Porous Anion Exchange Membrane for Effective Acid Recovery by Diffusion Dialysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of PT-PES Porous AEMs
2.3. Characterization
2.3.1. Membrane Morphology, Composition and Thermal Stability
2.3.2. Ion Exchange Capacity (IEC)
2.3.3. Water Uptake (WU)
2.3.4. Diffusion Dialysis (DD)
3. Results and Discussions
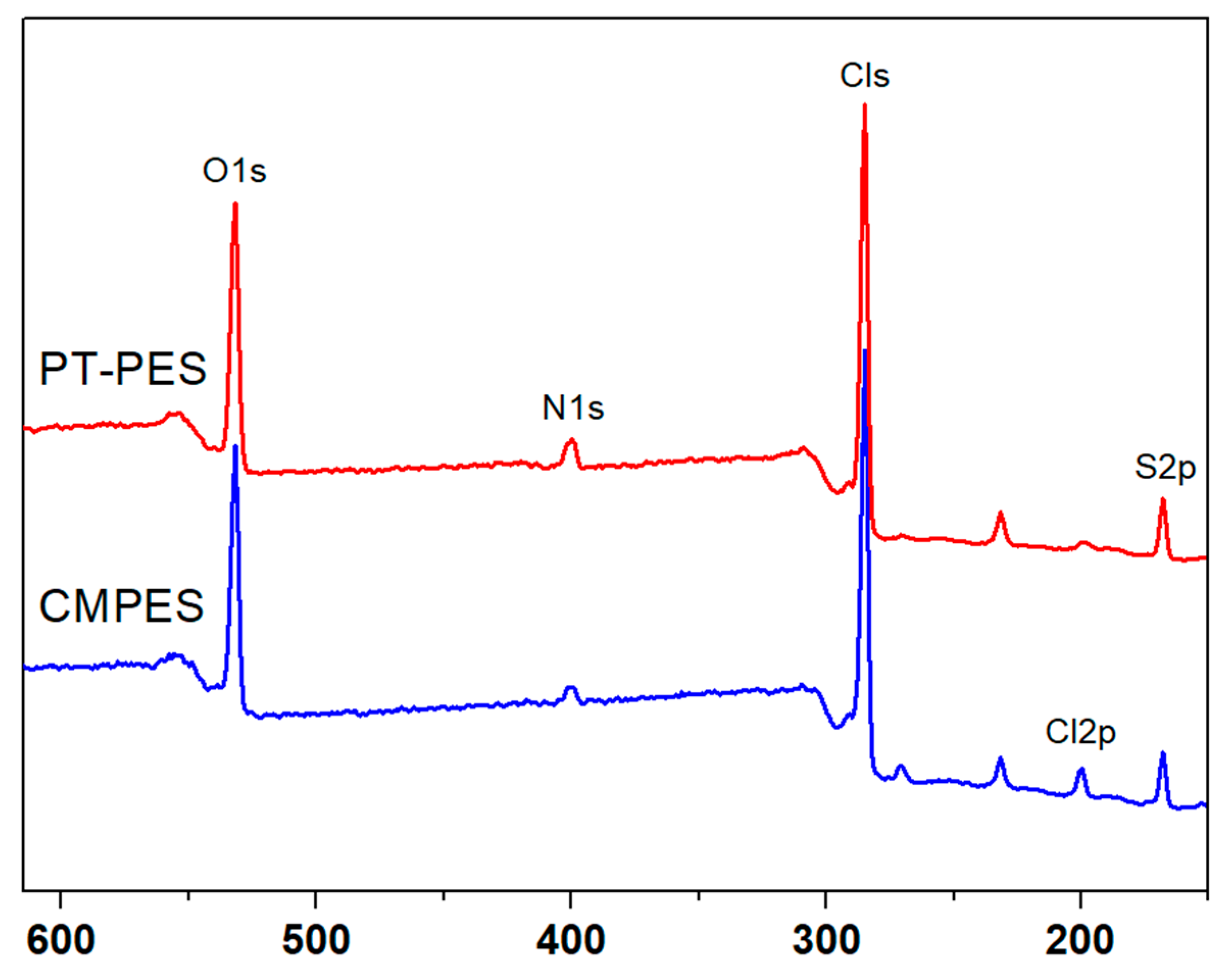
3.1. XPS
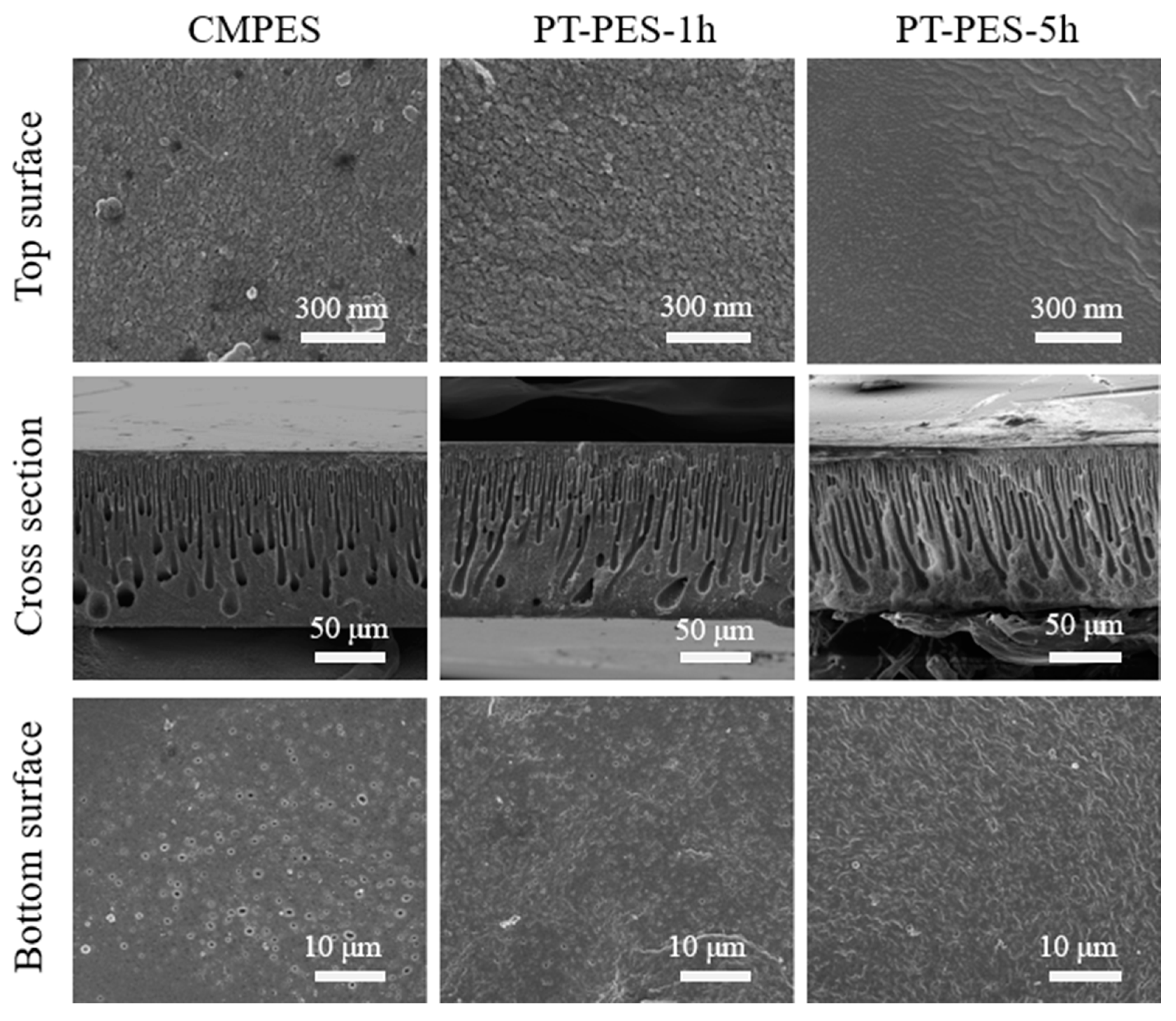
3.2. SEM
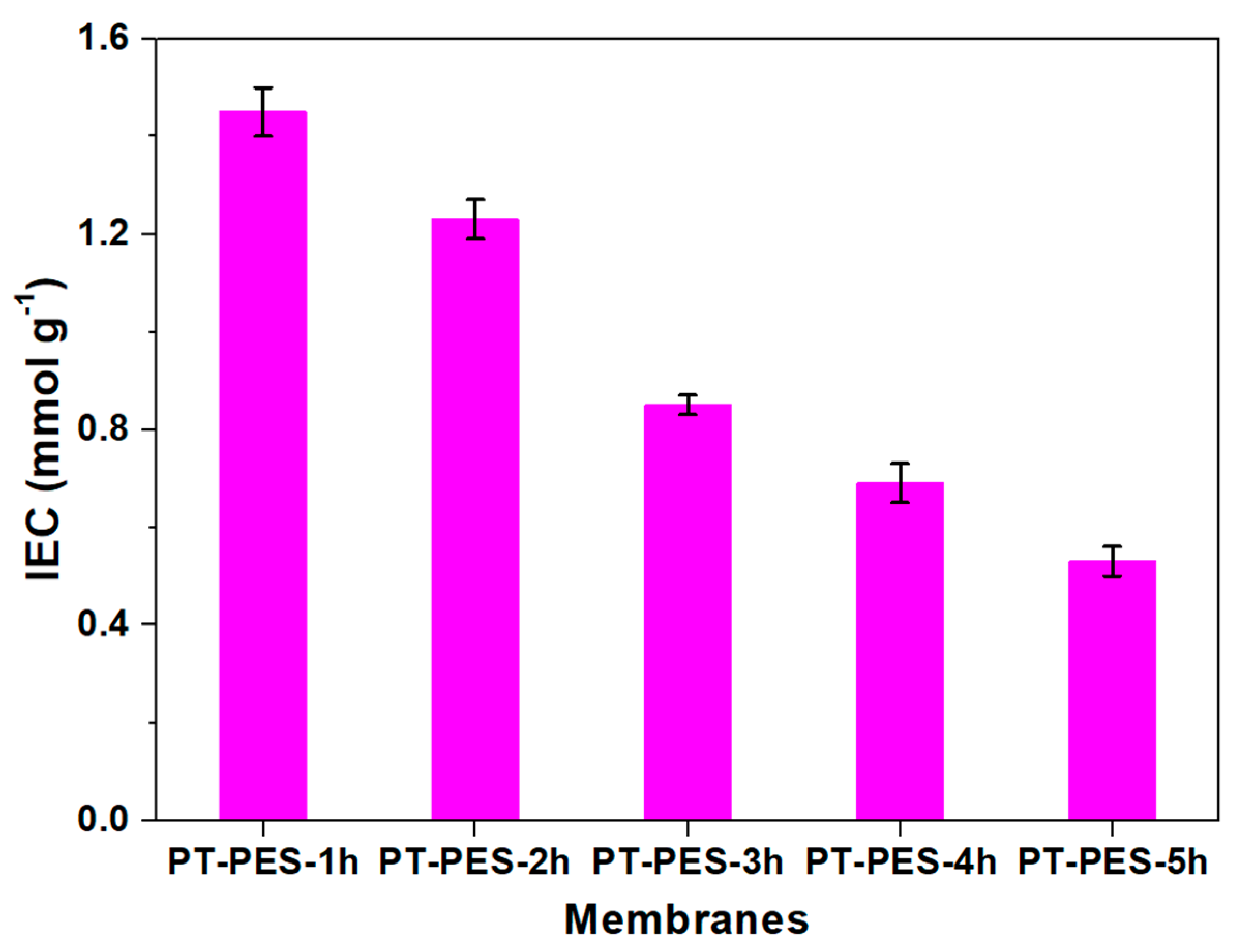
3.3. Ion Exchange Capacity (IEC)
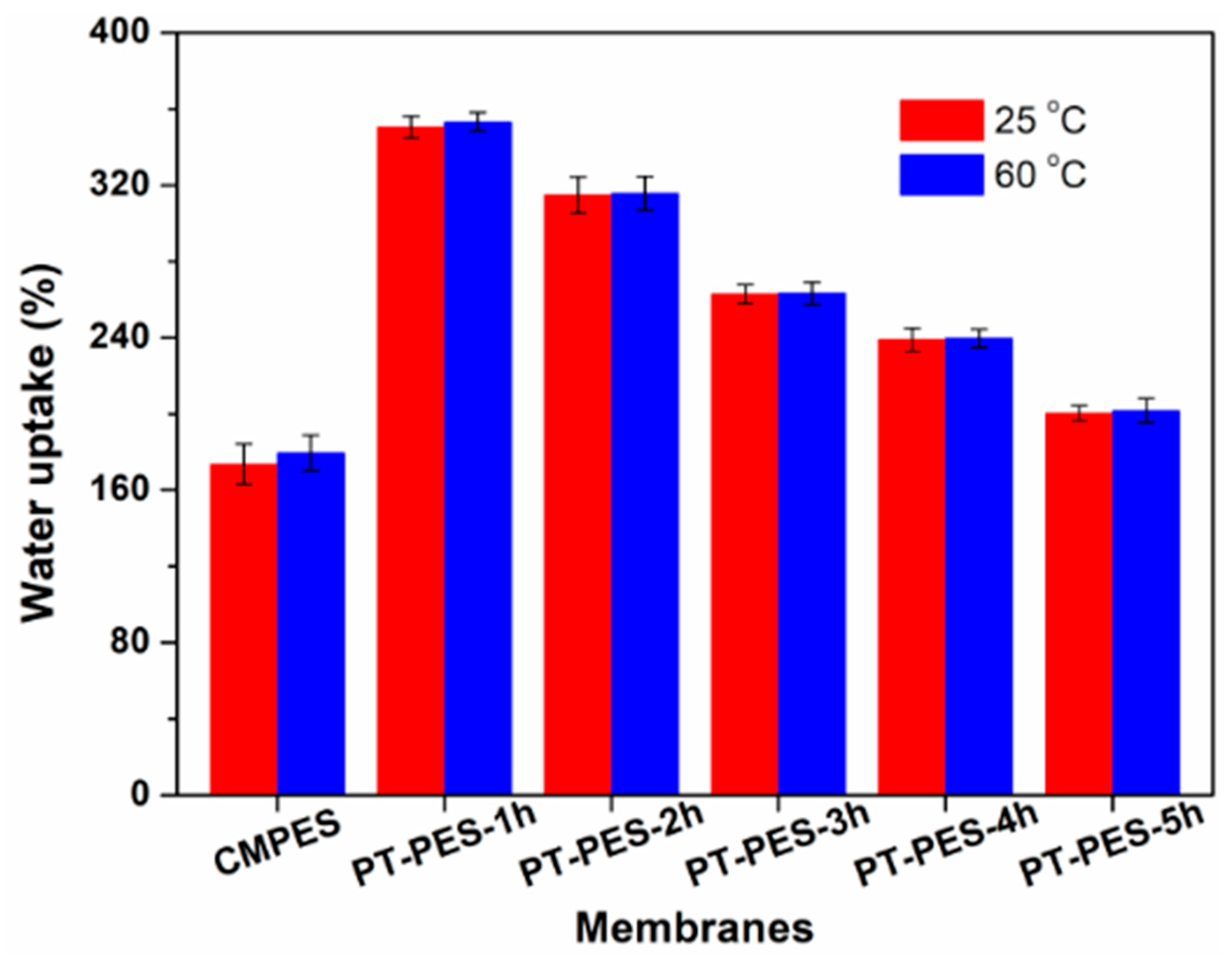
3.4. Water Uptake (WU)
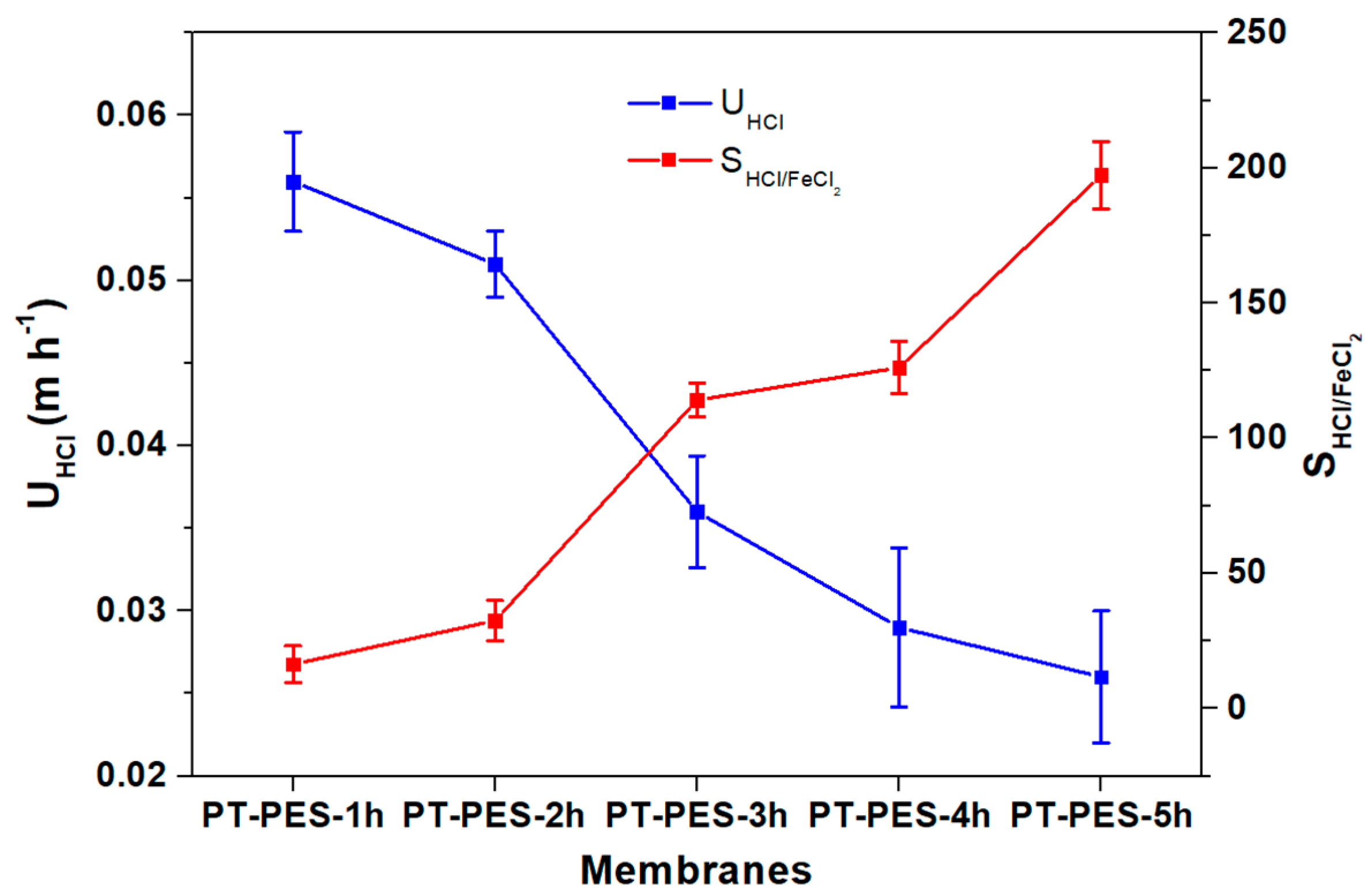
3.5. Diffusion Dialysis (DD) Performance
3.6. TGA
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rocchetti, L.; Vegliò, F.; Kopacek, B.; Beolchini, F. Environmental Impact Assessment of Hydrometallurgical Processes for Metal Recovery from WEEE Residues Using a Portable Prototype Plant. Environ. Sci. Technol. 2013, 47, 1581–1588. [Google Scholar] [CrossRef]
- Sahu, S.K.; Sahu, K.K.; Pandey, B.D. Leaching of zinc sulfide concentrate from the ganesh-himal deposit of nepal. Met. Mater. Trans. A 2006, 37, 541–549. [Google Scholar] [CrossRef]
- Kumari, A.; Sinha, M.K.; Sahu, S.K.; Pandey, B.D. Investigation of a novel ionic liquid, Cyphos IL 104 for the solvent ex-traction of mineral acids. Hydrometallurgy 2016, 165, 159–165. [Google Scholar] [CrossRef]
- Dean, J.G.; Bosqui, F.L.; Lanouette, K.H. Removing heavy metals from waste water. Environ. Sci. Technol. 1972, 6, 518–522. [Google Scholar] [CrossRef]
- Bramer, H.C. Pollution control in the steel industry. Environ. Sci. Technol. 1971, 5, 1004–1008. [Google Scholar] [CrossRef]
- Luo, J.; Wu, C.; Xu, T.; Wu, Y. Diffusion dialysis-concept, principle and applications. J. Membr. Sci. 2011, 366, 1–16. [Google Scholar] [CrossRef]
- Regel-Rosocka, M. A review on methods of regeneration of spent pickling solutions from steel processing. J. Hazard. Mater. 2010, 177, 57–69. [Google Scholar] [CrossRef]
- Oh, S.J.; Moon, S.-H.; Davis, T. Effects of metal ions on diffusion dialysis of inorganic acids. J. Membr. Sci. 2000, 169, 95–105. [Google Scholar] [CrossRef]
- Luo, J.; Wu, C.; Wu, Y.; Xu, T. Diffusion dialysis of hydrochloride acid at different temperatures using PPO–SiO2 hybrid anion exchange membranes. J. Membr. Sci. 2010, 347, 240–249. [Google Scholar] [CrossRef]
- Cheng, C.; Yang, Z.; Pan, J.; Tong, B.; Xu, T. Facile and cost effective PVA based hybrid membrane fabrication for acid recovery. Sep. Purif. Technol. 2014, 136, 250–257. [Google Scholar] [CrossRef]
- Wu, C.; Wu, Y.; Luo, J.; Xu, T.; Fu, Y. Anion exchange hybrid membranes from PVA and multi-alkoxy silicon copolymer tailored for diffusion dialysis process. J. Membr. Sci. 2010, 356, 96–104. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, C.; Li, Y.; Xu, T.; Fu, Y. PVA–silica anion-exchange hybrid membranes prepared through a copolymer cross-linking agent. J. Membr. Sci. 2010, 350, 322–332. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, J.; Yao, L.; Wu, C.; Mao, F.; Xu, T. PVA/SiO2 anion exchange hybrid membranes from multisilicon copolymers with two types of molecular weights. J. Membr. Sci. 2012, 399–400, 16–27. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, J.; Wu, C.; Xu, T.; Fu, Y. Bionic Multisilicon Copolymers Used as Novel Cross-Linking Agents for Preparing Anion Exchange Hybrid Membranes. J. Phys. Chem. B 2011, 115, 6474–6483. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, J.; Zhao, L.; Zhang, G.; Wu, C.; Xu, T. QPPO/PVA anion exchange hybrid membranes from double crosslinking agents for acid recovery. J. Membr. Sci. 2013, 428, 95–103. [Google Scholar] [CrossRef]
- Mondal, A.N.; Cheng, C.; Yao, Z.; Pan, J.; Hossain, M.; Khan, M.I.; Yang, Z.; Wu, L.; Xu, T. Novel quaternized aromatic amine based hybrid PVA membranes for acid recovery. J. Membr. Sci. 2015, 490, 29–37. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, M.; Cao, J.; Xu, T.; Mao, F. Combination of OH– ions and –OH groups within QPPO/PVA hybrid membranes for acid recovery. Desalin. Water Treat. 2015, 2015, 1–11. [Google Scholar] [CrossRef]
- Cheng, C.; Yang, Z.; He, Y.; Mondal, A.N.; Bakangura, E.; Xu, T. Diffusion dialysis membranes with semi-interpenetrating network for acid recovery. J. Membr. Sci. 2015, 493, 645–653. [Google Scholar] [CrossRef]
- Emmanuel, K.; Cheng, C.; Erigene, B.; Mondal, A.N.; Hossain, M.; Khan, M.I.; Afsar, N.U.; Liang, G.; Wu, L.; Xu, T. Imidazolium functionalized anion exchange membrane blended with PVA for acid recovery via diffusion dialysis process. J. Membr. Sci. 2016, 497, 209–215. [Google Scholar] [CrossRef]
- Emmanuel, K.; Erigene, B.; Cheng, C.; Mondal, A.N.; Hossain, M.; Khan, M.I.; Afsar, N.U.; Ge, L.; Wu, L.; Xu, T. Facile synthesis of pyridinium functionalized anion exchange membranes for diffusion dialysis application. Sep. Purif. Technol. 2016, 167, 108–116. [Google Scholar] [CrossRef]
- Irfan, M.; Bakangura, E.; Afsar, N.U.; Xu, T. Augmenting acid recovery from different systems by novel Q-DAN anion exchange membranes via diffusion dialysis. Sep. Purif. Technol. 2018, 201, 336–345. [Google Scholar] [CrossRef]
- George, S.C.; Thomas, S. Transport phenomena through polymeric systems. Prog. Polym. Sci. 2001, 26, 985–1017. [Google Scholar] [CrossRef]
- Koros, W.; Fleming, G.; Jordan, S.; Kim, T.; Hoehn, H. Polymeric membrane materials for solution-diffusion based permeation separations. Prog. Polym. Sci. 1988, 13, 339–401. [Google Scholar] [CrossRef]
- Lin, X.; Shamsaei, E.; Kong, B.; Liu, J.Z.; Zhao, D.; Xu, T.; Xie, Z.; Easton, C.D.; Wang, H. Asymmetrically porous anion exchange membranes with an ultrathin selective layer for rapid acid recovery. J. Membr. Sci. 2016, 510, 437–446. [Google Scholar] [CrossRef]
- Lin, X.; Shamsaei, E.; Kong, B.; Liu, J.Z.; Hu, Y.; Xu, T.; Wang, H. Porous diffusion dialysis membranes for rapid acid recovery. J. Membr. Sci. 2016, 502, 76–83. [Google Scholar] [CrossRef]
- Lin, X.; Shamsaei, E.; Kong, B.; Liu, J.Z.; Xu, T.; Wang, H. Fabrication of asymmetrical diffusion dialysis membranes for rapid acid recovery with high purity. J. Mater. Chem. A 2015, 3, 24000–24007. [Google Scholar] [CrossRef]
- Lin, X.; Wang, K.; Feng, Y.; Liu, J.Z.; Fang, X.; Xu, T.; Wang, H. Composite ultrafiltration membranes from polymer and its quaternary phosphoni-um-functionalized derivative with enhanced water flux. J. Membr. Sci. 2015, 482, 67–75. [Google Scholar] [CrossRef]
- Xu, T.; Yang, W. Sulfuric acid recovery from titanium white (pigment) waste liquor using diffusion dialysis with a new series of anion exchange membranes—Static runs. J. Membr. Sci. 2001, 183, 193–200. [Google Scholar]
- Brizzolara, R.A.; Stamper, D.M. The effect of covalent surface immobilization on the bactericidal efficacy of a quaternary ammonium compound. Surf. Interface Anal. 2007, 39, 559–566. [Google Scholar] [CrossRef]
- Xie, W.; Gao, Z.; Pan, W.-P.; Hunter, D.; Singh, A.; Vaia, R. Thermal Degradation Chemistry of Alkyl Quaternary Ammonium Montmorillonite. Chem. Mater. 2001, 13, 2979–2990. [Google Scholar] [CrossRef]



| Membrane | Compactness | (×10−3 m h−1) | S | Ref. |
|---|---|---|---|---|
| PT-PES-2h | Porous | 51.0 | 32.5 | This work |
| DF-120 commercial membrane | Dense | 8.5 | 18.5 | [9] |
| Quaternized PPO-based hybrid membranes | Dense | 5.0–11.0 | 17.0–32.0 | [9] |
| PVA and glycidyl trimethyl ammonium chloride (EPTAC) blending membranes | Dense | 11.0–18.0 | 18.5–21.0 | [10] |
| PVA and multi-alkoxy silicon copolymer blending membranes | Dense | 10.0–17.0 | 24.0–30.1 | [11] |
| PVA treated with alkoxysilanes membranes | Dense | 8.0–10.0 | 15.9–21.0 | [12] |
| Quaternized poly (VBC-co-γ-MPS) membranes | Dense | 24.0–43.0 | 22.0–26.0 | [13] |
| Quaternized bionic multisilicon copolymers | Dense | 7.2–7.5 | 25.9–42.8 | [14] |
| Quaternized blending of PPO and PVA membranes | Dense | 21.0–49.0 | 26.0–39.0 | [15] |
| Quaternized aromatic amine-based hybrid PVA membranes | Dense | 17.2–25.2 | 14.0–21.0 | [16] |
| Quaternized PPO blending with PVA and silanol | Dense | 9.5–14.5 | 45.0–67.5 | [17] |
| Quaternized blending of PVC and P (DMAM-co-DVB) | Dense | 12.0–40.0 | 36–61 | [18] |
| Imidazolium functionalized hybrid membrane blending with PVA | Dense | 18.7–48.3 | 12.72–52.5 | [19] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Dai, G.; Wang, J.; Pan, S.; Lu, G.; Shi, X.; Tang, D.; Chen, J.; Lin, X. Porous Anion Exchange Membrane for Effective Acid Recovery by Diffusion Dialysis. Processes 2021, 9, 1049. https://doi.org/10.3390/pr9061049
Yang J, Dai G, Wang J, Pan S, Lu G, Shi X, Tang D, Chen J, Lin X. Porous Anion Exchange Membrane for Effective Acid Recovery by Diffusion Dialysis. Processes. 2021; 9(6):1049. https://doi.org/10.3390/pr9061049
Chicago/Turabian StyleYang, Jinbei, Guangkai Dai, Jing Wang, Shuai Pan, Gang Lu, Xiaoke Shi, Danni Tang, Jinyi Chen, and Xiaocheng Lin. 2021. "Porous Anion Exchange Membrane for Effective Acid Recovery by Diffusion Dialysis" Processes 9, no. 6: 1049. https://doi.org/10.3390/pr9061049
APA StyleYang, J., Dai, G., Wang, J., Pan, S., Lu, G., Shi, X., Tang, D., Chen, J., & Lin, X. (2021). Porous Anion Exchange Membrane for Effective Acid Recovery by Diffusion Dialysis. Processes, 9(6), 1049. https://doi.org/10.3390/pr9061049







