Boosting Electrochemical Performance of Hematite Nanorods via Quenching-Induced Alkaline Earth Metal Ion Doping
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of α-Fe2O3@CC
2.3. Fabrication of Sr-Fe2O3@CC
2.4. Fabrication of Ba-Fe2O3@CC
2.5. Fabrication of Mg-Fe2O3@CC
2.6. Fabrication of Ca-Fe2O3@CC
2.7. Characterization
2.8. Electrochemical Measurements
3. Results
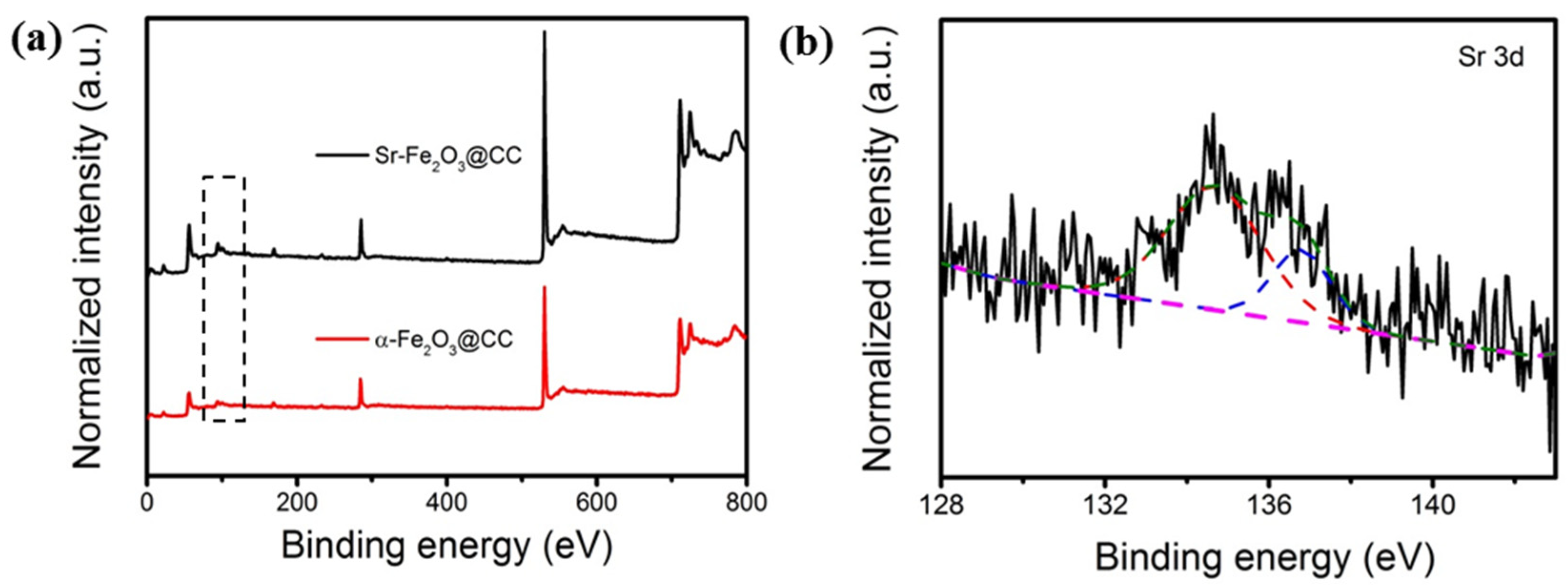
3.1. Characterization
3.2. Electrochemical Performances of M-Fe2O3@CC Electrode
3.3. Formatting of Mathematical Components
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.L.; Zhao, X.S. Carbon−based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhou, S.; Quan, H.; Zou, R.; Gao, W.; Luo, X.; Guo, L. Tetsubo−like α−Fe2O3/C nanoarrays on carbon cloth as negative electrode for high−performance asymmetric supercapacitors. Chem. Eng. J. 2018, 341, 102–111. [Google Scholar] [CrossRef]
- Choudhury, A.; Kim, J.-H.; Yang, K.-S.; Yang, D.-J. Facile synthesis of self−standing binder−free vanadium pentoxide−carbon nanofiber composites for high−performance supercapacitors. Electrochim. Acta 2016, 213, 400–407. [Google Scholar] [CrossRef]
- Kandalkar, S.G.; Gunjakar, J.L.; Lokhande, C.D. Preparation of cobalt oxide thin films and its use in supercapacitor application. Appl. Surf. Sci. 2008, 254, 5540–5544. [Google Scholar] [CrossRef]
- Huang, H.; Wang, X.; Tervoort, E.; Zeng, G.; Liu, T.; Chen, X.; Sologubenko, A.; Niederberger, M. Nano−Sized Structurally Disordered Metal Oxide Composite Aerogels as High−Power Anodes in Hybrid Supercapacitors. ACS Nano 2018, 12, 2753–2763. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Cui, X.; Chen, W.; Ivey, D.G. Manganese oxide−based materials as electrochemical supercapacitor electrodes. Chem. Soc. Rev. 2011, 40, 1697–1721. [Google Scholar] [CrossRef] [PubMed]
- Jyothibasu, J.P.; Wang, R.-H.; Ong, K.; Ong, J.H.L.; Lee, R.-H. Cellulose/carbon nanotube/MnO2 composite electrodes with high mass loadings for symmetric supercapacitors. Cellulose 2021, 28, 3549–3567. [Google Scholar] [CrossRef]
- Xia, H.; Shirley Meng, Y.; Yuan, G.; Cui, C.; Lu, L. A Symmetric RuO2/RuO2 Supercapacitor Operating at 1.6 V by Using a Neutral Aqueous Electrolyte. Electrochem. Solid State Lett. 2012, 15, A60–A63. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Q.; Kurra, N.; Alhabeb, M.; Gogotsi, Y.; Alshareef, H.N. All Pseudocapacitive MXene−RuO2Asymmetric Supercapacitors. Adv. Energy Mater. 2018, 8, 1703043. [Google Scholar] [CrossRef]
- Yang, Z.; Qiu, A.; Ma, J.; Chen, M. Conducting α−Fe2O3 nanorod/polyaniline/CNT gel framework for high performance anodes towards supercapacitors. Compos. Sci. Technol. 2018, 156, 231–237. [Google Scholar] [CrossRef]
- Li, Y.; Xu, J.; Feng, T.; Yao, Q.; Xie, J.; Xia, H. Fe2O3 Nanoneedles on Ultrafine Nickel Nanotube Arrays as Efficient Anode for High−Performance Asymmetric Supercapacitors. Adv. Funct. Mater. 2017, 27, 1606728. [Google Scholar] [CrossRef]
- Owusu, K.A.; Qu, L.; Li, J.; Wang, Z.; Zhao, K.; Yang, C.; Hercule, K.M.; Lin, C.; Shi, C.; Wei, Q.; et al. Low−crystalline iron oxide hydroxide nanoparticle anode for high−performance supercapacitors. Nat. Commun. 2017, 8, 14264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Q.; Xia, X.; He, W.; Huang, X.; Logan, B.E. Addition of conductive particles to improve the performance of activated carbon air−cathodes in microbial fuel cells. Environ. Sci. Water Res. Technol. 2017, 3, 806–810. [Google Scholar] [CrossRef]
- Ma, Z.; Huang, X.; Dou, S.; Wu, J.; Wang, S. One−Pot Synthesis of Fe2O3 Nanoparticles on Nitrogen−Doped Graphene as Advanced Supercapacitor Electrode Materials. J. Phys. Chem. C 2014, 118, 17231–17239. [Google Scholar] [CrossRef]
- Raut, S.S.; Sankapal, B.R. Comparative studies on MWCNTs, Fe2O3 and Fe2O3/MWCNTs thin films towards supercapacitor application. New J. Chem. 2016, 40, 2619–2627. [Google Scholar] [CrossRef]
- Shivakumara, S.; Penki, T.R.; Munichandraiah, N. Synthesis and Characterization of Porous Flowerlike Fe2O3 Nanostructures for Supercapacitor Application. ECS Electrochem. Lett. 2013, 2, A60–A62. [Google Scholar] [CrossRef]
- Nie, G.; Lu, X.; Lei, J.; Jiang, Z.; Wang, C. Electrospun V2O5−doped α−Fe2O3composite nanotubes with tunable ferromagnetism for high−performance supercapacitor electrodes. J. Mater. Chem. A 2014, 2, 15495–15501. [Google Scholar] [CrossRef]
- Ni, C.; Hou, J.; Li, L.; Li, Y.; Wang, M.; Yin, H.; Tan, W.J.C. The remarkable effect of alkali earth metal ion on the catalytic activity of OMS−2 for benzene oxidation. Chemosphere 2020, 250, 126211. [Google Scholar] [CrossRef]
- Reddy, M.V.; Yao, Q.C.; Adams, S. Mg, Cu, Zn doped Fe2O3 as an electrode material for Li−ion batteries. Mater. Lett. 2018, 2, 186–192. [Google Scholar] [CrossRef]
- Liu, S.; Li, Z.; Wang, C.; Tao, W.; Huang, M.; Zuo, M.; Yang, Y.; Yang, K.; Zhang, L.; Chen, S.; et al. Turning main−group element magnesium into a highly active electrocatalyst for oxygen reduction reaction. Nat. Commun. 2020, 11, 938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, S.; Wang, H.; Yang, W.; Fida, H.; You, L.; Zhou, K. Scalable synthesis of Ca−doped α−Fe2O3 with abundant oxygen vacancies for enhanced degradation of organic pollutants through peroxymonosulfate activation. Appl. Catal. B Environ. 2020, 2, 118250. [Google Scholar] [CrossRef]
- Su, M.; Pan, Z.; Chong, Y.; Ye, C.; Jin, X.; Wu, Q.; Hu, Z.; Ye, D.; Waterhouse, G.I.N.; Qiu, Y.; et al. Boosting the electrochemical performance of hematite nanorods via quenching−induced metal single atom functionalization. J. Mater. Chem. A 2021, 9, 3492–3499. [Google Scholar] [CrossRef]
- Poornajar, M.; Nguyen, N.; Ahn, H.−J.; Büchler, M.; Liu, N.; Kment, S.; Zboril, R.; Yoo, J.; Schmuki, P. Fe2O3 Blocking Layer Produced by Cyclic Voltammetry Leads to Improved Photoelectrochemical Performance of Hematite Nanorods. Surfaces 2019, 2, 131–144. [Google Scholar] [CrossRef] [Green Version]
- Bouhjar, F.; Derbali, L.; Marí, B.; Bessaïs, B. Electrodeposited Cr−Doped α−Fe2O3 thin films active for photoelectrochemical water splitting. Int. J. Hydrog. Energy 2020, 45, 11492–11501. [Google Scholar] [CrossRef]
- Kumar, P.; Sharma, P.; Shrivastav, R.; Dass, S.; Satsangi, V.R. Electrodeposited zirconium−doped α−Fe2O3 thin film for photoelectrochemical water splitting. Int. J. Hydrog. Energy 2011, 36, 2777–2784. [Google Scholar] [CrossRef]
- Shen, S.; Guo, P.; Wheeler, D.A.; Jiang, J.; Lindley, S.A.; Kronawitter, C.X.; Zhang, J.Z.; Guo, L.; Mao, S.S. Physical and photoelectrochemical properties of Zr−doped hematite nanorod arrays. Nanoscale 2013, 5, 9867. [Google Scholar] [CrossRef]
- Shen, S.; Jiang, J.; Guo, P.; Kronawitter, C.X.; Mao, S.S.; Guo, L. Effect of Cr doping on the photoelectrochemical performance of hematite nanorod photoanodes. Nano Energy 2012, 1, 732–741. [Google Scholar] [CrossRef]
- Wu, Y.; Dong, L.; Li, B. Effect of iron on physicochemical properties: Enhanced catalytic performance for novel Fe2O3 modified CuO/Ti0.5Sn0.5O2 in low temperature CO oxidation. Mol. Catal. 2018, 456, 65–74. [Google Scholar] [CrossRef]
- Ayastuy, J.L.; Gurbani, A.; Gutiérrez−Ortiz, M.A. Effect of calcination temperature on catalytic properties of Au/Fe2O3 catalysts in CO−PROX. Int. J. Hydrog. Energy 2016, 41, 19546–19555. [Google Scholar] [CrossRef]
- Li, M.; Liu, H.; Zhu, H.; Gao, H.; Zhang, S.; Chen, T. Kinetics and mechanism of Sr(II) adsorption by Al−Fe2O3: Evidence from XPS analysis. J. Mol. Liq. 2017, 2, 364–369. [Google Scholar] [CrossRef]
- Komai, S.; Hirano, M.; Ohtsu, N. Spectral analysis of Sr 3d XPS spectrum in Sr−containing hydroxyapatite. Surf. Interface Anal. 2020, 52, 823–828. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Cao, J.; Kong, F.; Xia, H.; Zhang, J.; Zhu, B.; Wang, S.; Wu, S. Low−temperature H2S sensors based on Ag−doped α−Fe2O3 nanoparticles. Sens. Actuators B Chem. 2008, 131, 183–189. [Google Scholar] [CrossRef]
- Banerjee, A.M.; Shirole, A.R.; Pai, M.R.; Tripathi, A.K.; Bharadwaj, S.R.; Das, D.; Sinha, P.K. Catalytic activities of Fe2O3 and chromium doped Fe2O3 for sulfuric acid decomposition reaction in an integrated boiler, preheater, and catalytic decomposer. Appl. Catal. B Environ. 2012, 127, 36–46. [Google Scholar] [CrossRef]
- Zhao, X.; Meng, J.; Yan, Z.; Cheng, F.; Chen, J. Nanostructured NiMoO4 as active electrocatalyst for oxygen evolution. Chin. Chem. Lett. 2019, 30, 319–323. [Google Scholar] [CrossRef]
- Szymanek, K.; Charmas, R.; Piasecki, W. A study on the mechanism of Ca(2+) adsorption on TiO2 and Fe2O3 with the usage of calcium ion−selective electrode. Chemosphere 2020, 242, 125162. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhao, X.; Su, R.; Hao, Z.; Jia, B.; Li, S.; Dong, L. Highly Porous Graphitic Activated Carbons from Lignite via Microwave Pretreatment and Iron−Catalyzed Graphitization at Low−Temperature for Supercapacitor Electrode Materials. Processes 2019, 7, 300. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Liu, X.X.; Han, J.; Hu, K.; Chen, J.S. Self−Supported Sheets−on−Wire CuO@Ni(OH)2/Zn(OH)2 Nanoarrays for High−Performance Flexible Quasi−Solid−State Supercapacitor. Processes 2021, 9, 680. [Google Scholar] [CrossRef]
- Zeng, Y.; Han, Y.; Zhao, Y.; Zeng, Y.; Yu, M.; Liu, Y.; Tang, H.; Tong, Y.; Lu, X. Advanced Ti−Doped Fe2O3@PEDOT Core/Shell Anode for High−Energy Asymmetric Supercapacitors. Adv. Energy Mater. 2015, 5, 1402176. [Google Scholar] [CrossRef]




| Sample | M2+ (mg/Kg) | M2+ (wt. %) |
|---|---|---|
| Sr-Fe2O3@CC | 3365 | 0.34 |
| Ba-Fe2O3@CC | 12,965 | 1.30 |
| Mg-Fe2O3@CC | 1691 | 0.17 |
| Ca-Fe2O3@CC | 9023 | 0.90 |
| Sample | BET Surface Area (m2 g−1) |
|---|---|
| α-Fe2O3@CC | 77.18 |
| Sr-Fe2O3@CC | 79.60 |
| Ba-Fe2O3@CC | 74.65 |
| Mg-Fe2O3@CC | 77.52 |
| Ca-Fe2O3@CC | 53.06 |
| Sample | CDL (mF) | ECSA (cm2) |
|---|---|---|
| α-Fe2O3@CC | 0.2050 | 5.12 |
| Sr-Fe2O3@CC | 0.4450 | 11.13 |
| Ba-Fe2O3@CC | 0.2800 | 7.00 |
| Mg-Fe2O3@CC | 0.3800 | 9.50 |
| Ca-Fe2O3@CC | 0.0046 | 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Chong, Y.; Rao, M.; Su, M.; Qiu, Y. Boosting Electrochemical Performance of Hematite Nanorods via Quenching-Induced Alkaline Earth Metal Ion Doping. Processes 2021, 9, 1102. https://doi.org/10.3390/pr9071102
Chen Q, Chong Y, Rao M, Su M, Qiu Y. Boosting Electrochemical Performance of Hematite Nanorods via Quenching-Induced Alkaline Earth Metal Ion Doping. Processes. 2021; 9(7):1102. https://doi.org/10.3390/pr9071102
Chicago/Turabian StyleChen, Qin, Yanan Chong, Mumin Rao, Ming Su, and Yongcai Qiu. 2021. "Boosting Electrochemical Performance of Hematite Nanorods via Quenching-Induced Alkaline Earth Metal Ion Doping" Processes 9, no. 7: 1102. https://doi.org/10.3390/pr9071102





