, sustainability, and energetic model of biodiesel production from microalgae. Bioresour. Technol. 2012, 111, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Hutner, S.H.; Provasoli, L.; Schatz, A.; Haskins, C.P. Some approaches to the study of the role of metals in the metabolism of microorganisms. Proc. Am. Philos. Soc. 1950, 94, 152–170. [Google Scholar]
- Pei, H.; Ma, C.; Hu, W.; Sun, F. The behaviors of Microcystis aeruginosa cells and extracellular microcystins during chitosan flocculation and flocs storage processes. Bioresour. Technol. 2014, 151, 314–322. [Google Scholar] [CrossRef]
- Arbib, Z.; Ruiz, J.; Alvarez, P.; Garrido-Pérez, C.; Barragan, J.; Perales, J.A. Photobiotreatment: Influence of nitrogen and phosphorus ratio in wastewater on growth kinetics of Scenedesmus obliquus. Int. J. Phytoremediat. 2013, 15, 774–788. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.; Fernandes, I.; Andrade, C.A.P.; Cordeiro, N. Assessing the impact of sulfur concentrations on growth and biochemical composition of three marine microalgae. J. Appl. Phycol. 2020, 32, 967–975. [Google Scholar] [CrossRef]
- Mera, R.; Torres, E.; Abalde, J. Effects of sodium sulfate on the freshwater microalga Chlamydomonas moewusii: Implications for the optimization of algal culture media. J. Phycol. 2016, 52, 75–88. [Google Scholar] [CrossRef]
- Xu, Y.; Purton, S.; Baganz, F. Chitosan flocculation to aid the harvesting of the microalga Chlorella sorokiniana. Bioresour. Technol. 2013, 129, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhu, Y.; Huang, W.; Zhang, C.; Li, T.; Zhang, T.; Li, A. Evaluation of flocculation induced by pH increase for harvesting microalgae and reuse of flocculated medium. Bioresour. Technol. 2012, 110, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.L.; Pires, J.C.M.; Simões, M. A review on the use of microalgal consortia for wastewater treatment. Algal. Res. 2017, 24, 403–415. [Google Scholar] [CrossRef]
- Halfhide, T.; Dalrymple, O.K.; Wilkie, A.C.; Trimmer, J.; Gillie, B.; Udom, I.; Zhang, Q.; Ergas, S.J. Growth of an Indigenous Algal Consortium on Anaerobically Digested Municipal Sludge Centrate: Photobioreactor Performance and Modeling. Bioenergy Res. 2015, 8, 249–258. [Google Scholar] [CrossRef]
- Moreno-García, A.F.; Neri-Torres, E.E.; Mena-Cervantes, V.Y.; Altamirano, R.H.; Pineda-Flores, G.; Luna-Sánchez, R.; García-Solares, M.; Vazquez-Arenas, J.; Suastes-Rivas, J.K. Sustainable biorefinery associated with wastewater treatment of Cr (III) using a native microalgae consortium. Fuel 2021, 290, 119040. [Google Scholar] [CrossRef]
- González-Camejo, J.; Robles, A.; Seco, A.; Ferrer, J.; Ruano, M.V. On-line monitoring of photosynthetic activity based on pH data to assess microalgae cultivation. J. Environ. Manag. 2020, 276, 111343. [Google Scholar] [CrossRef]
- Uggetti, E.; Sialve, B.; Hamelin, J.; Bonnafous, A.; Steyer, J.-P. CO2 addition to increase biomass production and control microalgae species in high rate algal ponds treating wastewater. J. CO2 Util. 2018, 28, 292–298. [Google Scholar] [CrossRef]
- Li, Y.; Ding, Y.; Li, D.; Miao, Z. Automatic carbon dioxide enrichment strategies in the greenhouse: A review. Biosyst. Eng. 2018, 171, 101–119. [Google Scholar] [CrossRef]
- Dineshkumar, R.; Kumaravel, R.; Gopalsamy, J.; Sikder, M.N.A.; Sampathkumar, P. Microalgae as Bio-fertilizers for Rice Growth and Seed Yield Productivity. Waste Biomass Valorization 2018, 9, 793–800. [Google Scholar] [CrossRef]
- Milledge, J.J.; Nielsen, B.V.; Maneein, S.; Harvey, P.J. A Brief Review of Anaerobic Digestion of Algae for Bioenergy. Energies 2019, 12, 1166. [Google Scholar] [CrossRef] [Green Version]
- Camacho, F.; Macedo, A.; Malcata, F. Potential Industrial Applications and Commercialization of Microalgae in the Functional Food and Feed Industries: A Short Review. Mar. Drugs 2019, 17, 312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novoveská, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.-F. Microalgal Carotenoids: A Review of Production, Current Markets, Regulations, and Future Direction. Mar. Drugs 2019, 17, 640. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Parameter | Effluent 1 | Effluent 2 | Effluent 3 | Effluent 4 |
|---|---|---|---|---|
| pH | 5.75 | 5.97 | 6.16 | 6.05 |
| Dry weight (g/L) | 0.01 | 0.01 | 0.01 | 0.01 |
| Carbon Oxygen Demand (COD) (mg/L) | 21.0 | 18.3 | - | - |
| Nitrate (mgN/L) | 235 | 292 | 170 | 144 |
| Phosphate (mgP/L) | 96.9 | 460 | 91.1 | 67.0 |
| Sulfate (mgS/L) | 140 | 182 | 43.0 | 144.9 |
| Ammonium (mgN/L) | ND 1 | ND 1 | ND 1 | ND 1 |
| Acetate (mg/L) | ND 1 | ND 1 | ND 1 | ND 1 |
| Chloride (mg/L) | 291 | 413 | 390 | 219 |
| Magnesium (mg/L) | 97.2 | 60.8 | 124 | 44.3 |
| Potassium (mg/L) | 70.1 | 644 | 59 | 79.6 |
| Sodium (mg/L) | 13.2 | 55.5 | ND1 | 139.2 |
| Strain | DCW after 5 Days (g/L) | ||
|---|---|---|---|
| Pure FWM | 50/50 FWM/HFWW | Pure HFWW | |
| NIES227 | 0.75 | 2.90 | 2.62 |
| NIES2152 | 1.06 | 2.31 | 3.04 |
| UTEX393 | 0.81 | 1.83 | 2.17 |
| UTEXB614 | 0.82 | 3.33 | 2.36 |
| Consortium B1 | 1.75 | 1.23 | 2.59 |
| Nitrate (mgN/L) | Phosphate (mgP/L) | Sulfate (mgS/L) | |
|---|---|---|---|
| Raw HFWW | 235 | 96.9 | 140 |
| NIES227 | 136.1 (42.0%) | 30.3 (68.8%) | 65.7 (52.9%) |
| NIES2152 | 71.5 (69.5%) | 0 (100%) | 111 (21.2%) |
| UTEX393 | 106 (54.7%) | 0 (100%) | 116 (17.1%) |
| UTEXB614 | 60 (74.4%) | 0 (100%) | 109 (22.3%) |
| Consortium B1 | 71.9 (69.4%) | 0 (100%) | 110 (19.1%) |
| Optimal Chitosan Dose (mg/g of Biomass) | Nitrate (mgN/L) (Removal Rate in %) | Phosphate (mgP/L) (Removal Rate in %) | COD (mgO2/L) | |
|---|---|---|---|---|
| Raw HFWW | 144 | 67.0 | - | |
| Cycle 1 | 29.65 | 0.26 (99.8%) | 10.0 (85.0%) | 523 |
| Cycle 2 | 9.63 | 5.8 (96.0%) | 0 (100%) | 471 |
| Cycle 3 | 8.89 | 1.85 (98.7%) | 16.0 (76.1%) | 298 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delrue, F.; Ribeiro de Jesus Cerqueira, M.; Compadre, A.; Alvarez, P.; Fleury, G.; Escoffier, C.; Sassi, J.-F. Hydroponic Farm Wastewater Treatment Using an Indigenous Consortium. Processes 2021, 9, 519. https://doi.org/10.3390/pr9030519
Delrue F, Ribeiro de Jesus Cerqueira M, Compadre A, Alvarez P, Fleury G, Escoffier C, Sassi J-F. Hydroponic Farm Wastewater Treatment Using an Indigenous Consortium. Processes. 2021; 9(3):519. https://doi.org/10.3390/pr9030519
Chicago/Turabian StyleDelrue, Florian, Matheus Ribeiro de Jesus Cerqueira, Ana Compadre, Pablo Alvarez, Gatien Fleury, Camille Escoffier, and Jean-François Sassi. 2021. "Hydroponic Farm Wastewater Treatment Using an Indigenous Consortium" Processes 9, no. 3: 519. https://doi.org/10.3390/pr9030519
APA StyleDelrue, F., Ribeiro de Jesus Cerqueira, M., Compadre, A., Alvarez, P., Fleury, G., Escoffier, C., & Sassi, J.-F. (2021). Hydroponic Farm Wastewater Treatment Using an Indigenous Consortium. Processes, 9(3), 519. https://doi.org/10.3390/pr9030519







