Efficacy of Different Waste and By-Products from Forest and Food Industries in the Removal/Retention of the Antibiotic Cefuroxime
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sorbent Materials
2.2. Chemical Reagents
2.3. Adsorption and Desorption Experiments
2.4. Data Treatment
3. Results and Discussion
3.1. Characteristics of the Sorbent Materials
3.2. Adsorption and Desorption of CFX
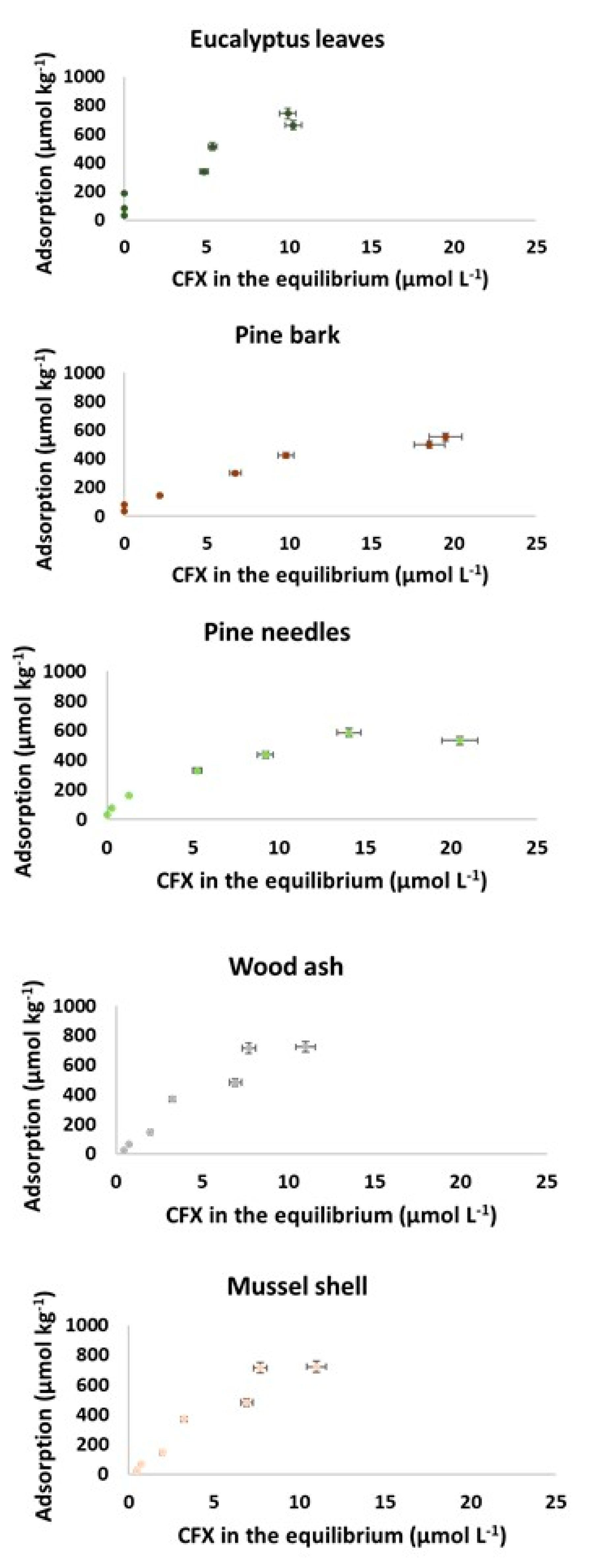
3.2.1. Adsorption
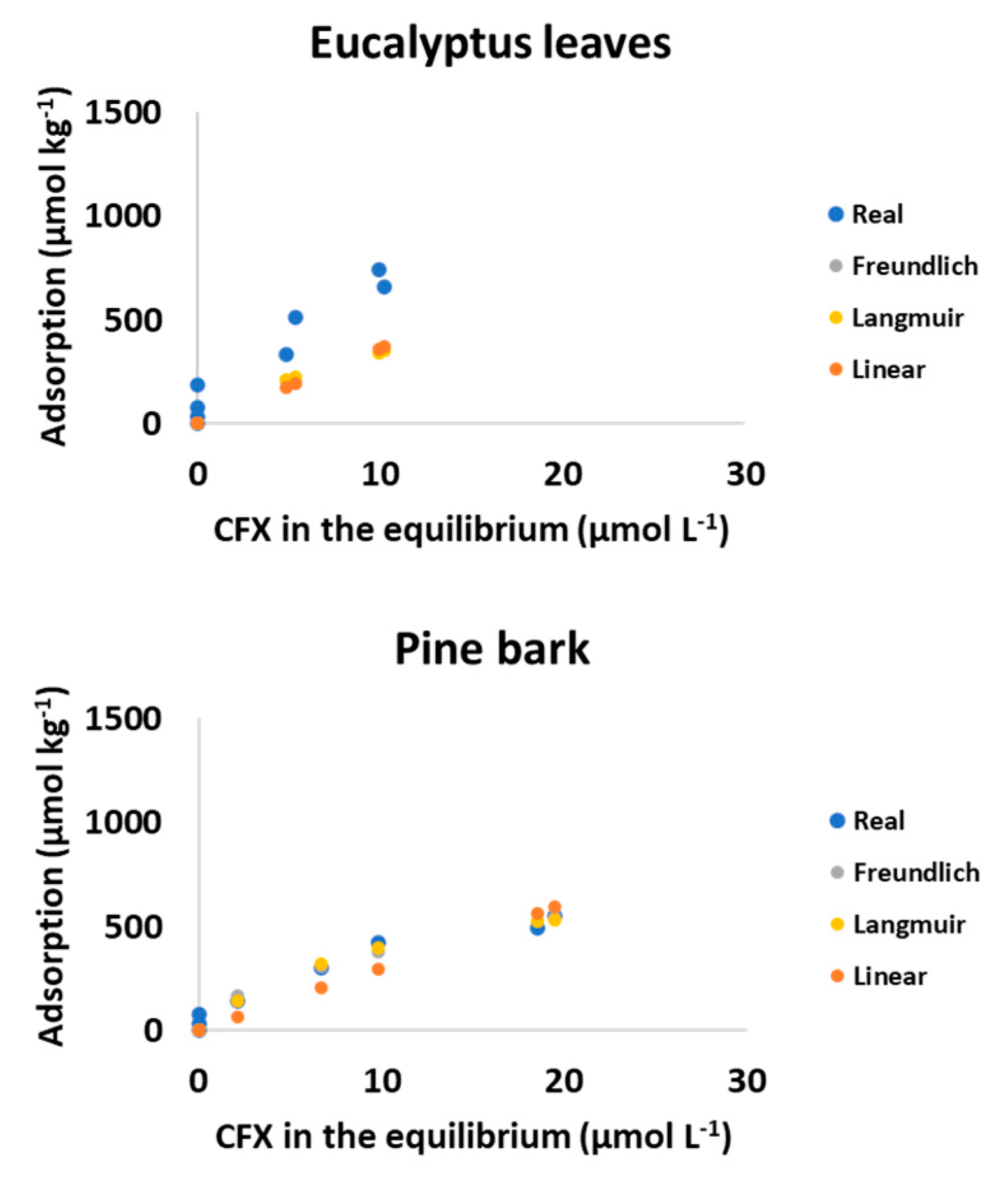
3.2.2. Modeling of Adsorption Data
3.2.3. Desorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Klein, E.Y.; Vanboeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, 3463–3470. [Google Scholar] [CrossRef] [Green Version]
- Wei, M.; Lv, D.; Cao, L.; Zhou, K.; Jiang, K. Adsorption behaviours and transfer simulation of levofloxacin in silty clay. Environ. Sci. Pollut. Res. 2021. [Google Scholar] [CrossRef]
- Zhao, R.X.; Feng, J.; Liu, J.; Fu, W.J.; Li, X.Y.; Li, B. Deciphering of microbial community and antibiotic resistance genes in activated sludge reactors under high selective pressure of different antibiotics. Water Res. 2019, 151, 388–402. [Google Scholar] [CrossRef]
- Kovalakova, P.; Cizmas, L.; McDonald, T.J.; Marsalek, B.; Feng, M.; Sharma, V.K. Occurrence and toxicity of antibiotics in the aquatic environment: A review. Chemosphere 2020, 251, 126351–126361. [Google Scholar] [CrossRef]
- Ren, X.; Liu, D.; Ding, N.; Huang, K.; Xiong, Y.; Du, G.; Zeng, F. Safety evaluation of cephalosporins based on utilization and adverse drug events: Analysis of two databases in china. Expert Opin. Drug Saf. 2012, 11, 689–697. [Google Scholar] [CrossRef]
- Hu, X.; Sun, T.; Jia, L.; Wei, J.; Sun, Z. Preparation of metal-organic framework based carbon materials and its application to adsorptive removal of cefepime from aqueous solution. J. Hazard. Mater. 2020, 390, 122190–122198. [Google Scholar] [CrossRef]
- Dancer, S.J. The problem with cephalosporins. J. Antimicrob. Chemother. 2001, 48, 463–478. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.R.; Sures, B.; Schmidt, T.C. Cephalosporin antibiotics in the aquatic environment: A critical review of occurrence, fate, ecotoxicity and removal technologies. Environ. Pollut. 2018, 241, 1153–1166. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.; Knowlton, K.F.; Shang, C.; Xia, K. Development and validation of a UPLC-MS/MS method to monitor Cephapirin excretion on dairy cows following intramammary infusion. PLoS ONE 2014, 9, 112343–112355. [Google Scholar] [CrossRef] [PubMed]
- EMA (European Medicines Agency). European Surveillance of Veterinary Antimicrobial Consumption. Sales of Veterinary Antimicrobial Agents in 29 European Countries in 2014. EMA/61769/2016. Available online: https://www.ema.europa.eu/en/documents/report/sixth-esvac-report-sales-veterinary-antimicrobial-agents-29-european-countries-2014_en.pdf (accessed on 10 June 2021).
- Versporten, A.; Coenen, S.; Adriaenssens, N.; Muller, A.; Minalu, G.; Faes, C.; Vankerckhoven, V.; Aerts, M.; Hens, N.; Molenberghs, G.; et al. European Surveillance of Antimicrobial Consumpetion (ESAC): Outpatient cephalosporin use in Europe (1997-2009). J. Antimicrob. Chemother. 2011, 66, 25–35. [Google Scholar]
- Sarmah, A.K.; Meyer, M.T.; Boxall, A.B.A. A global perspective on the use, sales exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 2006, 65, 725–759. [Google Scholar] [CrossRef]
- Duan, H.; Hu, X.; Sun, Z. Magnetic zeolite imidazole framework material-8 as an effective and recyclabe adsorbent for removal of ceftazidime from aqueous solution. J. Hazard. Mater. 2020, 384, 121406–121414. [Google Scholar] [CrossRef]
- Russell, J.N.; Yost, C.K. Alternative, environmentally conscious approaches for removing antibiotics from wastewater treatment systems. Chemosphere 2021, 263, 128177–128187. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Garg, A. Degradation of ciprofloxacin using Fenton’s oxidation: Effect of operating parameters, identification of oxidized by-products and toxicity assessment. Chemosphere 2018, 193, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Perini, J.A.L.; Tonetti, A.L.; Vidal, C.; Montagner, C.C.; Nogueira, R.F.P. Simultaneous degradation of ciprofloxacin, amoxicillin, sulfathiazole and sulfamethazine, and disinfection of hospital effluent after biological treatment via photo-Fenton process under ultraviolet germicidal irradiation. Appl. Catal. B 2018, 224, 761–771. [Google Scholar] [CrossRef] [Green Version]
- Becker, D.; Giustina, S.V.D.; Rodríguez-Mozaz, S.; Schoevaart, R.; Barceló, D.; de Cazes, M.; Belleville, M.; Sanchez-Marcano, J.; de Gunzburg, J.; Couillerot, O.; et al. Removal of antibiotics in wastewater by enzymatic treatment with fungal laccase—Degradation of compounds does not always eliminate toxicity. Bioresour. Technol. 2016, 219, 500–509. [Google Scholar] [CrossRef]
- Guo, R.; Chen, J. Application of alga-activated sludge combined system (AASCS) as a novel treatment to remove cephalosporins. Chem. Eng. J. 2015, 260, 550–556. [Google Scholar] [CrossRef]
- Iatrou, E.I.; Stasinakis, A.S.; Thomaidis, N.S. Consumption-based approach for predicting environmental risk in Greece due to the presence of antimicrobials in domestic wastewater. Environ. Sci. Pollut. Res. 2014, 21, 12941–12950. [Google Scholar] [CrossRef]
- Richmond, E.K.; Rosi, E.J.; Walters, D.M.; Fick, J.; Hamilton, S.K.; Brodin, T.; Sundelin, A.; Grace, M.R. A diverse suite of pharmaceuticals contaminates stream and riparian food webs. Nat. Commun. 2018, 9, 4491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchant, J. When antibiotics turn toxic. Nature 2018, 555, 431–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cela-Dablanca, R.; Nebot, C.; López, L.R.; Ferández-Calviño, D.; Arias-Estévez, M.; Núñez-Delgado, A.; Álvarez-Rodríguez, E.; Fernández-Sanjurjo, M.J. Retention of the Antibiotic Cefuroxime onto Agricultural and Forest Soils. Appl. Sci. 2021, 11, 4663. [Google Scholar] [CrossRef]
- Pan, M.; Chu, L.M. Occurrence of antibiotics and antibiotic resistance genes in soils from waste water irrigation areas in the Pearl River Delta region, southern China. Sci. Total Environ. 2017, 12, 145–152. [Google Scholar]
- Ding, H.; Wu, Y.; Zou, B.; Lou, Q.; Zhang, W.; Zhong, J.; Lu, L.; Dai, G. Simultaneous removal and degradation characteristics of sulfonamide, tetracycline, and quinolone antibiotics by laccase-mediated oxidation coupled with soil adsorption. J. Hazard. Mater. 2016, 307, 350–358. [Google Scholar] [CrossRef]
- Azhar, M.R.; Abid, H.R.; Periasamy, V.; Sun, H.; Tade, M.O.; Wang, S. Adsorptive removal of antibiotic sulfonamide by UiO-66 and ZIF-67 for wastewater treatment. J. Colloid Interface Sci. 2017, 500, 88–95. [Google Scholar] [CrossRef]
- Malakootian, M.; Yaseri, M.; Faraji, M. Removal of antibiotics from aqueous solutions by nanoparticles: A systematic review and meta-analysis. Environ. Sci. Pollut. Res. 2019, 26, 8444–8458. [Google Scholar] [CrossRef] [PubMed]
- Ata, R.; Sacco, O.; Vaiano, V.; Rizzo, L.; Tore, G.Y.; Sannino, D. Visible light active N- doped TiO2 immobilized on polystyrene as efficient system for wastewater treatment. J. Photochem. Photobiol. A 2017, 348, 255–262. [Google Scholar] [CrossRef]
- Crisafully, R.; Milhome, M.A.L.; Cavalcante, R.M.; Silveira, E.R.; De Keukeleire, D.; Nascimiento, R.F. Removal of some polycyclic aromatic hydrocarbons from petrochemical wastewater using low-cost adsorbents of natural origin. Bioresour. Technol. 2008, 99, 4515–4519. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Delgado, A.; Álvarez-Rodríguez, E.; Fernández-Sanjurjo, M.J.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M.; Fernández-Calviño, D. Perspectives on the use of by-products to treat soil and water pollution. Microporous Mesoporous Mat. 2015, 210, 199–201. [Google Scholar] [CrossRef]
- Fakhri, A.; Adami, S. Adsorption and thermodynamic study of Cephalosporins antibiotics from aqueous solution onto MgO nanoparticles. J. Taiwan Inst. Chem. Eng. 2014, 45, 1001–1006. [Google Scholar] [CrossRef]
- Kümmerer, K. The presence of pharmaceuticals in the environment due to human use—present knowledge and future challenges. J. Environ. Manag. 2009, 90, 2354–2366. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Schmidt, T.C. Determination of acid dissociation constants (pKa) of cephalosporin antibiotics: Computational and experimental approaches. Chemosphere 2017, 169, 524–533. [Google Scholar] [CrossRef]
- Evagelou, V.; Tsantili-Kakoulidou, A.; Koupparis, M. Determination of the dissociation constants of the cephalosporins cefepime and cefpirome using UV spectrometry and pH potentiometry. J. Pharmac. Biomed. Anal. 2003, 31, 1119–1128. [Google Scholar] [CrossRef]
- Legnoverde, M.S.; Simonetti, S.; Basaldella, E.I. Influence of pH on cephalexin adsorption onto SBA-15 mesoporous silica: Theoretical and experimental study. Appl. Surf. Sci. 2014, 300, 37–42. [Google Scholar] [CrossRef]
- Conde-Cid, M.; Ferreira-Coelho, G.; Arias-Estévez, M.; Álvarez-Esmorís, C.; Nóvoa-Muñoz, J.C.; Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E. Competitive adsorption/desorption of tetracycline, oxytetracycline and chlortetracycline on pine bark, oak ash and mussel shell. J. Environ. Manag. 2019, 250, 109509–109519. [Google Scholar] [CrossRef]
- Conde-Cid, M.; Cela-Dablanca, R.; Ferreira-Coelho, G.; Fernández-Calviño, D.; Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Arias-Estévez, M.; Álvarez-Rodríguez, E. Sulfadiazine, sulfamethazine and sulfachloropyridazine removal using three different porous materials: Pine bark, “oak ash” and mussel shell. Environ. Res. 2021, 195, 110814–110820. [Google Scholar] [CrossRef]
- Liu, H.; Liu, W.; Zhang, J.; Zhang, C.; Ren, L.; Li, Y. Removal of cephalexin from aqueous solutions by original and Cu (II)/Fe (III) impregnated activated carbons developed from stalks kinetics and equilibrium studies. J. Hazard. Mater. 2011, 185, 1528–1535. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Theydan, S.K. Adsorption of cephalexin onto active carbons from Albizia lebbeck seed pods by microwave induced KOH and K2CO3 activations. Chem. Eng. Sci. 2012, 211–212, 200–207. [Google Scholar] [CrossRef]
- Pouretedal, H.R.; Sadegh, N. Effective removal of Amoxicillin, Cephalexin, Tetracycline and Penicillin G from aqueous solutions using activated carbon nanoparticles prepared from vine wood. J. Water Process. Eng. 2014, 1, 64–73. [Google Scholar] [CrossRef]
- Mitchell, S.M.; Subbiah, M.; Ullman, J.L.; Frear, C.; Call, D.R. Evaluation of 27 different biochars for potential sequestration of antibiotic residues in food animal production environments. J. Environ. Chem. Eng. 2015, 3, 162–169. [Google Scholar] [CrossRef]
- Nazari, G.; Abolghasemi, H.; Esmaieli, M.; Pouya, E.S. Aqueous phase adsorption of cephalexin by walnut shell-based activated carbon: A fixed-bed column study. Appl. Surf. Sci. 2016, 375, 144–153. [Google Scholar] [CrossRef]
- Samarghandi, M.R.; Al-Musawi, T.J.; Mohsensi-Bandpi, A.; Zarrabi, M. Adsorption of cephalexin from aqueous solution using natural zeolite and zeolite coated with manganese oxide nanoparticles. J. Mol. Liq. 2015, 211, 431–441. [Google Scholar] [CrossRef]
- Taheran, M.; Naghdi, M.; Brar, S.K.; Knystautas, E.J.; Verma, M.; Ramirez, A.A.; Surampalli, R.Y.; Valero, J.R. Adsorption study of environmentally relevant concentrations of chlortetracycline on pinewood biochar. Sci. Total Environ. 2016, 571, 772–777. [Google Scholar] [CrossRef]
- Shimabuku, K.K.; Kearns, J.P.; Martinez, J.E.; Mahoney, R.B.; Moreno-Vasquez, L.; Summer, R.S. Biochar sorbents for sulfamethoxazole removal from surface stormwater, and wastewater effluent. Water Res. 2016, 96, 236–245. [Google Scholar] [CrossRef] [Green Version]
- Jafari, M.; Aghamiri, S.F.; Khaghanic, G. Batch Adsorption of Cephalosporins Antibiotics from Aqueous Solution by Means of Multi-Walled Carbon Nanotubes. World Appl. Sci. J. 2011, 14, 1642–1650. [Google Scholar]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Behnajady, M.A.; Bimeghdar, S. Synthesis of mesoporous NiO nanoparticles and their application in the adsorption of Cr (VI). Chem. Eng. J. 2014, 239, 105–113. [Google Scholar] [CrossRef]
- Kong, W.; Li, C.; Dolhi, J.M.; Li, S.; He, J.; Qiao, M. Characteristics of oxytetracycline sorption and potential bioavailability in soils with various physical–chemical properties. Chemosphere 2012, 87, 542–548. [Google Scholar] [CrossRef]
- De Arsénio, S.; Abreu, A.S.; Moura, I.; Vera-Machado, A. Polymeric materials for metal sorption from hydric resources. Water Purif. 2017, 289–322. [Google Scholar] [CrossRef]
- Conde-Cid, M.; Fernández-Calviño, D.; Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Arias-Estévez, M.; Álvarez-Rodríguez, E. Influence of mussel shell, oak ash and pine bark on the adsorption and desorption of sulfonamides in agricultural soils. J. Environ. Manag. 2020, 261, 110221–110231. [Google Scholar] [CrossRef]

| Parameter | Unit | Eucalyptus Leaves | Pine Bark | Pine Needles | Wood Ash | Mussel Shell |
|---|---|---|---|---|---|---|
| C | % | 53.05 | 48.70 | 50.31 | 13.23 | 11.43 |
| N | % | 1.35 | 0.08 | 1.08 | 0.22 | 0.21 |
| C/N | 39.18 | 608.75 | 46.76 | 60.14 | 55.65 | |
| pHwater | 4.88 | 3.99 | 3.68 | 11.31 | 9.39 | |
| pHKCl | 4.81 | 3.42 | 3.51 | 13.48 | 9.04 | |
| Cae | cmolc kg−1 | 7.95 | 5.38 | 2.13 | 95.0 | 24.75 |
| Mge | cmolc kg−1 | 8.53 | 2.70 | 7.15 | 3.26 | 0.72 |
| Nae | cmolc kg−1 | 1.36 | 0.46 | 1.42 | 12.17 | 4.37 |
| Ke | cmolc kg−1 | 12.93 | 4.60 | 11.09 | 250.65 | 0.38 |
| Ale | cmolc kg−1 | 0.13 | 1.78 | 2.15 | 0.07 | 0.03 |
| eCEC | cmolc kg−1 | 30.90 | 14.92 | 23.94 | 361.15 | 30.25 |
| Sat Al | % | 0.42 | 11.93 | 8.98 | 0.02 | 0.10 |
| Available-P | mg kg−1 | 262.84 | 70.45 | 217.95 | 462.83 | 54.17 |
| NaT | mg kg−1 | 242.31 | 68.92 | 271.54 | 2950 | 5174.00 |
| MgT | mg/kg | 840.96 | 473.55 | 653.40 | 26,171 | 980.66 |
| AlT | mg kg−1 | 80.58 | 561.50 | 246.95 | 14,966 | 433.24 |
| KT | mg kg−1 | 4464.10 | 737.84 | 4123.44 | 99,515 | 202.07 |
| CaT | mg kg−1 | 2262.96 | 2318.81 | 538.96 | 136,044 | 280,168 |
| CrT | mg kg−1 | 0.13 | 1.88 | 0.74 | 36.28 | 4.51 |
| MnT | mg kg−1 | 614.92 | 30.19 | 356.28 | 10,554 | 33.75 |
| FeT | mg kg−1 | 43.13 | 169.78 | 47.27 | 12,081 | 3535 |
| CoT | mg kg−1 | 0.03 | 0.20 | 0.38 | 17.25 | 1.02 |
| NiT | mg kg−1 | 2.17 | 1.86 | 0.93 | 69.25 | 8.16 |
| CuT | mg kg−1 | 2.80 | <LD | 3.81 | 146.33 | 6.72 |
| ZnT | mg kg−1 | 7.66 | 6.98 | 5.78 | 853.00 | 7.66 |
| AsT | mg kg−1 | 0.02 | <LD | 0.02 | 8.36 | 1.12 |
| CdT | mg kg−1 | 0.0 | 0.13 | 0.05 | 19.93 | 0.07 |
| Alo | mg kg−1 | 45.0 | 315.0 | 169.0 | 8323 | 178.33 |
| Feo | mg kg−1 | 77.0 | 74.0 | 15.0 | 4233 | 171.0 |
| Freundlich | Langmuir | Linear Model | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adsorbent | KF | Error | n | Error | R2 | KL | Error | qm | Error | R2 | Kd | Error | R2 |
| Eucalyptus leaves | 66.94 | 18.00 | 0.72 | 0.13 | 0.955 | 0.067 | 0.056 | 862.05 | 458.17 | 0.951 | 36.41 | 2.45 | 0.929 |
| Pine bark | 110.86 | 27.94 | 0.54 | 0.09 | 0.965 | 0.096 | 0.038 | 817.67 | 140.82 | 0.971 | 30.41 | 2.70 | 0.867 |
| Pine needles | 161.98 | 27.35 | 0.43 | 0.07 | 0.967 | 0.202 | 0.067 | 700.09 | 75.87 | 0.974 | 34.25 | 4.31 | 0.748 |
| Wood ash | - | - | - | - | - | - | - | - | - | - | 597.15 | 181.51 | 0.145 |
| Mussel shell | 93.25 | 34.91 | 0.77 | 0.18 | 0.909 | 0.077 | 0.067 | 1223.47 | 666.70 | 0.923 | 56.83 | 5.09 | 0.884 |
| C0 (µmol L−1) | |||||||
|---|---|---|---|---|---|---|---|
| Sorbent | 2.5 | 5 | 10 | 20 | 30 | 40 | 50 |
| Eucalyptus leaves | 6.4(19.8) | 16.3(20.2) | 41.1(22.2) | 65.9(19.5) | 141.1(27.5) | 164.5(24.9) | 212.2(28.6) |
| Pine bark | 8.4(25.9) | 36.2(44.8) | 84.0(59.1) | 133.3(44.5) | 157.0(37.1) | 141.8(28.6) | 146.5(26.6) |
| Pine needles | 0(0) | 15.4(20.5) | 20.7(13.0) | 19.5(5.9) | - | - | - |
| Wood ash | 0(0) | - | - | 0(0) | - | 0(0) | 0(0) |
| Mussel shell | 0.3(12.2) | 0.6(8.4) | 1.6(10.7) | 1.5(3.9) | 1.4(2.9) | 1.5(2.1) | 1.5(2.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cela-Dablanca, R.; Nebot, C.; Rodríguez López, L.; Fernández-Calviño, D.; Arias-Estévez, M.; Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E. Efficacy of Different Waste and By-Products from Forest and Food Industries in the Removal/Retention of the Antibiotic Cefuroxime. Processes 2021, 9, 1151. https://doi.org/10.3390/pr9071151
Cela-Dablanca R, Nebot C, Rodríguez López L, Fernández-Calviño D, Arias-Estévez M, Núñez-Delgado A, Fernández-Sanjurjo MJ, Álvarez-Rodríguez E. Efficacy of Different Waste and By-Products from Forest and Food Industries in the Removal/Retention of the Antibiotic Cefuroxime. Processes. 2021; 9(7):1151. https://doi.org/10.3390/pr9071151
Chicago/Turabian StyleCela-Dablanca, Raquel, Carolina Nebot, Lucia Rodríguez López, David Fernández-Calviño, Manuel Arias-Estévez, Avelino Núñez-Delgado, María J. Fernández-Sanjurjo, and Esperanza Álvarez-Rodríguez. 2021. "Efficacy of Different Waste and By-Products from Forest and Food Industries in the Removal/Retention of the Antibiotic Cefuroxime" Processes 9, no. 7: 1151. https://doi.org/10.3390/pr9071151