Enhanced Biomechanical Properties of Polyvinyl Alcohol-Based Hybrid Scaffolds for Cartilage Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
3. Biomechanical Implications in Cartilage Regeneration
3.1. The Peculiar Structure of Articular Cartilage
3.2. Tissue Biomechanics
3.3. PVA-Based Scaffolds for the Mechanical Support in Cartilage Healing
4. Design and Fabrication of PVA-Based Hybrid Scaffolds as Cartilage Substitutes
4.1. PVA Combined with Natural Materials
4.2. PVA Combined with Natural and Synthetic Materials
4.3. Fabrication Techniques
5. Mechanical Properties of PVA-Based Hybrid Scaffolds
5.1. Biomechanical Tests Performed on Composite Scaffolds
5.2. Main Experimental Outcomes
5.2.1. Collagen
5.2.2. Cartilage-Specific Components ± Synthetic Additives
5.2.3. Hydroxyapatite ± Synthetic Additives
5.2.4. Chitosan and Alginate ± Synthetic Additives
5.2.5. Nanocellulose
5.2.6. Biological ECM ± Synthetic Additives
5.2.7. Amino Acids and Acrylamide
6. Biocompatibility of Hybrid Scaffolds for Cartilage TE
6.1. In Vitro Cytotoxicity and Cell Seeding Studies
6.2. In Vivo Implant
7. Overall Considerations and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Temenoff, J.S.; Mikos, A.G. Review: Tissue engineering for regeneration of articular cartilage. Biomaterials 2000, 21, 431–440. [Google Scholar] [CrossRef]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef]
- Camarero-Espinosa, S.; Rothen-Rutishauser, B.; Foster, E.J.; Weder, C. Articular cartilage: From formation to tissue engineering. Biomater. Sci. 2016, 4, 734–767. [Google Scholar] [CrossRef]
- Glyn-Jones, S.; Palmer, A.J.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef]
- Roseti, L.; Desando, G.; Cavallo, C.; Petretta, M.; Grigolo, B. Articular Cartilage Regeneration in Osteoarthritis. Cells 2019, 8, 1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.H.; Xiao, R.; Li, J.B.; Zhu, Q. Regenerative approaches for cartilage repair in the treatment of osteoarthritis. Osteoarthr. Cartil. 2017, 25, 1577–1587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, W.; Li, H.; Zhang, Y.; Han, G. Identification of key biomarkers and immune infiltration in the synovial tissue of osteoarthritis by bioinformatics analysis. PeerJ 2020, 8, e8390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernhard, J.C.; Vunjak-Novakovic, G. Should we use cells, biomaterials, or tissue engineering for cartilage regeneration? Stem Cell Res. Ther. 2016, 7, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumagai, K.; Akamatsu, Y.; Kobayashi, H.; Kusayama, Y.; Saito, T. Mosaic Osteochondral Autograft Transplantation Versus Bone Marrow Stimulation Technique as a Concomitant Procedure with Opening-Wedge High Tibial Osteotomy for Spontaneous Osteonecrosis of the Medial Femoral Condyle. Arthroscopy 2018, 34, 233–240. [Google Scholar] [CrossRef]
- Kraeutler, M.J.; Aliberti, G.M.; Scillia, A.J.; McCarty, E.C.; Mulcahey, M.K. Microfracture Versus Drilling of Articular Cartilage Defects: A Systematic Review of the Basic Science Evidence. Orthop. J. Sports Med. 2020, 8, 2325967120945313. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Devi, D.; Mandal, B.B. Tissue-engineered cartilage: The crossroads of biomaterials, cells and stimulating factors. Macromol. Biosci. 2015, 15, 153–182. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, K.; Kim, S.; Jung, Y. Enhanced cartilaginous tissue formation with a cell aggregate-fibrin-polymer scaffold complex. Polymers 2017, 9, 348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makris, E.A.; Gomoll, A.H.; Malizos, K.N.; Hu, J.C.; Athanasiou, K.A. Repair and tissue engineering techniques for articular cartilage. Nat. Rev. Rheumatol. 2015, 11, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Hwang, N.S.; Varghese, S.; Lee, H.J.; Zhang, Z.; Ye, Z.; Bae, J.; Cheng, L.; Elisseeff, J. In vivo commitment and functional tissue regeneration using human embryonic stem cell-derived mesenchymal cells. Proc. Natl. Acad. Sci. USA 2008, 105, 20641–20646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.A.; Varghese, S.; Sharma, B.; Strehin, I.; Fermanian, S.; Gorham, J.; Fairbrother, D.H.; Cascio, B.; Elisseeff, J.H. Multifunctional chondroitin sulphate for cartilage tissue-biomaterial integration. Nat. Mater. 2007, 6, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Hydrogels for tissue engineering. Chem. Rev. 2001, 101, 1869–1880. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.D.; Lee, Y.; Kim, Y.; Hwang, Y.; Hwang, N.S. Biomimetically Reinforced Polyvinyl Alcohol-Based Hybrid Scaffolds for Cartilage Tissue Engineering. Polymers 2017, 9, 655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Chen, Q.; Deng, C.; Xu, B.; Zhang, Z.; Yang, Y.; Lu, T. Exquisite design of injectable Hydrogels in Cartilage Repair. Theranostics 2020, 10, 9843–9864. [Google Scholar] [CrossRef]
- Gaut, C.; Sugaya, K. Critical review on the physical and mechanical factors involved in tissue engineering of cartilage. Regen. Med. 2015, 10, 665–679. [Google Scholar] [CrossRef] [Green Version]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Mandal, A.; Clegg, J.R.; Anselmo, A.C.; Mitragotri, S. Hydrogels in the clinic. Bioeng. Transl. Med. 2020, 5, e10158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teodorescu, M.; Bercea, M.; Morariu, S. Biomaterials of PVA and PVP in medical and pharmaceutical applications: Perspectives and challenges. Biotechnol. Adv. 2019, 37, 109–131. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Téllez, D.A.; Téllez-Jurado, L.; Rodríguez-Lorenzo, L.M. Hydrogels for Cartilage Regeneration, from Polysaccharides to Hybrids. Polymers 2017, 9, 671. [Google Scholar] [CrossRef] [Green Version]
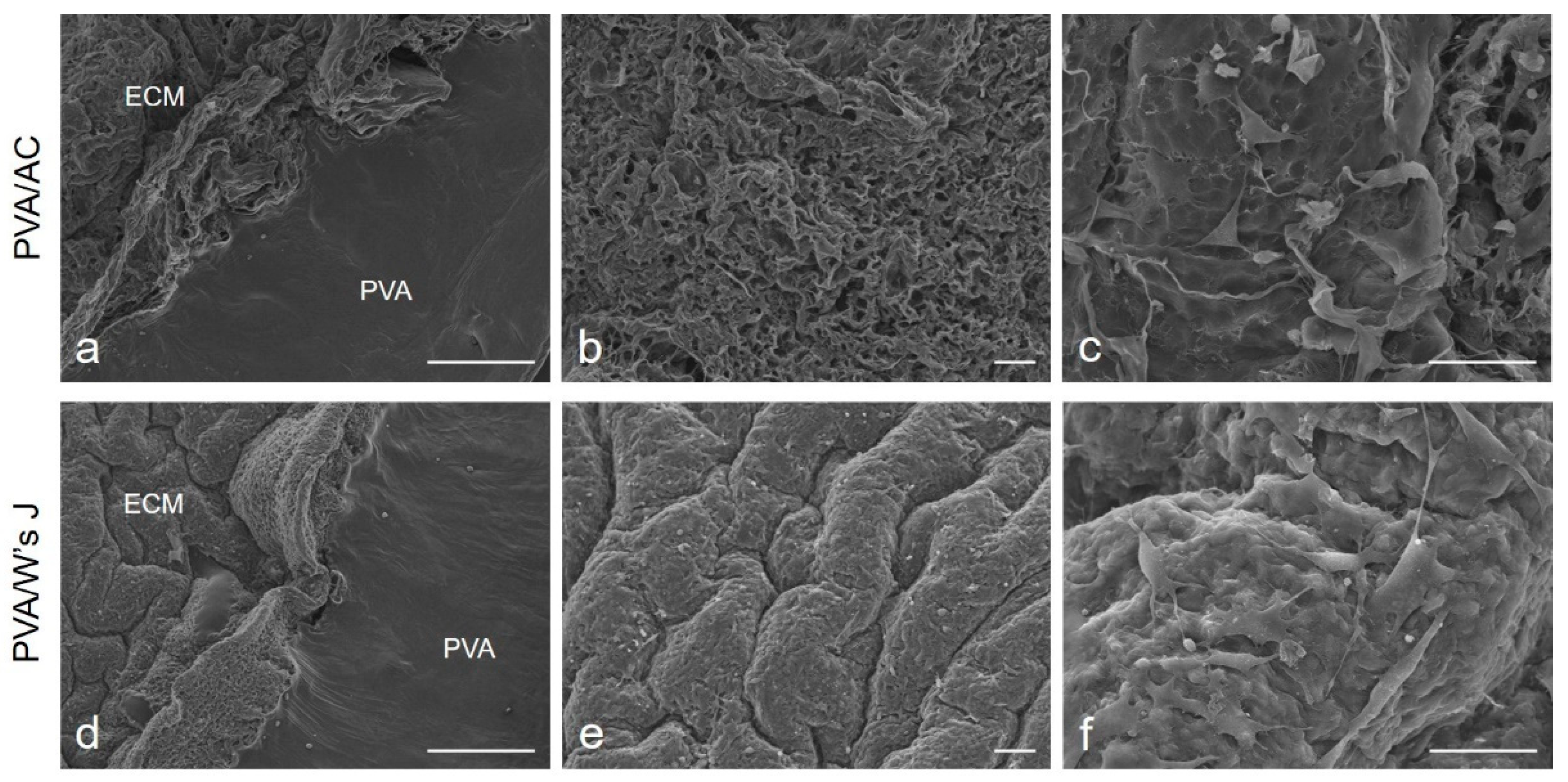
- Stocco, E.; Barbon, S.; Dalzoppo, D.; Lora, S.; Sartore, L.; Folin, M.; Parnigotto, P.P.; Grandi, C. Tailored PVA/ECM scaffolds for cartilage regeneration. BioMed Res. Int. 2014, 2014, 762189. [Google Scholar] [CrossRef] [PubMed]
- Stocco, E.; Barbon, S.; Radossi, P.; Rajendran, S.; Dalzoppo, D.; Bortolami, M.; Bagno, A.; Grandi, F.; Gamba, P.G.; Parnigotto, P.P.; et al. Autologous chondrocytes as a novel source for neo-chondrogenesis in haemophiliacs. Cell Tissue Res. 2016, 366, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Teodorescu, M.; Bercea, M.; Morariu, S. Biomaterials of Poly(vinyl alcohol) and Natural Polymers. Polym. Rev. 2018, 58, 247–287. [Google Scholar] [CrossRef]
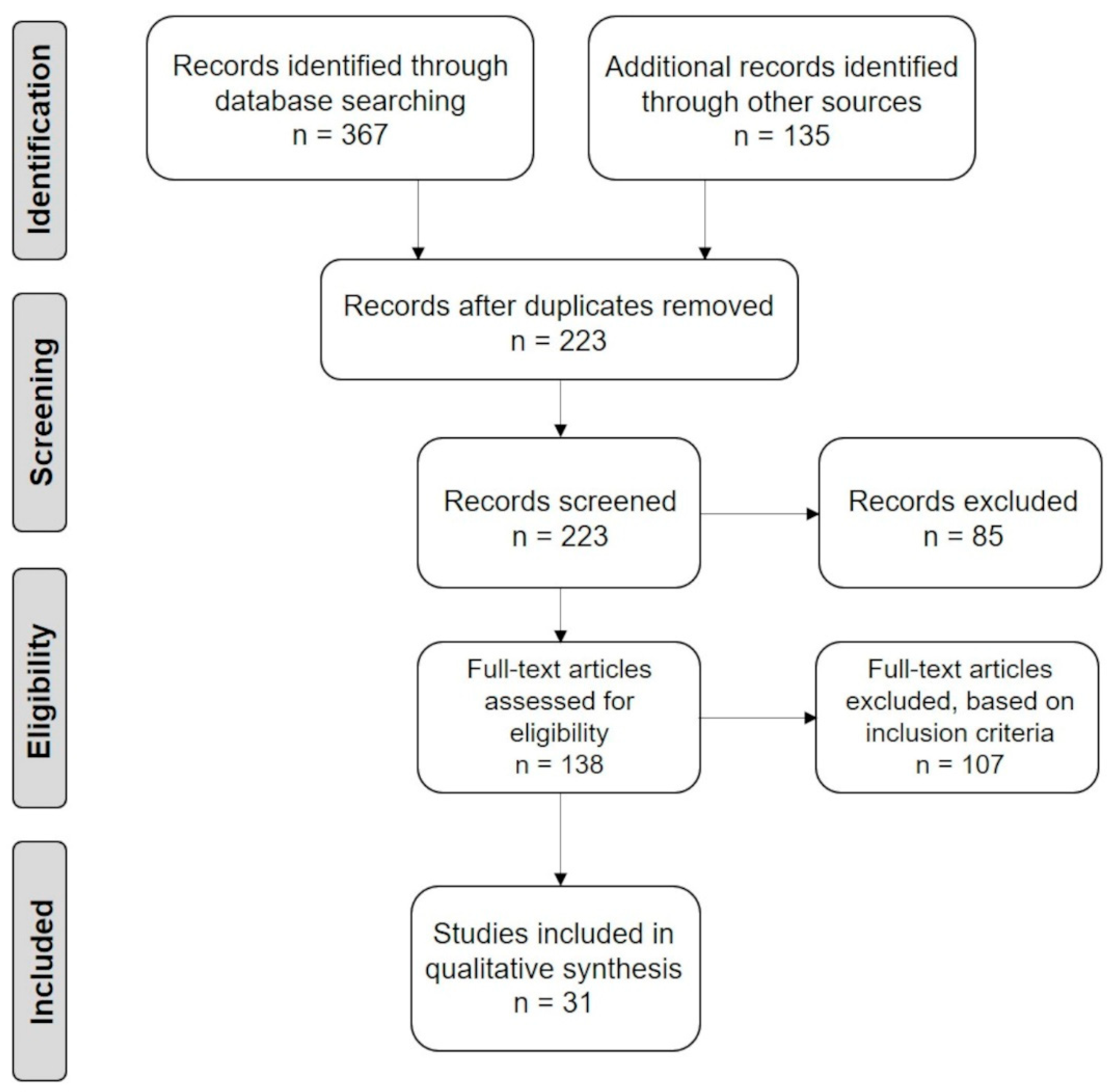
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef] [Green Version]
- Freed, L.E.; Engelmayr, G.C., Jr.; Borenstein, J.T.; Moutos, F.T.; Guilak, F. Advanced material strategies for tissue engineering scaffolds. Adv. Mater. 2009, 21, 3410–3418. [Google Scholar] [CrossRef] [Green Version]
- Becerra, J.; Andrades, J.A.; Guerado, E.; Zamora-Navas, P.; López-Puertas, J.M.; Reddi, A.H. Articular cartilage: Structure and regeneration. Tissue Eng. Part B Rev. 2010, 16, 617–627. [Google Scholar] [CrossRef]
- Klein, T.J.; Malda, J.; Sah, R.L.; Hutmacher, D.W. Tissue engineering of articular cartilage with biomimetic zones. Tissue Eng. Part B Rev. 2009, 15, 143–157. [Google Scholar] [CrossRef]
- Moutos, F.T.; Freed, L.E.; Guilak, F. A biomimetic three-dimensional woven composite scaffold for functional tissue engineering of cartilage. Nat. Mater. 2007, 6, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Antons, J.; Marascio, M.G.M.; Nohava, J.; Martin, R.; Applegate, L.A.; Bourban, P.E.; Pioletti, D.P. Zone-dependent mechanical properties of human articular cartilage obtained by indentation measurements. J. Mater. Sci. Mater. Med. 2018, 29, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.P.; Herzog, W.; Korhonen, R.K.; Jurvelin, J.S. The role of viscoelasticity of collagen fibers in articular cartilage: Axial tension versus compression. Med. Eng. Phys. 2005, 27, 51–57. [Google Scholar] [CrossRef]
- Bodugoz-Senturk, H.; Macias, C.E.; Kung, J.H.; Muratoglu, O.K. Poly(vinyl alcohol)-acrylamide hydrogels as load-bearing cartilage substitute. Biomaterials 2009, 30, 589–596. [Google Scholar] [CrossRef]
- Qazi, T.H.; Mooney, D.J.; Pumberger, M.; Geissler, S.; Duda, G.N. Biomaterials based strategies for skeletal muscle tissue engineering: Existing technologies and future trends. Biomaterials 2015, 53, 502–521. [Google Scholar] [CrossRef] [PubMed]
- Milner, P.E.; Parkes, M.; Puetzer, J.L.; Chapman, R.; Stevens, M.M.; Cann, P.; Jeffers, J.R.T. A low friction, biphasic and boundary lubricating hydrogel for cartilage replacement. Acta Biomater. 2018, 65, 102–111. [Google Scholar] [CrossRef]
- Baker, M.I.; Walsh, S.P.; Schwartz, Z.; Boyan, B.D. A review of polyvinyl alcohol and its uses in cartilage and orthopedic applications. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1451–1457. [Google Scholar] [CrossRef]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in regenerative medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef] [Green Version]
- Weller, W.J. Emerging Technologies in Upper Extremity Surgery: Polyvinyl Alcohol Hydrogel Implant for Thumb Carpometacarpal Arthroplasty and Processed Nerve Allograft and Nerve Conduit for Digital Nerve Repairs. Orthop. Clin. N. Am. 2019, 50, 87–93. [Google Scholar] [CrossRef]
- Hendriks, J.; Riesle, J.; van Blitterswijk, C.A. Co-culture in cartilage tissue engineering. J. Tissue Eng. Regen. Med. 2007, 1, 170–178. [Google Scholar] [CrossRef]
- Stocco, E.; Barbon, S.; Grandi, F.; Gamba, P.G.; Borgio, L.; Del Gaudio, C.; Dalzoppo, D.; Lora, S.; Rajendran, S.; Porzionato, A.; et al. Partially oxidized polyvinyl alcohol as a promising material for tissue engineering. J. Tissue Eng. Regen. Med. 2017, 11, 2060–2070. [Google Scholar] [CrossRef] [PubMed]
- Barbon, S.; Stocco, E.; Dalzoppo, D.; Todros, S.; Canale, A.; Boscolo-Berto, R.; Pavan, P.; Macchi, V.; Grandi, C.; De Caro, R.; et al. Halogen-Mediated Partial Oxidation of Polyvinyl Alcohol for Tissue Engineering Purposes. Int. J. Mol. Sci. 2020, 21, 801. [Google Scholar] [CrossRef] [Green Version]
- Alhosseini, S.N.; Moztarzadeh, F.; Kargozar, S.; Dodel, M.; Tahriri, M. Development of Polyvinyl Alcohol Fibrous Biodegradable Scaffolds for Nerve Tissue Engineering Applications: In Vitro Study. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 474–480. [Google Scholar] [CrossRef]
- Filová, E.; Rampichová, M.; Litvinec, A.; Držík, M.; Míčková, A.; Buzgo, M.; Košťáková, E.; Martinová, L.; Usvald, D.; Prosecká, E.; et al. A cell-free nanofiber composite scaffold regenerated osteochondral defects in miniature pigs. Int. J. Pharm. 2013, 447, 139–149. [Google Scholar] [CrossRef]
- Oh, S.H.; An, D.B.; Kim, T.H.; Lee, J.H. Wide-range stiffness gradient PVA/HA hydrogel to investigate stem cell differentiation behavior. Acta Biomater. 2016, 35, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Tsai, W.C.; Chang, S.H. Collagen-PVA aligned nanofiber on collagen sponge as bi-layered scaffold for surface cartilage repair. J. Biomater. Sci. Polym. Ed. 2017, 28, 664–678. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.; Xu, M.; Zhang, X.; Zhao, L.; Huang, D.; Wei, X.; Chen, W. Biomimetic polyvinyl alcohol/type II collagen hydrogels for cartilage tissue engineering. J. Biomater. Sci. Polym. Ed. 2020, 31, 1179–1198. [Google Scholar] [CrossRef]
- Chocholata, P.; Kulda, V.; Dvorakova, J.; Kolaja Dobra, J.; Babuska, V. Biological Evaluation of Polyvinyl Alcohol Hydrogels Enriched by Hyaluronic Acid and Hydroxyapatite. Int. J. Mol. Sci. 2020, 21, 5719. [Google Scholar] [CrossRef]
- Nayar, S.; Pramanick, A.K.; Sharma, B.K.; Das, G.; Ravi Kumar, B.; Sinha, A. Biomimetically synthesized polymer-hydroxyapatite sheet like nano-composite. J. Mater. Sci. Mater. Med. 2008, 19, 301–304. [Google Scholar] [CrossRef]
- Pan, Y.; Xiong, D.; Gao, F. Viscoelastic behavior of nano-hydroxyapatite reinforced poly(vinyl alcohol) gel biocomposites as an articular cartilage. J. Mater. Sci. Mater. Med. 2008, 19, 1963–1969. [Google Scholar] [CrossRef]
- Pan, Y.; Xiong, D. Study on compressive mechanical properties of nanohydroxyapatite reinforced poly(vinyl alcohol) gel composites as biomaterial. J. Mater. Sci. Mater. Med. 2009, 20, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Maiolo, A.S.; Amado, M.N.; Gonzalez, J.S.; Alvarez, V.A. Development and characterization of Poly (vinyl alcohol) based hydrogels for potential use as an articular cartilage replacement. Mater. Sci. Eng. C Mater. Biol. Appl. 2012, 32, 1490–1495. [Google Scholar] [CrossRef] [PubMed]
- Yusong, P.; Qianqian, S.; Chengling, P.; Jing, W. Prediction of mechanical properties of multilayer gradient hydroxyapatite reinforced poly(vinyl alcohol) gel biomaterial. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.S.; Alvarez, V.A. Mechanical properties of polyvinylalcohol/hydroxyapatite cryogel as potential artificial cartilage. J. Mech. Behav. Biomed. Mater. 2014, 34, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhang, D.; Yang, X.; Cui, X.; Zhang, X.; Wang, Q. Research on torsional friction behavior and fluid load support of PVA/HA composite hydrogel. J. Mech. Behav. Biomed. Mater. 2016, 62, 182–194. [Google Scholar] [CrossRef]
- Chen, K.; Yang, X.; Zhang, D.; Xu, L.; Zhang, X.; Wang, Q. Biotribology behavior and fluid load support of PVA/HA composite hydrogel as artificial cartilage. Wear 2017, 376–377, 329–336. [Google Scholar] [CrossRef]
- Su, C.; Su, Y.; Li, Z.; Haq, M.A.; Zhou, Y.; Wang, D. In situ synthesis of bilayered gradient poly(vinyl alcohol)/hydroxyapatite composite hydrogel by directional freezing-thawing and electrophoresis method. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 76–83. [Google Scholar] [CrossRef]
- Peng, L.; Zhou, Y.; Lu, W.; Zhu, W.; Li, Y.; Chen, K.; Zhang, G.; Xu, J.; Deng, Z.; Wang, D. Characterization of a novel polyvinyl alcohol/chitosan porous hydrogel combined with bone marrow mesenchymal stem cells and its application in articular cartilage repair. BMC Musculoskelet. Disord. 2019, 20, 257. [Google Scholar] [CrossRef] [Green Version]
- Scholten, P.M.; Ng, K.W.; Joh, K.; Serino, L.P.; Warren, R.F.; Torzilli, P.A.; Maher, S.A. A semi-degradable composite scaffold for articular cartilage defects. J. Biomed. Mater. Res. A 2011, 97, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Millon, L.E.; Oates, C.J.; Wan, W. Compression properties of polyvinyl alcohol--bacterial cellulose nanocomposite. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 90, 922–929. [Google Scholar] [CrossRef]
- Li, W.; Wu, Q.; Zhao, X.; Huang, Z.; Cao, J.; Li, J.; Liu, S. Enhanced thermal and mechanical properties of PVA composites formed with filamentous nanocellulose fibrils. Carbohydr. Polym. 2014, 113, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Shahruzzaman, M.; Biswas, S.; Nurus Sakib, M.; Rashid, T.U. Chitosan based bioactive materials in tissue engineering applications—A review. Bioact. Mater. 2020, 5, 164–183. [Google Scholar] [CrossRef] [PubMed]
- Gorgieva, S.; Trček, J. Bacterial Cellulose: Production, Modification and Perspectives in Biomedical Applications. Nanomaterials 2019, 9, 1352. [Google Scholar] [CrossRef] [Green Version]
- Dadgar, N.; Ghiaseddin, A.; Irani, S.; Rabbani, S.; Tafti, S.H.A.; Soufizomorrod, M.; Soleimani, M. Cartilage tissue engineering using injectable functionalized Demineralized Bone Matrix scaffold with glucosamine in PVA carrier; cultured in microbioreactor prior to study in rabbit model. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111677. [Google Scholar] [CrossRef]
- Mohanapriya, S.; Raj, V. Tuning biological properties of poly (vinyl alcohol) with amino acids and studying its influence on osteoblastic cell adhesion. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 86, 70–82. [Google Scholar] [CrossRef]
- Chen, K.; Liu, J.; Yang, X.; Zhang, D. Preparation, optimization and property of PVA-HA/PAA composite hydrogel. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Hu, Y.; Zeng, M.; Li, M.; Lin, S.; Zhou, Y.; Xie, J. Design and evaluation of nano-hydroxyapatite/poly(vinyl alcohol) hydrogels coated with poly(lactic-co-glycolic acid)/nano-hydroxyapatite/poly(vinyl alcohol) scaffolds for cartilage repair. J. Orthop. Surg. Res. 2019, 14, 446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gan, S.; Lin, W.; Zou, Y.; Xu, B.; Zhang, X.; Zhao, J.; Rong, J. Nano-hydroxyapatite enhanced double network hydrogels with excellent mechanical properties for potential application in cartilage repair. Carbohydr. Polym. 2020, 229, 115523. [Google Scholar] [CrossRef]
- Meng, Y.; Cao, J.; Chen, Y.; Yu, Y.; Ye, L. 3D printing of a poly(vinyl alcohol)-based nano-composite hydrogel as an artificial cartilage replacement and the improvement mechanism of printing accuracy. J. Mater. Chem. B 2020, 8, 677–690. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Zhang, F.; Wang, Q.; Wu, X. Fabrication of chitosan/graphene oxide polymer nanofiber and its biocompatibility for cartilage tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 697–701. [Google Scholar] [CrossRef]
- Chen, Q.; Cabanas-Polo, S.; Goudouri, O.M.; Boccaccini, A.R. Electrophoretic co-deposition of polyvinyl alcohol (PVA) reinforced alginate-Bioglass® composite coating on stainless steel: Mechanical properties and in-vitro bioactivity assessment. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 40, 55–64. [Google Scholar] [CrossRef]
- Yu, F.; Han, X.; Zhang, K.; Dai, B.; Shen, S.; Gao, X.; Teng, H.; Wang, X.; Li, L.; Ju, H.; et al. Evaluation of a polyvinyl alcohol-alginate based hydrogel for precise 3D bioprinting. J. Biomed. Mater. Res. A 2018, 106, 2944–2954. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zheng, Y.; Huang, X.; Xi, T.; Lin, X.; Han, D.; Song, W. Mineralization behavior and interface properties of BG-PVA/bone composite implants in simulated body fluid. Biomed. Mater. 2010, 5, 25003. [Google Scholar] [CrossRef]
- Manavi-Tehrani, I.; Rabiee, M.; Parviz, M.; Tahriri, M.R.; Fahimi, Z. Preparation, Characterization and Controlled Release Investigation of Biocompatible pH-Sensitive PVA/PAA Hydrogels. Macromol. Symp. 2010, 296, 457–465. [Google Scholar] [CrossRef]
- Guo, W.; Zheng, X.; Zhang, W.; Chen, M.; Wang, Z.; Hao, C.; Huang, J.; Yuan, Z.; Zhang, Y.; Wang, M.; et al. Mesenchymal Stem Cells in Oriented PLGA/ACECM Composite Scaffolds Enhance Structure-Specific Regeneration of Hyaline Cartilage in a Rabbit Model. Stem Cells Int. 2018, 2018, 6542198. [Google Scholar] [CrossRef]
- Ren, Y.; Lou, R.; Liu, X.; Gao, M.; Zheng, H.; Yang, T.; Xie, H.; Yu, W.; Ma, X. A self-healing hydrogel formation strategy via exploiting endothermic interactions between polyelectrolytes. Chem. Commun. 2016, 52, 6273–6276. [Google Scholar] [CrossRef]
- Gupta, S.; Goswami, S.; Sinha, A. A combined effect of freeze—Thaw cycles and polymer concentration on the structure and mechanical properties of transparent PVA gels. Biomed. Mater. 2012, 7, 015006. [Google Scholar] [CrossRef]
- Millon, L.E.; Wan, W.K. The polyvinyl alcohol-bacterial cellulose system as a new nanocomposite for biomedical applications. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 79, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Goodman, S.B.; Yao, Z.; Keeney, M.; Yang, F. The future of biologic coatings for orthopaedic implants. Biomaterials 2013, 34, 3174–3183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.C.; Ito, T.; Kim, K.O.; Kim, K.W.; Kim, B.S.; Khil, M.S.; Kim, H.Y.; Kim, I.S. Electrospun poly(vinyl alcohol) nanofibers: Effects of degree of hydrolysis and enhanced water stability. Polym. J. 2010, 42, 273–276. [Google Scholar] [CrossRef] [Green Version]
- Koh, Y.G.; Lee, J.A.; Kim, Y.S.; Lee, H.Y.; Kim, H.J.; Kang, K.T. Optimal mechanical properties of a scaffold for cartilage regeneration using finite element analysis. J. Tissue Eng. 2019, 10, 2041731419832133. [Google Scholar] [CrossRef] [Green Version]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Tian, L.; Shamirzaei-Jeshvaghani, E.; Dehghani, L.; Ramakrishna, S. Structural properties of scaffolds: Crucial parameters towards stem cells differentiation. World J. Stem Cells 2015, 7, 728–744. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, H.; Salmeron-Sanchez, M.; Dalby, M.J. Designing stem cell niches for differentiation and self-renewal. J. R. Soc. Interface 2018, 15, 20180388. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, J.; Athanasiou, K.A. The role of tissue engineering in articular cartilage repair and regeneration. Crit. Rev. Biomed. Eng. 2009, 37, 1–57. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.H.; Yeger-McKeever, M.; Stein, A.; Mauck, R.L. Tensile properties of engineered cartilage formed from chondrocyte- and MSC-laden hydrogels. Osteoarthr. Cartil. 2008, 16, 1074–1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blum, M.M.; Ovaert, T.C. Experimental and numerical tribological studies of a boundary lubricant functionalized poro-viscoelastic PVA hydrogel in normal contact and sliding. J. Mech. Behav. Biomed. Mater. 2012, 14, 248–258. [Google Scholar] [CrossRef]
- Michael, F.; Wolf, M.F.; Coleman, K.P.; Rankin, E.A.; Lewerenz, G.M. In Vitro Assessment of Cell and Tissue Compatibility. In Biomaterials Science, 4th ed.; Wagner, W.R., Sakiyama-Elbert, S.E., Zhang, G., Yaszemski, M.J., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 851–868. [Google Scholar] [CrossRef]
- Fini, M.; Giardino, R. In vitro and in vivo tests for the biological evaluation of candidate orthopedic materials: Benefits and limits. J. Appl. Biomater. Biomech. 2003, 1, 155–163. [Google Scholar] [PubMed]
- Stoppel, W.L.; Ghezzi, C.E.; McNamara, S.L.; Black, L.D., 3rd; Kaplan, D.L. Clinical applications of naturally derived biopolymer-based scaffolds for regenerative medicine. Ann. Biomed. Eng. 2015, 43, 657–680. [Google Scholar] [CrossRef]
- Stace, E.T.; Dakin, S.G.; Mouthuy, P.A.; Carr, A.J. Translating Regenerative Biomaterials into Clinical Practice. J. Cell Physiol. 2016, 231, 36–49. [Google Scholar] [CrossRef]
| Hybrid Scaffold Components | Component Concentrations | Fabrication Technique | Reference |
|---|---|---|---|
| PVA Hyaluronate Collagen type I Fibrin | PVA: 12.8% w/w | Electrospinning of PVA nanofibers embedded in biological components | [44] |
| PVA Hyaluronic acid | PVA: 5 wt% Hyaluronic acid: 2 wt% | Gradual freeze-thawing method: (1) the composite solution was put in contact with a surface of LN2 for 30 min at RT to enable gradual freezing from the bottom (LN2 side) to the top (2) the sample was subsequently stored at RT for 6 h (3) the gradual freeze-thawing procedure was repeated for 10 cycles | [45] |
| PVA Collagen type I | PVA: 10% w/v Collagen type I: 1% | Electrospinning of composite nanofibers | [46] |
| PVA Collagen type II | PVA: 10 wt% Collagen type II: 10% w/v added to PVA in different ratio | Freezing at −20 °C for 20 h/thawing at RT for 4 h 5 thermal cycles | [47] |
| PVA Hydroxyapatite | PVA: 11 wt% Hydroxyapatite: not reported | In situ synthesis of hydroxyapatite in PVA followed by lyophilization | [49] |
| PVA: not reported Hydroxyapatite: not reported | In situ synthesis of nanohydroxyapatite particles in PVA solution Freezing at −20 °C for 12 h/thawing at RT for 6 h 3, 5, or 7 thermal cycles | [50,51] | |
| PVA: 15 wt% Hydroxyapatite: 3 and 7.5 wt.% | In situ synthesis of nanohydroxyapatite Freezing at −18 °C for 12 h/thawing at 25 °C for 12 h 3 thermal cycles | [52] | |
| PVA: 77.6 g in 220 mL dH2O Hydroxyapatite: not reported | Layer-by-layer casting method Freezing at −20 °C for 12 h/thawing at RT for 10 h Repeated thermal cycles | [53] | |
| PVA: 15 wt% Hydroxyapatite: 1.5, 3, 6 and 7.5 wt% | Freezing at −18 °C for 1 h/thawing at 25 °C for 1 h 3 thermal cycles | [54] | |
| PVA: 15% Hydroxyapatite: 3% dH2O: 82% | Freezing at −20 °C for 6–10 h/thawing at RT for 2–3 h 9 thermal cycles F/T served for the crosslinking of the composite scaffold on UHMWPE acetabulum | [55,56] | |
| PVA: 10 wt% Hydroxyapatite: not reported | Directional freeze-thaw process to form the PVA hydrogel Gel electrophoresis method for the dispersion of hydroxyapatite particles within PVA | [57] | |
| PVA Chitosan | PVA: 10% weight ratio Chitosan: added to PVA in various proportion | Freezing at −20 °C for 20 h/thawing at RT for 8 h 7 thermal cycles | [58] |
| PVA Alginate | PVA: 10% or 20% w/v solution Alginate: microspheres formed using a standard water-in-oil emulsification technique | Freezing at −25 °C for 23 h/thawing at 25 °C for 1 h 5 thermal cycles | [59] |
| PVA Nanocellulose | PVA: 10 wt% BC: 0.30 and 0.85 wt% | Freezing at −20 °C/thawing at 20 °C 0.1 °C/min freeze-thawing rate 1, 3, 6 thermal cycles | [60] |
| PVA: 10 wt% NCF: 2, 6, 10, 14 wt% | Ultrasonication, degas and drying at 70 °C for 4 h | [61] | |
| PVA Human cartilage ECM Wharton’s jelly ECM | PVA: 16% and 25% w/w ECM: 1 g/15 mL | Freezing at −20 °C for 24 h/thawing at−2.5 °C for 24 h 5 thermal cycles | [24,25] |
| PVA Demineralized bone matrix | PVA: 1, 5, 10, 15, 20, 25, 30, 40% Bone matrix: 0.35 g/mL | Mixing DBM into PVA solutions to obtained injectable composite hydrogels | [64] |
| PVA Amino acids | PVA: 4 wt% Amino acids: varied from 5 to 20 wt% and optimized at 10 wt% | In situ crosslinking by citric acid | [65] |
| PVA Acrylamide | PVA: 15% (w/w) Acrylamide: 5, 10, 15% (w/w) | Freezing at −17 °C for 16 h/thawing for 8 h 2 thermal cycle separated by an 8 h polymerization process | [34] |
| Hybrid Scaffold Components | Component Concentrations | Fabrication Technique | Reference |
|---|---|---|---|
| PVA Hydroxyapatite PAA | PVA: 10%, 12%, 14%, 16% Hydroxyapatite: 2%, 2.5%, 3% PAA: 3%, 4%, 5% | Freezing at −20 °C for 12 h/thawing at RT for 6 h Repeated thermal cycles PEG dehydration Annealing treatment | [66] |
| PVA Hydroxyapatite PLGA | Not reported | Solvent extraction, evaporation technique Freezing at −20 °C for 21 h/thawing at RT for 3 h 5 thermal cycles | [67] |
| PVA Hydroxyapatite HACC | PVA: 15% w/v Hydroxyapatite: 2%, 3%, 4% w/v HACC: 5% w/v | Freezing at −20 °C for 12 h/thawing at 25 °C for 12 h 3 thermal cycles Immersion in Na3Cit for dual physical crosslinking | [68] |
| PVA Hydroxyapatite GO | PVA: 5, 16, 20, 25 wt% Hydroxyapatite: 10 mg/mL GO: 2.88 mg/mL | Extrusion 3D printing technique Freezing at −20 °C for 12 h/thawing at RT for 4 h 4 thermal cycles | [69] |
| PVA Chitosan GO | PVA: 20 wt% Chitosan: 8% weight GO: 20 mg/mL | Electron beam lithography treatment to produce chitosan solution Chemical crosslinking with glyoxal of PVA/chitosan solution Electrospinning of the final PVA/chitosan/GO blend | [70] |
| PVA Alginate Bioglass® | PVA: 10, 20, 30 g/L Alginate: 2 g/L Bioglass®: 10 g/L | Preparation of homogenous composite suspension by ultrasonication Electrophoretic deposition process of PVA/alginate/BG coatings on stainless steel | [71] |
| PVA Alginate PEGDA | PVA: 10% w/v Alginate: 10% w/v PEGDA: 10% w/v I-2959: 0.05% w/v | 3D bioprinting with simultaneous photopolymerization under UV light | [72] |
| PVA Chondroitin sulfate PEGDA | PVA: not reported Chondroitin sulfate: 10% w/v PEGDA: 10% w/v | Photocrosslinking with glycidyl methacrylate | [17] |
| PVA Hyaluronic acid PEGDA | PVA: not reported Hyaluronic acid: 1% w/v PEGDA: 10% w/v | ||
| PVA Bone BG | PVA: 20% (w/w) Pig spongy bone: not reported BG: 4%, 8% weight ratio with respect to PVA | Freezing at −20 °C for 8–12 h/thawing at RT for 4–6 h 4 thermal cycles | [73] |
| Mechanical Test | Description | Outcome Measures | References |
|---|---|---|---|
| Compression test | Assessment of the load-bearing capacity of the scaffolds by simulating the application of compressive load in the joint | - Stress-strain curves - Compressive elastic modulus (stiffness) determined from the stress-strain curves - Shear modulus - Compressive strength - Stress-at-break (σmax) - Strain-at-break (ε) - Ultimate compressive stress | [17,44,45,47,51,52,53,54,57,58,60,64,66,67,68,69,72,73] |
| Tensile test | Evaluation of scaffold response to tensile loads which simulate shear stress | - Stress-strain curves - Young’s modulus - Tensile strength - Stress-at-break (σmax) - Strain-at-break (ε) - Ultimate tensile stress - Ultimate tensile strength - Toughness | [17,24,25,46,47,49,51,52,54,57,58,61,64,65,67,68,70,72] |
| Friction test | Investigation of scaffold suitability to provide an ideal interface between bones by minimize frictional drag | - Friction coefficient - Friction torque-angular displacement (T-θ) curve - Average friction torques and friction coefficients under different loads - Swing friction coefficient - Torsion friction coefficient - Sliding friction coefficient - Rotation friction | [34,52,54,55,56,66,69] |
| Stress relaxation test | Simulation of the physiological condition of cartilage tissue when a constant deformation is applied due to the hydraulic pressure, which is reduced after liquid flow | - Elastic modulus - Poisson’s ratio (υ) - Aggregate modulus (Ha) - Dynamic modulus (ED) - Stress relaxation curve | [59,60,66] |
| Dynamic mechanical tests | Assessment of the viscoelastic properties of the composite scaffold | - Elastic modulus - Storage modulus - Loss modulus - Phase angle δ | [50,54] |
| Nanoindentation test | Analysis of the surface area of the composite scaffold which is expected to be in contact with bone | - Load-penetration curves | [54] |
| Creep test | Investigation of the improved ability of the composite scaffold to recover from creep deformation | - Total creep strain - Creep curve | [34,66] |
| Tear test | Measure of scaffold ability to withstand the effects of tearing which may occur in the joint | - Tear strength | [34] |
| Pull-off test of adhesion Cycled bending test | Measure of the adhesion strength between composite coatings and the substrate at the metallization/polymer interface | - Adhesion strength - Cracking and detachment behaviors | [71] |
| Shear strength test | Evaluation of the response of the composite scaffold to the shear loading condition which occurs in the joint | - Curves of the shear strength versus the immersion time | [73] |
| Material | Fabrication Technique | Effects on Composite Scaffold Biomechanics | References |
|---|---|---|---|
| Collagen | Electrospinning | - Electrospun PVA nanofiber increased the mechanical stiffness of the composite PVA/hyaluronan/collagen/fibrin scaffold. - In the bi-layered PVA/collagen type I composites, the aligned nanofibers had higher Young’s modulus (stiffness), ultimate strength, and strain than the random fibers. - Young’s modulus of seeded aligned scaffold was similar to the values reported for articular cartilage (0.35 MPa). | [44,46] |
| Freeze-thawing | - In the composite PVA/collagen type II/chondroitin sulfate scaffold, the collagen content affected the mechanical stiffness. - The addition of collagen into PVA matrix at 1:1 ratio led to an increase in elasticity modulus compared with pure PVA hydrogel (11 ± 1.7 vs. 4.9 ± 0.6 kPa). - Changing the proportion of PVA/collagen to 1:2 caused a decrease in elasticity modulus up to 2.3 ± 0.2 kPa. - The highest values of elastic modulus (12.9 ± 1.2 kPa) were shown with 2:1 PVA/collagen ratio. | [47] | |
| Hyaluronic acid | Gradual freeze-thawing | - The stiffness gradient of PVA/hyaluronic acid hydrogels (20–200 kPa) was significantly higher in comparison with pure PVA hydrogel (1–24 kPa). - PVA/hyaluronic acid composites exhibited a stiffness gradient which varied from 25.9 ± 14.4 kPa (on the top of the scaffold) to 186.6 ± 21.6 kPa (on the bottom layer). | [45] |
| Hyaluronic acid/PEGDA | Photocrosslinking | - In the absence of the PVA sponge, PEGDA and methacrylated chondroitin sulfate and hyaluronic acid hydrogels exhibited Young’s moduli similar to that of the PVA alone, but lower degree of elastic deformation. - The incorporation of photocrosslinkable hydrogels (PEGDA, chondroitin sulfate/PEGDA and hyaluronic acid/PEGDA) within the internal pores of the PVA sponges improved the matrix rigidity (Young’s modulus) and deformation without breakage (toughness) of the PVA-based hybrid scaffolds. | [17] |
| Chondroitin sulfate/PEGDA | |||
| Chitosan | Freeze-thawing | - In the PVA/chitosan composite, Young’s modulus and compressive strength increased as the PVA content increased. - The hydrogel with PVA/chitosan ratio of 6:4 demonstrated the best mechanical properties. | [58] |
| Chitosan/GO | Chemical crosslinking Electrospinning | - The addition of GO improved the mechanical strength of the composite scaffold. - The tensile strengths of electrospun PVA/chitosan/GO nanofibers were 2.78 MPa and 1.81 MPa for GO concentration of 0.4 and 0.6 wt%, respectively. - The elongations at break of composite nanofibers were 16.79 and 32.82% with GO concentration of 0.4 and 0.6 wt%, respectively. | [70] |
| Alginate | Freeze-thawing | - In the PVA/alginate composites, the mechanical properties varied according to the PVA concentration into the scaffold (i.e., 10 or 20 wt%). -Ha was significantly higher for 20% PVA constructs (0.16 ± 0.02 MPa) compared to that of the 10% PVA scaffolds (0.06 ± 0.01 MPa; p = 0.002), with the first kind of support showing similar values to bovine articular cartilage (0.21 ± 0.07 MPa). - The 20% PVA scaffold was significantly stiffer than the 10% PVA scaffold (Es: 0.14 ± 0.02 vs. 0.04 ± 0.01 MPa), with no significant difference between each scaffold type and articular cartilage (0.11 ± 0.06 MPa). - ED was significantly higher for articular cartilage when compared to that of the composite scaffolds. - Under confined compression conditions, the 20% PVA scaffolds showed no statistical differences in modulus values compared to articular cartilage at all tested strain levels. | [59] |
| Alginate/BG | Ultrasonication Electrophoresis deposition | - The adhesion strength of PVA/alginate/BG coating onto the substrate (stainless steel) increased significantly with PVA content. - Adding PVA at the proper concentration (i.e., 20 g/L) ensured no visible crack formation or coating detachment after 5 bending cycles. - Repeated depositions of PVA/alginate/BG onto the substrate produced coatings with higher thickness and suitable mechanical strength. | [71] |
| Alginate/PEGDA | 3D bioprinting Photopolymerization Freeze-thawing | - The PVA/alginate/PEGDA composite scaffold demonstrated better anticompression capability than alginate and alginate/PEGDA supports. - The Young’s modulus of PVA/alginate/PEGDA composite scaffold (6.77 ± 0.40 MPa) was higher than the modulus of alginate (3.08 ± 0.29 MPa) and alginate/PEGDA (5.06 ± 0.28 MPa) constructs. | [72] |
| Nanocellulose | Freeze-thawing | - The stiffness of the PVA/BC nanocomposite was higher than PVA and increased with the addition of higher BC concentrations. - The PVA/BC possesses both improved mechanical strength and increased strain-rate dependence. - The PVA/BC modulus ranged from 0.03 to 7.51 MPa, being rather close to the elastic modulus measured for articular cartilage (0.4–10 MPa). - Stress relaxation tests underlined the viscoelastic behavior of the nanocomposite. | [60] |
| Ultrasonication, degas and drying | - In the PVA/NCF composites, the mechanical performance was improved by the increasing NFC concentrations from 2 to 6 wt%. - At an NFC concentration of 6%, the tensile strength and Young’s modulus of the PVA composites reached maximum values, being 2.8 and 2.4 times higher than parameters measured for neat PVA, respectively. - When NCF content was higher than 6 wt%, the tensile strength and Young’s modulus showed a decrease trend, ascribable to less NCF dispersion into the hydrogel. | [61] | |
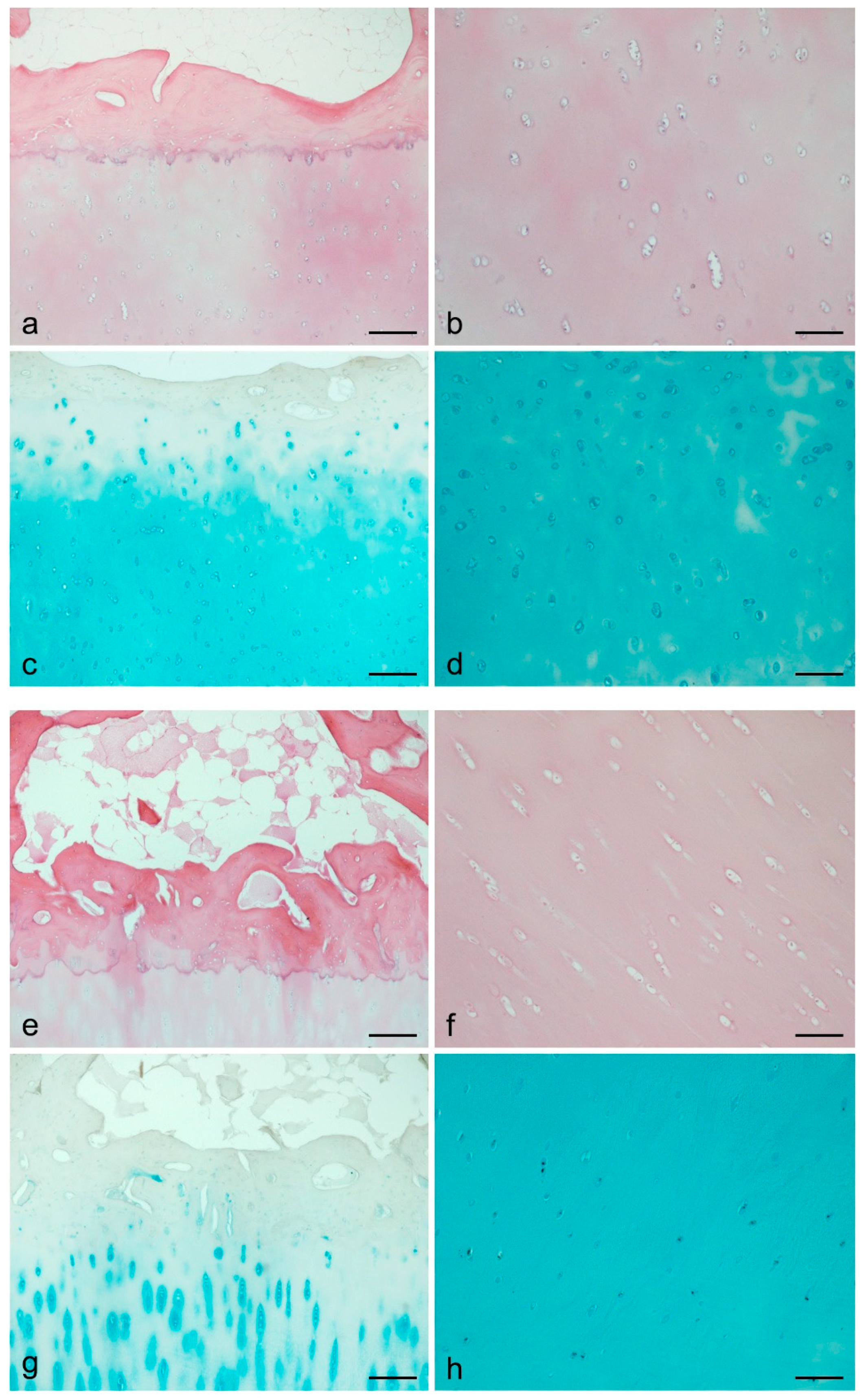
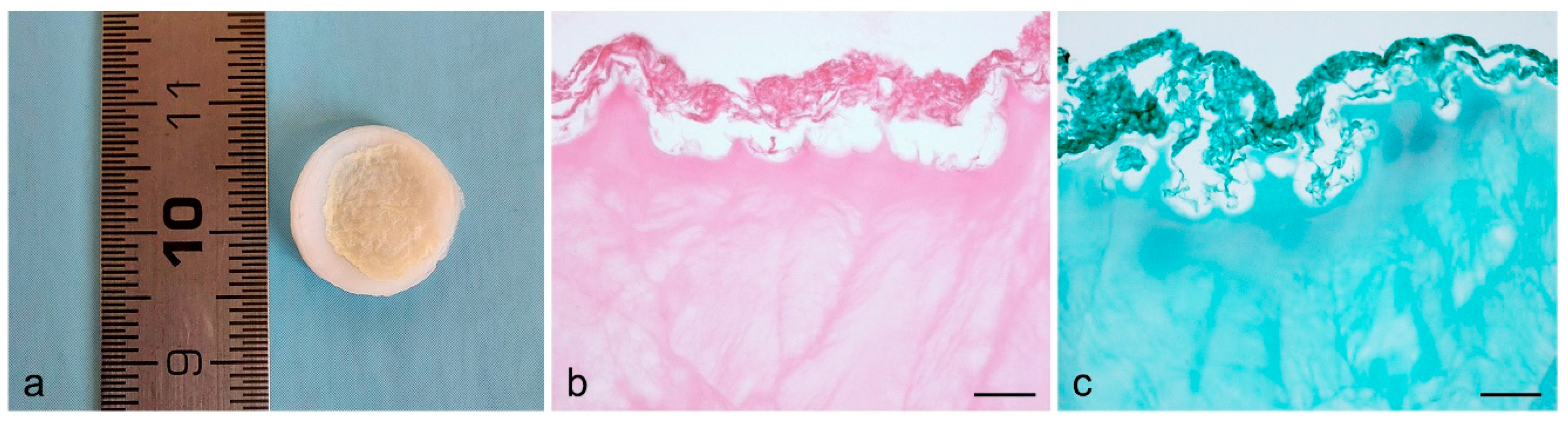
| Biological ECM | Freeze-thawing | - Stress-strain profiles showed stress values equal to 0.35 MPa for 16% PVA and 0.5 MPa for 25% PVA, at 100% strain. - 16% PVA did not maintain the residual strain when subjected to tensile strength, revealing to be more elastic than 25% PVA. - Comparing neat PVA and OxPVA, the stress-strain curves of both polymers during the loading/unloading cycle showed to overlap with no hysteresis, suggesting high resilience for both samples. - The elastic modulus decreased along with the degree of oxidation, showing a reduction in stiffness for OxPVA. - The elastic modulus did not differ between dried and swelled samples. | [24,25] |
| Injectable hydrogel | - Injectable composite scaffolds made of DBM combined with different PVA concentrations (10, 20, 25%) exhibited mechanical properties which depended on the PVA content. - PVA alone showed significantly stronger tensile mechanical characteristics than DBM/PVA. - Shear modulus showed that DBM/PVA 20% has significantly improved compressive mechanical properties in comparison with other scaffolds. - Young’s modulus of DBM/PVA 20% (5.86 ± 0.03 MPa) was significantly higher than other DBM/PVA groups. | [64] | |
| Bone/BG | Freeze-thawing | - The addition of BG to PVA/spongy bone hydrogel led to an increase in the compressive tangent modulus of the composite scaffold. - The shear properties of the PVA/spongy bone/BG composite were mainly affected by the porous size of the bone component. | [73] |
| Amino acids | In situ crosslinking by citric acid | - PVA/AA biocomposite membranes exhibited marginally improved tensile strength in comparison with PVA films. - Elongation-at-break was fairly decreased by AA blend. - PVA/lysine biocomposites showed the maximum tensile strength and good elongation characteristics, probably due to the strong interaction between lysine and PVA. | [65] |
| Acrylamide | Freeze-thawing | - Creep resistance of the PVA/acrylamide gels decreased with increasing acrylamide content. - A strong correlation was found between elastic strain and EWC and viscoelastic strain and EWC. - A weak correlation was observed between viscoelastic recovery and EWC and elastic recovery and EWC. - Tear strength and RCOF of the PVA/acrylamide composites decreased with the rise in acrylamide content. | [34] |
| Hydroxyapatite | Lyophilization | - PVA/hydroxyapatite sheets showed a ductile failure at a stress of 0.30 MPa. - The Young’s modulus calculated as the slope of the initial linear portion of stress-strain curve was 7.5 MPa. | [49] |
| Freeze-thawing | - The compressive strength, compressive modulus, storage modulus, and loss modulus of the composite scaffold first increased and then decreased with the rise in hydroxyapatite content in the PVA matrix. - The compressive strength, storage modulus, and loss modulus increased with the rise in PVA concentration in the composite scaffold. - The compressive strength, compressive modulus, and elastic and loss modulus increased with the number of freeze-thawing cycles, with the increase rate reducing at higher freeze-thaw cycle times. - The tensile test revealed an increase in the tensile Young’s modulus (up to 460 ± 20 kPa) with the addition of hydroxyapatite in comparison with neat PVA (250 ± 50 kPa). - Nanoindentation tests confirmed the contribution of the hydroxyapatite to increase the hardness of the composite, since a decrease in the work of adhesion to the indenter tip was observed. - Scaffolds with lower concentrations of hydroxyapatite were found to have the lowest friction coefficient (0.067 ± 0.049) together with a high resistance (721 ± 25 kPa). - Evaluating the torsional friction behavior of the composites against CoCrMo femoral head, the average friction torques were 0.74, 3.69, and 9.89 Nmm during the torsional friction with 10, 30, and 50 N loads, respectively. - Fluid loss of the composite hydrogel increased as the friction time, load, and torsional angle increased, leading to the decrease in fluid load support and the increase in friction coefficient. - PVA/hydroxyapatite composite hydrogel crosslinked on UHMWPE acetabulum showed negligibly small swing and torsion friction coefficients and largest sliding friction coefficient. | [50,51,52,53,54,55,56] | |
| Directional freeze-thawing | - The bi-layered gradient PVA/hydroxyapatite hydrogel presented gradient mechanical strength with tensile modulus ranging from 0.18 to 0.27 MPa. - The gradient compressive modulus ranged from 0.33 to 0.51 MPa. | [57] | |
| Hydroxyapatite/PAA | Freeze-thawing Annealing treatment | - The creep deformation of the composite scaffold decreased with the rise in PVA concentration. - The creep deformation of the hydrogel increased first and then decreased with the rise in PAA content. - Hydroxyapatite had a minor effect on the creep properties. - The stress relaxation rate decreased with the decrease in PVA content. At the same time, it first decreased and then increased with the rise in PAA. - Hydroxyapatite seemed to have the weakest effect on stress relaxation properties. - The stress relaxation rate decreased with increasing annealing temperature. - According to the compressive stress-strain curve, the composite hydrogel had a typical viscoelastic behavior, resembling natural AC. - The elastic modulus of the composite hydrogel increased with the annealing temperature. - The elastic modulus of the composite hydrogel decreased first and then increased with increasing freeze-thawing cycles. - Increasing the content of hydroxyapatite and PAA, the elastic modulus showed a trend of increasing first and then decreasing. - The friction coefficient of the composite hydrogels was relatively low and shown to increase with the contact load. - The friction coefficient of the composite hydrogels increased and then decreased with the increase in sliding rate. | [66] |
| Hydroxyapatite/PLGA | Freeze-thawing | - The double-layer PVA/hydroxyapatite hydrogel coated with PVA/hydroxyapatite/PLGA scaffold demonstrated to be a viscoelastic material. - The compressive stress and tensile stress of PVA/hydroxyapatite alone was higher than that of the PVA/hydroxyapatite/PLGA scaffold. - The ultimate compressive stress and ultimate tensile stress of the PVA/hydroxyapatite/PLGA scaffold-modified PVA/hydroxyapatite hydrogel was lower than that of the HA/PVA hydrogel and higher than that of the PVA/hydroxyapatite/PLGA hydrogel. - The compressive/tensile stress and the ultimate compressive/tensile stress increased along with the increase in the composition ratio of the PVA/hydroxyapatite hydrogel. | |
| Hydroxyapatite/PAA | Freeze-thawing Annealing treatment | - The creep deformation of the composite scaffold decreased with the rise in PVA concentration. - The creep deformation of the hydrogel increased first and then decreased with the rise in PAA content. - Hydroxyapatite had the minor effect on the creep properties. - The stress relaxation rate decreased with the decrease in PVA content. At the same time, it first decreased and then increased with the rise in PAA. - Hydroxyapatite seemed to have the weakest effect on stress relaxation properties. - The stress relaxation rate decreased with the increasing of annealing temperature. - According to the compressive stress-strain curve, the composite hydrogel had a typical viscoelastic behavior, resembling natural AC. - The elastic modulus of the composite hydrogel increased with the annealing temperature. - The elastic modulus of the composite hydrogel decreased first and then increased with the increasing of freeze-thawing cycles. - Increasing the content of hydroxyapatite and PAA, the elastic modulus showed a trend of increasing first and then decreasing. - The friction coefficient of the composite hydrogels was relatively low and showed to increase with the contact load. - The friction coefficient of the composite hydrogels increased and then decreased with the increase in sliding rate. | [66] |
| Hydroxyapatite/PLGA | Freeze-thawing | - The double-layer PVA/hydroxyapatite hydrogel coated with PVA/hydroxyapatite/PLGA scaffold was demonstrated to be a viscoelastic material. - The compressive stress and tensile stress of PVA/hydroxyapatite alone was higher than that of the PVA/hydroxyapatite/PLGA scaffold. - The ultimate compressive stress and ultimate tensile stress of the PVA/hydroxyapatite/PLGA scaffold-modified PVA/hydroxyapatite hydrogel was lower than that of the HA/PVA hydrogel and higher than that of the PVA/hydroxyapatite/PLGA hydrogel. - The compressive/tensile stress and the ultimate compressive/tensile stress increased along with the increase in the composition ratio of the PVA/hydroxyapatite hydrogel. | [67] |
| Hydroxyapatite/HACC | Freeze-thawing Immersion in Na3Cit for dual physical crosslinking | - The addition of hydroxyapatite nanoparticles led to a slight decrease in the ultimate tensile stress and elongation and a slight increase in the compressive stress of the composite scaffold. - After crosslinking with Na3Cit, the PVA/hydroxyapatite/HACC-Cit scaffold exhibited improved load-bearing and self-recovery ability. - The ultimate tensile stress and compressive stress of composites after dual crosslinking were nearly 10 and 12 times higher, respectively, in comparison with composites which were not treated with Na3Cit. - The fracture toughness and compressive Young’s modulus of the composite significantly increased after dual crosslinking. | [68] |
| Hydroxyapatite/GO | Extrusion 3D printing Freeze-thawing | - Higher concentration of PVA (25 wt%) better matched the mechanical properties of AC in terms of compressive strength (0.93 MPa) and compressive modulus (0.96 MPa). - The addition of hydroxyapatite and GO significantly improved the printability and printing accuracy of PVA solution, ensuring the structure integration and better mechanical properties. - The 3D-printed construct appeared to be difficult to be destroyed under cyclic compression, suggesting excellent antifatigue ability from the perspective of being used during joint motion (i.e., jumping and running). - 3D-printed PVA/hydroxyapatite/GO samples displayed a time-dependent friction response which was similar to that of AC, reaching a final value as low as 0.0698. - No furrows were detected on the surface morphologies for the friction area of the composite hydrogel, suggesting good wear resistance of the structure. | [69] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbon, S.; Contran, M.; Stocco, E.; Todros, S.; Macchi, V.; Caro, R.D.; Porzionato, A. Enhanced Biomechanical Properties of Polyvinyl Alcohol-Based Hybrid Scaffolds for Cartilage Tissue Engineering. Processes 2021, 9, 730. https://doi.org/10.3390/pr9050730
Barbon S, Contran M, Stocco E, Todros S, Macchi V, Caro RD, Porzionato A. Enhanced Biomechanical Properties of Polyvinyl Alcohol-Based Hybrid Scaffolds for Cartilage Tissue Engineering. Processes. 2021; 9(5):730. https://doi.org/10.3390/pr9050730
Chicago/Turabian StyleBarbon, Silvia, Martina Contran, Elena Stocco, Silvia Todros, Veronica Macchi, Raffaele De Caro, and Andrea Porzionato. 2021. "Enhanced Biomechanical Properties of Polyvinyl Alcohol-Based Hybrid Scaffolds for Cartilage Tissue Engineering" Processes 9, no. 5: 730. https://doi.org/10.3390/pr9050730
APA StyleBarbon, S., Contran, M., Stocco, E., Todros, S., Macchi, V., Caro, R. D., & Porzionato, A. (2021). Enhanced Biomechanical Properties of Polyvinyl Alcohol-Based Hybrid Scaffolds for Cartilage Tissue Engineering. Processes, 9(5), 730. https://doi.org/10.3390/pr9050730












