Abstract
The enzymatic transesterification of Atlantic salmon (Salmo salar) oil was carried out using Novozym 435 (immobilized lipase from Candida antartica) to produce biodiesel. A response surface modelling design was performed to investigate the relationship between biodiesel yield and several critical factors, including enzyme concentration (5, 10, or 15%), temperature (40, 45, or 50 °C), oil/alcohol molar ratio (1:3, 1:4, or 1:5) and time (8, 16, or 24 h). The results indicated that the effects of all the factors were statistically significant at p-values of 0.000 for biodiesel production. The optimum parameters for biodiesel production were determined as 10% enzyme concentration, 45 °C, 16 h, and 1:4 oil/alcohol molar ratio, leading to a biodiesel yield of 87.23%. The step-wise addition of methanol during the enzymatic transesterification further increased the biodiesel yield to 94.5%. This is the first study that focused on Atlantic salmon oil-derived biodiesel production, which creates a paradigm for valorization of Atlantic salmon by-products that would also reduce the consumption and demand of plant oils derived from crops and vegetables.
1. Introduction
Biodiesel has been studied for decades as a promising alternative to traditional fossil fuel [1,2,3]. Biodiesel is a processed fuel that is produced via transesterification of triglycerides derived from naturally occurring plant oils and animal fats. During the transesterification, the triglycerides are converted into fatty acid methyl esters, with glycerol produced as a by-product. Three methods have been traditionally used to produce biodiesel, including acid catalysis, base catalysis, and non-catalytic transesterification using supercritical alcohol [4]. The most commonly used commercial process for biodiesel production is alkali-catalyzed transesterification using sodium hydroxide or potassium hydroxide due to their relatively low cost and high conversion efficiency [3]. However, chemical transesterification has multiple disadvantages [5], such as the high dependency of conversion efficiency on the content of water and free fatty acids in the raw materials and the tremendous energy consumption due to the high reaction temperature and product separation process. Moreover, the acid or base catalysts are not reusable, and extra steps of neutralization are required to dispose of them as an aqueous salt waste stream, which is less environmentally hazardous [6].
In recent years, biocatalytic transesterification, which is catalyzed by lipases, has been intensively studied for biodiesel production [5,7,8,9]. As lipases catalyze the reaction through interactions with the molecules at specific sites, the conversion efficiency and product purity are high, with little need for downstream processing. The biocatalytic process avoids the use of alkaline, so there is no soap formation or generation of alkaline wastewater, which reduces the workload for wastewater treatment [6]. In addition, the enzymatic transesterification can be carried out under mild operation conditions [10]. Among various lipases, the immobilized lipase from Candida antarctica has been widely used due to its extraordinary efficiency for biodiesel conversion [11]. After the transesterification, the immobilized lipase can be easily separated from the reaction mixture through vacuum filtration and washed for reuse. It has been reported that the enzyme can be reused for at least 10 cycles with a slight decrease in biodiesel yield [12,13].
A variety of vegetable oils extracted from corn, canola, palm, soybean, sunflower, rapeseed, coconut, and groundnut have been converted into biodiesel [14,15,16]. However, as most of these plant-derived oils are initially used for food, livestock feed, and oleochemical industries, biodiesel production results in increased consumption of these resources and increased crop demand [10]. Therefore, oils derived from alternative resources have been studied, such as fish oil extracted from fish by-products [17,18,19,20]. Atlantic salmon aquaculture has played an essential role in the Newfoundland aquaculture industry for decades, accounting for above 80% of the total aquaculture landing [21]. In 2017, the aquaculture landing of salmonids (mainly Atlantic salmon) in Newfoundland and Labrador was 18,822 tonnes (87%) [21]. In the Atlantic salmon processing industry, 40–50% of the fish body ends up as by-products (heads, frames, and viscera), which have been reported to be abundant in lipids (15–25%) [22]. Several studies have been reported on oil extraction from these by-products [23,24,25,26,27]. The extracted oil has been reported with high yield and good quality with a low extent of oxidation or hydrolysis [23]. To the authors’ best knowledge, there has not been any research reported on biodiesel production from Atlantic salmon oil. This is the first study that converted enzymatically extracted Atlantic salmon oil to biodiesel. The aim of this work was to maximize the biodiesel production from the salmon oil, which can be developed as an effective way to valorize the large amounts of Atlantic salmon by-products.
The statistical design of experiments plays a vital role in determination of optimal parameters in many research areas of science and industry. In a multivariable system, only one factor can be studied in each set of experiments using the conventional protocols, and such methods require many experiments and do not represent the combined effect. Therefore, different types of design of experiment techniques, including Latin squares, full factorial, fractional factorial, response surface methodology, and Plackett–Burman and Taguchi methods, have been implemented in various studies [28,29]. The response surface methodology (RSM) is a widely used statistical modelling system to analyze a process in which the response of interest might be affected by various variables. The main objective of response surface methodology is to optimize the response as per the needs of the study. Response surface methodology reduces the number of experiments needed to determine the optimal parameters for the process [30,31]. In the present study, the transesterification process was optimized using response surface methodology to maximize the biodiesel yield, and the influence of the main reaction parameters, including enzyme concentration, oil/alcohol molar ratio, temperature, and time, was investigated.
2. Materials and Methods
2.1. Materials
Farmed Atlantic salmon (Salmo salar) by-products, including heads, frames, and viscera, were collected in Styrofoam boxes on ice from a salmon aquaculture processing plant, Atlantic Canada. The by-products were shipped overnight to the Marine Bioprocessing Facility, Marine Institute of Memorial University of Newfoundland, St. John’s, NL, Canada, and immediately processed once received. The salmon raw materials were minced three times using a Hobart grinder (Model 4146 The Hobart MFG. Co. Ltd., Peterborough, UK) using different plate sizes (17, 15, and 13 mm). The raw materials were vacuum-packed in plastic bags with each weighing approximately 500 g, and stored at −28 °C until use.
Alcalase 2.4 L, boron trifluoride, tricosanoic acid methyl ester, Novozym 435 (immobilized lipase from Candida antarctica), tert-butanol, tetrahydrofuran, N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA), and PUFA No. 3 (from menhaden oil) analytical standard were purchased from Sigma-Aldrich, Oakville, ON, Canada. Hexane and methanol were purchased from Fisher Scientific, Nepean, ON, Canada.
2.2. Salmon Oil Extraction
The frozen ground salmon materials were thawed in cold running water. An amount of 500 g salmon by-products was weighed in a 1 L Mason jar. To the weighed raw material, 500 mL water was added (1:1 w/w). The mixture was gently stirred using a magnetic stirrer, and the pH was adjusted to the optimal value of 8 for Alcalase by adding a 10% sodium hydroxide solution. The mixture was heated in a water bath to 80 °C for 5 min to deactivate the endogenous enzymes in the fish materials. Afterwards, the mixture was cooled down to the optimal temperature of 55 °C for Alcalase. The enzymatic hydrolysis was initiated by adding 1% (w/w of the raw fish materials) of Alcalase, and the mixture was digested in an incubator shaker (Thermo-Scientific Max Q 6000, Marietta, OH, USA) at 140 rpm for 2 h. The hydrolysis was stopped by heating the mixture at 90 °C for 10 min to deactivate the enzyme. The mixture was cooled down to room temperature and centrifuged at 10,000 rpm for 20 min. After centrifugation, four layers were obtained: the oil layer on top, an emulsion layer, an aqueous layer of protein hydrolysates, and the sludge on the bottom. The oil was collected and stored at −80 °C for subsequent analyses.
2.3. Enzymatic Transesterification of Salmon Oil
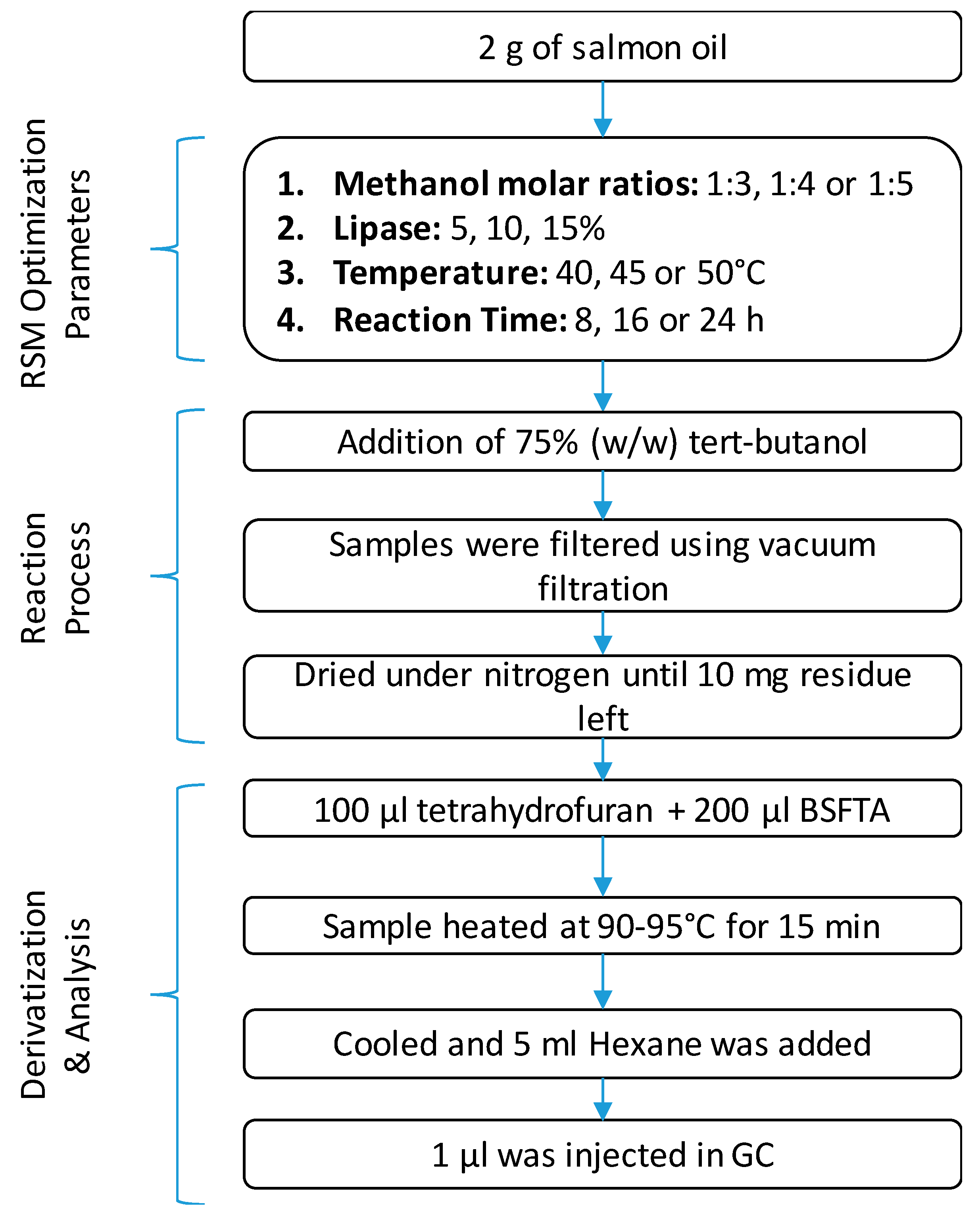
The concentration of Novozym 435 used for the enzymatic transesterification was investigated at three different levels (5, 10, or 15% w/w). Other factors studied included reaction temperature (40, 45, or 50 °C), time (8, 16, or 24 h), and oil/alcohol molar ratio (1:3, 1:4, or 1:5) for the optimization of biodiesel production (Figure 1). The homogenized salmon oil (2 g) was placed into a 50 mL conical flask, and an appropriate amount of methanol was added based on the stoichiometric oil/alcohol molar ratio (1:3, 1:4, or 1:5), followed by the addition of tert-butanol (75 wt% based on oil weight). The mixture was heated at an appropriate temperature (40, 45, or 50 °C) in a reciprocal shaking water bath at 200 rpm for 5–10 min. The reaction was initiated by adding the appropriate amount of immobilized enzyme (5, 10, or 15% w/w). After the desired reaction time (8, 16, or 24 h), the enzyme was filtered by vacuum filtration as per the method reported by Nelson et al. [32]. Then, 100 μL of the filtrate was taken and analyzed using a gas chromatograph (GC) (Section 2.5).
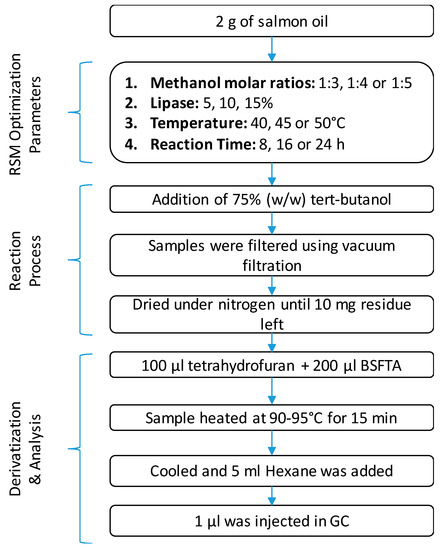
Figure 1.
Experiment design of enzymatic transesterification of salmon oil for production of biodiesel.
2.4. Fatty Acid Composition Analysis of Salmon Oil
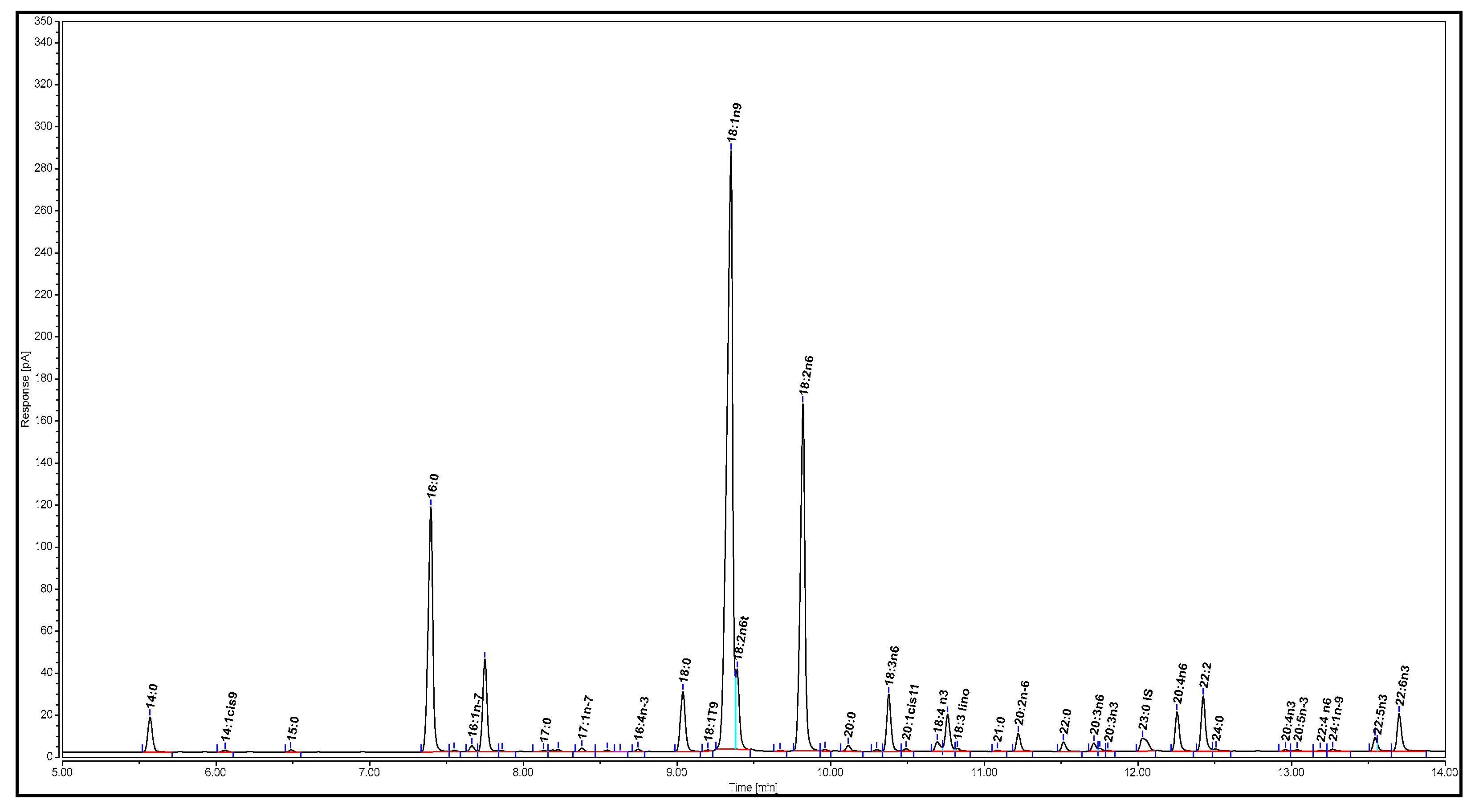
The extracted salmon oil was analyzed using the AOAC Official Method 991.39 [33]. First, 25 ± 0.1 mg of tricosanoic acid methyl ester (the internal standard) was weighed into a 25 mL volumetric flask and diluted to the line with hexane. Next, 1.0 mL portions of this stock solution were pipetted into screw cap glass test tubes, and the solvent was evaporated in a gentle stream of nitrogen (N2). The tubes were stored in the freezer (−80 °C) if not used immediately. A total of 25 ± 0.1 mg of the extracted salmon oil was weighed into a glass test tube containing the internal standard, followed by the addition of 1.5 mL of 0.5 M methanolic NaOH. The mixture was blanketed with N2, capped, and heated at 100 °C for 5 min. After the test tube was cooled, 2 mL of boron trifluoride (in 14% methanol) was added. The solution was blanketed with N2, capped, and heated at 100 °C for 30 min. Afterwards, the mixture was cooled to 30–40 °C, and 1 mL of hexane was added. The solution was blanketed with N2, capped, and mixed vigorously for 30 s while still warm. Next, 5 mL of saturated NaCl solution was immediately added to the test tube, and the mixture was blanketed with N2, capped, and mixed thoroughly. The sample was cooled to room temperature and left still for the formation of two layers. The hexane layer was transferred to another test tube, blanketed with N2, and capped tightly. The aqueous layer was extracted again with 1 mL of hexane. Afterwards, the hexane extracts were collected together and concentrated to approximately 1 mL under N2. Finally, 1 µL of the sample was injected into the GC for analysis. The gas chromatogram of the salmon oil is illustrated in Figure 2.
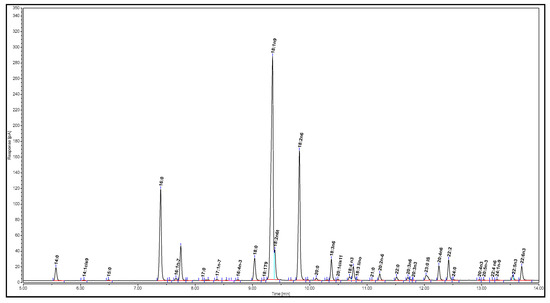
Figure 2.
Gas chromatogram of Atlantic salmon oil.
The area percentage of the fatty acid was calculated as follows:
where
Area percent of the fatty acid (%) = AX/(AT − AIS)*100
- AX = area counts of individual methyl ester;
- AT = total area counts for chromatogram;
- AIS = area counts of the internal standard;
- The fatty acid composition of the salmon oil.
2.5. Fatty Acid Methyl Ester (FAME) Composition Analysis of Biodiesel
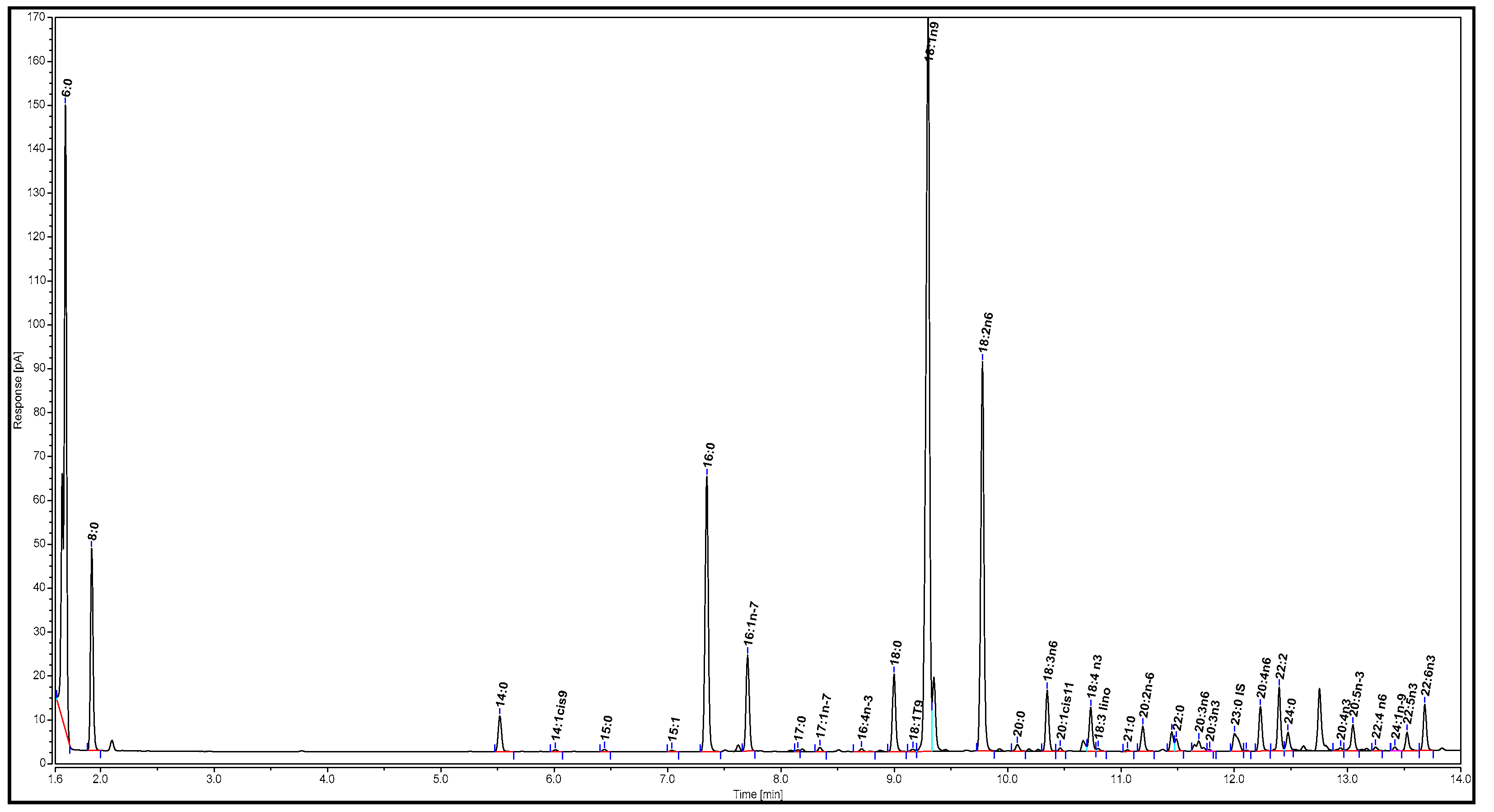
The sample for analysis was prepared as described by Nelson et al. [32]. First, 100 µL of the filtrate after reaction and filtration (Section 2.3) was concentrated under N2 until approximately 10 mg was left. Then, the sample was dissolved in 100 µL tetrahydrofuran, followed by the addition of 200 µL BSTFA. The mixture was heated in a water bath at 90–95 °C for 15 min. After the mixture was cooled to room temperature, 5 mL hexane was added. An aliquot of 1 µL of the mixture was injected into the GC for analysis. The gas chromatogram of the biodiesel is illustrated in Figure 3.
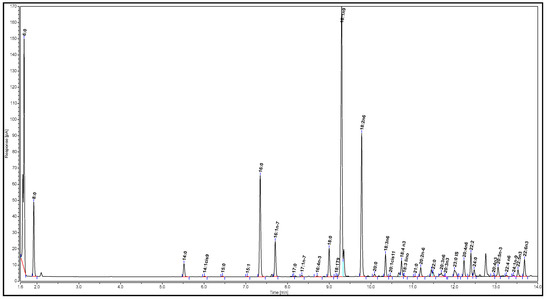
Figure 3.
Gas chromatogram of biodiesel.
The conversion yield of FAME was calculated as follows:
FAME Conversion(%) = Total methyl ester peak area after enzymatic reaction/Total methyl ester peak area of fish oil
2.6. GC Settings for Analysis
The composition analysis of fish oil and biodiesel was carried out using Trace 1300 gas chromatograph (GC) with flame ionization detector (FID) (Thermo Fisher, Canada). The column used for the analysis was the TR-FAME Trace GC capillary column with an internal diameter of 0.22 mm, film thickness of 0.25 µm, and a length of 30 m. The inlet temperature was maintained at 255 °C under split mode. The split flow of the carrier gas was maintained at 100 mL/min, and the purge flow was 2 mL/min. The inlet pressure was maintained at 81.7 kPa. The temperature of the flame ionization detector was maintained at 270 °C. The ignition threshold was 1 pA, and the data collection rate was 10 Hz. The gas settings included an airflow at 350 mL/min, makeup gas flow at 35 mL/min, and hydrogen flow at 35 mL/min. The GC oven temperature was initially maintained at 130 °C for 3 min, ramped to 250 °C at the rate of 10 °C/min, and held for 15 min. The total run time for one sample was 30 min.
2.7. Statistical Analysis
The response surface methodology was used for the experimental modelling design and analysis to describe the relationship between the response (yield) and critical factors (enzyme concentration, oil/alcohol molar ratio, time, and temperature). The experimental design consisted of four factors, generating 24 = 16 runs. The reactions were carried out in duplicates, and the design contained six center points, therefore yielding 38 runs. The initial design was modelled to fit linear modelling. However, curvature was observed in data analysis, which indicated that the average response points detected at the center points were higher or lower than the average response at the corner cube points. Therefore, the linear model was inadequate to describe the relationship between the response and the factors completely. A total of 11 curvature points were added to modify the model, and the experiments were carried out. All the data were analyzed with a one-way analysis of variance (ANOVA) at a 95% confidence level. The effect of each factor was determined by estimating the main products and two-way interactions. All experimental design and data analyses were performed using Minitab 17.1.
3. Results
3.1. Statistical Modeling of Biodiesel Production
The experiment conditions and corresponding biodiesel yield of the forty-nine transesterification reactions are shown in Table 1. The highest yield of 91.86% was obtained from the 16 h reaction at 45 °C with 15% enzyme concentration and 1:4 oil/alcohol molar ratio (Run order 41). A quadratic model fits the results with an R2 value of 99.31%, suggesting that this model can explain 99.31% of the yield variation. The regression equation that was developed for fitting the quadratic model for the biodiesel conversion was as follows:
Yield = −722.4 + 1.629 EC + 28.31 Temp − 0.582 Time + 83.02 Ratio + 0.0018 EC*EC − 0.3296 Temp×Temp − 0.1065 Time×Time − 9.516 Ratio×Ratio + 0.00417 EC×Temp − 0.05352 EC×Time − 0.0354 EC×Ratio + 0.08611 Temp×Time − 0.1679 Temp×Ratio + 0.3099 Time×Ratio

Table 1.
Biodiesel conversion yield from enzymatic transesterification of Atlantic salmon oil.
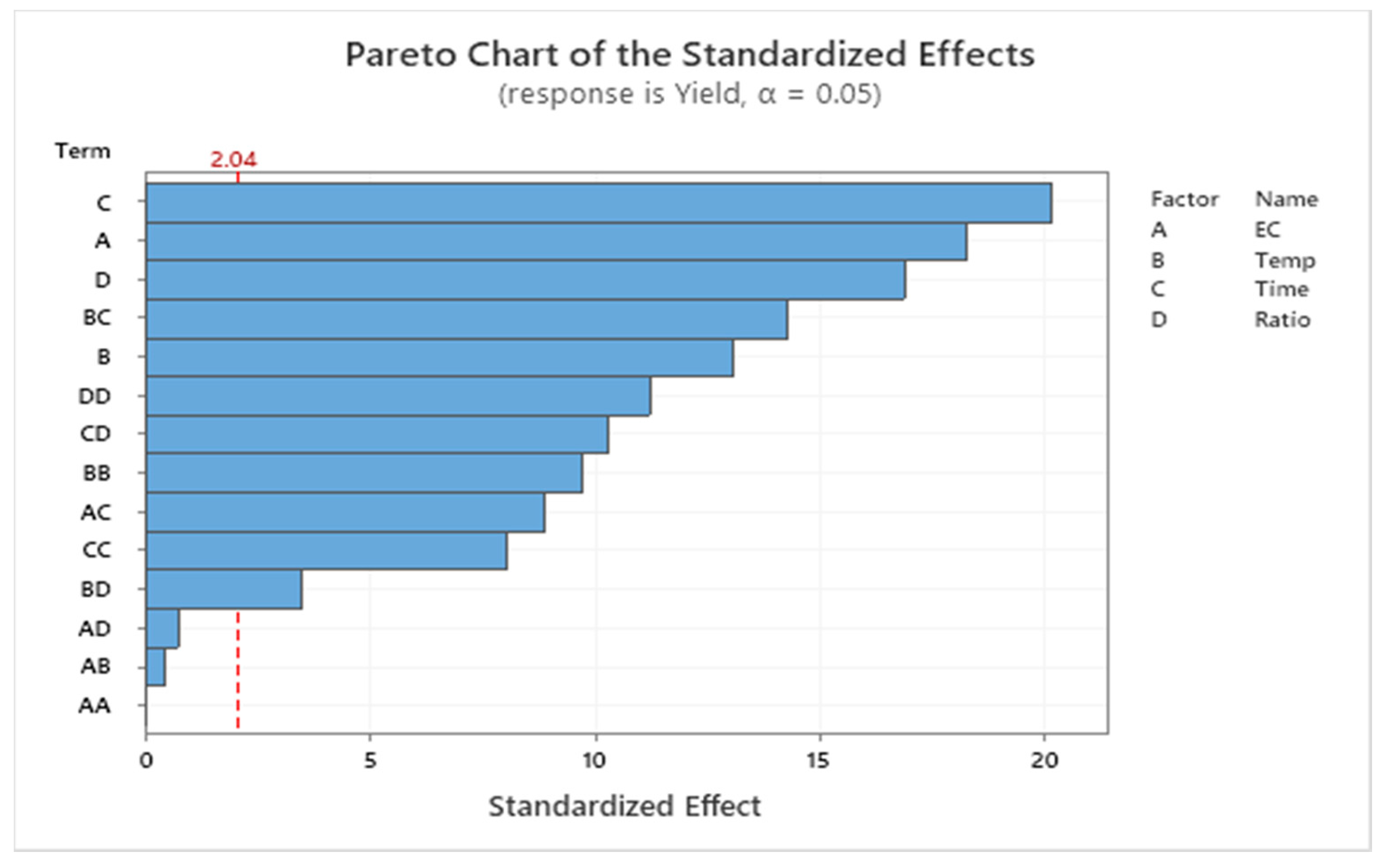
The model indicated a relationship between biodiesel yield and the factors at a 0.05 level of significance. As indicated in the Pareto chart (Figure 4), the most influential factor was time (C), followed by enzyme concentration (A), oil/alcohol molar ratio (D), the interaction between temperature and time (BC), temperature (B), and other interactions between factors. The analysis of variance of the model summarized the linear terms, square terms, and interaction of factors (Table 2). The effects of enzyme concentration (p-value: 0.000), temperature (p-value: 0.000), time (p-value: 0.000), and molar ratio (p-value: 0.000) on biodiesel conversion yield were statistically significant (p-value < 0.05).
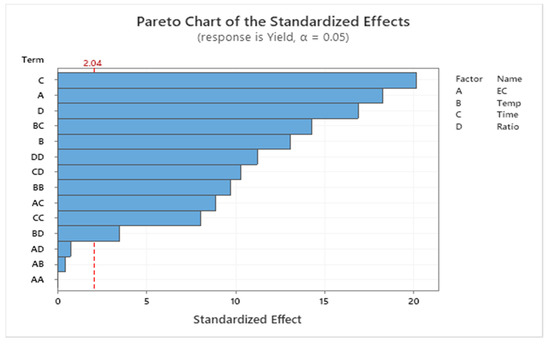
Figure 4.
Pareto chart for the effect of factors on biodiesel conversion yield (EC: enzyme concentration; Temp: temperature; Ratio: oil/alcohol molar ratio).

Table 2.
Analysis of variance on biodiesel conversion yield.
As indicated in Table 2, interaction terms including EC*Time, Temp*Time, Temp*Ratio, and Time*Ratio had a significant influence (p < 0.05) on the biodiesel conversion yield. However, EC*Temp and EC*Ratio did not significantly affect the biodiesel yield (p > 0.05). The square terms Temp*Temp, Time*Time, and Ratio*Ratio had p-values of 0.000, suggesting a significant quadratic effect and the relationship between temperature, time, and ratio of biodiesel yield followed a curved line rather than a straight line. The square term EC*EC had a p-value of 0.959, so it did not significantly contribute to the biodiesel conversion yield.
The p-values of 0.181 and 0.087 for blocks 1 and 2 (Table 2), respectively, indicated no significant block effect. Therefore, the data collected in those two blocks did not significantly affect the yield. The small p-values for linear terms (p-value: 0.000), square terms (p-value: 0.000), and interactions (p-value: 0.000) indicated there was curvature in the response surface. The ANOVA results showed a p-value of 0.230 for the lack of fit, suggesting a significant model fit with the data. The s-value (standard error of the regression) in a model indicates the standard distance that data values fall from the regression line or the standard deviation of the residuals. The s-value of the current model was 1.362, which was quite low and therefore suggested a good fit.
As presented in Table 2, the variance inflation factors (VIFs) for most of the predictors were close to 1, which suggested that the predictors were not correlated. The VIFs of four interactions (EC×EC, Temp×Temp, Time×Time and Ratio×Ratio) were 4.03, suggesting that they were moderately correlated. None of the results showed VIF values higher than 5, which would result in severe multicollinearity, and the model would become unstable and difficult to interpret.
3.2. Effect of Operating Parameters
3.2.1. Temperature
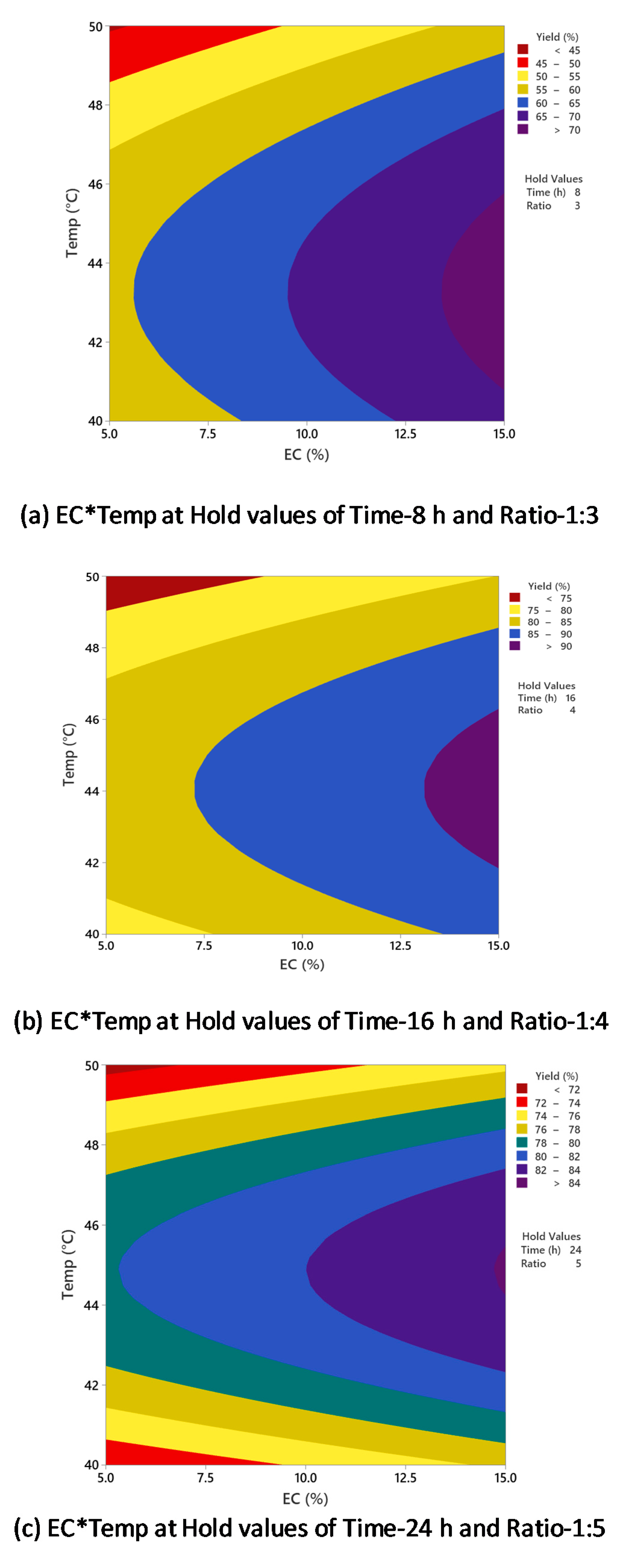
Figure 5 shows counterplots of biodiesel yield vs. enzyme concentration and temperature, with time and oil/alcohol molar ratio held constant at different conditions. In Figure 5a, which had a hold value of 8 h (time) and 1:3 (oil/alcohol molar ratio), the highest biodiesel yield (>70%) was obtained at 43–45 °C and 13–15% enzyme concentration. When the hold values were changed to 16 h (time) and 1:4 (oil/alcohol molar ratio) (Figure 5b), the highest yield was >90%, with the optimal setting of around 45 °C and 13% enzyme concentration. In Figure 5c, which had a hold value of 24 h (time) and 1:5 (oil/alcohol molar ratio), the highest biodiesel yield was >84% at around 45 °C and 14% enzyme concentration. In all the three plots, it is evident that when the temperature was increased to above 45 °C, the yield decreased significantly. The highest biodiesel yield was always obtained at around 45 °C regardless of the enzyme concentration. This effect was further supported in Table 2, which indicated no significant interaction between the two factors (enzyme concentration and temperature) for biodiesel yield (p-value of 0.668).
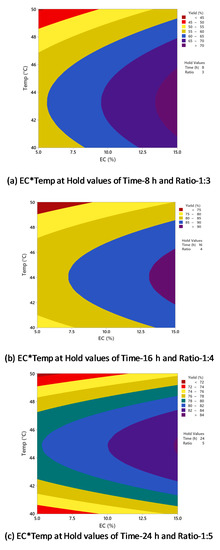
Figure 5.
Counterplots of biodiesel yield vs. enzyme concentration and temperature at (a) 8 h and 1:3; (b) 16 h and 1:4; (c) 24 h and 1:5.
As observed in the present study, the effect of temperature on biodiesel conversion agrees with previously reported research. Chen et al. studied the enzymatic conversion of waste cooking oils into biodiesel and reported the highest biodiesel yield of 87% at 40 °C using immobilized Rhizopus oryzae lipase [34]. It was observed that the further increase of temperature to above 40 °C decreased the biodiesel yield. Dizge and Keskinler produced biodiesel from canola oil using immobilized Thermomyces lanuginosus lipase [35]. The effect of temperature on enzymatic transesterification for biodiesel production was studied from 30 to 70 °C, and 40 °C was the optimal temperature, resulting in the highest biodiesel yield of 85.8%. With a further increase of temperature to above 50 °C, the biodiesel yield was decreased drastically since the enzyme lost its activity significantly above 50 °C. Nie et al. performed lipase-catalyzed methanolysis of salad oil using immobilized Candida sp. 99–125 lipase [36]. The highest yield was observed at 40 °C and decreased drastically above that. As claimed by the authors, high temperatures can accelerate the reaction process; however, the enzyme will be denatured if the temperatures are too high. This was supported by Pinyaphong et al. in their study of methanolysis of fish oil catalyzed by Carcia papaya lipase [37]. The reaction was performed at 30–60 °C, and a maximum biodiesel conversion yield of 83% was obtained at 40 °C. The results showed that reaction temperature significantly influenced enzyme activity and stability, and a high temperature can lead to deactivation of the lipase.
3.2.2. Reaction Time
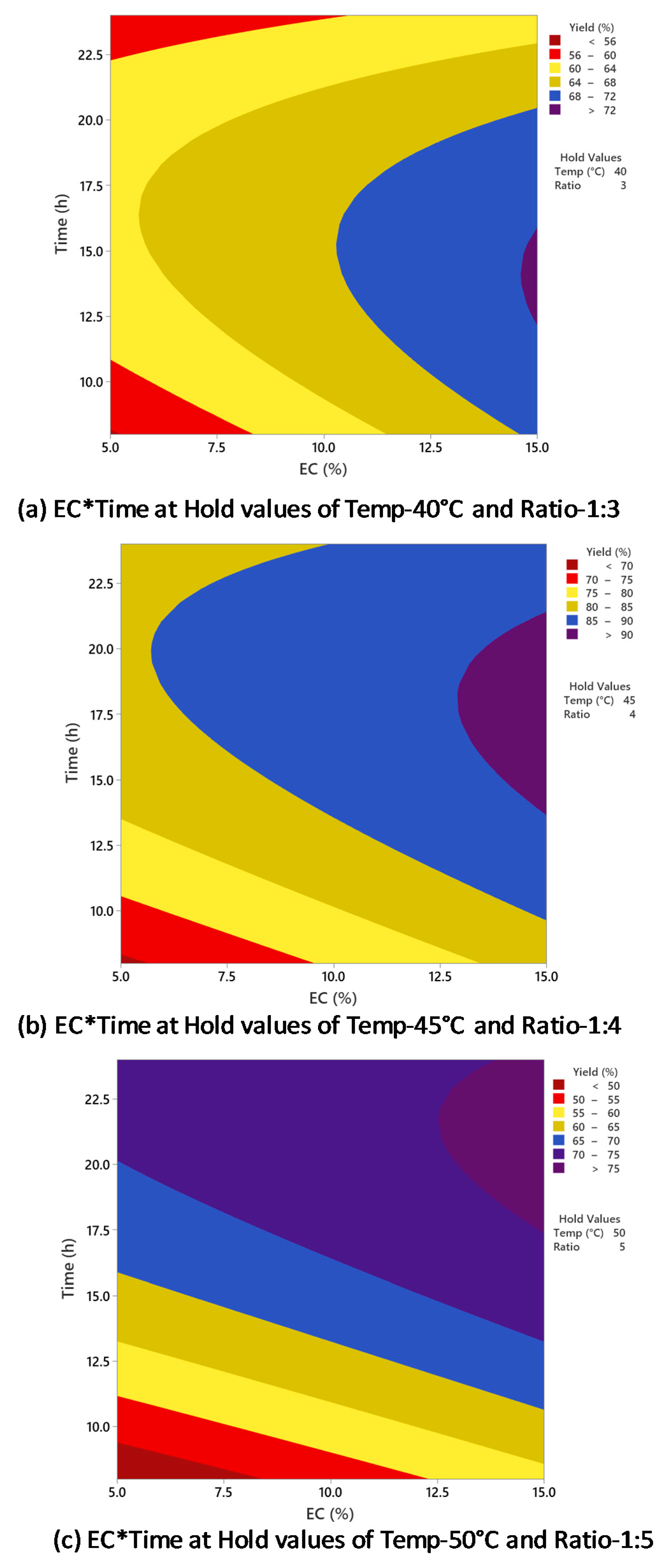
Figure 6 indicates the relationship between biodiesel yield and enzyme concentration and time, with temperature and oil/alcohol molar ratio held constant at different conditions. In Figure 6a, the biodiesel yield was increased when enzyme concentration was increased from 5 to 15% and time was increased from 8 to 15 h, and the highest yield (>72%) was achieved at 14.5% enzyme concentration within 13–15 h of reaction time. However, Figure 6b indicates that biodiesel yield >90% was obtained when the reaction time was 18 h and the enzyme concentration was 14.5%. Figure 6c shows that further increase in time did not increase biodiesel yield, which was >75% after 22 h of reaction with 13% enzyme concentration. Therefore, the optimal setting for the maximum biodiesel yield (>90%) could be achieved after around 18 h of reaction with 14.5% enzyme concentration. The biodiesel yield was significantly affected by the interaction between enzyme concentration and time, supported by the p-value of 0.000 (Table 2).
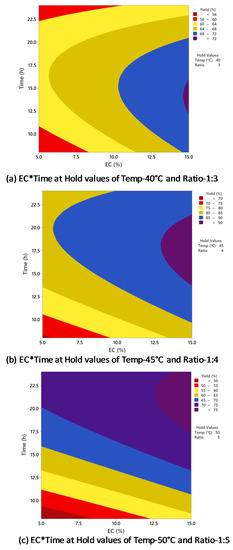
Figure 6.
Counterplots of biodiesel yield vs. enzyme concentration and time at (a) 40 °C and 1:3; (b) 45 °C and 1:4; (c) 50 °C and 1:5.
In the present study, the increase of time resulted in the increase of biodiesel production yield in most of the parameters. However, when the time was increased from 16 to 24 h, there was no significant increase in biodiesel yield, and the optimum time for biodiesel production was limited to 16 h. Pinyaphong et al. reported a maximum biodiesel conversion yield of 83% from fish oil from a reaction of 18 h [37]. Nelson et al. carried out biodiesel production from tallow oil and reported the highest yield of 83.8% after 16 h of reaction [32]. Li et al. performed transesterification of rapeseed oil for biodiesel production using Novozyme 435, and a 12 h reaction resulted in the highest biodiesel yield of 95% [38]. Azcar et al. studied the biodiesel production from waste frying oil using Novozyme 435 and reported the highest biodiesel yield of 100% after 4 h of reaction [39]. Compared to the optimized reaction time of 16 h in the present study, their reaction time was four times shorter. The biodiesel yield was higher, mainly due to the lower content of unsaturated fatty acids in frying oil than that of fish oil.
3.2.3. Oil/Alcohol Molar Ratio
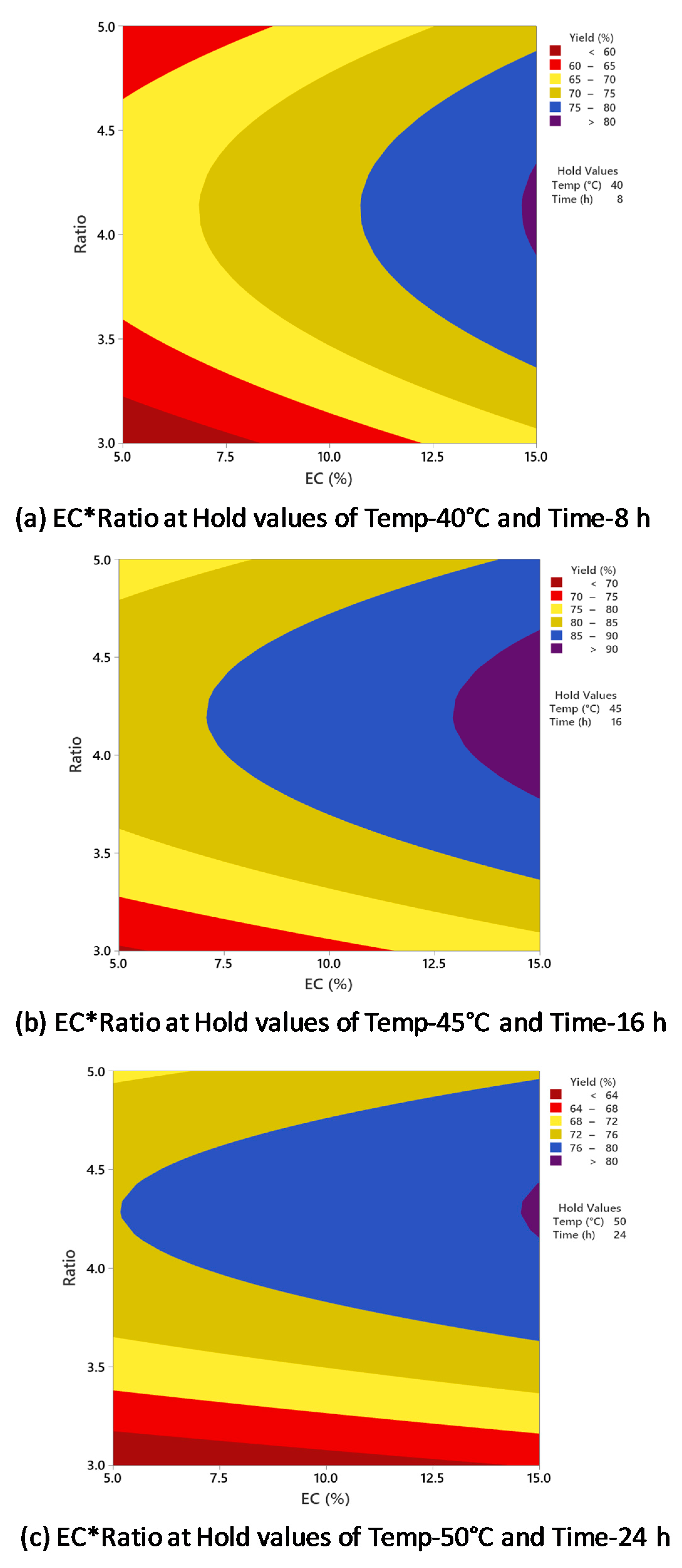
As indicated in Table 1, the increase in the oil/alcohol molar ratio positively affected biodiesel production from 1:3 to 1:4. However, there was no significant increase when the molar ratio was increased to 1:5, and in some experiments, the biodiesel yield was reduced. Figure 7 indicates the influence of enzyme concentration and oil/alcohol molar ratio on biodiesel yield with temperature and time held constant at different conditions. The results from Figure 7a–c indicate that whether the enzyme concentration was increased or decreased, the highest biodiesel yield of >90% was achieved with around 1:4 oil/alcohol molar ratio. Further increase in the molar ratio did not increase the biodiesel yield. The interaction between enzyme concentration and oil/alcohol molar ratio had no significant effect on biodiesel yield, as supported by the p-value of 0.468 (Table 2).
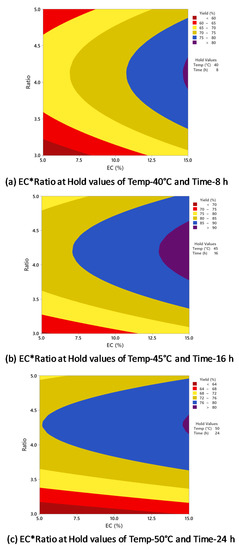
Figure 7.
Counterplots of yield vs. enzyme concentration and oil/alcohol ratio at (a) 40 °C and 8 h; (b) 45 °C and 16 h; (c) 50 °C and 24 h.
Li et al. reported in their study of lipase-catalyzed transesterification of rapeseed oil using Novozyme 435 that the highest biodiesel conversion yield of 95% was obtained with 1:4 oil/alcohol molar ratio with tert-butanol as a solvent [38]. Nelson et al. reported a maximum biodiesel conversion of 83.8% with a 1:3 oil/alcohol molar ratio using tallow and 25% Candida antarctica (SP 435) with hexane as the solvent [32]. Ognjanovic et al. reported biodiesel conversion of above 99% with 1:3 oil/methanol molar ratio after 50 h of reaction, but the enzyme activity rapidly decreased [40]. To stabilize the enzyme, methyl acetate was used as an alternative to methanol in the ratio of 1:12, and 93.6% biodiesel yield was achieved from sunflower oil using Novozyme 435 enzyme. Chen et al. reported a maximum biodiesel conversion yield of 89% at 1:4 oil/alcohol molar ratio from waste cooking oil [34]. As suggested by Hernández-Martín and Otero, the amount of alcohol used for biodiesel conversion should be slightly higher than the stoichiometric amount equal to the number of fatty acids in the oil to compensate for the thermodynamic or kinetic constraints [41]. Furthermore, the use of excess alcohol in biodiesel production has been claimed to maintain a uniform suspension of catalysts in the reaction medium, increase reaction rates, minimize diffusion limitations, and retain glycerol formation without deactivating the catalytic pores [42,43].
3.2.4. Enzyme Concentration
The effect of enzyme concentration (5, 10, and 15% w/w) of Novozyme 435 on biodiesel conversion yield was studied. The highest biodiesel yield was obtained using 15% enzyme. The results from Figure 5, Figure 6 and Figure 7 suggest that while visualizing the biodiesel yield with respect to time, temperature, and molar ratio, the highest yield was always obtained with above 10% enzyme concentration.
Marín-Suárez et al. studied the transesterification of waste fish oil for biodiesel production using three commercial immobilized enzymes: Lipozyme RM IM, Lipozyme TL IM, and Novozym 435 [44]. They reported the highest biodiesel yield of 82.91% with 50% Novozyme 435 using excess ethanol per mol of oil. Pinyaphong et al. reported a maximum biodiesel conversion yield of 83% from fish oil using 20% Carica papaya lipase [37]. Chen et al. reported a maximum biodiesel conversion yield of 89% from waste cooking oil using 30% Rhizopus oryzae lipase [34]. Nelson et al. carried out biodiesel production from tallow oil using 25% Candida antartica (SP 435) and reported the highest yield of 83.8% [32].
3.3. Optimization of Biodiesel Production
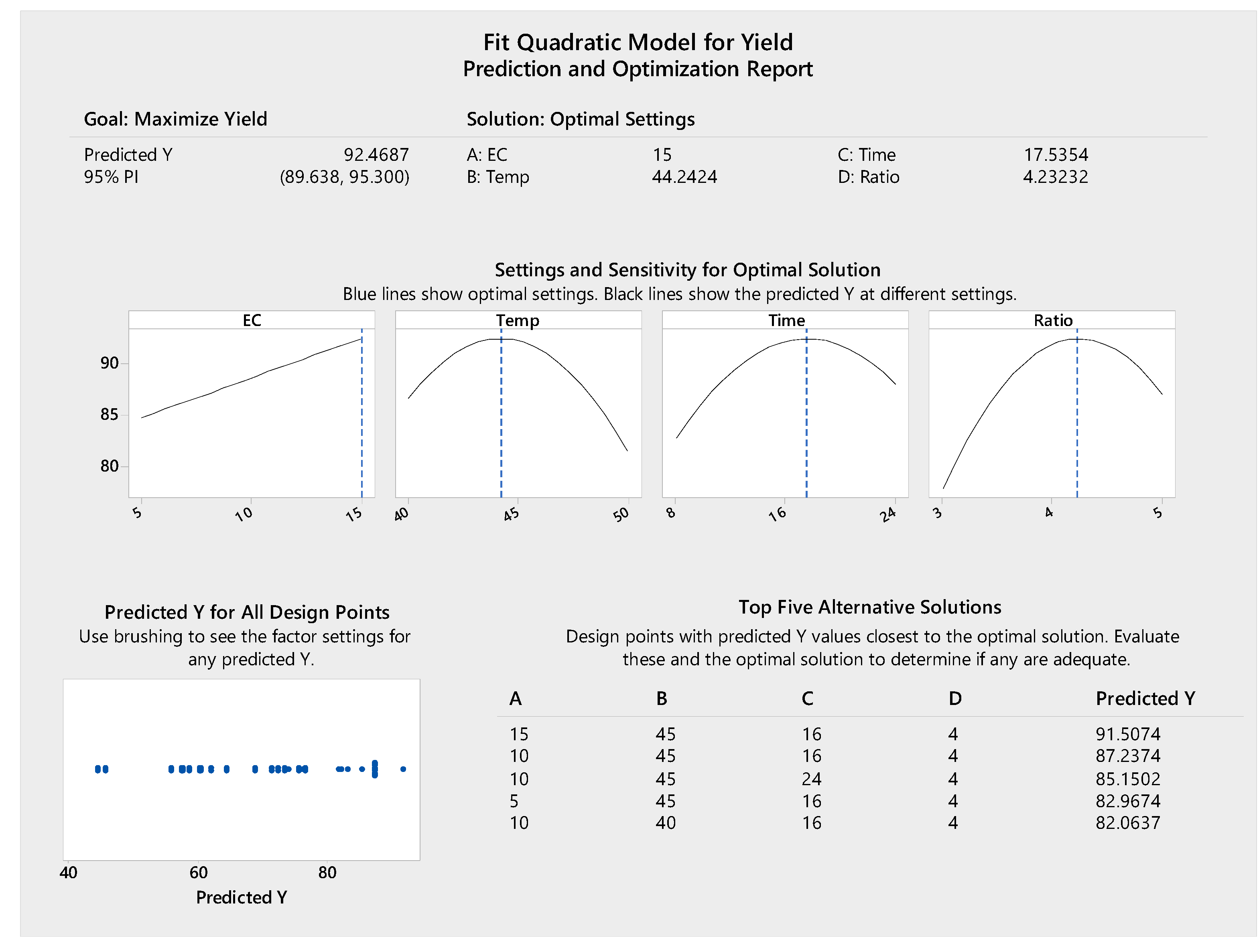
The response surface methodology was used to model the experiments statistically and optimize the parameters for biodiesel production. As indicated in Figure 8, the optimized reaction conditions were 17.53 h of transesterification at 44.24 °C with 15% enzyme concentration and oil/alcohol molar ratio of 1:4.23, resulting in a predicted maximum biodiesel yield of 92.46%. The highest experimental yield was 91.86% from 16 h of reaction at 45 °C with 15% enzyme concentration and oil/alcohol molar ratio of 1:4 (Table 1, Run order 41), which was in agreement with the prediction. In addition to the maximum biodiesel yield conditions, the modelling also provided five alternative solutions for optimizing the biodiesel production process (Figure 8). With 10% enzyme concentration, a biodiesel yield of 87.24% can be obtained. The increase of 10% to 15% enzyme concentration (50% increase) to achieve only a 5% higher yield (87.24% to 92.46%) may not be economically feasible. Therefore, the optimum parameters for biodiesel production were determined as 10% enzyme concentration, 45 °C, 16 h and 1:4 oil/alcohol molar ratio for a predicted biodiesel yield of 87.24%. The experiment performed under these conditions resulted in a biodiesel yield of 88.24% (Table 1, Run order 49), which was consistent with the predicted value.
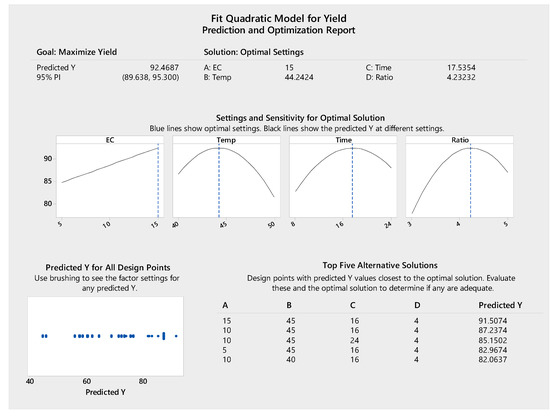
Figure 8.
Prediction and optimization report for biodiesel conversion yield.
Step-Wise Addition of Methanol
As indicated in several previously reported studies about biodiesel production, methanol would lead to lipase inactivation during the transesterification [45,46,47]. Therefore, step-wise addition of methanol has been adopted to reduce its concentration in the system, thereby minimizing its influence on lipase activity and improving the oils’ conversion to biodiesel [48,49,50]. In the present study, based on the optimized conditions for biodiesel production, the efficiency of step-wise addition of methanol was evaluated to further improve biodiesel yield. Instead of a one-time addition of methanol as 1:4 oil/alcohol molar ratio, the experiment was started with 1:2 oil/methanol molar ratio for the first 8 h, and then another portion of methanol was added following the 1:2 oil/methanol molar ratio for the subsequent 8 h of reaction. The biodiesel yield obtained from this experiment was 94.5%, which was approximately 6% higher than the results with a one-time addition of methanol (88.24%, Table 1, Run order 49). Shimada et al. produced biodiesel from waste edible oil with Candida antartica lipase by step-wise addition of methanol [51] and reported more than 90% biodiesel yield. They also reported the lipase catalyst retained activity for more than 100 cycles of production. The same group also performed biodiesel production using vegetable oil with Candida antartica lipase and reported a 98.4% biodiesel yield after 72 h by step-wise addition of methanol [52]. Watanable et al. successfully produced biodiesel from degummed soybean oil using a three-step methanolysis with a yield of 93.8% in the presence of Candida antartica lipase [53]. Hajar et al. applied a three-step methanolysis of canola oil using Candida antartica lipase in a solvent-free system and produced biodiesel with a yield of 99.57% [54].
3.4. Investigation of Glycerol as the By-Product
In the present study, free glycerol was not detected from reactions at all the oil/alcohol molar ratios, reaction temperatures, and times studied. This could be due to the low alcohol concentration present in the reaction system and the fact that the whole reaction was carried out using a small amount of starting material. Theoretically, 3 moles of alcohol reacts with 1 mole of triglycerides to give 3 moles of FAME and 1 mole of glycerol (by-product). According to the optimization, 2 g of oil was reacted with methanol with a 1:4 molar ratio and 15% enzyme concentration at 45 °C for 17.53 h to result in a maximum biodiesel conversion yield of 92.46%. The remaining balance of 7.54% observed in this study was made of intermediates and bound glycerols, such as monoacylglycerol (monoglycerides), diacylglycerol (diglycerides), and triacylglycerol (triglycerides). Glycerol is immiscible with oil and biodiesel and has a higher density than that of any other component in the liquid phase of the reaction system. However, it was difficult to identify and separate the glycerol phase on a laboratory scale using immobilized enzymes because the glycerol phase was relatively thin and colorless, as reported in many studies in the literature [55,56]. It has also been reported that the formation of glycerol during transesterification can inhibit the reaction. The free glycerol can clog the lipase’s active sites, and the bound glycerol can restrict the mass transfer between the substrate and enzyme. Therefore, it is essential to remove glycerol from the reaction system to improve transesterification efficiency. In the present study, tert-butanol dissolved both methanol and glycerol, which eliminated the adverse effects of glycerol accumulation during the transesterification process. As reported in previous studies, tert-butanol is not a substrate for lipases, and it does not act on tertiary alcohols [57,58,59].
4. Conclusions
In the present study, biodiesel production from salmon oil extracted from Atlantic salmon by-products was optimized using response surface methodology. The four parameters, reaction time, temperature, enzyme concentration and oil/alcohol molar ratio, significantly influenced biodiesel production. The maximum biodiesel yield was 92.46% from transesterification for 17.53 h at 44.24 °C with 15% enzyme concentration, oil/alcohol molar ratio of 1:4.23, and the use of 75% (w/w) tert-butanol as the solvent. In comparison to the five alternative optimization solutions provided by the modelling, an increase of 10% to 15% enzyme concentration (50% increase) resulted in only a 5% higher yield (87.24% to 92.46%), which may not be economically feasible. Therefore, the optimum parameters of biodiesel production were determined as 10% enzyme concentration, 45 °C, 16 h, and 1:4 oil/alcohol molar ratio, resulting in a biodiesel yield of 87.24%. By applying step-wise addition of methanol, the biodiesel yield was further increased to 94.5%.
The present study was the first to produce biodiesel from Atlantic salmon oil, which can be a potential replacement of plant-derived oils in conventional biodiesel production. As the salmon oil is extracted from Atlantic salmon heads and frames, this will also be a promising way to valorize the salmon by-products. The results of the present study are promising for the biocatalytic production of biodiesel on an industrial scale. However, there are still some disadvantages in the optimized process, such as the use of tert-butanol as the solvent. Future studies will be focused on improving the process by reducing the amount of the solvent, recovery of the solvent for reuse, or completely removing the solvent from the system. The physicochemical properties of FAMEs in the biodiesel obtained were not analyzed in the present study, since the whole process was performed on a small scale and the amount of biodiesel obtained was small. Future studies should be carried out on a larger scale to produce biodiesel using the optimized parameters, and its properties should be assessed and compared against traditional fuel. In addition, the reusability of the lipase and reaction kinetics should be studied as they are essential if the process will be implemented on a larger scale.
Author Contributions
Conceptualization, D.D. and W.M.; methodology, V.V.R.; validation, V.V.R.; formal analysis, V.V.R. and W.R.; investigation, V.V.R.; resources, W.M.; data curation, V.V.R. and Y.L.; writing—original draft preparation, V.V.R.; writing—review and editing, Y.L., V.V.R., D.D., W.R. and W.M.; visualization, V.V.R. and Y.L.; supervision, D.D.; project administration, D.D.; funding acquisition, D.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Ocean Frontier Institute, through an award from the Canada First Research Excellence Fund.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Ambat, I.; Srivastava, V.; Sillanpää, M. Recent advancement in biodiesel production methodologies using various feedstock: A review. Renew. Sustain. Energy Rev. 2018, 90, 356–369. [Google Scholar] [CrossRef]
- Manaf, I.S.A.; Embong, N.H.; Khazaai, S.N.M.; Rahim, M.H.A.; Yusoff, M.M.; Lee, K.T.; Maniam, G.P. A review for key challenges of the development of biodiesel industry. Energy Convers. Manag. 2019, 185, 508–517. [Google Scholar] [CrossRef]
- Thangaraj, B.; Solomon, P.R.; Muniyandi, B.; Ranganathan, S.; Lin, L. Catalysis in biodiesel production-A review. Clean Energy 2019, 3, 2–23. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, D.; Soni, S.L.; Sharma, S.; Sharma, P.K.; Jhalani, A. A review on feedstocks, production processes, and yield for different generations of biodiesel. Fuel 2020, 262, 116553. [Google Scholar] [CrossRef]
- Lv, L.; Dai, L.; Du, W.; Liu, D. Progress in enzymatic biodiesel production and commercialization. Processes 2021, 9, 355. [Google Scholar] [CrossRef]
- de Lima, A.L.; Mota, C.J.A. Biodiesel: A Survey on Production Methods and Catalysts. In Jatropha, Challenges for a New Energy Crop; Mulpuri, S., Carels, N., Bahadur, B., Eds.; Springer: Singapore, 2019; pp. 475–491. [Google Scholar]
- Sendzikiene, E.; Santaraite, M.; Makareviciene, V. Lipase-Catalysed In Situ Transesterification of Waste Rapeseed Oil to Produce Diesel-Biodiesel Blends. Processes 2020, 8, 1118. [Google Scholar] [CrossRef]
- Zhong, L.; Feng, Y.; Wang, G.; Wang, Z.; Bilal, M.; Lv, H.; Jia, S.; Cui, J. Production and use of immobilized lipases in/on nanomaterials: A review from the waste to biodiesel production. Int. J. Biol. Macromol. 2020, 152, 207–222. [Google Scholar] [CrossRef]
- Ching-Velasquez, J.; Fernandez-Lafuente, R.; Rodrigues, R.C.; Plata, V.; Rosales-Quintero, A.; Torrestiana-Sanchez, B.; Tacias-Pascacio, V.G. Production and characterization of biodiesel from oil of fi sh waste by enzymatic catalysis. Renew. Energy 2020, 153, 1346–1354. [Google Scholar] [CrossRef]
- Moazeni, F.; Chen, Y.C.; Zhang, G. Enzymatic transesterification for biodiesel production from used cooking oil, a review. J. Clean. Prod. 2019, 216, 117–128. [Google Scholar] [CrossRef]
- Ortiz, C.; Ferreira, M.L.; Barbosa, O.; Santos, J.C.S.; Rodrigues, R.C.; Berenguer-murcia, Á.; Briand, L.E.; Fernandez-lafuente, R. Novozym 435: The “perfect” lipase immobilized biocatalyst? Catal. Sci. Technol. 2019, 9, 2380–2420. [Google Scholar] [CrossRef]
- Vijayan, G. Enzymatic Transesterification of Fish Oil for the Biodiesel Production; Dalhousie University: Halifax, NS, Canada, 2013. [Google Scholar]
- Kumar, S. Enzymatic Transesterification of Waste Animal Fats for Production of Biodiesel; Dalhousie University: Halifax, NS, Canada, 2013. [Google Scholar]
- Kianimanesh, H.R.; Abbaspour-aghdam, F.; Derakhshan, M.V. Biodiesel production from vegetable oil: Process design, evaluation and optimization. Pol. J. Chem. Technol. 2017, 19, 49–55. [Google Scholar] [CrossRef]
- Santaraite, M.; Sendzikiene, E.; Makareviciene, V.; Kazancev, K. Biodiesel Production by Lipase-Catalyzed In Situ Transesterification of Rapeseed Oil Containing a High Free Fatty Acid Content with Ethanol in Diesel Fuel Media. Energies 2020, 13, 2588. [Google Scholar] [CrossRef]
- Veljkovi, V.B.; Biberd, M.O.; Bankovi, I.B.; Djalovi, I.G.; Tasi, M.B.; Nje, Z.B.; Stamenkovi, O.S. Biodiesel production from corn oil: A review. Renew. Sustain. Energy Rev. 2018, 91, 531–548. [Google Scholar] [CrossRef]
- Kara, K.; Ouanji, F.; Lotfi, E.M.; El Mahi, M.; Kacimi, M.; Ziyad, M. Biodiesel production from waste fish oil with high free fatty acid content from Moroccan fish-processing industries. Egypt. J. Pet. 2018, 27, 249–255. [Google Scholar] [CrossRef]
- Yari, N.; Mostafaei, M.; Naderloo, L.; Ardebili, S.M.S. Energy indicators for microwave-assisted biodiesel production from waste fish oil. Energy Sources Part A Recover. Util. Environ. Eff. 2019, 1–12. [Google Scholar] [CrossRef]
- Makoure, D.; Arhaliass, A.; Echchelh, A.; Legrand, J. Valorization of Fish By-Products Using Reactive Extrusion for Biodiesel Production and Optimization. Waste Biomass Valoriz. 2020, 11, 6285–6293. [Google Scholar] [CrossRef]
- Samat, A.F.; Muhamad, N.A.S.; Rasib, N.A.A.; Hassan, S.A.M.; Sohaimi, K.S.A.; Iberahim, N.I. The Potential of Biodiesel Production derived from Fish Waste. IOP Conf. Ser. Mater. Sci. Eng. 2018, 318, 012017. [Google Scholar] [CrossRef]
- Fisheries and Land Resources Seafood Industry Year in Review. 2019. Available online: https://www.gov.nl.ca/ffa/files/2019-SIYIR-WEB.pdf (accessed on 22 January 2021).
- Liu, Y.; Ramakrishnan, V.V.; Dave, D. Lipid class and fatty acid composition of oil extracted from Atlantic salmon by-products under different optimization parameters of enzymatic hydrolysis. Biocatal. Agric. Biotechnol. 2020, 30, 101866. [Google Scholar] [CrossRef]
- Liu, Y.; Ramakrishnan, V.V.; Dave, D. Enzymatic hydrolysis of farmed Atlantic salmon by-products: Investigation of operational parameters on extracted oil yield and quality. Process. Biochem. 2021, 100, 10–19. [Google Scholar] [CrossRef]
- Dave, D.; Ramakrishnan, V.R.; Pohling, J.; Cheema, S.K.; Trenholm, S.; Manuel, H.; Murphy, W. Investigation on oil extraction methods and its influence on omega-3 content from cultured salmon. J. Food Process. Technol. 2014, 5, 1000401. [Google Scholar] [CrossRef]
- Routray, W.; Dave, D.; Ramakrishnan, V.V.; Murphy, W. Production of high quality fish oil by enzymatic protein hydrolysis from cultured Atlantic salmon by-products: Investigation on effect of various extraction parameters using central composite rotatable design. Waste Biomass Valoriz. 2018, 9, 2003–2014. [Google Scholar] [CrossRef]
- Rubio-Rodríguez, N.; Sara, M.; Beltrán, S.; Jaime, I.; Sanz, M.T.; Rovira, J. Supercritical fluid extraction of fish oil from fish by-products: A comparison with other extraction methods. J. Food Eng. 2012, 109, 238–248. [Google Scholar] [CrossRef]
- Głowacz-Różyńska, A.; Tynek, M.; Malinowska-Pańczyk, E.; Martysiak-Żurowska, D.; Pawłowicz, R.; Kołodziejska, I. Comparison of oil yield and quality obtained by different extraction procedures from salmon (Salmo salar) processing byproducts. Eur. J. Lipid Sci. Technol. 2016, 118, 1759–1767. [Google Scholar] [CrossRef]
- Zieniuk, B.; Wołoszynowska, M.; Białecka-Florjańczyk, E. Enzymatic Synthesis of Biodiesel by Direct Transesterification of Rapeseed Cake. Int. J. Food Eng. 2020, 16, 1–10. [Google Scholar] [CrossRef]
- Behera, S.K.; Meena, H.; Chakraborty, S.; Meikap, B.C. Application of response surface methodology (RSM) for optimization of leaching parameters for ash reduction from low-grade coal. Int. J. Min. Sci. Technol. 2018, 28, 621–629. [Google Scholar] [CrossRef]
- Kumari, M.; Gupta, S.K. Response surface methodological (RSM) approach for optimizing the removal of trihalomethanes (THMs) and its precursor’s by surfactant modified magnetic nanoadsorbents (sMNP)-An endeavor to diminish probable cancer risk. Sci. Rep. 2019, 9, 18339. [Google Scholar] [CrossRef] [PubMed]
- Chumuang, N.; Punsuvon, V. Response Surface Methodology for Biodiesel Production Using Calcium Methoxide Catalyst Assisted with Tetrahydrofuran as Cosolvent. J. Chem. 2017, 2017, 4190818. [Google Scholar] [CrossRef]
- Nelson, L.A.; Foglia, T.A.; Marmer, W.N. Lipase-catalyzed production of biodiesel. JAOCS J. Am. Oil Chem. Soc. 1996, 73, 1191–1195. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Methods of Analysis of AOAC International, 17th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Chen, G.; Ying, M.; Li, W. Enzymatic Conversion of Waste Cooking Oils Into Alternative Fuel—Biodiesel. Appl. Biochem. Biotechnol. 2006, 132, 911–921. [Google Scholar] [CrossRef]
- Dizge, N.; Keskinler, B. Enzymatic production of biodiesel from canola oil using immobilized lipase. Biomass Bioenergy 2008, 32, 1274–1278. [Google Scholar] [CrossRef]
- Nie, K.; Xie, F.; Wang, F.; Tan, T. Lipase catalyzed methanolysis to produce biodiesel: Optimization of the biodiesel production. J. Mol. Catal. B Enzym. 2006, 43, 142–147. [Google Scholar] [CrossRef]
- Pinyaphong, P.; Sriburi, P.; Phutrakul, S. Biodiesel fuel production by methanolysis of fish oil derived from the discarded parts of fish catalyzed by Carica papaya lipase. World Acad. Sci. Eng. Technol. 2011, 76, 466–472. [Google Scholar]
- Li, L.; Du, W.; Liu, D.; Wang, L.; Li, Z. Lipase-catalyzed transesterification of rapeseed oils for biodiesel production with a novel organic solvent as the reaction medium. J. Mol. Catal. B Enzym. 2006, 43, 58–62. [Google Scholar] [CrossRef]
- Azcar, L.; Ciudad, G.; Muoz, R.; Jeison, D.; Toro, C.; Navi, R. Feasible Novozym 435-Catalyzed Process to Fatty Acid Methyl Ester Production from Waste Frying Oil: Role of Lipase Inhibition. In Enzyme Inhibition and Bioapplications; InTech: Philadelphia, PA, USA, 2012. [Google Scholar]
- Ognjanovic, N.; Bezbradica, D.; Knezevic-Jugovic, Z. Enzymatic conversion of sunflower oil to biodiesel in a solvent-free system: Process optimization and the immobilized system stability. Bioresour. Technol. 2009, 100, 5146–5154. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Martín, E.; Otero, C. Different enzyme requirements for the synthesis of biodiesel: Novozym® 435 and Lipozyme® TL IM. Bioresour. Technol. 2008, 99, 277–286. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Volpato, G.; Wada, K.; Ayub, M.A.Z. Enzymatic synthesis of biodiesel from transesterification reactions of vegetable oils and short chain alcohols. J. Am. Oil Chem. Soc. 2008, 85, 925–930. [Google Scholar] [CrossRef]
- Noureddini, H.; Gao, X.; Philkana, R.S. Immobilized Pseudomonas cepacia lipase for biodiesel fuel production from soybean oil. Bioresour. Technol. 2005, 96, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Marín-Suárez, M.; Méndez-Mateos, D.; Guadix, A.; Guadix, E.M. Reuse of immobilized lipases in the transesterification of waste fish oil for the production of biodiesel. Renew. Energy 2019, 140, 1–8. [Google Scholar] [CrossRef]
- Lotti, M.; Pleiss, J.; Valero, F.; Ferrer, P. Effects of methanol on lipases: Molecular, kinetic and process issues in the production of biodiesel. Biotechnol. J. 2015, 10, 22–30. [Google Scholar] [CrossRef]
- Lotti, M.; Pleiss, J.; Valero, F.; Ferrer, P. Enzymatic Production of Biodiesel: Strategies to Overcome Methanol Inactivation. Biotechnol. J. 2018, 13, 1700155. [Google Scholar] [CrossRef] [PubMed]
- Hama, S.; Noda, H.; Kondo, A. How lipase technology contributes to evolution of biodiesel production using multiple feedstocks. Curr. Opin. Biotechnol. 2018, 50, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Andrade, T.A.; Errico, M.; Christensen, K.V. Influence of the reaction conditions on the enzyme catalyzed transesterification of castor oil: A possible step in biodiesel production. Bioresour. Technol. 2017, 243, 366–374. [Google Scholar] [CrossRef]
- Al Basir, F.; Roy, P.K. Study on enzyme inhibition in biodiesel synthesis: Effect of stepwise addition of methanol and removal of glycerol. Energy Ecol. Environ. 2019, 4, 75–84. [Google Scholar] [CrossRef]
- Norjannah, B.; Ong, H.C.; Masjuki, H.H. Effects of methanol and enzyme pretreatment to Ceiba pentandra biodiesel production. Energy Sources Part A Recover. Util. Environ. Eff. 2017, 39, 1548–1555. [Google Scholar] [CrossRef]
- Shimada, Y.; Watanabe, Y.; Sugihara, A.; Tominaga, Y. Enzymatic alcoholysis for biodiesel fuel production and application of the reaction to oil processing. J. Mol. Catal. B Enzym. 2002, 17, 133–142. [Google Scholar] [CrossRef]
- Shimada, Y.; Watanabe, Y.; Samukawa, T.; Sugihara, A.; Noda, H.; Fukuda, H.; Tominaga, Y. Conversion of vegetable oil to biodiesel using immobilized Candida antarctica lipase. JAOCS J. Am. Oil Chem. Soc. 1999, 76, 789–793. [Google Scholar] [CrossRef]
- Watanabe, Y.; Shimada, Y.; Sugihara, A.; Tominaga, Y. Conversion of degummed soybean oil to biodiesel fuel with immobilized Candida antarctica lipase. J. Mol. Catal. B Enzym. 2002, 17, 151–155. [Google Scholar] [CrossRef]
- Hajar, M.; Shokrollahzadeh, S.; Vahabzadeh, F.; Monazzami, A. Solvent-free methanolysis of canola oil in a packed-bed reactor with use of Novozym 435 plus loofa. Enzym. Microb. Technol. 2009, 45, 188–194. [Google Scholar] [CrossRef]
- Xu, Y.; Nordblad, M.; Nielsen, P.M.; Brask, J.; Woodley, J.M. In situ visualization and effect of glycerol in lipase-catalyzed ethanolysis of rapeseed oil. J. Mol. Catal. B Enzym. 2011, 72, 213–219. [Google Scholar] [CrossRef]
- Watanabe, Y.; Shimada, Y.; Sugihara, A.; Tominaga, Y. Stepwise ethanolysis of tuna oil using immobilized Candida antarctica lipase. J. Biosci. Bioeng. 1999, 88, 622–626. [Google Scholar] [CrossRef]
- Tiosso, P.C.; Carvalho, A.K.F.; De Castro, H.F.; De Moraes, F.F.; Zanin, G.M. Utilization of immobilized lipases as catalysts in the transesterification of non-edible vegetable oils with ethanol. Braz. J. Chem. Eng. 2014, 31, 839–847. [Google Scholar] [CrossRef][Green Version]
- Taher, H.; Al-Zuhair, S.; Al-Marzouqi, A.H.; Haik, Y.; Farid, M.M. A review of enzymatic transesterification of microalgal oil-based biodiesel using supercritical technology. Enzym. Res. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Royon, D.; Daz, M.; Ellenrieder, G.; Locatelli, S. Enzymatic production of biodiesel from cotton seed oil using t-butanol as a solvent. Bioresour. Technol. 2007, 98, 648–653. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).