Techno Economic Analysis of the Modified MixAlco Process
Abstract
:1. Introduction
2. Methodology
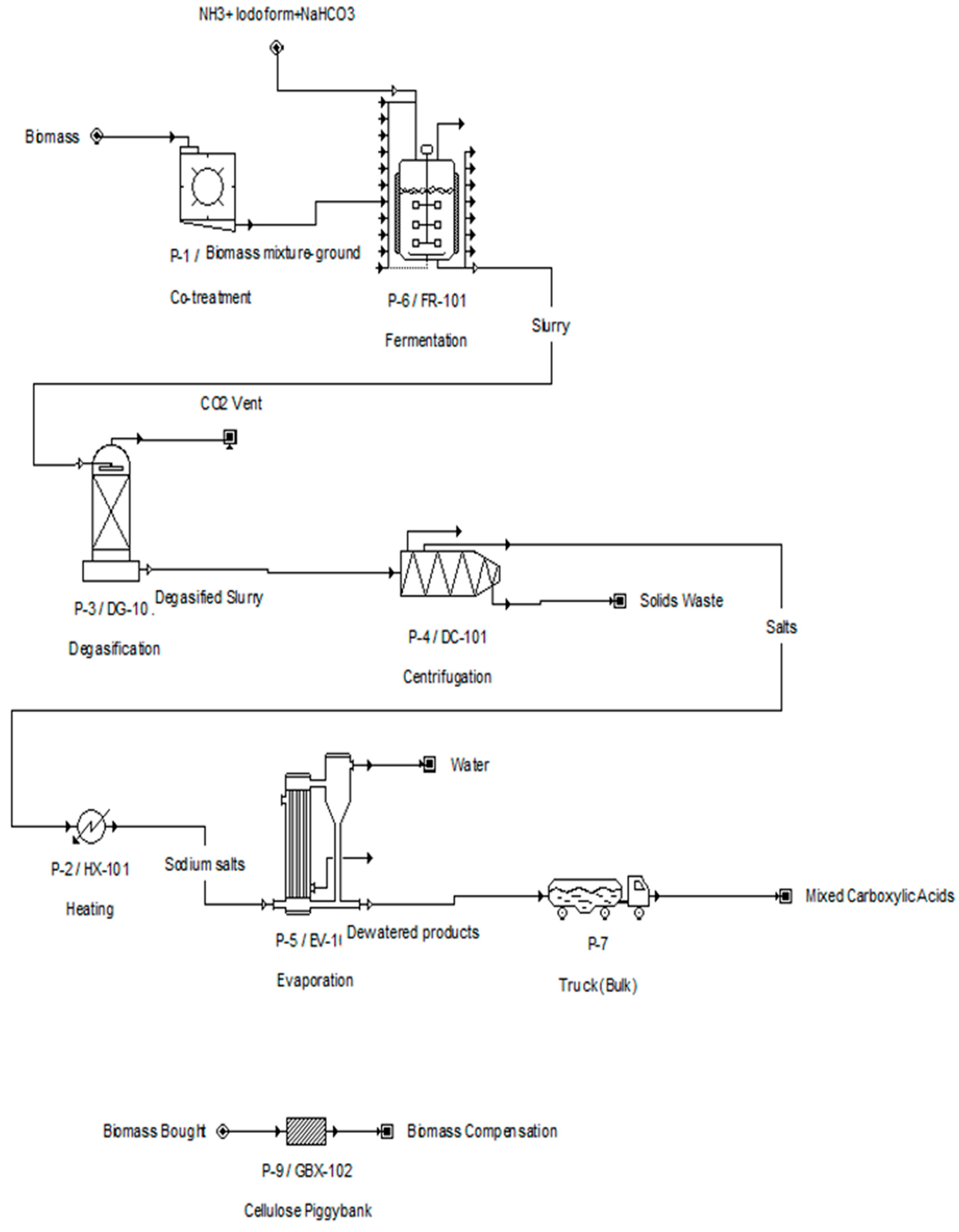
2.1. Proposed Process Description
2.1.1. Cotreatment
2.1.2. Fermentation
2.1.3. Degasification
2.1.4. Centrifugation
2.1.5. Evaporator
2.1.6. Transportation
2.1.7. Cellulose Piggybank
2.1.8. Assumptions Used in the Proposed Design
2.2. Sensitivity Analysis
3. Results and Discussion
3.1. Base Case
3.2. Sensitivity Analysis
3.2.1. Biomass Feed Rate
3.2.2. Selling Price of the Carboxylate Salt Mixture
3.2.3. Distribution Cost
3.2.4. Biomass Composition
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fasahati, P.; Liu, J.J. Application of MixAlco® processes for mixed alcohol production from brown algae: Economic, energy, and carbon footprint assessments. Fuel Process. Technol. 2016, 144, 262–273. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef]
- Holtzapple, M.T.; Davison, R.R.; Ross, M.K.; Aldrett-Lee, S.; Nagwani, M.; Lee, C.-M.; Lee, C.; Adelson, S.; Kaar, W.; Gaskin, D.; et al. Biomass Conversion to Mixed Alcohol Fuels Using the MixAlco Process. Appl. Biochem. Biotechnol. 1999, 79, 609–631. [Google Scholar] [CrossRef]
- Vasquez, S.T.; Dunkleman, J.; Chaudhuri, S.K.; Bond, A.; Holtzapple, M.T. Biomass conversion to hydrocarbon fuels using the MixAlco™ process at a pilot-plant scale. Biomass Bioenergy 2014, 62, 138–148. [Google Scholar] [CrossRef]
- Holtzapple, M.T.; Granda, C.B. Carboxylate Platform: The MixAlco Process Part 1: Comparison of Three Biomass Conversion Platforms. Appl. Biochem. Biotechnol. 2009, 156, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Schaidle, J.A.; Humbird, D.; Baddour, F.G.; Sahir, A. Conceptual Process Design and Techno-Economic Assessment of Ex Situ Catalytic Fast Pyrolysis of Biomass: A Fixed Bed Reactor Implementation Scenario for Future Feasibility. Top. Catal. 2016, 59, 2–18. [Google Scholar] [CrossRef] [Green Version]
- Pham, V.; Holtzapple, M.; El-Halwagi, M. Techno-economic analysis of biomass to fuel conversion via the MixAlco process. J. Ind. Microbiol. Biotechnol. 2010, 37, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- Holtzapple, M. History of the MixAlco Process and Future Challenges; Lecture presented at Rewiring Anaerobic Digestion; Advanced Research Projects Agency-Energy (ARPA-E): Washington, DC, USA, 2016. Available online: https://arpa-e.energy.gov/sites/default/files/Mark_Holtzapple_MixAlco_History.pdf (accessed on 25 May 2021).
- Badea, G.I.; Radu, G.L. Introductory Chapter: Carboxylic Acids—Key Role in Life Sciences. In Carboxylic Acid—Key Role in Life Sciences; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.-T.; Huang, H.; Tay, A.; Qin, W.; De Guzman, L.; Nicolas, E.C.S. Extractive Fermentation for the Production of Carboxylic Acids. In Bioprocessing for Value-Added Products from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2007; pp. 421–446. [Google Scholar] [CrossRef]
- Solubilizers & Emulsifiers—Excipients for R&D Use Only: Sigma-Aldrich. 2020. Available online: https://www.sigmaaldrich.com/life-science/biochemicals/biochemical-products.html?TablePage=105243119 (accessed on 24 June 2020).
- Granda, C.B.; Holtzapple, M.T.; Luce, G.; Searcy, K.; Mamrosh, D.L. Carboxylate Platform: The MixAlco Process Part 2: Process Economics. Appl. Biochem. Biotechnol. 2009, 156, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Crocker, M. Thermochemical Conversion of Biomass to Liquid Fuels and Chemicals; RSC Publishing: Cambridge, UK, 2010. [Google Scholar] [CrossRef] [Green Version]
- Advanced BioFuels USA. Available online: https://advancedbiofuelsusa.info/the-mixalco-process-green-energy-for-the-future/ (accessed on 20 March 2018).
- Newcombe, G.; Dixon, D. Interface Science in Drinking Water Treatment—Theory and Application. In Interface Science and Technology; Academic Press: London, UK, 2006. [Google Scholar] [CrossRef]
- Crude Oil vs. Gasoline Prices—10 Year Daily Chart. Available online: https://www.macrotrends.net/2501/crude-oil-vs-gasoline-prices-chart (accessed on 8 May 2020).
- Acid Carboxylic Hot Sell Stearic Acid Manufacturer Price Carboxylic Acid. Mixed Carboxylic Acid-Mixed Carboxylic Acid Manufacturers, Suppliers and Exporters on Alibaba.com Pharmaceutical Intermediates. 2020. Available online: www.alibaba.com/products/mixed_carboxylic_acid.html?spm=a2700.7724857.0.0.4b15226eXDYDz5 (accessed on 6 May 2020).
- National Overview: Facts and Figures on Materials, Wastes and Recycling (13 March 2020). Available online: https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/national-overview-facts-and-figures-materials (accessed on 9 July 2020).
- Lee, U.; Han, J.; Wang, M. Evaluation of landfill gas emissions from municipal solid waste landfills for the life-cycle analysis of waste-to-energy pathways. J. Clean. Prod. 2017, 166, 335–342. [Google Scholar] [CrossRef]
- Staff, W.T. Waste Today. EREF Releases Analysis on National Landfill Tipping Fees. Available online: www.wastetodaymagazine.com/article/eref-releases-analysis-national-msw-landfill-tipping-fees/#:~:text=Virtual%20Trade%20Show-,EREF%20releases%20analysis%20on%20national%20landfill%20tipping%20fees,percent%2C%20to%20%2455.36%20per%20ton (accessed on 29 October 2019).
- Alibaba. Available online: https://www.alibaba.com/products/carboxylate_salts.html (accessed on 14 May 2021).
- Thamkittikasem, J. Mayor’s Management Report. 2020. Available online: www1.nyc.gov/assets/operations/downloads/pdf/pmmr2020/2020_pmmr.pdf (accessed on 14 October 2020).
- Oden, M.W. City-Serviced Residential Waste Characterization Study; CB&I Environmental & Infrastructure, Inc.: Baton Rouge, LA, USA, 2015; p. 8. Available online: https://www.texasdisposal.com/sites/default/files/uploads/2015AustinCommunityDiversionStudyFinal04.29.16.pdf (accessed on 14 October 2020).
- Weaver, D.; Najarian, A. General Counsel-Public Works; Resolution No. 11-241; Glendale, CA, USA, 2011; pp. 1–14. Available online: https://www.glendaleca.gov/home/showdocument?id=6864 (accessed on 14 October 2020).
- U.S. EPA. Green Waste Solutions; SMART Waste Management: Atlanta, GA, USA, 2009; p. 6. Available online: https://ilsr.org/wp-content/uploads/2012/02/atlanta-payt.pdf (accessed on 14 October 2020).


| Ethanol Production (gal/ton) | Hydrocarbon Production (gal/ton) | |
|---|---|---|
| Thermochemical Platform | 145 | 95.8 |
| Sugar Platform | 175 | 115 |
| Carboxylate Platform | 175 | 115 |
| Carboxylic Acid Salt | Weight Percent (%) |
|---|---|
| C2-Acetic | 41 |
| C3-Propionic | 15 |
| C4-Butyric | 21 |
| C5-Valeric | 8 |
| C6-Caproic | 12 |
| C7-Heptanoic | 3 |
| Project Lifetime | 15 years |
| US Dollar | November 2019 |
| Depreciation model | $2247 for 10 years |
| Piggybank output stream | 16,782.92 kg/h $55.00/US ton biomass |
| Financing | 100% equity |
| Construction period | 30 months |
| Start-up time | 4 months |
| Income tax rate | 40% |
| Operating season | 7920 h/year, 330 days/year |
| Working capital investment | 4,108,722 (thousand $) |
| Unit | Parameter | Value |
|---|---|---|
| Cellulose Piggybank | Inlet biomass flow rate | 18.5 US ton/h |
| Co-treatment | Specific Power | 0.1 kW/(kg/h) |
| Fermentation | Final Temperature Reaction Time Conversion Selectivity | 40 °C 5 weeks 0.8 g digested/g volatile solids fed 0.65 g carboxylic acids/g volatile solids digested |
| Degasification | % Carbon Dioxide Removed | 100% |
| Centrifugation | Particulate concentration | 1034.73 g/L |
| Heating | Temperature of the exit product stream Heating Duty | 98 °C 2,500,442.74 kJ/h |
| Evaporation | Relative Mass Evaporation Rate of water | 100,000 |
| Transportation | Quantity per Shipment Shipping Distance | 20.00 m3 1000 km |
| Unit | Parameter | Value |
|---|---|---|
| Co-treatment | Operating Throughput | 16,782.92 kg/h |
| Fermentation | Working Volume | 15,324.12 m3 |
| Degasification | Throughput Steam Temperature | 17,830.75 L/h 152.00 °C |
| Centrifugation | Throughput | 17,706.06 L/h |
| Heating | Heating Agent (Steam) Mass Flow Rate Temperature | 1156 kg/h 152 °C |
| Evaporation | Vacuum Steam Temperature Flow (Heating agent) | 152 °C 9201 kg/h |
| Transportation | Shipping Frequency | 2759 shipments/year |
| Cellulose Piggybank | Throughput | 16,782.92 kg/h |
| Component | Selling Price [$/kg] | Weight [US ton/h] | Annual Profit [$] |
|---|---|---|---|
| Biomass tipping fee [19] | 0.06 | 18.50 | 7,975,242 |
| Sodium acetate | 0.73 | 1.16 | 6,110,227 |
| Sodium propionate | 3.00 | 0.40 | 8,721,347 |
| Sodium butyrate | 5.00 | 0.55 | 19,605,435 |
| Sodium valerate | 5.07 | 0.20 | 7,366,066 |
| Sodium hexanoate | 10 | 0.30 | 21,326,929 |
| Sodium heptanoate | 5 | 0.07 | 2,620,356 |
| Indices | Value |
|---|---|
| Total capital investment | $28,949,000 |
| Fixed capital investment | $23,657,000 |
| Annual operating cost | $54,618,000 |
| Annual revenues | $73,809,000 |
| Gross margin | 26.00% |
| ROI | 47.54% |
| Payback time | 2.10 years |
| IRR (after tax) | 35.28% |
| NPV at 7% | $69,803,000 |
| Cost Component | US $/kg of Product | Contribution (%) |
|---|---|---|
| Raw Materials | 1.30 | 54.72 |
| Labor | 0.12 | 5.00 |
| Facility Dependent Cost (maintenance, overhead, depreciation, and local tax) | 0.16 | 6.79 |
| Waste Treatment and Disposal | 0.12 | 4.94 |
| Utilities | 0.10 | 4.19 |
| Transportation | 0.18 | 7.56 |
| Income Tax | 0.28 | 11.76 |
| Total | $2.38 | 94.96 |
| Total Plant Direct Cost (TPDC) | Total Plant Indirect Cost (TPIC) | ||
|---|---|---|---|
| Equipment purchase cost | $3,956,000 | Engineering | $3,214,000 |
| Installation | $1,463,000 | Construction | $4,500,000 |
| Process piping | $1,385,000 | ||
| Instrumentation | $1,583,000 | ||
| Insulation | $119,000 | ||
| Electrical | $396,000 | ||
| Buildings | $1,780,000 | ||
| Yard improvement | $593,000 | ||
| Auxiliary facilities | $1,583,000 | ||
| City | Population | Waste Generated (US ton/h) |
|---|---|---|
| New York (NYC) | 8,600,000 | 451.1 |
| Austin | 950,715 | 24.3 |
| Glendale, CA | 201,361 | 18.5 |
| Atlanta | 486,290 | 11.4 |
| Biomass Feed Rate (US ton/h) | Total Capital Investment ($) | NPV @ 7% ($) | Annual Operating Cost ($/year) | Payback Time (Years) |
|---|---|---|---|---|
| 11.4 | 22,707,000 | −21,095,000 | 50,159,000 | 205.22 |
| 18.5 | 28,949,000 | 69,808,000 | 54,618,000 | 2.10 |
| 24.28 | 33,668,000 | 138,468,000 | 59,411,000 | 1.40 |
| 451.1 | 280,116,000 | 5,247,480,000 | 352,325,000 | 0.36 |
| Selling Price ($/kg) | Total Capital Investment ($) | NPV @ 7% ($) | Annual Operating Cost ($/yr) | Payback Time (Years) |
|---|---|---|---|---|
| 1.79 | 28,949,000 | 185,000 | 54,618,000 | 7.35 |
| 1.90 | 28,949,000 | 12,337,000 | 54,618,000 | 5.03 |
| 2.38 | 28,949,000 | 69,803,000 | 54,618,000 | 2.10 |
| 6.00 | 28,949,000 | 494,891,000 | 54,618,000 | 0.39 |
| 10.00 | 28,949,000 | 963,812,000 | 54,618,000 | 0.21 |
| Shipping Distance (km) | Total Capital Investment ($) | NPV @ 7% ($) | Annual Operating Cost ($/yr) | Payback Time (Years) |
|---|---|---|---|---|
| 100 | 28,949,000 | 80,499,000 | 52,135,000 | 1.90 |
| 250 | 28,949,000 | 78,716,000 | 52,549,000 | 1.93 |
| 500 | 28,949,000 | 75,745,000 | 53,239,000 | 1.98 |
| 1000 | 28,949,000 | 69,803,000 | 54,618,000 | 2.10 |
| 1500 | 28,949,000 | 63,861,000 | 55,997,000 | 2.24 |
| Biomass Composition | Total Capital Investment ($) | NPV @ 7% ($) | Annual Operating Cost ($/year) | Payback Time (Years) |
|---|---|---|---|---|
| 1–27 wt.% cellulose, 5.4 wt.% lignin, 67.6 wt.% water | $28,949,000 | $69,803,000 | $54,618,000 | 2.10 |
| 2–24.3 wt.% cellulose, 8.1 wt.% lignin, 67.6 wt.% water | $29,036,000 | $42,148,000 | $54,395,000 | 2.93 |
| 3–21.6 wt.% cellulose, 10.8 wt.% lignin, 67.6 wt.% water | $29,114,000 | $14,776,000 | $54,174,000 | 4.76 |
| 4–24.3 wt.% cellulose, 5.4 wt.% lignin, 70.3 wt.% water | $29,040,000 | $42,712,000 | $54,265,000 | 2.90 |
| 5–29.7 wt.% cellulose, 5.4 wt.% lignin, 64.9 wt.% water | $28,839,000 | $95,555,000 | $55,317,000 | 1.66 |
| Input | Total Capital Investment | NPV @ 7% | Annual Operating Cost | Payback Time |
|---|---|---|---|---|
| Biomass Feed Rate | Increases with increased feed rate/city population size | Increases with increased feed rate/city population size | Increases with increased feed rate/city population size | Decreases with increased feed rate/city population size |
| Mixed Carboxylic Acid Salt Selling Price | No impact | Increases with increased selling price | No impact | Decreases with increased selling price |
| Distribution Cost | No impact | Decreases with increased distribution cost/shipping distance | Minimal impact with increased distribution cost/shipping distance | Minimal impact with increased distribution cost/shipping distance |
| Biomass Composition | Minimal impact with changes in cellulose % | Increases with increase in cellulose % | Minimal impact with changes in cellulose % | Decreases with increase in cellulose % |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simchick, C.; Zidek, B.; Moder, D.; Madson, D.; Payne, M.; Kelsey, J.; Shaikh, F.; Ozturk, S. Techno Economic Analysis of the Modified MixAlco Process. Processes 2021, 9, 958. https://doi.org/10.3390/pr9060958
Simchick C, Zidek B, Moder D, Madson D, Payne M, Kelsey J, Shaikh F, Ozturk S. Techno Economic Analysis of the Modified MixAlco Process. Processes. 2021; 9(6):958. https://doi.org/10.3390/pr9060958
Chicago/Turabian StyleSimchick, Chloe, Brooke Zidek, Devin Moder, Dustin Madson, Michael Payne, Jonathon Kelsey, Faisal Shaikh, and Serdar Ozturk. 2021. "Techno Economic Analysis of the Modified MixAlco Process" Processes 9, no. 6: 958. https://doi.org/10.3390/pr9060958
APA StyleSimchick, C., Zidek, B., Moder, D., Madson, D., Payne, M., Kelsey, J., Shaikh, F., & Ozturk, S. (2021). Techno Economic Analysis of the Modified MixAlco Process. Processes, 9(6), 958. https://doi.org/10.3390/pr9060958






