Extraction of Added-Value Triterpenoids from Acacia dealbata Leaves Using Supercritical Fluid Extraction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Acacia Dealbata Biomass
2.3. Soxhlet Extraction
2.4. Supercritical Fluid Extraction
2.5. Gas Chromatography Coupled to Mass Spectrometry
3. Results and Discussion
3.1. Total Extraction Yield
3.2. Volatile Extractives
3.3. Triterpenoid Extraction Yields
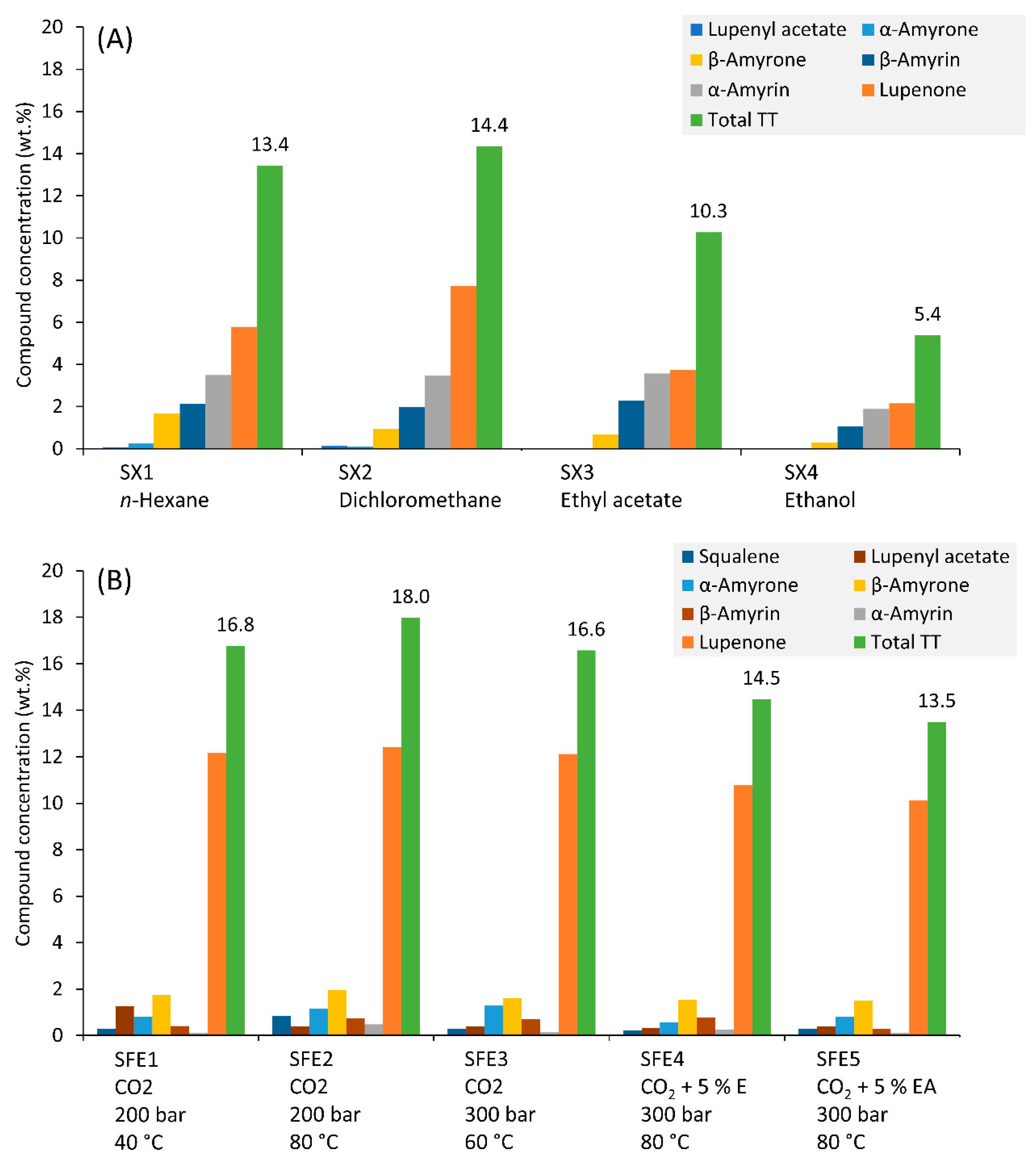
3.4. Triterpenoid Concentration in Extracts
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lorenzo, P.; Gonzalez, L.O.; Reigosa, M.J. The genus Acacia as invader: The characteristic case of Acacia dealbata Link in Europe. Ann. For. Sci. 2010, 67, 101. [Google Scholar] [CrossRef] [Green Version]
- Kull, C.A.; Shackleton, C.M.; Cunningham, P.J.; Ducatillon, C.; Dufour-Dror, J.-M.; Esler, K.J.; Friday, J.B.; Gouveia, A.C.; Griffin, A.R.; Marchante, E.; et al. Adoption, use and perception of Australian acacias around the world. Divers. Distrib. 2011, 17, 822–836. [Google Scholar] [CrossRef] [Green Version]
- Le Maitre, D.C.; Gaertner, M.; Marchante, E.; Ens, E.-J.; Holmes, P.M.; Pauchard, A.; O’Farrell, P.J.; Rogers, A.M.; Blanchard, R.; Blignaut, J.; et al. Impacts of invasive Australian acacias: Implications for management and restoration. Divers. Distrib. 2011, 17, 1015–1029. [Google Scholar] [CrossRef]
- Instituto da Conservação da Natureza e das Florestas. Inventário Florestal Nacional (IFN6)–Principais Resultados; 2019. [Google Scholar]
- European Commission Directive (EU) 2018/2002 of the European Parliament and of the Council of 11 December 2018 amending Directive 2012/27/EU on energy efficiency. Off. J. Eur. Union 2018, 61, 1–230.
- Oliveira, C.S.D.; Moreira, P.; Resende, J.; Cruz, M.T.; Pereira, C.M.F.; Silva, A.M.S.; Santos, S.A.O.; Silvestre, A.J.D. Characterization and Cytotoxicity Assessment of the Lipophilic Fractions of Different Morphological Parts of Acacia dealbata. Int. J. Mol. Sci. 2020, 21, 1814. [Google Scholar] [CrossRef] [Green Version]
- Freire, C.; Coelho, D.S.C.; Santos, N.M.; Silvestre, A.J.D.; Neto, C. Identification of Δ7 phytosterols and phytosteryl glucosides in the wood and bark of several Acacia species. Lipids 2005, 40, 317–322. [Google Scholar] [CrossRef]
- Luís, A.; Gil, N.; Amaral, M.E.; Duarte, A.P. Antioxidant activities of extracts from Acacia melanoxylon, Acacia dealbata and Olea europaea and alkaloids estimation. Int. J. Pharm. Pharm. Sci. 2012, 4, 225–231. [Google Scholar]
- Yáñez, R.; Gómez, B.; Martínez, M.; Gullón, B.; Alonso, J.L.; Estévez, B.G. Valorization of an invasive woody species, Acacia dealbata, by means of Ionic liquid pretreatment and enzymatic hydrolysis. J. Chem. Technol. Biotechnol. 2014, 89, 1337–1343. [Google Scholar] [CrossRef]
- Yildiz, S.; Gürgen, A.; Can, Z.; Tabbouche, S.A.; Kiliç, A.O. Some bioactive properties of Acacia dealbata extracts and their potential utilization in wood protection. Drewno 2018, 61, 81–97. [Google Scholar] [CrossRef]
- Devi, S.R.; Prasad, M.N.V. Tannins and related polyphenols from ten common Acacia species of India. Bioresour. Technol. 1991, 36, 189–192. [Google Scholar] [CrossRef]
- Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P. Demonstration of long-chain n-alkyl caffeates and Δ7-steryl glucosides in the bark of Acacia species by gas chromatography-mass spectrometry. Phytochem. Anal. 2007, 18, 151–156. [Google Scholar] [CrossRef]
- Lisperguer, J.; Saraiva, Y.; Vergara, E. Structure and thermal behavior of tannins from Acacia dealbata bark and their reactivity toward formaldehyde. J. Chil. Chem. Soc. 2016, 61, 3188–3190. [Google Scholar] [CrossRef] [Green Version]
- Neiva, D.M.; Luís, Â.; Gominho, J.; Domingues, F.; Duarte, A.P.; Pereira, H. Bark residues valorization potential regarding antioxidant and antimicrobial extracts. Wood Sci. Technol. 2020, 54, 559–585. [Google Scholar] [CrossRef]
- Casas, M.P.; Conde, E.; Ribeiro, D.; Fernandes, E.; Domínguez, H.; Torres, M.D. Bioactive properties of Acacia dealbata flowers extracts. Waste Biomass Valoriz. 2020, 11, 2549–2557. [Google Scholar] [CrossRef]
- López-Hortas, L.; Falqué, E.; Domínguez, H.; Torres, M.D. Microwave hydrodiffusion and gravity versus conventional distillation for Acacia dealbata flowers. Recovery of bioactive extracts for cosmetic purposes. J. Clean. Prod. 2020, 274. [Google Scholar] [CrossRef]
- Pereira, F.B.M.; Domingues, F.M.J.; Silva, A.M.S. Triterpenes from Acacia dealbata. Nat. Prod. Lett. 1996, 8, 97–103. [Google Scholar] [CrossRef]
- Imperato, F. A chalcone glycoside from Acacia dealbata. Phytochemistry 1982, 21, 480–481. [Google Scholar] [CrossRef]
- Rodolphe, P.; Katharina, B.; Meierhenrich, U.J.; Elise, C.; Georges, F.; Nicolas, B. Chemical composition of french mimosa absolute oil. J. Agric. Food Chem. 2010, 58, 1844–1849. [Google Scholar] [CrossRef]
- Soto, M.L.; Parada, M.; Falqué, E.; Domínguez, H. Personal-care products formulated with natural antioxidant extracts. Cosmetics 2018, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Silva, E.; Fernandes, S.; Bacelar, E.; Sampaio, A. Antimicrobial activity of aqueous, ethanolic and methanolic leaf extracts from Acacia spp. and Eucalyptus nicholii. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 130–134. [Google Scholar] [CrossRef] [Green Version]
- Borges, A.; José, H.; Homem, V.; Simões, M. Comparison of Techniques and Solvents on the Antimicrobial and Antioxidant Potential of Extracts from Acacia dealbata and Olea europaea. Antibiotics 2020, 9, 48. [Google Scholar] [CrossRef]
- de Melo, M.M.R.; Oliveira, E.L.G.; Silvestre, A.J.D.; Silva, C.M. Supercritical fluid extraction of vegetable matrices: Applications, trends and future perspectives of a convincing green technology. J. Supercrit. Fluids 2014, 92, 115–176. [Google Scholar] [CrossRef]
- Reverchon, E.; Marco, I. De Supercritical fluid extraction and fractionation of natural matter. J. Supercrit. Fluids 2006, 38, 146–166. [Google Scholar] [CrossRef]
- Rodrigues, V.H.; de Melo, M.M.R.; Portugal, I.; Silva, C.M. Extraction of Eucalyptus leaves using solvents of distinct polarity. Cluster analysis and extracts characterization. J. Supercrit. Fluids 2018, 135, 263–274. [Google Scholar] [CrossRef]
- Rodrigues, V.H.; de Melo, M.M.R.; Portugal, I.; Silva, C.M. Supercritical fluid extraction of Eucalyptus globulus leaves. Experimental and modelling studies of the influence of operating conditions and biomass pretreatment upon yields and kinetics. Sep. Purif. Technol. 2018, 191, 173–181. [Google Scholar] [CrossRef]
- Domingues, R.M.A.; de Melo, M.M.R.; Oliveira, E.L.G.; Neto, C.P.; Silvestre, A.J.D.; Silva, C.M. Optimization of the supercritical fluid extraction of triterpenic acids from Eucalyptus globulus bark using experimental design. J. Supercrit. Fluids 2013, 74, 105–114. [Google Scholar] [CrossRef]
- de Melo, M.M.R.; Oliveira, E.L.G.; Silvestre, A.J.D.; Silva, C.M. Supercritical fluid extraction of triterpenic acids from Eucalyptus globulus bark. J. Supercrit. Fluids 2012, 70, 137–145. [Google Scholar] [CrossRef]
- Domingues, R.M.A.; Oliveira, E.L.G.; Freire, C.S.R.; Couto, R.M.; Simões, P.C.; Neto, C.P.; Silvestre, A.J.D.; Silva, C.M. Supercritical fluid extraction of Eucalyptus globulus bark-A promising approach for triterpenoid production. Int. J. Mol. Sci. 2012, 13, 7648–7662. [Google Scholar] [CrossRef] [Green Version]
- de Melo, M.M.R.; Carius, B.; Simões, M.M.Q.; Portugal, I.; Saraiva, J.; Silva, C.M. Supercritical CO2 extraction of V. vinifera leaves: Influence of cosolvents and particle size on removal kinetics and selectivity to target compounds. J. Supercrit. Fluids 2020, 165, 104959. [Google Scholar] [CrossRef]
- de Melo, M.M.R.; Vieira, P.G.; Şen, A.; Pereira, H.; Portugal, I.; Silva, C.M. Optimization of the supercritical fluid extraction of Quercus cerris cork towards extraction yield and selectivity to friedelin. Sep. Purif. Technol. 2020, 238, 116395. [Google Scholar] [CrossRef]
- Martins, P.F.; de Melo, M.M.R.; Sarmento, P.; Silva, C.M. Supercritical fluid extraction of sterols from Eichhornia crassipes biomass using pure and modified carbon dioxide. Enhancement of stigmasterol yield and extract concentration. J. Supercrit. Fluids 2016, 107, 441–449. [Google Scholar] [CrossRef]
- Evans, C.S.; Qureshi, M.Y.; Bell, E.A. Free amino acids in the seeds of Acacia species. Phytochemistry 1977, 16, 565–570. [Google Scholar] [CrossRef]
- Domingues, R.; Guerra, A.; Duarte, M.; Freire, C.; Neto, C.; Silva, C.; Silvestre, A. Bioactive Triterpenic Acids: From Agroforestry Biomass Residues to Promising Therapeutic Tools. Mini Rev. Org. Chem. 2014, 11, 382–399. [Google Scholar] [CrossRef]
- de Melo, M.M.R.; Domingues, R.M.A.; Silvestre, A.J.D.; Silva, C.M. Extraction and purification of triterpenoids using supercritical fluids: From lab to exploitation. Mini Rev. Org. Chem. 2014, 362–381. [Google Scholar] [CrossRef]
- Xu, F.; Huang, X.; Wu, H.; Wang, X. Beneficial health effects of lupenone triterpene: A review. Biomed. Pharmacother. 2018, 103, 198–203. [Google Scholar] [CrossRef]
- Saleem, M. Lupeol, a novel anti-inflammatory and anti-cancer dietary triterpene. Cancer Lett. 2009, 285, 109–115. [Google Scholar] [CrossRef] [Green Version]
- Meneses-Sagrero, S.E.; Navarro-Navarro, M.; Ruiz-Bustos, E.; Del-Toro-Sánchez, C.L.; Jiménez-Estrada, M.; Robles-Zepeda, R.E. Antiproliferative activity of spinasterol isolated of Stegnosperma halimifolium (Benth, 1844). Saudi Pharm. J. 2017, 25, 1137–1143. [Google Scholar] [CrossRef]
- Lou-Bonafonte, J.M.; Martínez-Beamonte, R.; Sanclemente, T.; Surra, J.C.; Herrera-Marcos, L.V.; Sanchez-Marco, J.; Arnal, C.; Osada, J. Current Insights into the Biological Action of Squalene. Mol. Nutr. Food Res. 2018, 62, 1–16. [Google Scholar] [CrossRef]
- Aragão, G.F.; Carneiro, L.M.V.; Junior, A.P.F.; Vieira, L.C.; Bandeira, P.N.; Lemos, T.L.G.; de B. Viana, G.S. A possible mechanism for anxiolytic and antidepressant effects of alpha- and beta-amyrin from Protium heptaphyllum (Aubl.) March. Pharmacol. Biochem. Behav. 2006, 85, 827–834. [Google Scholar] [CrossRef]
- Aragão, G.F.; Cunha Pinheiro, M.C.; Nogueira Bandeira, P.; Gomes Lemos, T.L.; de Barros Viana, G.S. Analgesic and anti-inflammatory activities of the isomeric mixture of alpha- and beta-amyrin from protium heptaphyllum (Aubl.) March. J. Herb. Pharmacother. 2007, 7, 31–47. [Google Scholar] [CrossRef] [PubMed]
- da Silva Júnior, W.F.; Lima Bezerra de Menezes, D.; Calvarho de Oliveira, L.; Scherer Koester, L.; Olveira de Almeida, P.D.; Lima, E.S.; Pereira de Azevedo, E.; da Veiga Júnior, V.F.; Neves de Lima, Á.A. Inclusion complexes of β and HPβ-cyclodextrin with α, β amyrin and in vitro anti-inflammatory activity. Biomolecules 2019, 9, 241. [Google Scholar] [CrossRef] [Green Version]
- Holanda Pinto, S.A.; Pinto, L.M.S.; Cunha, G.M.A.; Chaves, M.H.; Santos, F.A.; Rao, V.S. Anti-inflammatory effect of α, β-Amyrin, a pentacyclic triterpene from Protium heptaphyllum in rat model of acute periodontitis. Inflammopharmacology 2008, 16, 48–52. [Google Scholar] [CrossRef]
- Oliveira, F.A.; Vieira-Júnior, G.M.; Chaves, M.H.; Almeida, F.R.C.; Florêncio, M.G.; Lima, R.C.P., Jr.; Silva, R.M.; Santos, F.A.; Rao, V.S.N. Gastroprotective and anti-inflammatory effects of resin from Protium heptaphyllum in mice and rats. Pharmacol. Res. 2004, 49, 105–111. [Google Scholar] [CrossRef]
- Okoye, N.N.; Ajaghaku, D.L.; Okeke, H.N.; Ilodigwe, E.E.; Nworu, C.S.; Okoye, F.B.C. Beta-Amyrin and alpha-Amyrin acetate isolated from the stem bark of Alstonia boonei display profound anti-inflammatory activity. Pharm. Biol. 2014, 52, 1478–1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jæger, D.; O’Leary, M.C.; Weinstein, P.; Møller, B.L.; Semple, S.J. Phytochemistry and bioactivity of Acacia sensu stricto (Fabaceae: Mimosoideae). Phytochem. Rev. 2019, 18, 129–172. [Google Scholar] [CrossRef]
- Subhan, N.; Burrows, G.E.; Kerr, P.G.; Obied, H.K. Phytochemistry, Ethnomedicine, and Pharmacology of Acacia. Stud. Nat. Prod. Chem. 2018, 57, 247–326. [Google Scholar] [CrossRef]
- Pitzer, K.S.; Schreiber, D.R. Improving equation-of-state accuracy in the critical region; equations for carbon dioxide and neopentane as examples. Fluid Phase Equilib. 1988, 41, 1–17. [Google Scholar] [CrossRef]
- Pöhler, H.; Kiran, E. Volumetric properties of carbon dioxide + ethanol at high pressures. J. Chem. Eng. Data 1997, 42, 384–388. [Google Scholar] [CrossRef]
- Falco, N.; Kiran, E. Volumetric properties of ethyl acetate + carbon dioxide binary fluid mixtures at high pressures. J. Supercrit. Fluids 2012, 61, 9–24. [Google Scholar] [CrossRef]
- Gujar, A.; Anderson, T.; Cavagnino, D.; Patel, A. Comparative analysis of mass spectral matching for confident compound identification using the Advanced Electron Ionization source for GC-MS. Thermoscientific 2018, 10598, 1–7. [Google Scholar]
- Zuluaga, A.M.; Mena-García, A.; Soria Monzón, A.C.; Rada-Mendoza, M.; Chito, D.M.; Ruiz-Matute, A.I.; Sanz, M.L. Microwave assisted extraction of inositols for the valorization of legume by-products. LWT 2020, 133, 109971. [Google Scholar] [CrossRef]
- Zuluaga, A.M.; Mena-García, A.; Chito-Trujillo, D.; Rada-Mendoza, M.; Sanz, M.L.; Ruiz-Matute, A.I. Development of a microwave-assisted extraction method for the recovery of bioactive inositols from lettuce ( Lactuca sativa) byproducts. Electrophoresis 2020, 41, 1804–1811. [Google Scholar] [CrossRef] [PubMed]
- Mena-García, A.; Rodríguez-Sánchez, S.; Ruiz-Matute, A.I.; Sanz, M.L. Exploitation of artichoke byproducts to obtain bioactive extracts enriched in inositols and caffeoylquinic acids by Microwave Assisted Extraction. J. Chromatogr. A 2020, 1613, 460703. [Google Scholar] [CrossRef]
- Ruiz-Aceituno, L.; García-Sarrió, M.J.; Alonso-Rodriguez, B.; Ramos, L.; Sanz, M.L. Extraction of bioactive carbohydrates from artichoke (Cynara scolymus L.) external bracts using microwave assisted extraction and pressurized liquid extraction. Food Chem. 2016, 196, 1156–1162. [Google Scholar] [CrossRef] [PubMed]
- Chóez-Guaranda, I.; Ruíz-Barzola, O.; Ruales, J.; Manzano, P. Antioxidant activity optimization and GC-MS profile of aqueous extracts of Vernonanthura patens (Kunth) H. Rob. leaves. Nat. Prod. Res. 2020, 34, 2505–2509. [Google Scholar] [CrossRef]
- Somsub, W.; Kongkachuichai, R.; Sungpuag, P.; Charoensiri, R. Effects of three conventional cooking methods on vitamin C, tannin, myo-inositol phosphates contents in selected Thai vegetables. J. Food Compos. Anal. 2008, 21, 187–197. [Google Scholar] [CrossRef]
- Warren, C.R.; Aranda, I.; Cano, F.J. Responses to water stress of gas exchange and metabolites in Eucalyptus and Acacia spp. Plant Cell Environ. 2011, 34, 1609–1629. [Google Scholar] [CrossRef]
- Croze, M.L.; Soulage, C.O. Potential role and therapeutic interests of myo-inositol in metabolic diseases. Biochimie 2013, 95, 1811–1827. [Google Scholar] [CrossRef] [PubMed]
- Pütter, K.M.; van Deenen, N.; Müller, B.; Fuchs, L.; Vorwerk, K.; Unland, K.; Bröker, J.N.; Scherer, E.; Huber, C.; Eisenreich, W.; et al. The enzymes OSC1 and CYP716A263 produce a high variety of triterpenoids in the latex of Taraxacum koksaghyz. Sci. Rep. 2019, 9, 5942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yendo, A.C.A.; De Costa, F.; Gosmann, G.; Fett-Neto, A.G. Production of plant bioactive Triterpenoid saponins: Elicitation strategies and target genes to improve yields. Mol. Biotechnol. 2010, 46, 94–104. [Google Scholar] [CrossRef]
- Fukushima, E.O.; Seki, H.; Ohyama, K.; Ono, E.; Umemoto, N.; Mizutani, M.; Saito, K.; Muranaka, T. CYP716A Subfamily Members are Multifunctional Oxidases in Triterpenoid Biosynthesis. Plant Cell Physiol. 2011, 52, 2050–2061. [Google Scholar] [CrossRef] [PubMed]
- Brendolise, C.; Yauk, Y.K.; Eberhard, E.D.; Wang, M.; Chagne, D.; Andre, C.; Greenwood, D.R.; Beuning, L.L. An unusual plant triterpene synthase with predominant α-amyrin—Producing activity identified by characterizing oxidosqualene cyclases from Malus × domestica. FEBS J. 2011, 278, 2485–2499. [Google Scholar] [CrossRef] [PubMed]







| Run | Method | Solvent | T (°C) | P (bar) | |
|---|---|---|---|---|---|
| SX1 | Soxhlet | n-Hexane | 68.5 * | 1 | - |
| SX2 | Dichloromethane | 39.6 * | 1 | - | |
| SX3 | Ethyl acetate | 77.1 * | 1 | - | |
| SX4 | Ethanol | 78.4 * | 1 | - | |
| SFE1 | SFE | CO2 | 40 | 200 | 840.6 [48] |
| SFE2 | CO2 | 80 | 200 | 594.9 [48] | |
| SFE3 | CO2 | 60 | 300 | 830.4 [48] | |
| SFE4 | CO2:Ethanol (95:5 wt.%) | 80 | 300 | 764.6 [49] | |
| SFE5 | CO2:Ethyl acetate (95:5 wt.%) | 80 | 300 | 761.4 [50] |
| Rt (min) | Compound | Family | RSI | SX1 | SX2 | SX3 | SX4 | SFE1 | SFE2 | SFE3 | SFE4 | SFE5 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10.77 | Glycerol | P | 909 | - | + | + | + | - | - | - | - | - |
| 18.67 | Erythritol | P | 942 | - | - | + | + | - | - | - | - | - |
| 20.6 | 5-Hydroxypipecolic acid | CA | 770 | - | - | - | + | - | - | - | - | - |
| 24.17 | Xylitol | P | 800 | - | - | + | + | - | - | - | - | - |
| 24.79 | Ribitol | P | 887 | - | - | + | + | - | - | - | - | - |
| 27.55 | myo-Inositol | P | 803 | - | - | + | + | - | - | - | - | - |
| 28.19 | Tyramine | A | 890 | - | - | - | + | - | - | - | - | - |
| 28.79 | D-Mannose | M | 803 | - | - | + | + | - | - | - | - | - |
| 30.13 | Mannitol | P | 851 | - | - | + | + | - | - | - | - | - |
| 31.27 | Glucose | M | 827 | - | - | + | + | - | - | - | - | - |
| 44.37 | Docosanoic acid | FA | 729 | + | + | - | - | + | + | + | + | + |
| 46.09 | Squalene | TT | 833 | - | - | - | - | + | + | + | + | + |
| 47.76 | Pentacosanoic acid | FA | 829 | + | + | + | + | + | + | + | + | + |
| 48.61 | Hexadecanoic acid | FA | 705 | + | + | + | - | - | - | - | - | - |
| 50.98 | Octacosanoic acid | FA | 849 | + | + | + | + | + | + | + | + | + |
| 51.82 | Octacosan-1-ol | LCAA | 811 | + | - | + | - | - | - | - | - | - |
| 52.66 | 4’-OH,5-OH,7-Di-O-Glucoside | F | 688 | + | + | + | + | + | + | + | + | + |
| 52.86 | α-Amyrone | TT | 786 | + | + | - | - | + | + | + | + | + |
| 53.06 | β-Amyrone | TT | 738 | + | + | + | + | + | + | + | + | + |
| 53.66 | Lupenone | TT | 843 | + | + | + | + | + | + | + | + | + |
| 54.92 | β-Amyrin | TT | 755 | + | + | + | + | + | + | + | + | + |
| 55.16 | α-Amyrin | TT | 691 | + | + | + | + | + | + | + | + | + |
| 56.28 | Lupenyl acetate | TT | 742 | + | + | - | - | + | + | + | + | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, V.H.; de Melo, M.M.R.; Portugal, I.; Silva, C.M. Extraction of Added-Value Triterpenoids from Acacia dealbata Leaves Using Supercritical Fluid Extraction. Processes 2021, 9, 1159. https://doi.org/10.3390/pr9071159
Rodrigues VH, de Melo MMR, Portugal I, Silva CM. Extraction of Added-Value Triterpenoids from Acacia dealbata Leaves Using Supercritical Fluid Extraction. Processes. 2021; 9(7):1159. https://doi.org/10.3390/pr9071159
Chicago/Turabian StyleRodrigues, Vítor H., Marcelo M. R. de Melo, Inês Portugal, and Carlos M. Silva. 2021. "Extraction of Added-Value Triterpenoids from Acacia dealbata Leaves Using Supercritical Fluid Extraction" Processes 9, no. 7: 1159. https://doi.org/10.3390/pr9071159
APA StyleRodrigues, V. H., de Melo, M. M. R., Portugal, I., & Silva, C. M. (2021). Extraction of Added-Value Triterpenoids from Acacia dealbata Leaves Using Supercritical Fluid Extraction. Processes, 9(7), 1159. https://doi.org/10.3390/pr9071159