Numerical Investigation of the Deformable Porous Media Treated by the Intermittent Microwave
Abstract
:1. Introduction
2. Materials and Methods
2.1. Process Description and Assumptions
2.2. Descriptions of the Variables
2.3. Mass Conservation
2.3.1. Conservation Equations
2.3.2. The Non-Equilibrium Description of Phase Change
2.3.3. Initial and Boundary Conditions
2.4. Continuity Equation for Pressure
2.4.1. Conservation Equations
2.4.2. Initial and Boundary Conditions
2.5. Energy Conservation
2.5.1. Conservation Equations
2.5.2. Heat Fluxes and Sources
2.5.3. Initial and Boundary Conditions
2.6. Solid Mechanics Formulation
2.6.1. Solid Momentum Balance
2.6.2. Constitutive Law
2.6.3. Initial and Boundary Conditions
2.7. Input Parameters
2.7.1. Transport Properties
2.7.2. Dielectric Properties
2.7.3. Mechanical Properties
2.8. Validation Experiments
2.8.1. Overview of the Validation Experiments
2.8.2. Description of the Validation Experiments
2.8.3. Statistical Analysis and Measurement Uncertainty
2.9. Solution Procedure
3. Results and Discussion
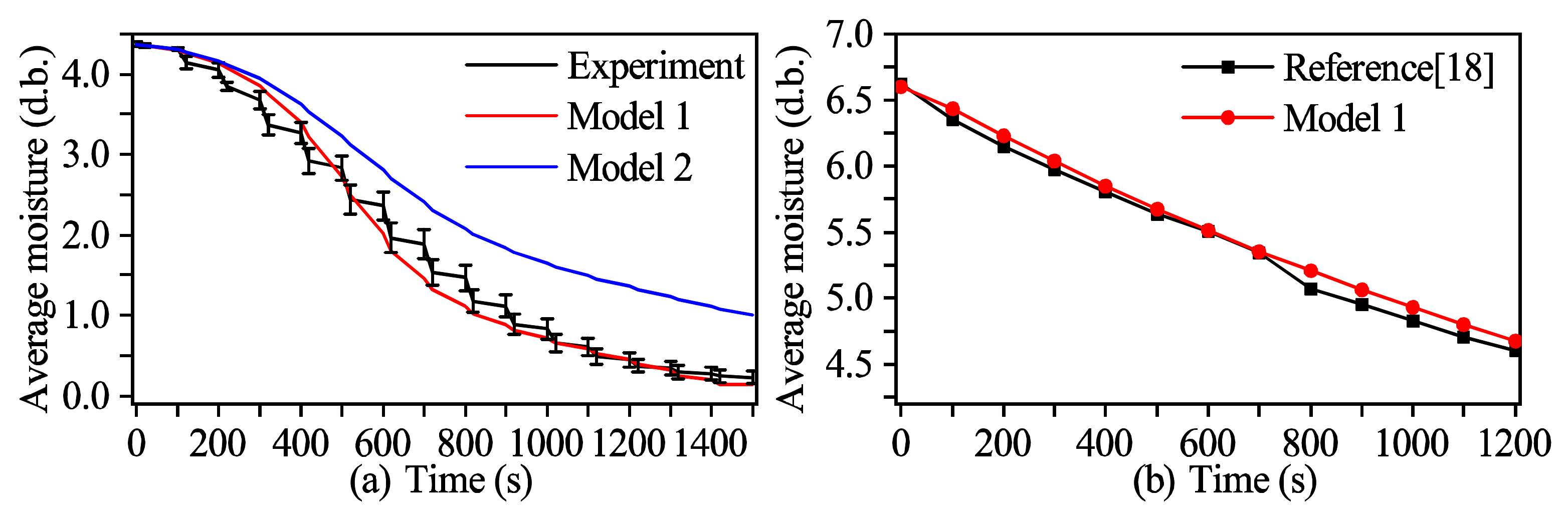
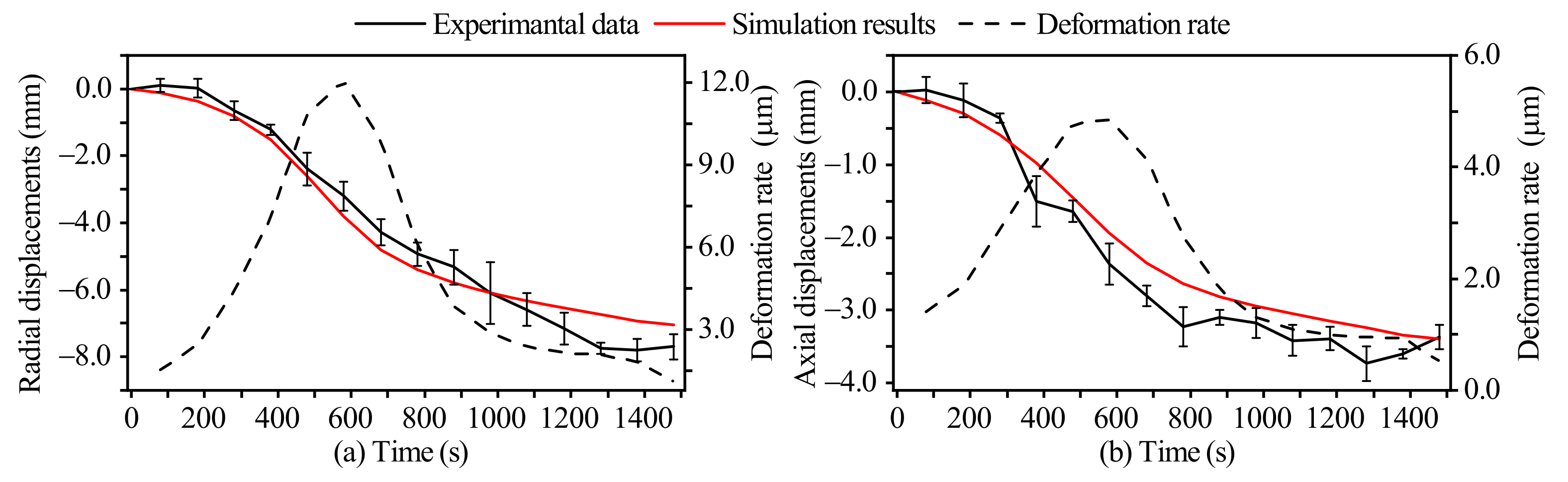
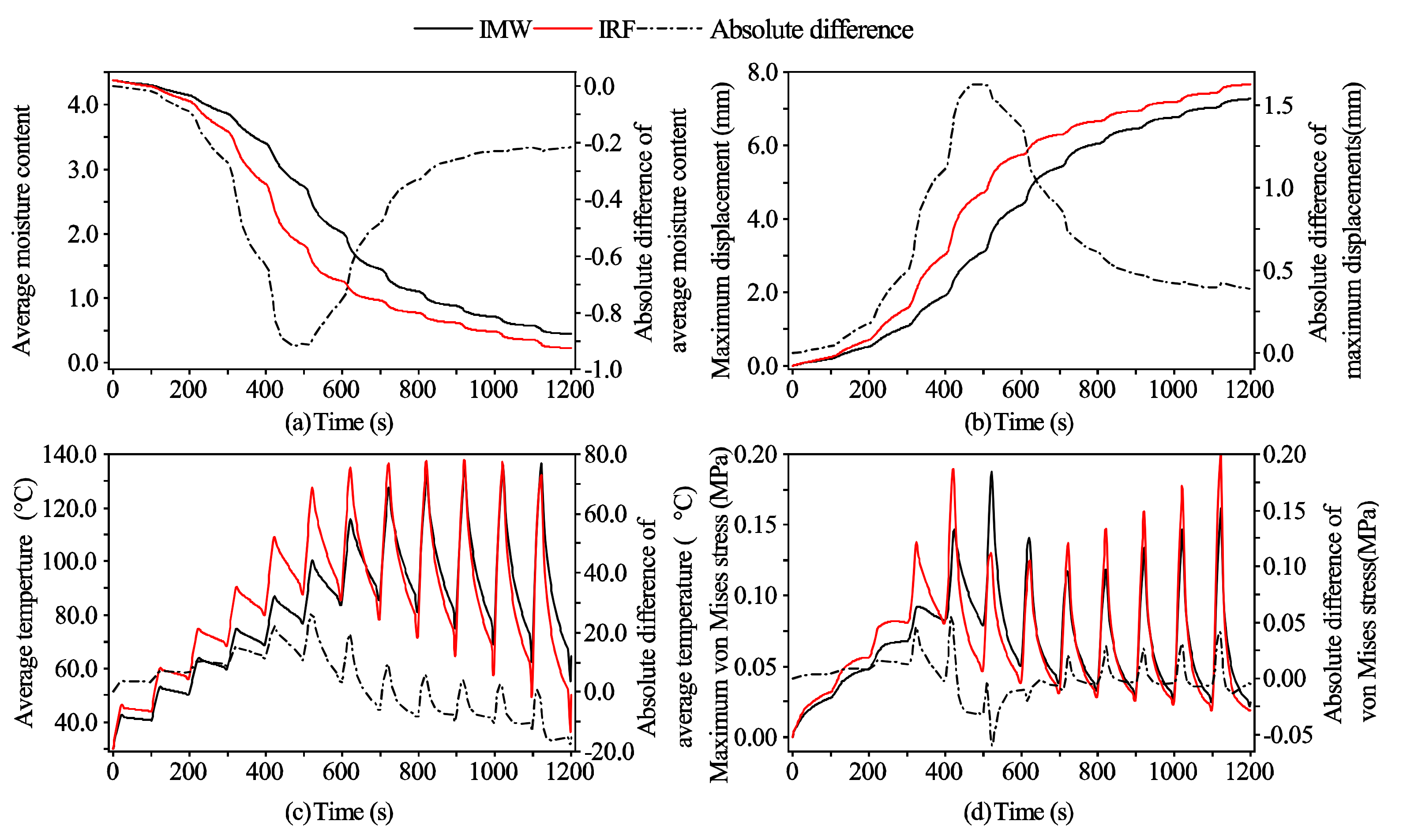
3.1. Model Validation
3.1.1. Moisture Content
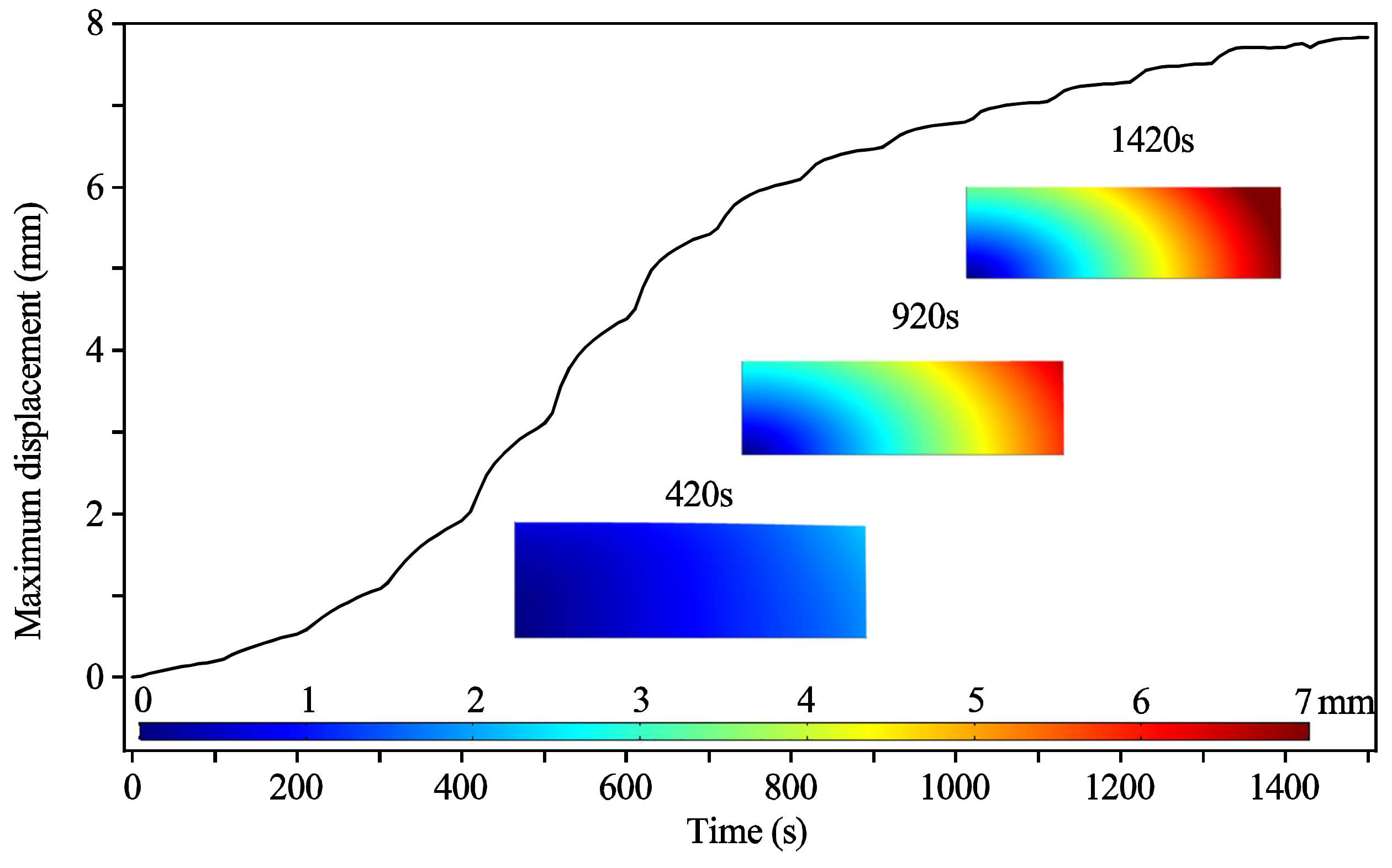
3.1.2. Deformation Displacements
3.1.3. Temperature
3.2. Discussions about the Mechanisms of Different Phenomena
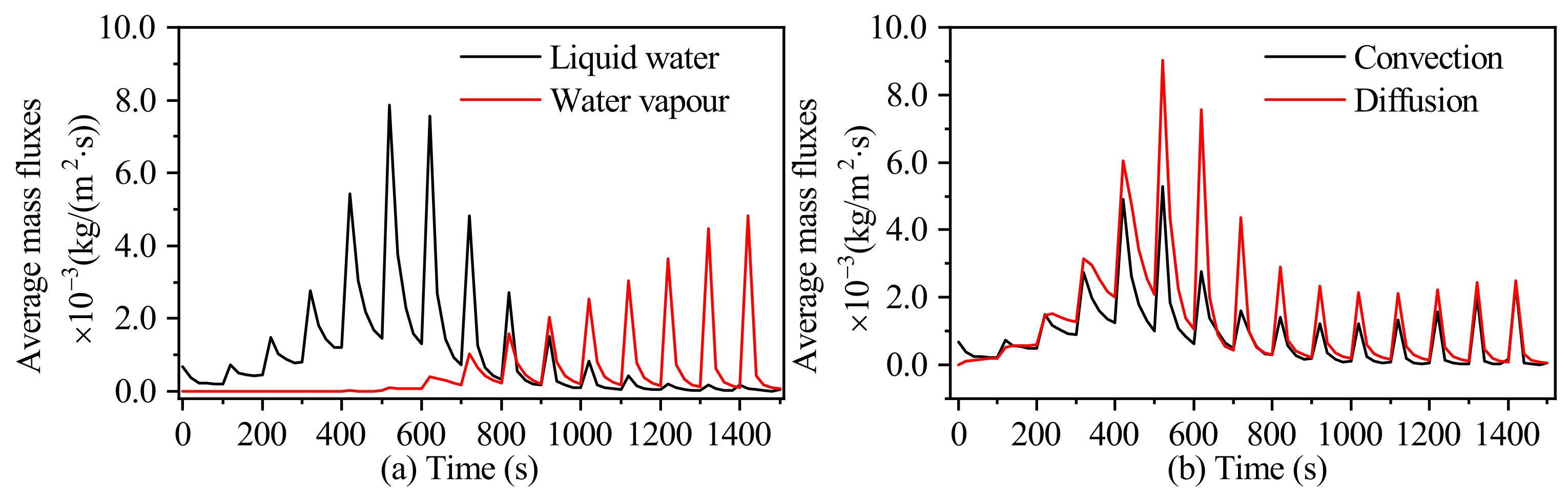
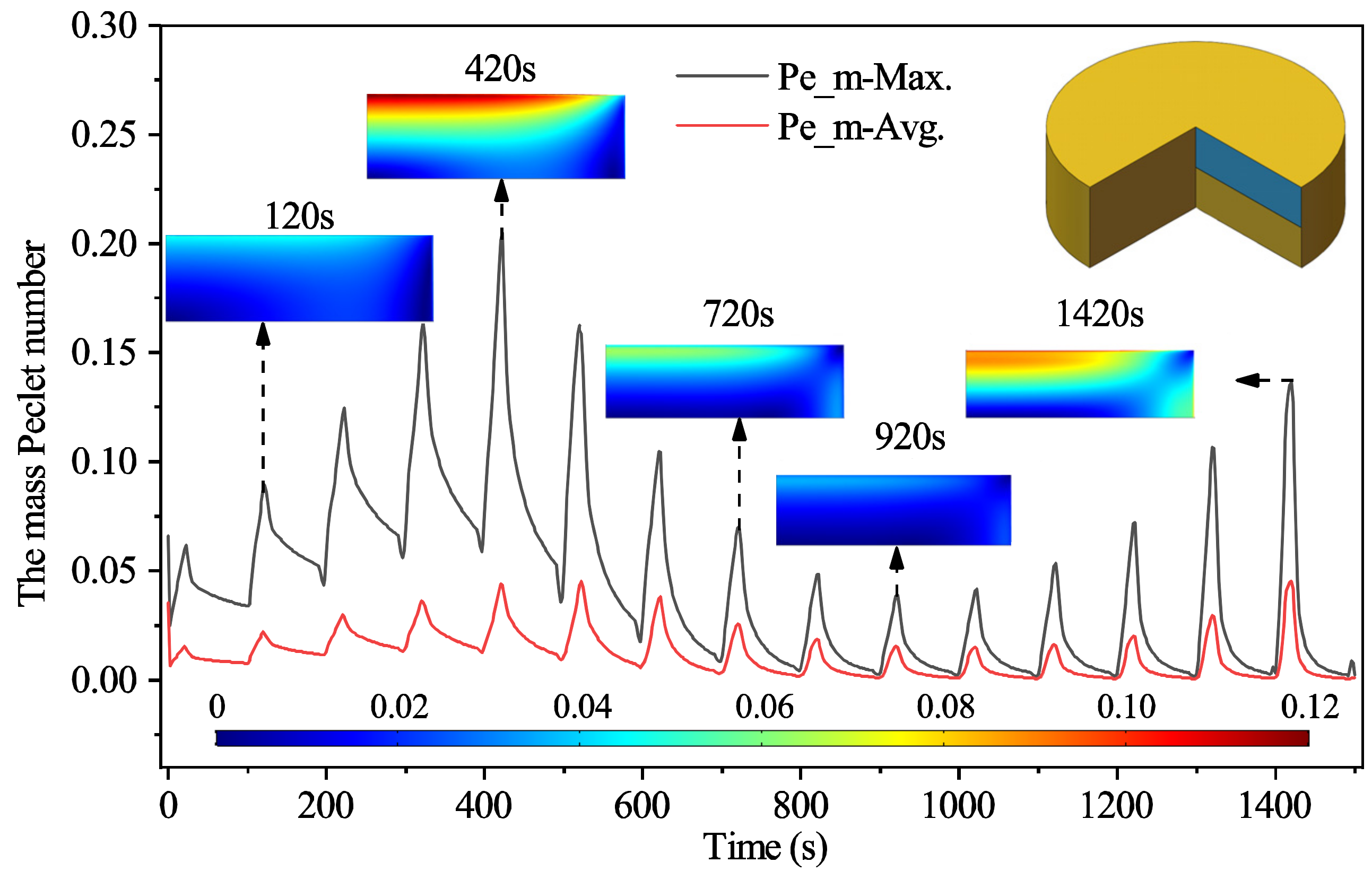
3.2.1. The Mass Transport Mechanism
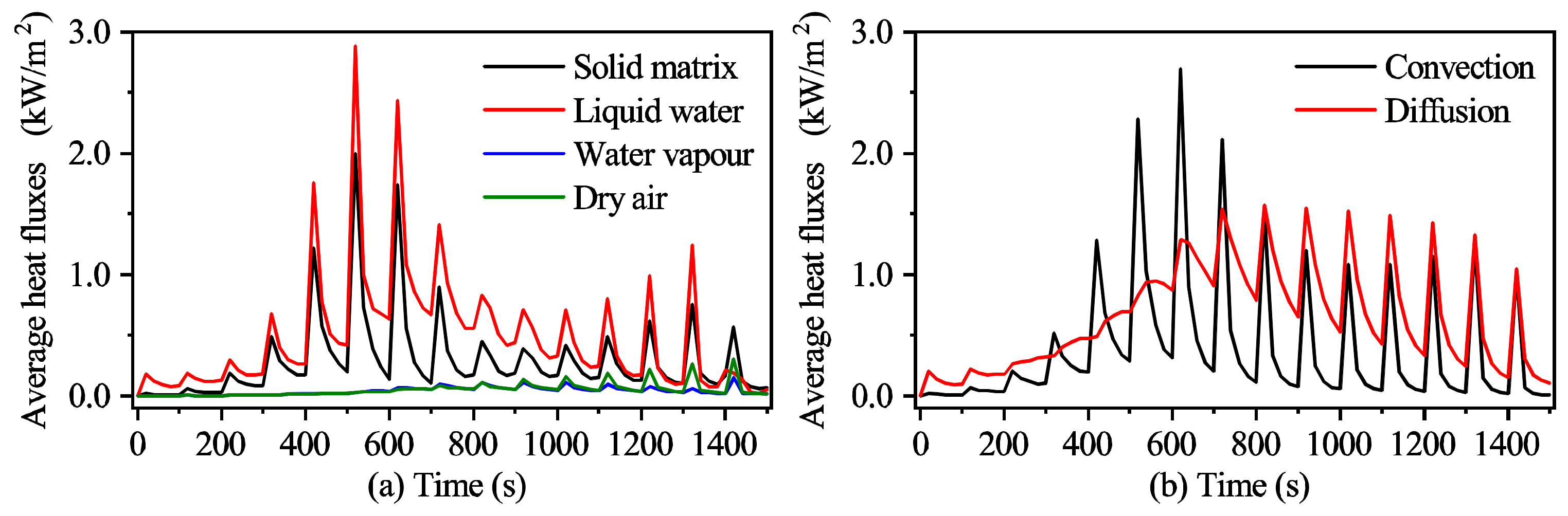
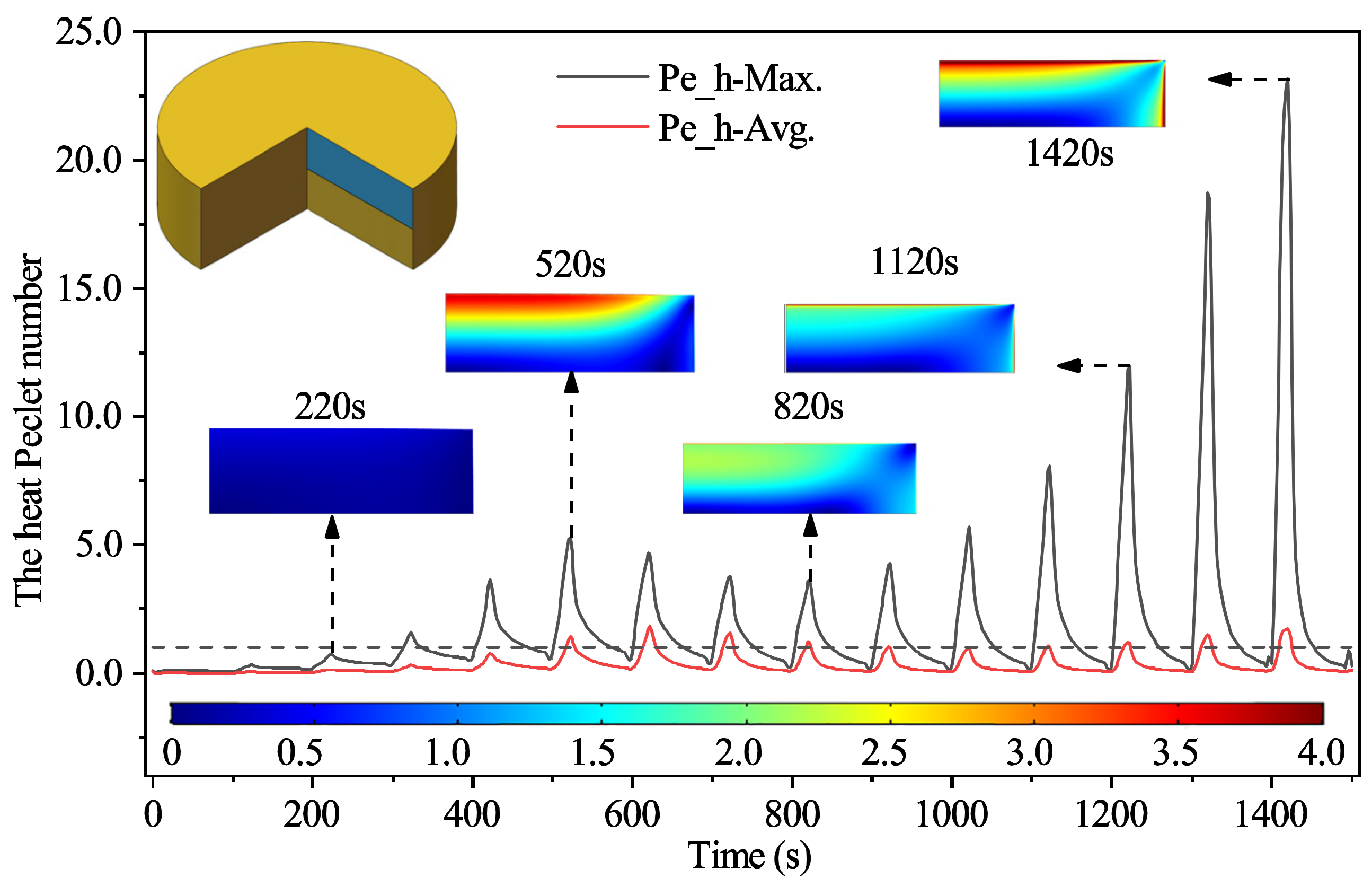
3.2.2. The Heat Transport Mechanism
3.2.3. The von Mises Stress
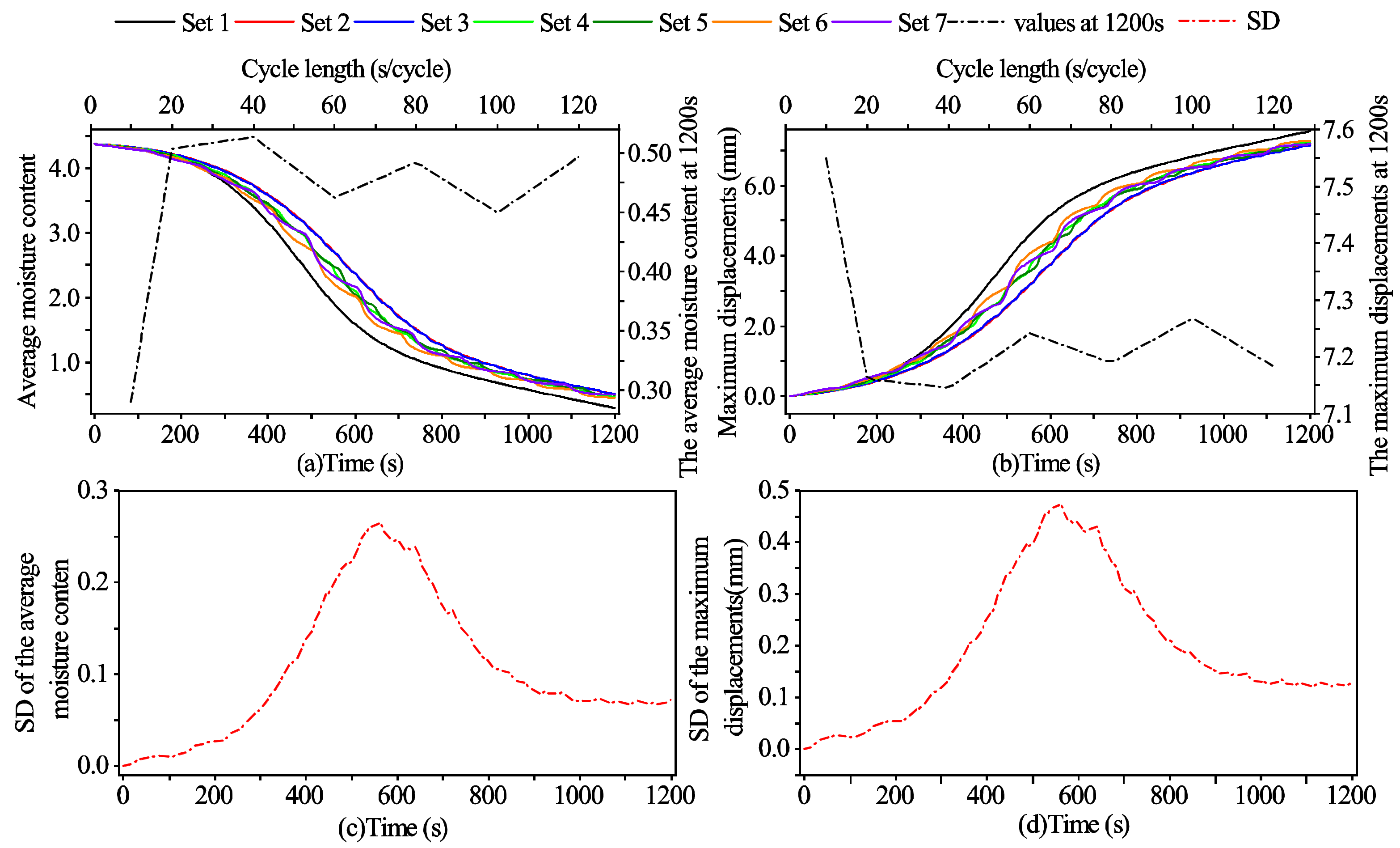
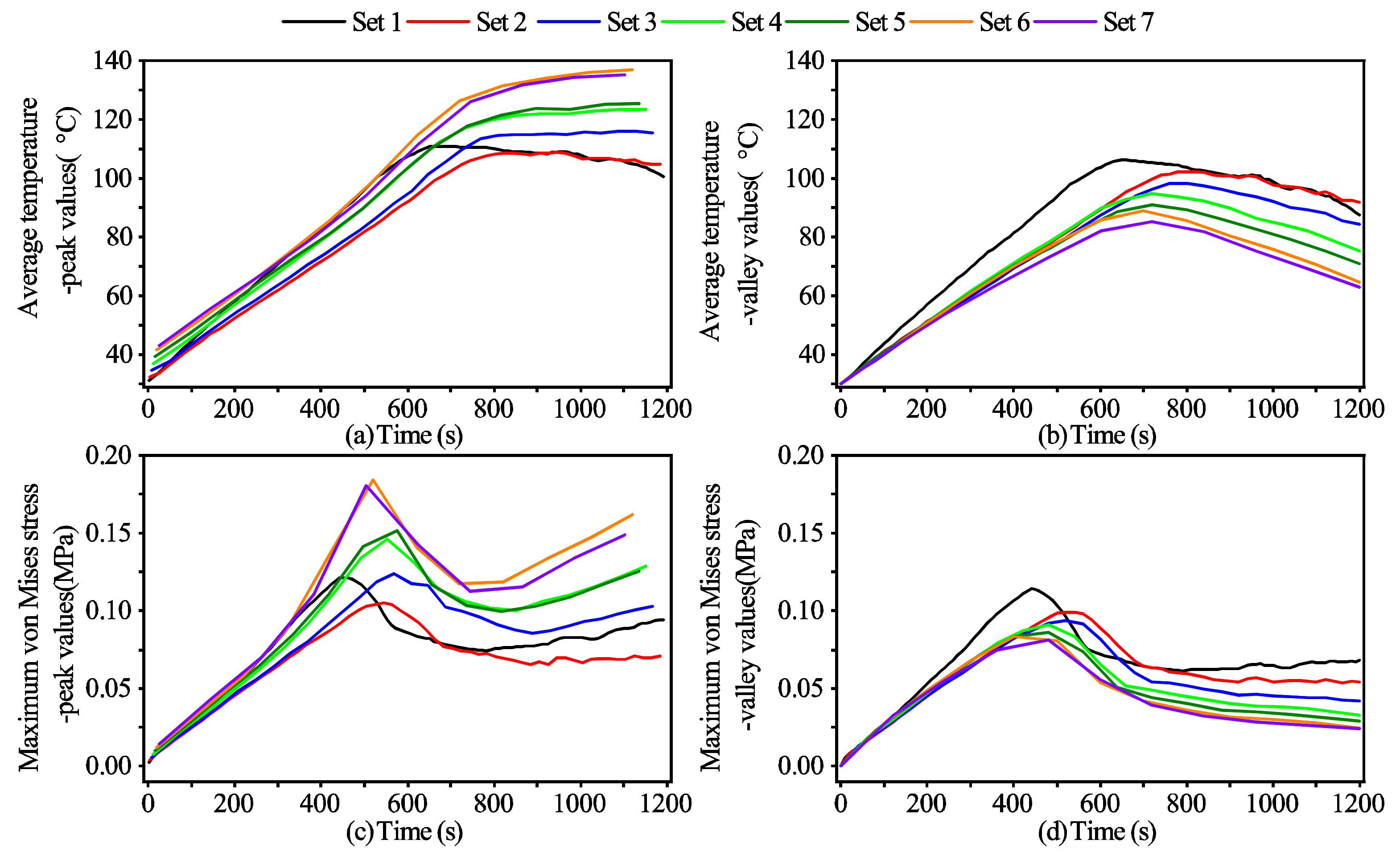
3.3. The Effects of the Cycle Length on the Outcomes
3.4. The Effects of the Frequency on the Outcomes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| representative elementary volume: | |
| saturation of a fluid phase | |
| mass concentration, | |
| molecular weight, | |
| universal gas constant, | |
| temperature, | |
| pressure, | |
| moisture content | |
| evaporation rate, | |
| molar flux of a fluid phase, | |
| velocity, | |
| capillary diffusivity, | |
| molecular diffusivity of water vapor, | |
| evaporation rate constant, | |
| water activity | |
| mass transfer coefficient, | |
| heat transfer coefficient, | |
| intrinsic permeability, | |
| relative permeability | |
| specific heat capacity, | |
| heat conductivity, | |
| heat source, | |
| mass fraction of a phase | |
| latent heat, | |
| microwave power, | |
| radius of the sample, | |
| height of the sample, | |
| deformation displacement, | |
| velocity of light, | |
| microwave frequency, | |
| inward normal heat flux, | |
| deformation tensor | |
| Piola–Kirchhoff stress tensor, | |
| Creen–Lagrange strain tensor | |
| identity tensor | |
| strain energy density, | |
| Jacobian determinant | |
| Reynolds number | |
| Prandtl number | |
| Lewis number | |
| Young’s modulus | |
| glass transition temperature, | |
| a specific physical quantity | |
| average of a specific physical quantity | |
| the maximum mesh size | |
| Greek symbols | |
| attenuation coefficient | |
| dielectric constant | |
| dielectric loss factor | |
| emissivity | |
| porosity | |
| Stefan–Boltzmann constant, | |
| viscosity of gas phase, | |
| viscosity of liquid water, | |
| shear modulus, | |
| Lamé’s constant, | |
| free space wavelength of the electromagnetic wave | |
| uncertainty of A class | |
| uncertainty of B class | |
| measurement uncertainty | |
| Subscripts | |
| ith phase | |
| solid phase | |
| liquid water | |
| gas phase | |
| dry air | |
| water vapor | |
| initial state | |
| dry basis | |
| wet basis | |
| state of equilibrium | |
| state of saturation | |
| ambient | |
| effective | |
| microwave | |
| radial direction | |
| axial direction | |
| surface of the sample | |
| elastic | |
| moisture | |
| simulation values | |
| experimental values |
References
- Schubert, H.; Regier, M. The Microwave Processing of Foods; CRC Press LLC: Boca Raton, FL, USA; Woodhead Publisher Limited: Cambridge, UK, 2005. [Google Scholar]
- Zhao, P.; Liu, C.; Qu, W.; He, Z.; Gao, J.; Jia, L.; Ji, S.; Ruan, R. Effect of Temperature and Microwave Power Levels on Microwave Drying Kinetics of Zhaotong Lignite. Processes 2019, 7, 74. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Liu, D.; Yu, H.; Wang, D.; Li, J. Optimization of Microwave Coupled Hot Air Drying for Chinese Yam Using Response Surface Methodology. Processes 2019, 7, 745. [Google Scholar] [CrossRef] [Green Version]
- Soni, A.; Smith, J.; Thompson, A.; Brightwell, G. Microwave-induced thermal sterilization—A review on history, technical progress, advantages and challenges as compared to the conventional methods. Trends Food Sci. Technol. 2020, 97, 433–442. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, L.; Wang, S. Computer simulation of radio frequency selective heating of insects in soybeans. Int. J. Heat Mass Transf. 2015, 90, 406–417. [Google Scholar] [CrossRef]
- Kumar, C.; Karim, M.A.; Joardder, M.U.H. Intermittent drying of food products: A critical review. J. Food Eng. 2014, 121, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Rosa, A.R.D.; Bressan, F.; Leone, F.; Falqui, L.; Chiofalo, V. Radio frequency heating on food of animal origin: A review. Eur. Food Res. Technol. 2019, 245, 1787–1797. [Google Scholar] [CrossRef]
- Ramaswamy, H.S. Radio-Frequency Heating in Food Processing Principles and Applications; CRC Press: Boca Raton, FL, USA, 2014; p. 422. [Google Scholar]
- Thuto, W.; Banjong, K. Investigation of Heat and Moisture Transport in Bananas during Microwave Heating Process. Processes 2019, 7, 545. [Google Scholar] [CrossRef] [Green Version]
- Hou, L.; Zhou, X.; Wang, S. Numerical analysis of heat and mass transfer in kiwifruit slices during combined radio frequency and vacuum drying. Int. J. Heat Mass Transf. 2020, 154, 119704. [Google Scholar] [CrossRef]
- Fu, B.A.; Chen, M.Q. Linear non-equilibrium thermal and deformation transport model for hematite pellet in dielectric and magnetic heating. Appl. Therm. Eng. 2020, 173, 115197. [Google Scholar] [CrossRef]
- Li, H.; Shi, S.; Lin, B.; Lu, J.; Lu, Y.; Ye, Q.; Wang, Z.; Hong, Y.; Zhu, X. A fully coupled electromagnetic, heat transfer and multiphase porous media model for microwave heating of coal. Fuel Process. Technol. 2019, 189, 49–61. [Google Scholar] [CrossRef]
- Itaya, Y.; Uchiyama, S.; Mori, S. Internal Heating Effect and Enhancement of Drying of Ceramics by Microwave Heating with Dynamic Control. Transp. Porous Media 2006, 66, 29–42. [Google Scholar] [CrossRef]
- Jalili, M.; Anca-Couce, A.; Zobel, N. On the Uncertainty of a Mathematical Model for Drying of a Wood Particle. Energy Fuels 2013, 27, 6705–6717. [Google Scholar] [CrossRef]
- Rattanadecho, P.; Keangin, P. Numerical study of heat transfer and blood flow in two-layered porous liver tissue during microwave ablation process using single and double slot antenna. Int. J. Heat Mass Transf. 2013, 58, 457–470. [Google Scholar] [CrossRef]
- Datta, A.K. Porous media approaches to studying simultaneous heat and mass transfer in food processes. I: Problem formulations. J. Food Eng. 2007, 80, 80–95. [Google Scholar] [CrossRef]
- Datta, A.K. Porous media approaches to studying simultaneous heat and mass transfer in food processes. II: Property data and representative results. J. Food Eng. 2007, 80, 96–110. [Google Scholar] [CrossRef]
- Kumar, C.; Joardder, M.U.H.; Farrell, T.W.; Karim, M.A. Multiphase porous media model for intermittent microwave convective drying (IMCD) of food. Int. J. Therm. Sci. 2016, 104, 304–314. [Google Scholar] [CrossRef]
- Mahiuddin, M.; Khan, M.I.H.; Kumar, C.; Rahman, M.M.; Karim, M.A. Shrinkage of Food Materials during Drying: Current Status and Challenges. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1113–1126. [Google Scholar] [CrossRef] [Green Version]
- Datta, A.K. Toward computer-aided food engineering: Mechanistic frameworks for evolution of product, quality and safety during processing. J. Food Eng. 2016, 176, 9–27. [Google Scholar] [CrossRef]
- Pabis, S. Theoretical models of vegetable drying by convection. Transp. Porous Media 2007, 66, 77–87. [Google Scholar] [CrossRef]
- Onwude, D.I.; Hashim, N.; Abdan, K.; Janius, R.; Chen, G. Numerical modeling of radiative heat and mass transfer for sweet potato during drying. J. Food Process. Preserv. 2018, 42, e13741. [Google Scholar] [CrossRef]
- Onwude, D.I.; Hashim, N.; Abdan, K.; Janius, R.; Chen, G. Experimental studies and mathematical simulation of intermittent infrared and convective drying of sweet potato (Ipomoea batatas L.). Food Bioprod. Process. 2019, 114, 163–174. [Google Scholar] [CrossRef]
- Joardder, M.U.H.; Karim, M.A. Development of a porosity prediction model based on shrinkage velocity and glass transition temperature. Dry. Technol. 2019, 37, 1988–2004. [Google Scholar] [CrossRef] [Green Version]
- Joardder, M.U.H.; Kumar, C.; Karim, M.A. Multiphase transfer model for intermittent microwave-convective drying of food: Considering shrinkage and pore evolution. Int. J. Multiph. Flow 2017, 95, 101–119. [Google Scholar] [CrossRef]
- Rakesh, V.; Datta, A. Microwave puffing: Mathematical modeling and optimization. In Proceedings of the 11th International Congress on Engineering and Food (ICEF), Athens, Greece, 22–26 May 2011; pp. 762–769. [Google Scholar]
- Rakesh, V.; Datta, A.K. Microwave puffing: Determination of optimal conditions using a coupled multiphase porous media-Large deformation model. J. Food Eng. 2011, 107, 152–163. [Google Scholar] [CrossRef]
- Rakesh, V.; Datta, A.K. Transport in Deformable Hygroscopic Porous Media during Microwave Puffing. AIChE J. 2012, 59, 33–45. [Google Scholar] [CrossRef]
- Zhang, J.; Datta, A.K.; Mukherjee, S. Transport Processes and Large Deformation During Baking of Bread. AIChE J. 2005, 51, 2569–2580. [Google Scholar] [CrossRef]
- Niamnuy, C.; Devahastin, S.; Soponronnarit, S.; Raghavan, G.S.V. Modeling coupled transport phenomena and mechanical deformation of shrimp during drying in a jet spouted bed dryer. Chem. Eng. Sci. 2008, 63, 5503–5512. [Google Scholar] [CrossRef]
- Timoumi, S.; Mihoubi, D.; Zagrouba, F. Modelling of Moisture Content, β-Carotene and Deformation Variation during Drying of Carrot. Int. J. Food Eng. 2019, 15, 20180155. [Google Scholar] [CrossRef]
- Curcio, S.; Aversa, M. Influence of shrinkage on convective drying of fresh vegetables: A theoretical model. J. Food Eng. 2014, 123, 36–49. [Google Scholar] [CrossRef]
- Yang, H.; Sakai, N.; Watanabe, M. Drying model with non-isotropic shrinkage deformation undergoing simultaneous heat and mass transfer. Dry. Technol. 2001, 19, 1441–1460. [Google Scholar] [CrossRef]
- Aregawi, W.A.; Defraeye, T.; Verboven, P.; Herremans, E.; Roeck, G.D.; Nicolai, B.M. Modeling of Coupled Water Transport and Large Deformation During Dehydration of Apple Tissue. Food Bioprocess Technol. 2013, 6, 1963–1978. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Llave, Y.; Mao, W.; Fukuoka, M.; Sakai, N. Heat and mass transfer, shrinkage, and thermal protein denaturation of kuruma prawn (Marsupenaeus japonicas) during water bath treatment: A computational study with experimental validation. J. Food Eng. 2018, 238, 30–43. [Google Scholar] [CrossRef]
- Llave, Y.; Takemori, K.; Fukuoka, M.; Takemori, T.; Tomita, H.; Sakai, N. Mathematical modeling of shrinkage deformation in eggplant undergoing simultaneous heat and mass transfer during convection-oven roasting. J. Food Eng. 2016, 178, 124–136. [Google Scholar] [CrossRef]
- Kowalski, S.J.; Smoczkiewicz-Wojciechowska, A. Stresses in dried wood. Modelling and experimental identification. Transp. Porous Media 2006, 66, 145–158. [Google Scholar] [CrossRef]
- Heydari, M.; Khalili, K. Investigation on the Effect of Young’s Modulus Variation on Drying-Induced Stresses. Transp. Porous Media 2016, 112, 519–540. [Google Scholar] [CrossRef]
- Mihoubi, D.; Bellagi, A. Stress Generated During Drying of Saturated Porous Media. Transp. Porous Media 2009, 80, 519–536. [Google Scholar] [CrossRef]
- Kowalski, S.J.; Rybicki, A. Drying stress formation by inhomogeneous moisture and temperature distribution. Transp. Porous Media 1996, 24, 139–156. [Google Scholar] [CrossRef]
- Defraeye, T.; Radu, A. Insights in convective drying of fruit by coupled modeling of fruit drying, deformation, quality evolution and convective exchange with the airflow. Appl. Therm. Eng. 2018, 129, 1026–1038. [Google Scholar] [CrossRef]
- Gulati, T.; Datta, A.K. Mechanistic understanding of case-hardening and texture development during drying of food materials. J. Food Eng. 2015, 166, 119–138. [Google Scholar] [CrossRef]
- Gulati, T.; Zhu, H.; Datta, A.K. Coupled electromagnetics, multiphase transport and large deformation model for microwave drying. Chem. Eng. Sci. 2016, 156, 206–228. [Google Scholar] [CrossRef] [Green Version]
- Aregawi, W.A.; Abera, M.K.; Fanta, S.W.; Verboven, P.; Nicolai, B. Prediction of water loss and viscoelastic deformation of apple tissue using a multiscale model. J. Phys. Condens. Matter 2014, 26, 464111. [Google Scholar] [CrossRef]
- Dhall, A.; Datta, A.K. Transport in deformable food materials: A poromechanics approach. Chem. Eng. Sci. 2011, 66, 6482–6497. [Google Scholar] [CrossRef]
- Benczedi, D.; Tomka, I.; Escher, F. 46. D. Benczedi; I. Tomka; Escher, F. Thermodynamics of amorphous starch-water systems. Macromolecules 1998, 31, 3055–3061. [Google Scholar] [CrossRef]
- Kasapis, S. Glass transition phenomena in dehydrated model systems and foods: A review. Dry. Technol. 2007, 23, 731–757. [Google Scholar] [CrossRef]
- Katekawa, M.E.; Silva, M.A. On the influence of glass transition on shrinkage in convective drying of fruits: A case study of banana drying. Dry. Technol. 2007, 25, 1659–1666. [Google Scholar] [CrossRef]
- Oyinloye, T.M.; Yoon, W.B. Effect of Freeze-Drying on Quality and Grinding Process of Food Produce: A Review. Processes 2020, 8, 354. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wang, M.; Guo, M.; Ye, X.; Ding, T.; Liu, D. Numerical Simulation of Water Absorption and Swelling in Dehulled Barley Grains during Canned Porridge Cooking. Processes 2018, 6, 230. [Google Scholar] [CrossRef] [Green Version]
- Ratti, C.; Crapiste, G.; Rotstein, E. A new water sorption equilibrium expression for solid foods based on thermodynamic considerations. J. Food Sci. 1989, 54, 738–742. [Google Scholar] [CrossRef]
- Gulati, T.; Datta, A.; Doona, C.J.; Ruan, R.R.; Feeherry, F.E. Modeling moisture migration in a multi-domain food system: Application to storage of a sandwich system. Food Res. Int. 2015, 76, 427–438. [Google Scholar] [CrossRef] [PubMed]
- El Abrach, H.; Dhahri, H.; Mhimid, A. Numerical Simulation of Drying of a Saturated Deformable Porous Media by the Lattice Boltzmann Method. Transp. Porous Media 2013, 99, 427–452. [Google Scholar] [CrossRef]
- El Abrach, H.; Dhahri, H.; Mhimid, A. Effects of Anisotropy and Drying Air Parameters on Drying of Deformable Porous Media Hydro-Dynamically and Thermally Anisotropic. Transp. Porous Media 2014, 104, 181–203. [Google Scholar] [CrossRef]
- Song, C.; Wu, T.; Li, Z.; Li, J.; Chen, H. 2-C14. Analysis of the heat transfer characteristics of blackberries during microwave vacuum heating. J. Food Eng. 2018, 223, 70–78. [Google Scholar] [CrossRef]
- Kumar, V.; Devi, M.K.; Panda, B.K.; Shrivastava, S.L. Shrinkage and rehydration characteristics of vacuum assisted microwave dried green bell pepper. J. Food Process. Eng. 2019, 42, 1–9. [Google Scholar] [CrossRef]
- Yang, H.W.; Gunasekaran, S. Comparison of temperature distribution in model food cylinders based on Maxwell’s equations and Lambert’s law during pulsed microwave heating. J. Food Eng. 2004, 64, 445–453. [Google Scholar] [CrossRef]
- Kumar, C.; Karim, M.A. Microwave-convective drying of food materials: A critical review. Crit. Rev. Food Sci. Nutr. 2019, 59, 379–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nissan, A.; Berkowitz, B. Anomalous transport dependence on Péclet number, porous medium heterogeneity, and a temporally varying velocity field. Phys. Rev. E 2019, 99, 033108. [Google Scholar] [CrossRef]
- Cherepanov, G.P. Two-dimensional Convective Heat/Mass Transfer for Low Prandtl and Any Peclet Numbers. SIAM J. Appl. Math. 1998, 58, 942–960. [Google Scholar] [CrossRef] [Green Version]
- Onwude, D.I.; Hashim, N.; Abdan, K.; Janius, R.; Chen, G. The effectiveness of combined infrared and hot-air drying strategies for sweet potato. J. Food Eng. 2019, 241, 75–87. [Google Scholar] [CrossRef]
- Soysal, Y.; Ayhan, Z.; Eştürk, O.; Arıkan, M.F. Intermittent microwave–convective drying of red pepper: Drying kinetics, physical (colour and texture) and sensory quality. Biosyst. Eng. 2009, 103, 455–463. [Google Scholar] [CrossRef]
- Pham, N.D.; Khan, M.I.H.; Karim, M.A. A mathematical model for predicting the transport process and quality changes during intermittent microwave convective drying. Food Chem. 2020, 325, 126932. [Google Scholar] [CrossRef]




| Parameters, Unit | Value | Reference | Value | Parameters | Reference |
|---|---|---|---|---|---|
| [43] | |||||
| [43] | |||||
| [43] | |||||
| [43] | |||||
| [43] | |||||
| [43] | |||||
| [43] | |||||
| [43] | |||||
| [43] | [43] | ||||
| [43] | |||||
| [43] | |||||
| [43] | [43] | ||||
| [43] |
| Set 1 | Set 2 | Set 3 | Set 4 | Set 5 | Set 6 | Set 7 | |
|---|---|---|---|---|---|---|---|
| Cycle length | 10 s/cycle | 20 s/cycle | 40 s/cycle | 60 s/cycle | 80 s/cycle | 100 s/cycle | 120 s/cycle |
| Heating, s | 2 | 4 | 8 | 12 | 16 | 20 | 24 |
| Tempering, s | 8 | 16 | 32 | 48 | 64 | 80 | 96 |
| PR | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Cycles | 120 | 60 | 30 | 20 | 15 | 12 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, T.; Zhang, W.; Zhang, Z.; Wang, X.; Zhang, S. Numerical Investigation of the Deformable Porous Media Treated by the Intermittent Microwave. Processes 2021, 9, 757. https://doi.org/10.3390/pr9050757
Su T, Zhang W, Zhang Z, Wang X, Zhang S. Numerical Investigation of the Deformable Porous Media Treated by the Intermittent Microwave. Processes. 2021; 9(5):757. https://doi.org/10.3390/pr9050757
Chicago/Turabian StyleSu, Tianyi, Wenqing Zhang, Zhijun Zhang, Xiaowei Wang, and Shiwei Zhang. 2021. "Numerical Investigation of the Deformable Porous Media Treated by the Intermittent Microwave" Processes 9, no. 5: 757. https://doi.org/10.3390/pr9050757






