Oxidative Extractive Desulfurization System for Fuel Oil Using Acidic Eutectic-Based Ionic Liquid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Acidic EIL
2.3. Characterization of EILs
2.4. Oxidative Extraction Desulfurization Method
3. Results and Discussion
3.1. Characterization of EILs
3.1.1. Thermogravimetry Analysis
3.1.2. Differential Scanning Calorimetry Analysis
3.2. Removal Efficiency of Sulfur Compounds
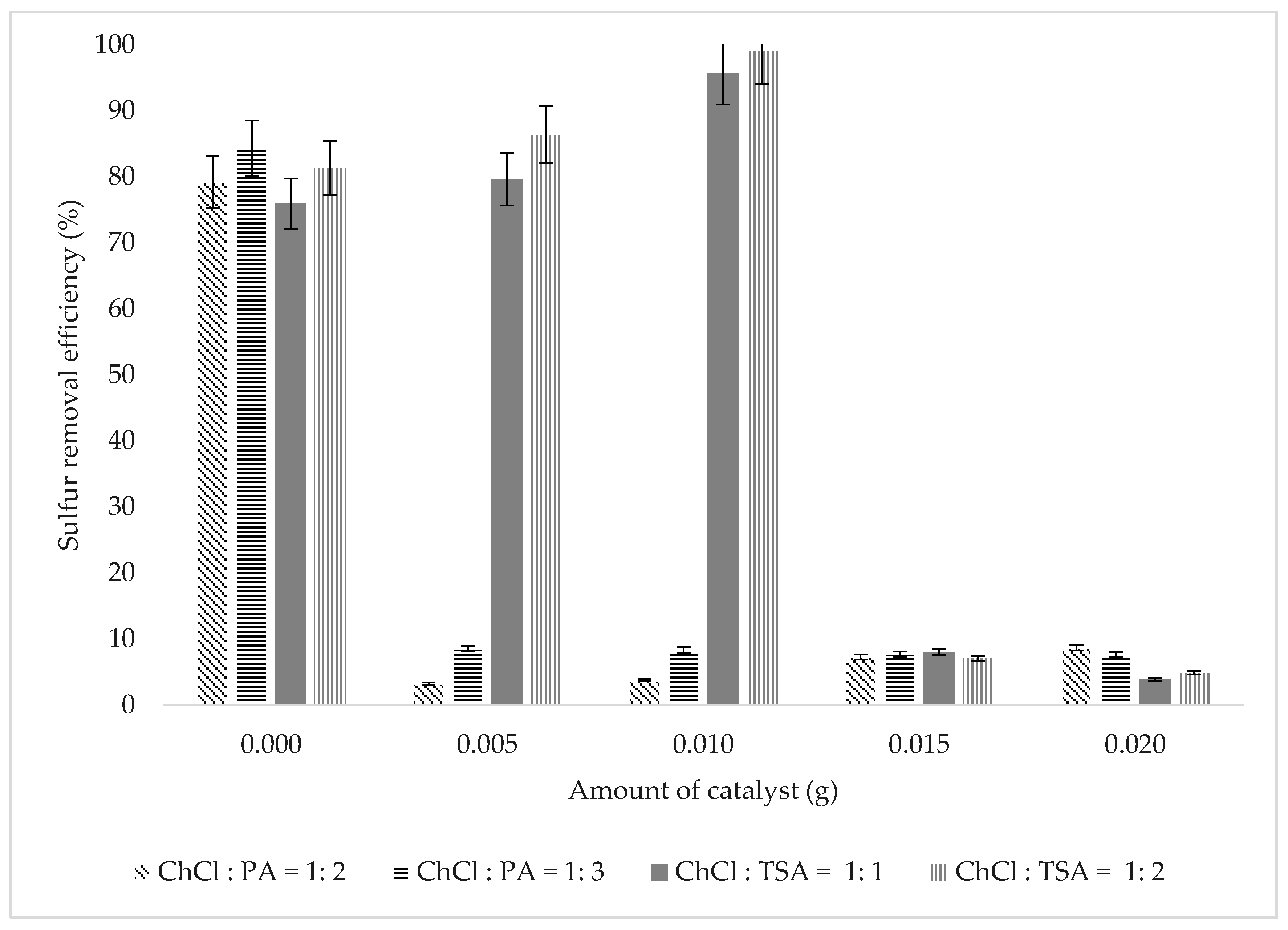
3.2.1. Effect of Catalyst Dosage
3.2.2. Effect of H2O2:DBT Ratio
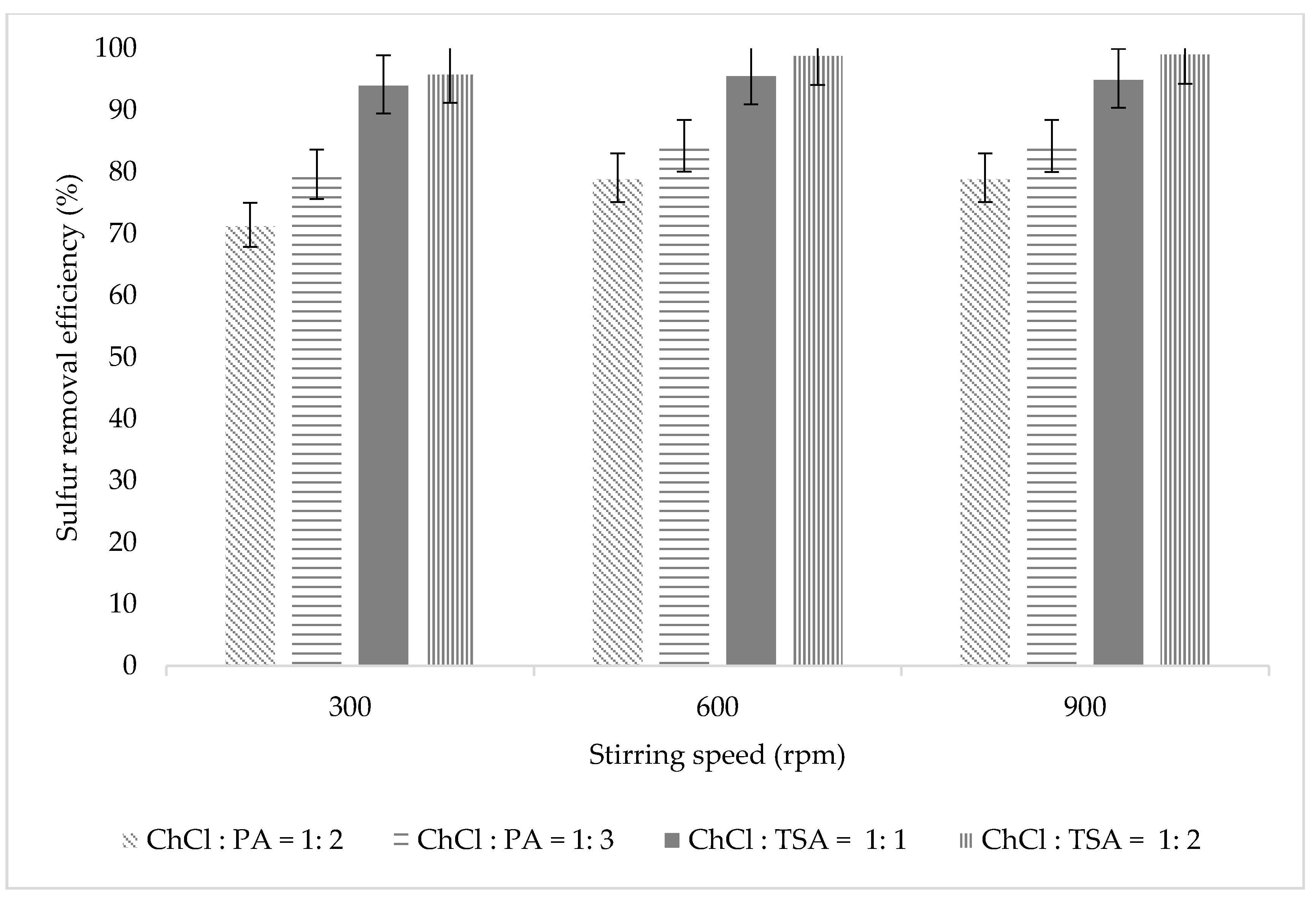
3.2.3. Effect of Stirring Speed
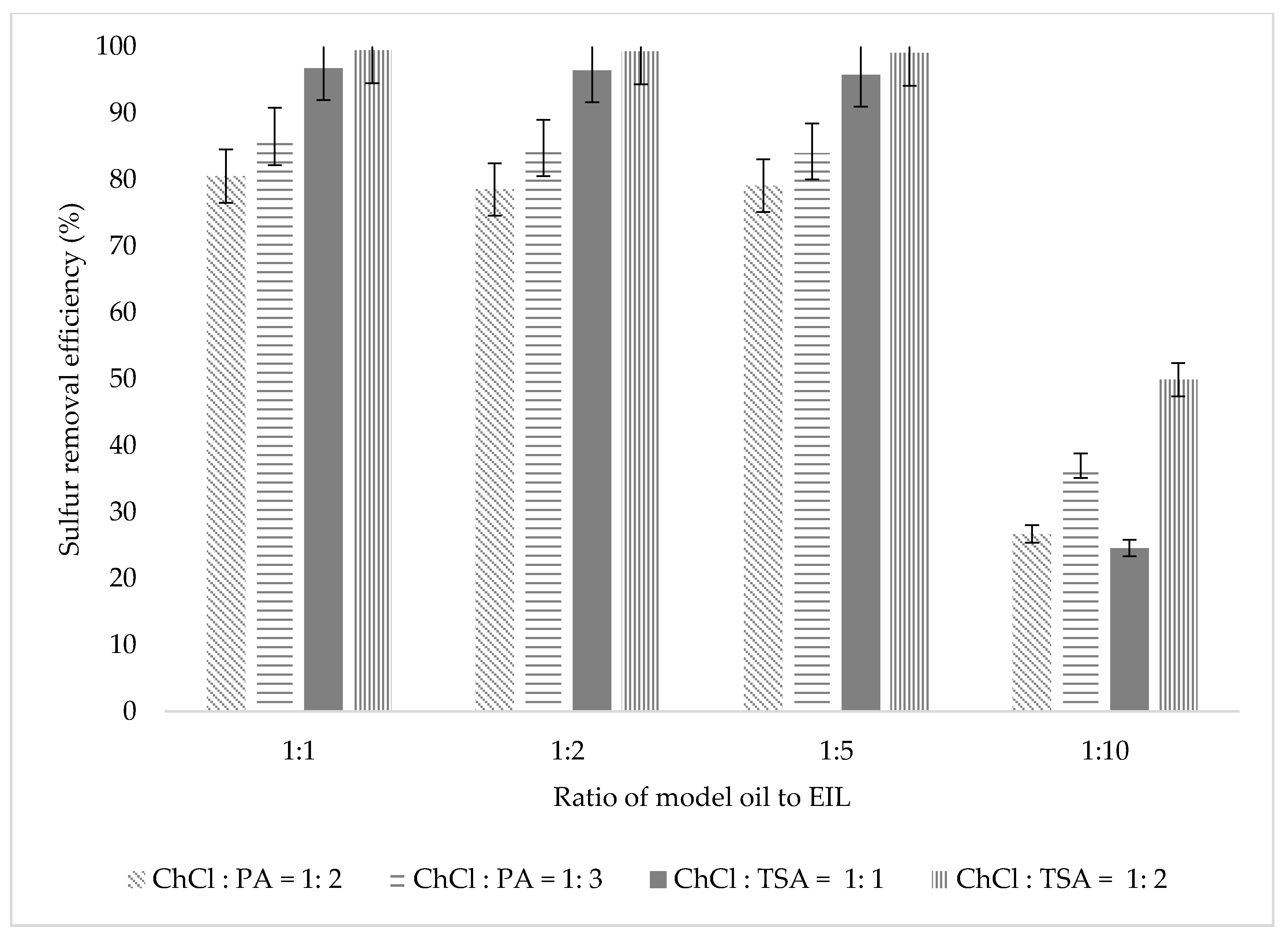
3.2.4. Effect of Oil to EIL Ratio
3.2.5. Effect of Types of EIL
3.2.6. Effect of Concentration of Sulfur Species
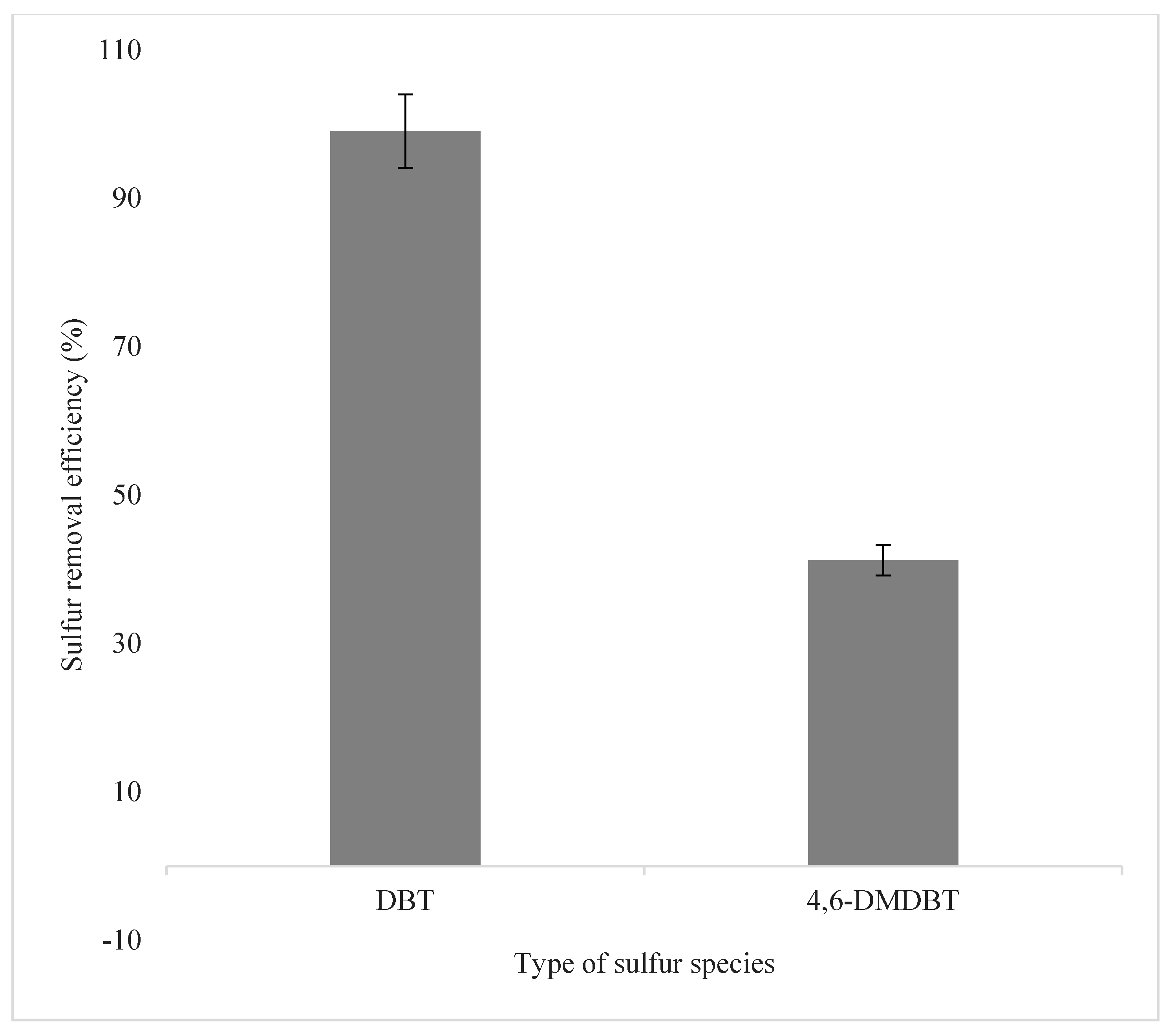
3.2.7. Effect of Different Sulfur Species
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhutto, A.W.; Abro, R.; Gao, S.; Abbas, T.; Chen, X.; Yu, G. Oxidative desulfurization of fuel oils using ionic liquids: A review. J. Taiwan Inst. Chem. Eng. 2016, 62, 84–97. [Google Scholar] [CrossRef]
- Srivastava, V.C. An evaluation of desulfurization technologies for sulfur removal from liquid fuels. RSC Adv. 2012, 2, 759–783. [Google Scholar] [CrossRef]
- Chandran, D.; Khalid, M.; Walvekar, R.; Mubarak, N.M.; Dharaskar, S.; Wong, W.Y.; Gupta, T. Deep eutectic solvents for extraction-desulphurization: A review. J. Mol. Liq. 2019, 275, 312–322. [Google Scholar] [CrossRef]
- Ibrahim, R.K.; Hayyan, M.; AlSaadi, M.A.; Ibrahim, S.; Hayyan, A.; Hashim, M.A. Physical properties of ethylene glycol-based deep eutectic solvents. J. Mol. Liq. 2019, 276, 794–800. [Google Scholar] [CrossRef]
- Alkhalili, B.E.; Yahya, A.; Abrahim, N.; Ganapathy, B. Biodesulfurization of sour crude oil. Mod. Appl. Sci. 2017, 11, 104. [Google Scholar] [CrossRef]
- Majid, M.F.; Mohd Zaid, H.F.; Kait, C.F.; Jumbri, K.; Lim, J.W.; Masri, A.N.; Mat Ghani, S.M.; Yamagishi, H.; Yamamoto, Y.; Yuliarto, B. Liquid Polymer Eutectic Mixture for Integrated Extractive-Oxidative Desulfurization of Fuel Oil: An Optimization Study via Response Surface Methodology. Processes 2020, 8, 848. [Google Scholar] [CrossRef]
- Gano, Z.S.; Mjalli, F.S.; Al-Wahaibi, T.; Al-Wahaibi, Y.; Alnashef, I.M. Chemical Engineering and Processing: Process Intensification Extractive desulfurization of liquid fuel with FeCl3 -based deep eutectic solvents: Experimental design and optimization by central-composite design. Chem. Eng. Process. Process Intensif. 2015, 93, 10–20. [Google Scholar] [CrossRef]
- Dancila, A.M.; Caprarescu, S.; Bobirica, C.; Purcar, V.; Garleanu, G.; Vasile, E.; Modrogan, C.; Borda, C.; Dobrota, D. Optimization of the Technological Parameters for Obtaining Zn-Ti Based Composites to Increase the Performance of H2S Removal from Syngas. Processes 2020, 8, 562. [Google Scholar] [CrossRef]
- Mohd Zaid, H.F.; Chong, F.K.; Abdul Mutalib, M.I. Photooxidative-extractive deep desulfurization of diesel using Cu-Fe/TiO2 and eutectic ionic liquid. Fuel 2015, 156, 54–62. [Google Scholar] [CrossRef]
- Almashjary, K.H.; Khalid, M.; Dharaskar, S.; Jagadish, P.; Walvekar, R.; Gupta, T.C.S.M. Optimisation of extractive desulfurization using Choline Chloride-based deep eutectic solvents. Fuel 2018, 234, 1388–1400. [Google Scholar] [CrossRef]
- EU: Fuels: Diesel and Gasoline. Int. Counc. Clean Transp. Diesel Emiss. 2019. Available online: www.transportpolicy.net/standard/eu-fuels-diesel-and-gasoline/ (accessed on 10 May 2021).
- Zhao, D.; Wang, J.; Zhou, E. Oxidative desulfurization of diesel fuel using a Brønsted acid room temperature ionic liquid in the presence of H2O2. Green Chem. 2007, 9, 1219–1222. [Google Scholar] [CrossRef]
- Maugeri, Z.; Dominguez De Maria, P. Novel choline-chloride-based deep-eutectic-solvents with renewable hydrogen bond donors: Levulinic acid and sugar-based polyols. RSC Adv. 2012, 2, 421–425. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [Green Version]
- Larriba, M.; Ayuso, M.; Navarro, P.; Mellado, N.D.; Miquel, G.M.; Garcia, J.; Rodrigues, F. Choline chloride-based deep eutectic solvents in the dearomatization of gasolines. ACS Sustain. Chem. Eng. 2018, 6, 1039–1047. [Google Scholar] [CrossRef]
- Mellado, D.N.; Larriba, M.; Navarro, P.; Rigual, V.; Ayuso, M.; Garcia, J.; Rodriguez, F. Thermal stability of choline chloride Deep eutectic solvents by TGA/FTIR-ATR analysis. J. Mol. Liq. 2018, 260, 37–43. [Google Scholar] [CrossRef]
- Gao, H.; Guo, C.; Xing, J.; Zhao, J.; Liu, H. Extraction and oxidative desulfurization of diesel fuel catalyzed by a Brønsted acidic ionic liquid at room temperature. Green Chem. 2010, 12, 1220–1224. [Google Scholar] [CrossRef]
- Mohd Zaid, H.F.; Chong, F.K.; Abdul Mutalib, M.I. Extractive deep desulfurization of diesel using choline chloride-glycerol eutectic-based ionic liquid as a green solvent. Fuel 2017, 192, 10–17. [Google Scholar] [CrossRef]
- Yin, J.; Wang, J.; Li, Z.; Li, D.; Yang, G.; Cui, Y.; Wang, A.; Li, C. Deep desulfurization of fuels based on an oxidation/extraction process with acidic deep eutectic solvents. Green Chem. 2015, 17, 4552–4559. [Google Scholar] [CrossRef]
- Li, Z.; Cui, Y.; Li, C.; Shen, Y. Deep desulfurization of fuels based on deep eutectic theory. Sep. Purif. Technol. 2019, 219, 9–15. [Google Scholar] [CrossRef]
- Liu, H.; Bao, S.; Cai, Z.; Xu, T.; Li, N.; Wang, L.; Chen, H.; Lu, W.; Chen, W. A novel method for ultra-deep desulfurization of liquid fuels at room temperature. Chem. Eng. J. 2017, 317, 1092–1098. [Google Scholar] [CrossRef]
- Jiang, W.; Zhu, W.; Chang, Y.; Chao, Y.; Yin, S.; Liu, H.; Zhu, F.; Li, H. Ionic liquid extraction and catalytic oxidative desulfurization of fuels using dialkylpiperidinium tetrachloroferrates catalysts. Chem. Eng. J. 2014, 250, 48–54. [Google Scholar] [CrossRef]
- Zheng, D.; Zhu, W.; Xun, S.; Zhou, M.; Zhang, M.; Jiang, W.; Qin, Y.; Li, H. Deep oxidative desulfurization of dibenzothiophene using low-temperature-mediated titanium dioxide catalyst in ionic liquids. Fuel 2015, 159, 446–453. [Google Scholar] [CrossRef]
- Hao, L.; Sun, L.; Su, T.; Hao, D.; Liao, W.; Deng, C.; Ren, W.; Zhang, Y.; Lu, H. Polyoxometalate-based ionic liquid catalyst with unprecedented activity and selectivity for oxidative desulfurization of diesel in [Omim] BF4. Chem. Eng. J. 2019, 358, 419–426. [Google Scholar] [CrossRef]
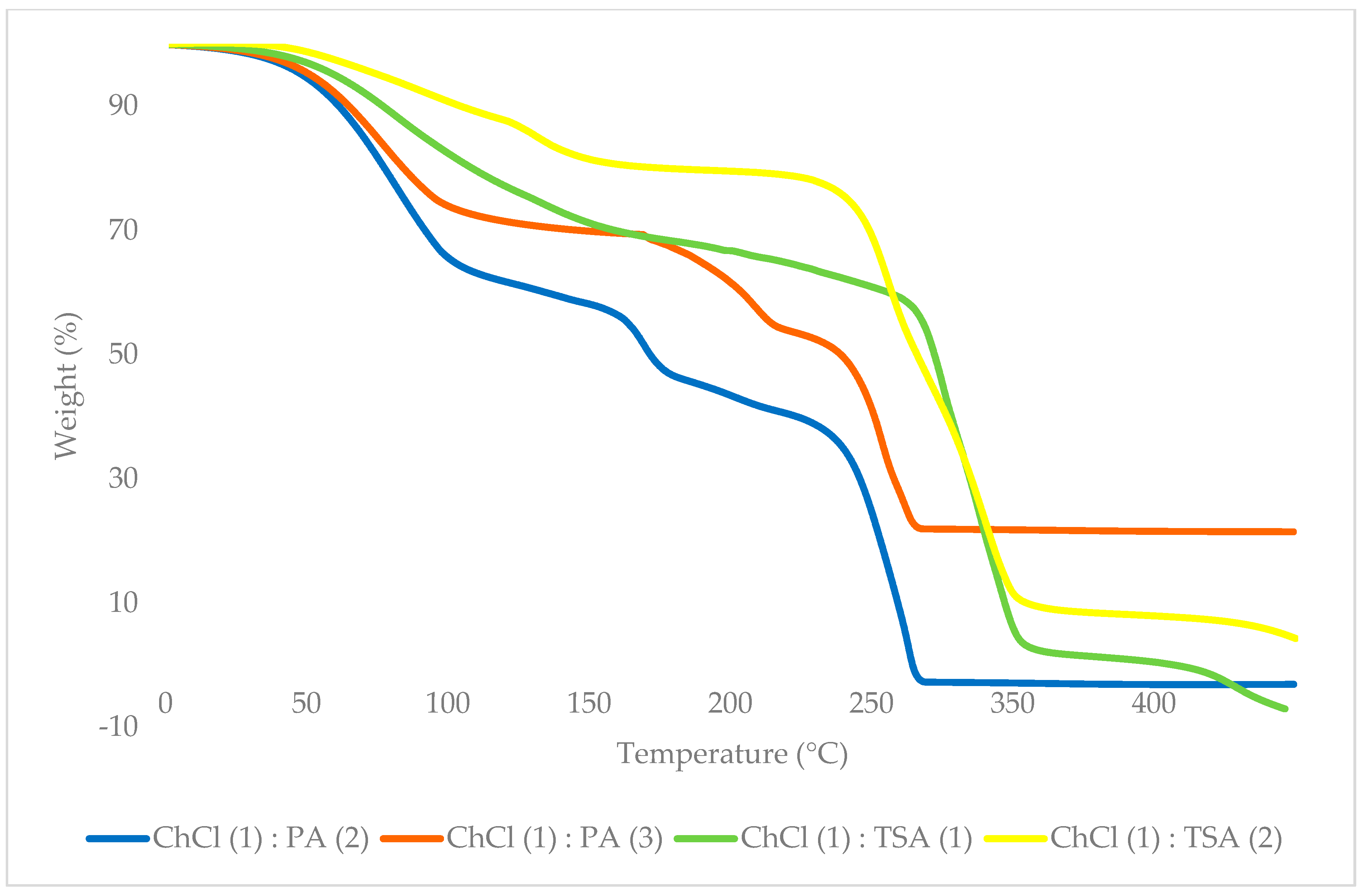



| EIL | ChCl: HBD (Molar Ratio) | Onset Temperature/Tonset (°C) | Weight Loss at 250 °C (%) | Complete Decomposition Temperature (°C) |
|---|---|---|---|---|
| ChCl: PA | 1:2 | 28.3 | 58.9 | 312.5 |
| ChCl: PA | 1:3 | 28.0 | 45.3 | - |
| ChCl: TSA | 1:1 | 25.2 | 34.9 | 444.1 |
| ChCl: TSA | 1:2 | 33.9 | 21.1 | - |
| Component | ChCl: HBD (Molar Ratio) | Melting Point (°C) |
|---|---|---|
| ChCl | Pure | 302 |
| PA | Pure | −21 |
| TSA | Pure | 38 |
| ChCl: PA | 1:2 | −23 |
| ChCl: PA | 1:3 | −24 |
| ChCl: TSA | 1:1 | 24 |
| ChCl: TSA | 1:2 | 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajasuriyan, S.; Mohd Zaid, H.F.; Majid, M.F.; Ramli, R.M.; Jumbri, K.; Lim, J.W.; Mohamad, M.; Show, P.L.; Yuliarto, B. Oxidative Extractive Desulfurization System for Fuel Oil Using Acidic Eutectic-Based Ionic Liquid. Processes 2021, 9, 1050. https://doi.org/10.3390/pr9061050
Rajasuriyan S, Mohd Zaid HF, Majid MF, Ramli RM, Jumbri K, Lim JW, Mohamad M, Show PL, Yuliarto B. Oxidative Extractive Desulfurization System for Fuel Oil Using Acidic Eutectic-Based Ionic Liquid. Processes. 2021; 9(6):1050. https://doi.org/10.3390/pr9061050
Chicago/Turabian StyleRajasuriyan, Sarrthesvaarni, Hayyiratul Fatimah Mohd Zaid, Mohd Faridzuan Majid, Raihan Mahirah Ramli, Khairulazhar Jumbri, Jun Wei Lim, Mardawani Mohamad, Pau Loke Show, and Brian Yuliarto. 2021. "Oxidative Extractive Desulfurization System for Fuel Oil Using Acidic Eutectic-Based Ionic Liquid" Processes 9, no. 6: 1050. https://doi.org/10.3390/pr9061050









