Continuous Biodiesel Production from Waste Soybean Oil Using a Nano-Fe3O4 Microwave Catalysis
Abstract
:1. Introduction
2. Materials and Methods
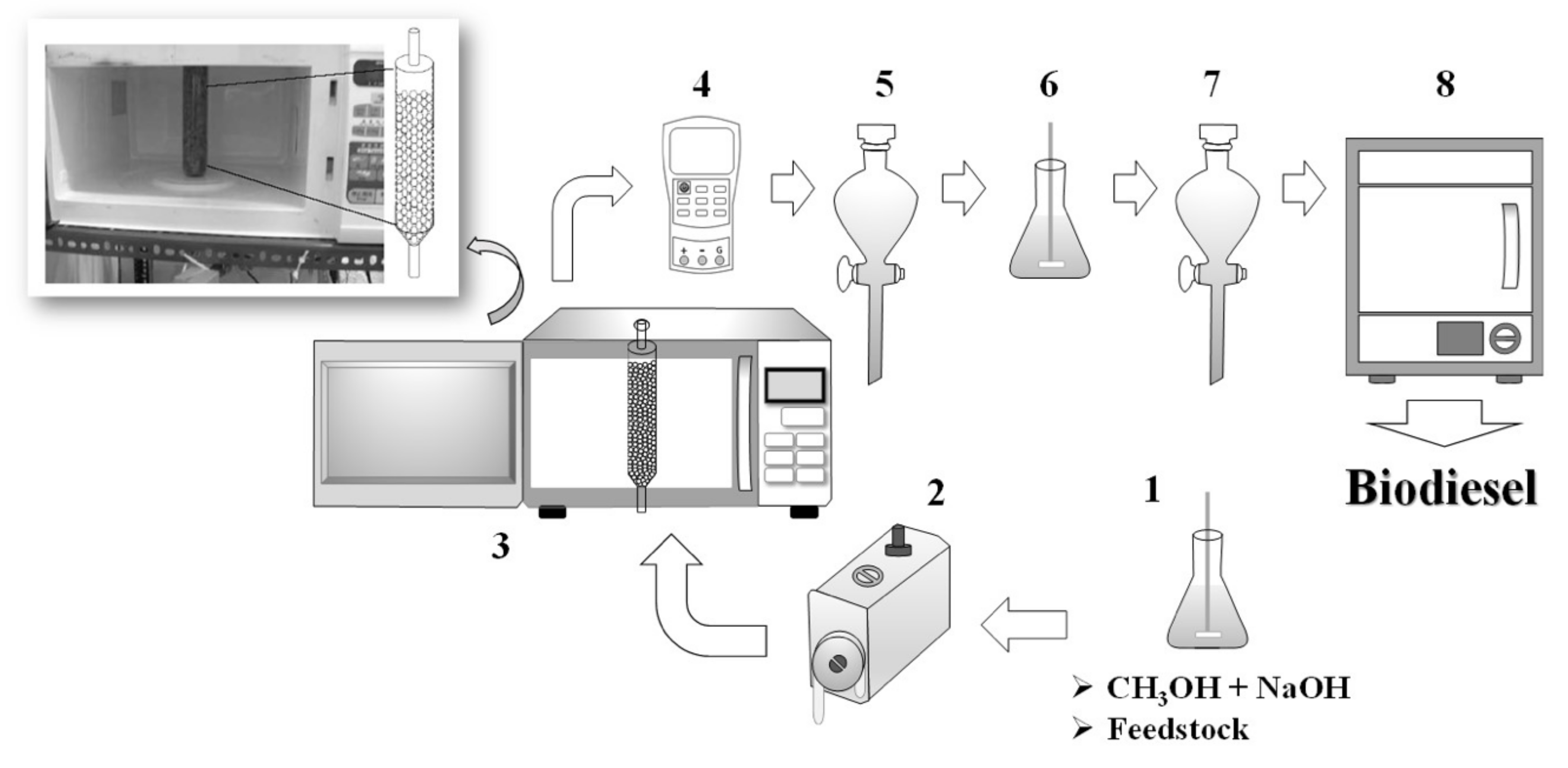
2.1. Microwave Catalytic Reactor Design
2.2. Manufacturing of the Fe3O4 Microwave Co-Catalyst
2.3. Performance Testing of the Microwave Catalytic Reaction System
2.3.1. Processes in Batch Mode
2.3.2. Processes in Continuous Mode
2.4. Analysis of the Physical and Chemical Properties of the Biodiesel
3. Results and Discussion
3.1. Surface Morphology of the Fe3O4 Co-Catalyst
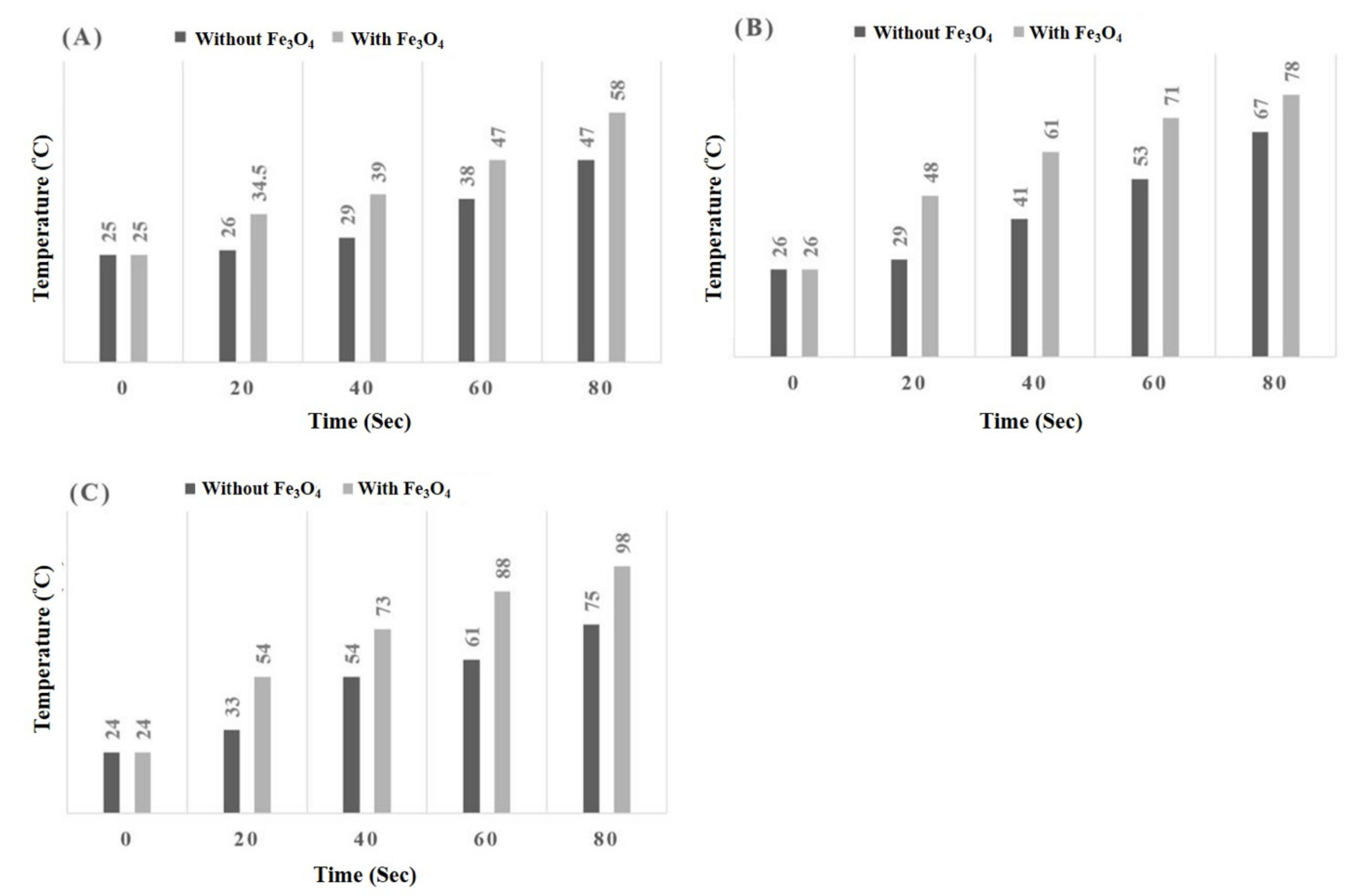
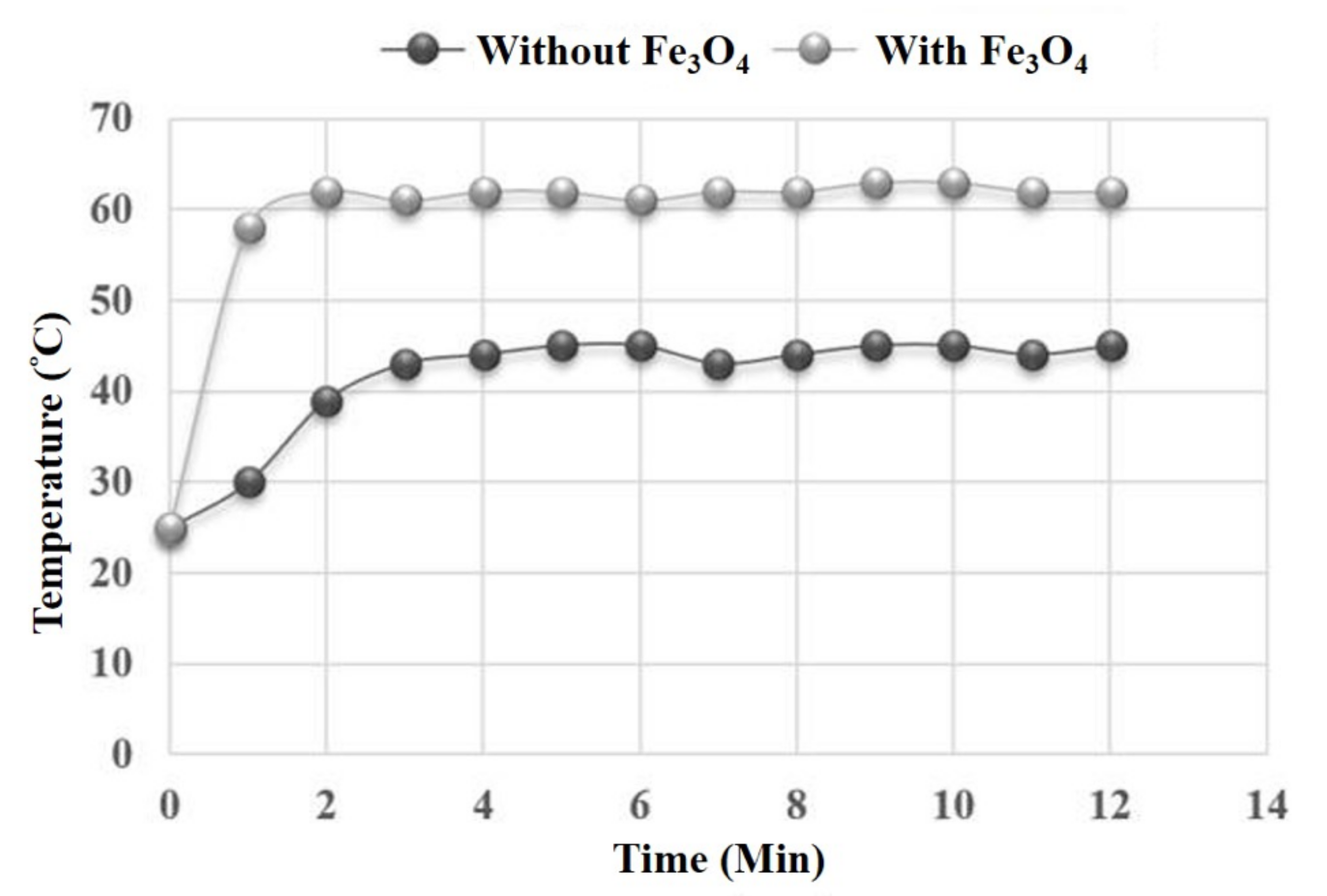
3.2. Temperature Comparisons of the Microwave Process with and without Fe3O4 Co-Catalyst
3.3. The Performance of Fe3O4 Microwave Catalysis during Continuous Biodiesel Production
3.4. Analysis of the Physical and Chemical Properties of the Biodiesel
3.5. Comparisons in Terms of Energy Consumption
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ren, Y.; He, B.Q.; Yan, F.; Wang, H.; Cheng, Y.; Lin, L.; Feng, Y.H.; Li, J.X. Continuous biodiesel production in a fixed bed reactor packed with anion-exchange resin as heterogeneous catalyst. Bioresour. Technol. 2012, 113, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Hajjari, M.; Tabatabaei, M.; Aghbashlod, M.; Ghanavatia, H. A review on the prospects of sustainable biodiesel production: A global scenario with an emphasis on waste-oil biodiesel utilization. Renew. Sustain. Energy Rev. 2017, 72, 445–464. [Google Scholar] [CrossRef]
- Gebremariam, S.N.; Marchetti, J.M. Economics of biodiesel production: Review. Energy Convers. Manag. 2018, 168, 74–84. [Google Scholar] [CrossRef]
- Sinha, S.; Agarwal, A.K.; Garg, S. Biodiesel development from rice bran oil: Transesterification process optimization and fuel characterization. Energy Convers. Manag. 2008, 49, 1248–1257. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Wu, X.; Leung, M.K.H. A review on biodiesel production using catalyzed transesterification. Appl. Energy 2010, 87, 1083–1095. [Google Scholar] [CrossRef]
- Jain, S.; Sharma, M.P.; Rajvanshi, S. Acid base catalyzed transesterification kinetics of waste cooking oil. Fuel Process Technol. 2011, 92, 32–38. [Google Scholar] [CrossRef]
- Bajpai, D.; Tyagi, V.K. Biodiesel: Source, production, composition, properties and its benefits. J. Oleo Sci. 2006, 55, 487–502. [Google Scholar] [CrossRef] [Green Version]
- Ma, F.; Hanna, M.A. Biodiesel production: A review. Bioresour. Technol. 1999, 70, 1–15. [Google Scholar] [CrossRef]
- Verma, P.; Sharma, M.P. Review of process parameters for biodiesel production from different feedstocks. Renew. Sustain. Energy Rev. 2016, 62, 1063–1071. [Google Scholar] [CrossRef]
- Veljković, V.B.; Biberdžić, M.O.; Banković-Ilić, I.B.; Djalović, I.G.; Tasić, M.B.; Nježić, Z.B.; Stamenković, O.S. Biodiesel production from corn oil: A review. Renew. Sustain. Energy Rev. 2018, 91, 531–548. [Google Scholar] [CrossRef]
- Freedman, B.; Butterfield, R.; Pryde, E. Transesterification kinetics of soybean oil. J. Am. Oil Chem. Soc. 1986, 63, 1375–1380. [Google Scholar] [CrossRef]
- Shahid, E.M.; Jamal, Y. Production of biodiesel: A technical review. Renew. Sustain. Energy Rev. 2011, 15, 4732–4745. [Google Scholar] [CrossRef]
- Go, A.W.; Sutanto, S.; Ong, L.K.; Tran-Nguyen, P.L.; Ismadji, S.; Ju, Y.H. Developments in in-situ (trans) esterification for biodiesel production: A critical review. Renew. Sustain. Energy Rev. 2016, 60, 284–305. [Google Scholar] [CrossRef] [Green Version]
- Meher, L.C.; Sagar, D.V.; Naik, S.N. Technical aspects of biodiesel production by transesterification-a review. Renew. Sustain. Energy Rev. 2006, 10, 248–268. [Google Scholar] [CrossRef]
- Demirbas, A. Importance of biodiesel as transportation fuel. Energy Policy 2007, 35, 4661–4670. [Google Scholar] [CrossRef]
- Wang, Y.; Ou, P.L.S.; Zhang, Z. Preparation of biodiesel from waste cooking oil via two-step catalyzed process. Energy Convers. Manag. 2007, 48, 184–188. [Google Scholar] [CrossRef]
- Casas, A.; Fernandez, C.M.; Ramos, M.J.; Perez, A.; Rodriguez, J.F. Optimization of the reaction parameters for fast pseudo single-phase transesterification of sunflower oil. Fuel 2010, 89, 650–658. [Google Scholar] [CrossRef]
- Padhi, S.K.; Singh, R.K. Optimization of esterification and transesterification of Mahua (Madhuca Indica) oil for production of biodiesel. J. Chem. Pharm. Res. 2010, 2, 599–608. [Google Scholar]
- Thangaraj, B.; Solomon, P.R.; Muniyandi, B.; Ranganathan, S.; Lin, L. Catalysis in biodiesel production—A review. Clean Energy 2019, 3, 2–23. [Google Scholar] [CrossRef] [Green Version]
- Azcan, N.; Yilmaz, O. Microwave assisted transesterification of waste frying oil and concentrate methyl ester content of biodiesel by molecular distillation. Fuel 2013, 104, 614–619. [Google Scholar] [CrossRef]
- Quirino, M.R.; Oliveira, M.J.C.; Keyson, D.; Lucena, G.L.; Oliveira, J.B.L.; Gama, L. Synthesis of zinc oxide by microwave hydrothermal method for application to transesterification of soybean oil (biodiesel). Mater. Chem. Phys. 2017, 185, 24–30. [Google Scholar] [CrossRef]
- Milano, J.; Ong, H.C.; Masjuki, H.H.; Silitonga, A.S.; Chen, W.H.; Kusumo, F.; Dharma, S.; Sebayang, A.H. Optimization of biodiesel production by microwave irradiation-assisted transesterification for waste cooking oil-Calophyllum inophyllum oil via response surface methodology. Energy Convers. Manag. 2018, 158, 400–415. [Google Scholar] [CrossRef]
- Lin, Y.C.; Lin, J.F.; Hsiao, Y.H.; Hsu, K.H. Soybean oil for biodiesel production assisted by a microwave system and sodium methoxide catalyst. Sustain. Environ. Res. 2012, 22, 247–254. [Google Scholar]
- Azcan, N.; Danisman, A. Alkali catalyzed transesterification of cottonseed oil by microwave irradiation. Fuel 2007, 86, 2639–2644. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, G.R.; Chandrashekar, N. Microwave assisted alkali-catalyzed transesterification of Pongamia pinnata seed oil for biodiesel production. Bioresour. Technol. 2011, 102, 6617–6620. [Google Scholar] [CrossRef]
- Patil, P.D.; Gude, V.G.; Mannarswamy, A.; Cooke, P.; Nirmalakhandan, N.; Lammers, P.; Deng, S. Comparison of direct transesterification of algal biomass under supercritical methanol and microwave irradiation conditions. Fuel 2012, 97, 822–831. [Google Scholar] [CrossRef]
- Lertsathapornsuka, V.; Pairintrab, R.; Aryusukb, K.; Krisnangkura, K. Microwave assisted in continuous biodiesel production from waste frying palm oil and its performance in a 100 kW diesel generator. Fuel Process. Technol. 2008, 89, 1330–1336. [Google Scholar] [CrossRef]
- Khedri, B.; Mostafaei, M.; Safieddin Ardebili, S.M. A review on microwave-assisted biodiesel production. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 41, 2377–2395. [Google Scholar] [CrossRef]
- Refaat, A.A. Different techniques for the production of biodiesel from waste vegetable oil. Int. J. Environ. Sci. Technol. 2010, 7, 183–213. [Google Scholar] [CrossRef] [Green Version]
- Saydut, A.; Kafadar, A.B.; Aydin, F.; Erdogan, S. Effect of homogeneous alkaline catalyst type on biodiesel production from soybean [Glycine max (L.) Merrill] oil. Indian J. Biotechnol. 2016, 15, 596–600. [Google Scholar]
- Standish, N.; Worner, H. Microwave application in the reduction of metal oxides with carbon. J. Microwave Power Electromagn. Energy 1990, 25, 177–180. [Google Scholar] [CrossRef]
- Chang, C.Y.; Lin, C.H.; Hwa, M.Y.; Lee, Y.C.; Tseng, W.M. Microwave catalytic process for the production of biodiesel. J. Biobased Mater. Bioenergy 2013, 7, 198–201. [Google Scholar] [CrossRef]
- Thangaraj, B.; Jia, Z.; Dai, L.; Liu, D.; Du, W. Lipase NS81006 immobilized on Fe3O4 magnetic nanoparticles for biodiesel production. Ovidius Univ. Ann. Chem. 2016, 27, 13–21. [Google Scholar] [CrossRef] [Green Version]
- ASTM International. Available online: https://www.astm.org/ (accessed on 21 April 2021).
- Reddy, A.N.R.; Saleh, A.A.; Isalm, M.S.; Hamdan, S.; Maleque, M.A. Biodiesel production from crude Jatropha oil using a highly active heterogeneous nanocatalyst by optimizing transesterification reaction parameters. Energy Fuels 2016, 30, 334–343. [Google Scholar]
- Shahbazi, M.R.; Khoshandam, B.; Nasiri, M.; Ghazvini, M. Biodiesel production via alkali-catalyzed transesterification of Malaysian RBD palm oil-Characterization, kinetics model. J. Taiwan Inst. Chem. Energy 2012, 43, 504–510. [Google Scholar] [CrossRef]
- Zhong, G.; Xu, S.; Chen, C.; Kline, D.J.; Giroux, M.; Pei, Y.; Jiao, M.; Liu, D.; Mi, R.; Xie, H.; et al. Synthesis of metal oxide nanoparticles by rapid, high-temperature 3D microwave heating. Adv. Funct. Mater. 2019, 29, 1904282. [Google Scholar] [CrossRef]
- Zhang, S.; Zu, Y.G.; Fu, Y.J.; Luo, M.; Zhang, D.Y.; Efferth, T. Rapid microwave-assisted transesterification of yellow horn oil to biodiesel using a heteropolyacid solid catalyst. Bioresour. Technol. 2010, 101, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Moser, B.R. Biodiesel production, properties, and feedstocks. In Vitro Cell. Dev. Biol. Plant 2009, 45, 229–266. [Google Scholar] [CrossRef]
- Sakthivela, R.; Rameshb, K.; Purnachandrana, R.; Mohamed Shameera, P. A review on the properties, performance and emission aspects of the third generation biodiesels. Renew. Sustain. Energy Rev. 2018, 82, 2970–2992. [Google Scholar] [CrossRef]
- Jham, G.N.; Moser, B.R.; Shah, S.N.; Holser, R.A.; Dhingra, O.D.; Vaughn, S.F.; Berhow, M.A.; Winkler-Moser, J.K.; Isbell, T.A.; Holloway, R.K.; et al. Wild Brazilian mustard (Brassica juncea L.) seed oil methyl esters as biodiesel fuel. J. Am. Oil Chem. Soc. 2009, 86, 917–926. [Google Scholar] [CrossRef]
- Patil, P.D.; Gude, V.G.; Camacho, L.M.; Deng, S. Microwave-assisted catalytic transesterification of Camelina sativa oil. Energy Fuels 2010, 24, 1298–1304. [Google Scholar] [CrossRef]
- Moseley, J.D.; Woodman, E.K. Energy efficiency of microwave- and conventionally heated reactors compared at meso scale for organic reactions. Energy Fuels 2009, 23, 5438–5447. [Google Scholar] [CrossRef]

| Feedstock | Alcohol | Molar Ratio (Alcohol/Oil) | Catalyst | Reaction Time | Microwave Power (W) | Temperature (°C) | References |
|---|---|---|---|---|---|---|---|
| Sunflower oil | Methanol | 6:1 | NaOCH3 (1 wt%) | 5 min | - | 60 | [20] |
| Mixture of waste cooking oil and Calophyllum inophyllum oil (7:3) | Methanol | 5.96:1 | KOH (0.774 wt%) | 7.15 min | 850 | 100 | [22] |
| Soybean oil | Methanol | 6:1 | CH3ONa (0.75 wt%) | 3 min | 750 | - | [23] |
| Cottonseed oil | Methanol | - | KOH (1.5 wt%) | 7 min | 252 | 60 | [24] |
| Pongamia pinnata oil | Methanol | 6:1 | NaOH (0.5 wt%), KOH (1 wt%) | 3–10 min | 125–300 | 60 | [25] |
| Camelina Sativa oil | Methanol | 9:1 | KOH (1 wt%), NaOH (0.5 wt%), BaO (1.5 wt%), SrO (2.0 wt%) | 60 s (KOH, NaOH), 4 min (BaO, SrO) | 800 | - | [26] |
| Palm oil (fried) | Ethanol | 12:1 | NaOH (3 wt%) | 30 s | 800 | 78 | [27] |
| Yellow horn oil | Methanol | 12:1 | Cs2.5H0.5PW12O40 (1 wt%) | 10 min | 500 | 60 | [38] |
| Waste soybean oil | Methanol | 6:1 | CH3ONa (1 wt%) | 30 s | 560 | 60–61 | This study * |
| Items | ASTM D6751 | EN 14214 | Biodiesel (This Study) |
|---|---|---|---|
| Density at 15 °C (kg/m3) | 889 | 860–900 | 898.1 |
| Flash point (°C) | >130 | >101 | 182 |
| Viscosity at 40 °C (mm2/s) | 1.9–6.0 | 3.5–5.0 | 5.356 |
| Sulfur (ppm) | <15 | <10 | 0.18 |
| Copper strip corrosion *2 | Class 3 max. | Class 1 rating | Class 1 |
| Cetane number | >47 | >51 | 51.1 |
| Water content and sediment | <0.005 (vol%) | <500 (mg/kg) | 0.05 (vol%) |
| Distillation temperature (°C) | 360 | - *3 | 345–346 |
| Carbon residue (wt%) | <0.05 | <0.3 | 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-H.; Chang, Y.-T.; Lai, M.-C.; Chiou, T.-Y.; Liao, C.-S. Continuous Biodiesel Production from Waste Soybean Oil Using a Nano-Fe3O4 Microwave Catalysis. Processes 2021, 9, 756. https://doi.org/10.3390/pr9050756
Lin C-H, Chang Y-T, Lai M-C, Chiou T-Y, Liao C-S. Continuous Biodiesel Production from Waste Soybean Oil Using a Nano-Fe3O4 Microwave Catalysis. Processes. 2021; 9(5):756. https://doi.org/10.3390/pr9050756
Chicago/Turabian StyleLin, Ching-Hsing, Yi-Tang Chang, Mei-Chou Lai, Tai-Ying Chiou, and Chien-Sen Liao. 2021. "Continuous Biodiesel Production from Waste Soybean Oil Using a Nano-Fe3O4 Microwave Catalysis" Processes 9, no. 5: 756. https://doi.org/10.3390/pr9050756






