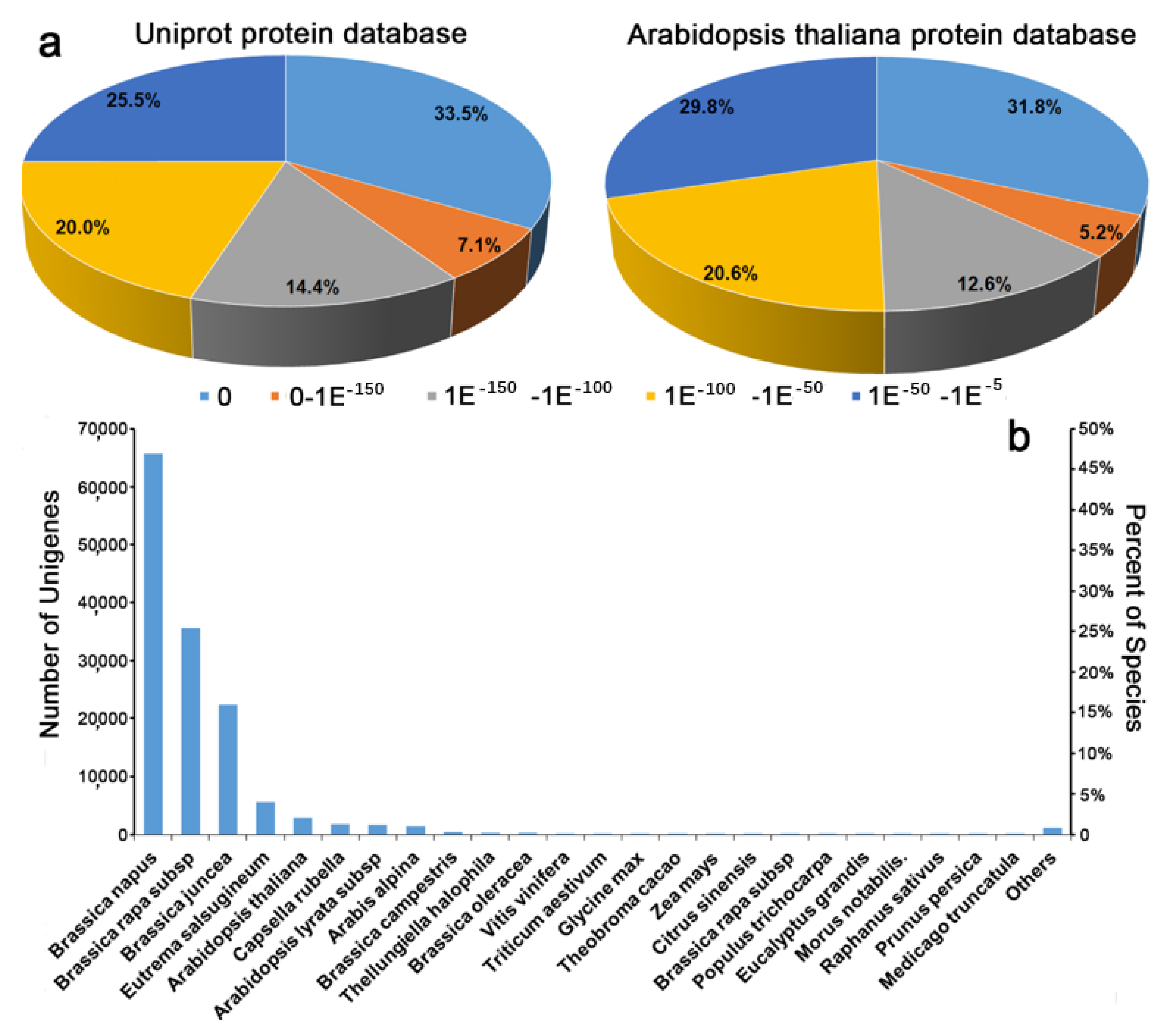
1. Introduction
Heavy metals have been found to be a kind of widespread pollutant and to pose a threat to organisms. Vast areas of agricultural soil are contaminated by heavy metals via atmospheric deposition or anthropogenic activities, such as animal manure, the direct application of phosphate fertilizers, sewage sludge, and irrigation water [
1,
2]. Cadmium (Cd), is one of the most prevalent and poisonous elements in agricultural soil, and jeopardizes the environment and human health. Toxicology studies have been undertaken of Cd stress in plants affecting tissue morphological, biochemical and physiological changes, as well as altered gene expressions [
3,
4,
5,
6]. The presence of 40 mg L
−1 Cd in soil has already been found to affect yield in some crops [
7]. Cadmium ions are easily taken in by plant roots and then transported to other organs, given Cd’s high mobility and water solubility, and this severely disturbs physiological processes in plants [
8] and inhibits plant development by affecting respiration, photosynthesis and nitrogen metabolism [
9].
Brassica juncea L. (AABB, 2
n = 36) is an amphidiploid species that originated from interspecies crosses between
Brassica nigra (AA, 2
n = 16) and
Brassica rapa (BB, 2
n = 20), which is another important annual or biennial oil crop and leafy vegetable used worldwide [
10,
11].
Brassica juncea is also a cruciferous plant capable of accumulating significant quantities of metals, including Cd, Pb, zinc (Zn), chromium (Cr), copper (Cu), aurum (Au) and selenium (Se) [
12,
13,
14]. The plant can accumulate >400 μg Cd/g dry weight in its leaves over 96 h, and it therefore holds promise for the remediation of Cd-contaminated soils, given its high biomass, moderate metal accumulation capacity, ease of harvesting, and metal tolerance [
15].
MicroRNAs (miRNAs) are a class of small endogenous non-coding regulatory RNA molecules containing approximately 18–30 nucleotides (nt), which can regulate gene expression through guiding translational repression and can also target mRNA cleavage, mainly at the post-transcriptional level [
16,
17,
18]. An increasing number of studies have indicated that miRNAs play important regulatory in the response to heavy metals (Cd iron) in different species, such as
Brassica napus [
5,
19],
Arabidopsis [
20,
21],
Ipomoea aquatic Forsk [
22],
Typha angustifolia [
23],
Raphanus sativus L. [
24], maize [
25], wheat [
26,
27] and rice [
28,
29,
30]. Exploring Cd-regulated gene expression and the regulatory relationship between miRNA and target genes are both areas of interest as regards the molecular mechanism of metal homeostasis and accumulation [
21,
27,
31]. Numerous studies have indicated the importance of miRNAs in biotic and abiotic stress responses to ion metals at the post-transcriptional level. For example, 39 differentially expressed miRNAs were identified via a comprehensive analysis of miRNA expression profiles after Cd treatment in
Brassica napus [
19]. Moreover, a Cd treatment of
Brassica napus significantly affected the expression of 22 miRNAs belonging to 11 families in the roots, and 29 miRNAs belonging to 14 miRNA families in the shoots, of
Brassica napus [
5]. In total, 39 known and eight novel miRNAs were aberrantly expressed in response to Cd treatment in rice [
28]. About 199 miRNAs were identified in xylem sap from maize seedlings, including 97 newly discovered miRNAs and 102 known miRNAs [
25]. Plants overexpressing miR156 accumulated significantly less Cd in the shoots than the roots, and showed higher tolerance against Cd-stress [
20]. The miR398 is involved in oxidative stress tolerance via the regulation of its target CSD, which is active in wheat seedlings exposed to Cd stress [
26]. miR166 and miR268 act as negative regulators, modulating Cd tolerance and accumulation in rice [
29,
30]. miRNA395 is an up-regulator that enhances Cd retention and detoxification in the roots of
Ipomoea aquatic Forsk, and miR5139, miR1511 and miR8155 contribute to Cd’s translocation into the shoots of low-shoot-Cd cultivars [
22]. This evidence strongly suggests the key roles of miRNAs in plants’ Cd stress responses, and comprehensive analyses of plants’ transcriptome and miRNA profiles are widely used to screen important miRNAs involved in biotic stress.
Our previous study has investigated the physiological responses of
Brassica juncea L. to 30 and 50 mg kg
−1 Cd stress, and demonstrated the activities of catalase enzymes, the contents of soluble sugar and chlorophyll were reduced, the content of the soluble protein malondialdehyde increased [
6]. Moreover, comparative transcriptomic analysis indicated that the downregulation of
HMA3 and the upregulation of
Nramp3,
HMA2 and
Nramp1 also play roles in reducing Cd toxicity in the roots of
Brassica juncea L. under Cd stress. However, the molecular activity of Cd in response to miRNAs and their corresponding pathways in
Brassica juncea have yet to be fully elucidated. A combined analysis of microRNA and mRNA expression to infer Cd-induced regulation has not been performed for
Brassica juncea. In the current study, with the aim of identifying the Cd-regulated unigenes and miRNAs in roots and leaves and developing a Cd-associated miRNA regulatory network, we constructed mRNA and sRNA libraries of Cd-treated and Cd-free
Brassica juncea, which were then sequenced via an Illumina Hiseq2500 system. These results could provide a new perspective for studying the regulation of miRNAs in root and leaf under Cd stress, and facilitate genetic breeding of Cd tolerance in
Brassica juncea.
4. Discussion
Cadmium has been considered with increasing frequency worldwide as a toxicant, and it is a serious and urgent environmental problem. Meanwhile, Cd also poses a threat to human health as a result of accumulation in the human body via the food chain, leading to chronic toxicity in bones, lungs, kidney and other organs [
45,
46]. Considering these detriments of Cd, many studies have applied a high-throughput sequencing approach to identify dysregulated genes and miRNAs associated with Cd stress in several species [
9,
33]. Notably, a number of miRNAs and target genes responding to Cd stress have been identified in some species. However, few studies have analyzed Cd stress-associated mRNAs and miRNAs in
Brassica juncea. In this report, approximate 16 and eight million raw reads were obtained from transcriptome and sRNA libraries of
Brassica juncea leaves and roots with or without Cd treatment, respectively.
High-throughput sequencing technologies have been applied to obtain comprehensive sequencing data for transcriptome and non-coding RNA analysis, which may help elucidate the molecular mechanisms at play in associated biological processes and pathways [
33,
47]. In the current study, 4 transcriptomes and four sRNA libraries were constructed from the leaves and roots of Cd-treated and normal control in
Brassica juncea, and sequence data were generated by the Illumina platform to analyse the miRNA–unigene regulatory pathway and genes networks in response to Cd stress. Transcriptomic pooling from the leaves and roots of
Brassica juncea with or without Cd treatments has provided more valuable information to facilitate the screening of Cd-responsive miRNAs and their targets [
6]. We found that the differentially expressed unigenes identified from roots and leaves were mainly down-regulated after Cd treatment, which was consistent with previous studies [
24]. Importantly, we found that aberrantly expressed unigenes were significantly enriched in a metabolic process that was severely affected by Cd stress. The unigenes affect metabolic processes by interacting with organic compounds when Cd
2+ enters plant cells or interacts with lipids and proteins through oxidative stress [
48]. These findings suggest that the down-regulation of unigenes might play a crucial role in plants’ responses to Cd stress.
Moreover, 59 conserved miRNAs and 93 novel miRNAs were successfully screened as associated with Cd stress in
Brassica juncea. Among them, 57 miRNAs were down-regulated, while 95 were up-regulated, in response to Cd exposure, indicating that miRNAs are differentially regulated under heavy metal stress. Various published reports have identified the many responses of miRNAs to diverse heavy metals, such as Cd, Hg, As and Al in soybean [
42], rice [
43],
Brassica juncea [
44] and
M. truncatula [
17], which has provided valuable information regarding the regulatory mechanisms of plant miRNAs in response to heavy metal stresses.
In this report, conserved microRNA families, including miR156, miR159, miR166 and miR398, were differentially regulated in response to Cd stress, which agreed with the findings for rice and
Brassica napus [
43,
44]. We also observed that some conserved miRNA families, such as miR172, miR161 and miR162, were dysregulated in response to Cd stress, but there was no significantly differential expression after Cd treatment in radishes [
24]. Notably, some previously reported miRNA families in responses to heavy metal stress showed temporal organ-specific expression patterns. In a previous report, miR161, miR171, miR398, miR319 and miR385 were differentially expressed in roots and shoots [
17]. Similarly, we found that only the miR857 family was expressed in the root, while miR9408 was expressed in the leaf. Therefore, further studies will be needed to explore the molecular mechanisms of those miRNAs in plants’ responses to Cd stress.
miRNA is a major regulator of target gene expression at the post-transcriptional level. A great number of dysregulated miRNAs and their target genes exert key roles in response to plant organs under heavy metal stress [
24,
42,
44]. One miRNA named bra-miR172b-3p was found to target 15 transcripts, and also showed significant differential expression between two contrasting Finger millet genotypes while under salinity stress [
49], which was in line with our results in this study. Moreover, bra-miR172b-3p has been found to be down-regulated under Turnip mosaic virus stress in non-heading Chinese cabbage, providing a better way to understand the relationship between plants and virus [
50].
Surprisingly, several key enzymes associated with heavy metal (Cd
2+, Hg
2+, Mn
2+, Pb
2+ or Na
+) uptake and translocation were found to be target genes for a few conserved miRNAs. bra-miR172b-3p targeted several gene-encoding enzymes related to abiotic stress, such as
DOX1, ATCCS, ATCAT3 and
ATKT1 (
Figure 7a). Functional annotation analysis indicated that these target genes participate in various stress responses, including abiotic stress. It is well known that production of reactive oxygen species in plants is closely related to the phytotoxicity caused by heavy metals, and toxic concentrations of high Cd also induce oxidative stress in plant [
51,
52].
Although Cd is not a redox-active element, increased reactive oxygen species (ROS) levels may be induced via indirect stress or a maladjustment of the antioxidant system, such as the induction of enzymatic lipid peroxidation and the activation of ROS-producing enzymes [
53,
54]. Moreover, superoxide dismutase (SOD) and catalase have been widely considered as important defense systems of plants against oxygen free radicals. SOD as an antioxidant enzyme in physiological mechanism and defense system against high concentrations of ROS, and is associated with enhanced tolerance and resistance to oxidative stress [
55,
56], indicating the critical role of
ATCCS in abiotic stress regulated by miRNAs. A previous study demonstrated the effects of aluminum on lipid peroxidation and the activities of enzymes associated with the production of activated oxygen stress [
6].
Notably, a certain degree of Cd accumulation is involved in the induction of oxidative response in
Holcuslanatus and
Saccharomyces cerevisiae, immediately activated antioxidant enzyme to resist the stress response [
57,
58]. Recently, the key role of SOD in the detoxification process against Cd in
Paxillusinvolutus has been demonstrated using high-throughput sequencing [
59]. Meanwhile,
CCS delivers heavy metals to SOD in cytosol [
60]. Similar to copper chaperone for superoxide dismutase, ATCCS plays an important role in the Cd defense by regulating the levels of SOD in plants. These findings suggest that bra-miR172b-3p could be involved in the Cd defense against oxygen free radicals by directing the regulation of
DOX1,
ATCCS, ATCAT3 and
ATKT1 in
Brassica juncea. Notably, a recent study has demonstrated the role of miR172 in regulating tobacco tolerance to high salt environment (NaCl), which was consistent with our qRT-PCR results showing that the level of bra-miR172b-3p significantly decreased after NaCl treatment [
61]. By targeting complementary sequences, miRNA negatively regulates targeted gene expression, the identified miRNA-mediated gene expression of
DOX1, ATCCS, ATCAT3, and
ATKT1 could play a vital role in the regulatory networks of Cd detoxification in
Brassica juncea.
Although RNA-seq generally offers higher detection sensitivity and opens a new avenue in the research on gene fusions, novel alternative transcripts and allele-specific expression, some limitations also exist. The nonconformity between RNA-seq and qRT-PCR verification for bra-miR398-3p suggests a gene-specific biases in high-throughput sequencing, but qRT-PCR should be considered for the final detection of interesting miRNAs or target genes [
62]. Meanwhile, only one sample group was detected in the RNA-seq experiment, and three replicate tests were conducted in qRT-PCR due to limited funds. Therefore, this nonconformity may be a false positive because of the individual differences in various samples.