UPLC-QToF Nanospray MS and NMR Analysis of Ficus sycomorus Stem Bark and Its Effects on Rabbit
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Plant Materials and Preparation of the Extracts
2.2. UPLC-QToF-MS Analysis and Molecular Networking for Screening of Secondary Metabolites
2.3. NMR Analysis
2.4. Experimental Evaluation of the Effect of F. sycomorus on Rabbit
2.5. Histopathological Examination
2.6. Statistical Analysis
3. Results
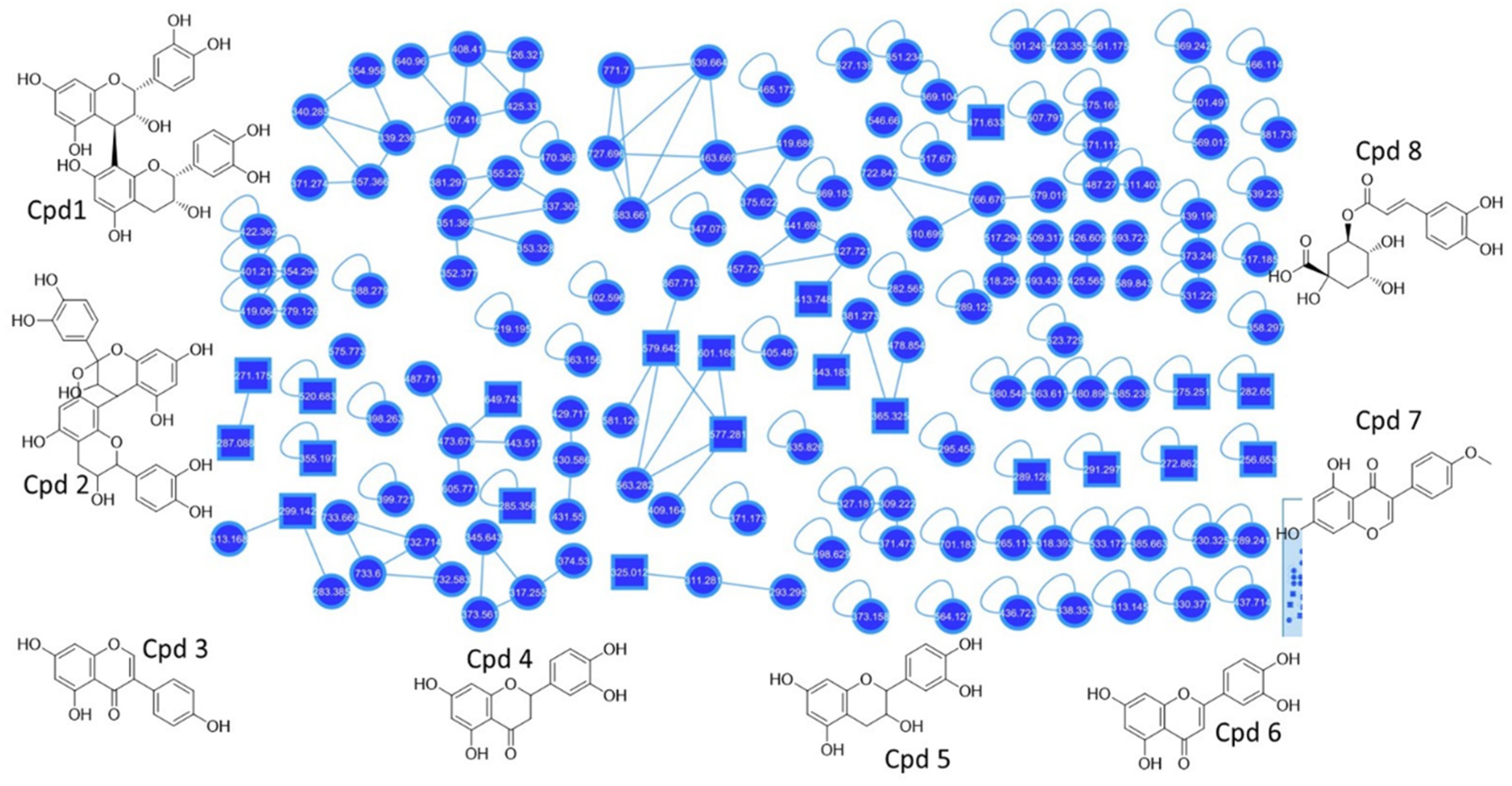
3.1. UPLC-QToF-MS and Molecular Networking Analysis
3.2. NMR Analysis
3.3. Growth Performance
3.4. Serum Biochemical Analysis and Hemogram
3.5. Internal Organs and Carcass Quality
3.6. Histopathological Findings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Noort, S.; Gardiner, A.J.; Tolley, K.A. New records of Ficus (Moraceae) species emphasize the conservation significance of inselbergs in Mozambique. S. Afr. J. Bot. 2007, 73, 642–649. [Google Scholar] [CrossRef] [Green Version]
- Sandabe, U.; Onyeyili, P.; Chibuzo, G. Phytochemical screening, and effect of aqueous extract of Ficus sycomorus L. (Moraceae) stembark on muscular activity in laboratory animals. J. Ethnopharmacol. 2006, 104, 283–285. [Google Scholar] [CrossRef]
- Zaku, S.G.; Abdulrahaman, F.A.; Onyeyili, P.A.; Aguzue, O.C.; Thomas, S.A. Phytochemical constituents, and effects of aqueous root-bark extract of Ficus sycomorus L. (Moracaea) on muscular relaxation, anaesthetic and sleeping time on laboratory animals. Afr. J. Biotechnol. 2009, 8, 6004–6006. [Google Scholar]
- Arnold, H.-J.; Gulumian, M. Pharmacopoeia of traditional medicine in Venda. J. Ethnopharmacol. 1984, 12, 35–74. [Google Scholar] [CrossRef]
- Konai, N.; Raidandi, D.; Pizzi, A.; Meva’a, L. Characterization of Ficus sycomorus tannin using ATR-FT MIR, MALDI-TOF MS and 13C NMR methods. Eur. J. Wood Wood Prod. 2017, 75, 807–815. [Google Scholar] [CrossRef]
- Hassan, S.W.; Muhammad, L.M.; Umar, B.Y.; Bilbis, R.A.; Saidu, L.S.; Saidu, Y. Effects of anthraquinone glycosides and aqueous ethanol extracts of Ficus sycomorus L. (Moraceae) on rat liver and kidney functions. Asian J. Biochem. 2007, 2, 136–141. [Google Scholar] [CrossRef] [Green Version]
- Al-Matani, S.K.; Al-Wahaibi, R.N.S.; Hossain, M.A. Total flavonoids content and antimicrobial activity of crude extract from leaves of Ficus sycomorus native to Sultanate of Oman. Karbala Int. J. Mod. Sci. 2015, 1, 166–171. [Google Scholar] [CrossRef] [Green Version]
- El-Sayyad, S.M.; Makboul, M.A.; Ali, R.M.; El-Amir, J.O.; Farag, S.F. Hepatoprotective activity of Ficus sycomorus L. against N-nitrosodiethylamine and CCL4 induced hepatocarcinogenesis in experimental rats. Res. Rev. J. Pharmacogn. Phytochem. 2015, 3, 1–5. [Google Scholar]
- Igbokwe, N.A.; Igbokwe, I.; Sandabe, U. Effect of prolonged oral administration of aqueous Ficus sycomorus stem-bark Extract on testicular size of growing albino rat. Int. J. Morphol. 2010, 28, 1315–1322. [Google Scholar] [CrossRef] [Green Version]
- Okpara, J.O.; Ochai, C.Z.; Shwarpshakka, S.Y.; Kaigoma, G.J.; Salihu, A. Phytochemical, and antibacterial activities of Ficus sycomorus (linneaus) leaves and unriped fruits extracts. IJASR 2017, 2, 43–56. [Google Scholar]
- Offiah, N.V.; Makama, S.; Elisha, I.L.; Makoshi, M.S.; Gotep, J.G.; Dawurung, C.J.; Oladipo, O.O.; Lohlum, A.S.; Shamaki, D. Ethnobotanical survey of medicinal plants used in the treatment of animal diarrhoea in Plateau State, Nigeria. BMC Vet. Res. 2011, 7, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayed, W.F.; Salem, W.M.; Haridy, M.A.; Hassan, N.H. Efficacy of Caltropis procera and Ficus sycomorus extracts in treating MRSA (methicillin-resistant Staphylococcus aureus)-keratitis in rabbit. EXCLI J. 2015, 14, 747–757. [Google Scholar] [PubMed]
- Nenaah, G. Antimicrobial activity of Calotropis procera Ait. (Asclepiadaceae) and isolation of four flavonoid glycosides as the active constituents. World J. Microbiol. Biotechnol. 2013, 29, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Oh, I.; Yang, W.Y.; Chung, S.C.; Kim, T.Y.; Oh, K.B.; Shin, J. In vitro sortase A inhibitory and antimicrobial activity of flavonoids isolated from the roots of Sophora flavescens. Arch. Pharmacal. Res. 2011, 34, 217–222. [Google Scholar] [CrossRef]
- Arunachalam, K.; Parimelazhagan, T. Anti-inflammatory, wound healing and in-vivo antioxidant properties of the leaves of Ficus amplissima Smith. J. Ethnopharmacol. 2013, 145, 139–145. [Google Scholar] [CrossRef]
- Salem, M.Z.; Salem, A.Z.M.; Camacho, L.M.; Hayssam, A.M. Antimicrobial activities and phytochemical composition of extracts of Ficus species: An overview. Afr. J. Microbiol. Res. 2013, 7, 4207–4219. [Google Scholar]
- Mousa, O.; Vuorela, P.; Kiviranta, J.; Wahab, S.A.; Hiltunen, R.; Vuorela, H. Bioactivity of certain Egyptian Ficus species. J. Ethnopharmacol. 1994, 41, 71–76. [Google Scholar] [CrossRef]
- Ibrahim, G.; Yaro, A.H.; Abdurahman, M. Analgesic and anti-inflammatory activities of Ficus sycomorus (mor acea e). Niger J. Nat. Prod. Med. 2006, 10, 66–68. [Google Scholar]
- Njagi, J.M.; Piero, M.N.; Ngeranwa, J.J.N.; Njagi, E.N.M.; Kibiti, C.M.; Njue, W.M.; Maina, D.; Gathumbi, P.K. Assessment of antidiabetic potential of Ficus sycomorus on alloxan-induced diabetic mice. Int. J. Diabetes Res. 2012, 1, 47–51. [Google Scholar]
- Ahmadu, A.; Grougnet, R.; Magiatis, P.; Skaltsounis, A.L.; Tillequin, F. New peltogynoids from Ficus sycomorus L. Planta Med. 2007, 73, 385. [Google Scholar] [CrossRef]
- El-Sayed, M.; Mahmoud, M.A.; Abel-Khalik, H.; El-Toumy, S.; El-Wakil, E.; Ahmed, M. Bio-guided isolation, and structure elucidation of antioxidant compounds from the leaves of Ficus sycomorus. Pharmacologyonline 2010, 3, 317–332. [Google Scholar]
- Romeh, A. Phytochemicals from Ficus sycomorus L. leaves act as insecticides and acaricides. Afr. J. Agric. Res. 2013, 8, 3571–3579. [Google Scholar] [CrossRef] [Green Version]
- Garba, S.; Prasad, J.; Sandabe, U. Hepatoprotective effect of the aqueous root bark extract of Ficus sycomorus (Linn) on carbon tetrachloride induced hepatotoxicity in rats. J. Biol. Sci. 2007, 7, 276–281. [Google Scholar] [CrossRef] [Green Version]
- El-Sayed, M.M.; Abdel-Hadi, A.M.; Sabra, A.N.; Mahmoud, M.A.; El-Wakil, E.A.; Ghareeb, M.A. Effect of Ficus sycomorus and Azadirachta indica extracts on liver state of mice infected with Schistosoma mansoni. J. Egypt Soc. Parasitol. 2011, 41, 77–88. [Google Scholar] [PubMed]
- Igbokwe, N.A.; Sandabe, U.K.; Sanni, S.; Wampana, B.; Wiam, I.M.; Igbokwe, I.O. Aqueous stem-bark extract of Ficus sycomorus increases sperm production and pH of sperm microenvironment in growing albino rat. Anim. Reprod. 2009, 6, 509. [Google Scholar]
- Foyet, H.S.; Tchinda Deffo, S.; Koagne Yewo, P.; Antioch, I.; Zingue, S.; Asongalem, E.A.; Kamtchouing, P.; Ciobica, A. Ficus sycomorus extract reversed behavioral impairment and brain oxidative stress induced by unpredictable chronic mild stress in rats. BMC Complement. Altern. Med. 2017, 17, 502. [Google Scholar]
- Post, J.; Rebel, J.M.; ter Huurne, A.A. Automated blood cell count: A sensitive and reliable method to study corticosterone-related stress in broilers. Poult. Sci. 2003, 82, 591–595. [Google Scholar] [CrossRef]
- Figueroa-Pérez, M.G.; Pérez-Ramírez, I.F.; Paredes-López, O.; Mondragón-Jacobo, C.; Reynoso-Camacho, R. Phytochemical composition and in vitro analysis of nopal (O. Ficus-indica) cladodes at different stages of maturity. Int. J. Food Prop. 2018, 21, 1728–1742. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.-Q.; Lei, C.; Gao, L.-X.; Liao, H.-B.; Li, J.-Y.; Li, J.; Hou, A.J. Isoprenylated flavonoids with PTP1B inhibition from Ficus tikoua. Nat. Prod. Commun. 2015, 10, 2105–2107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.P.; Lv, H.; Li, Z.; Jiang, K.; Lee, M.-R. Identification, and characterization of major chemical compounds in the ethyl acetate extract from Ficus pandurata Hance aerial roots by HPLC-Q-TOF MS. J. Chin. Mass Spectrom. Soc. 2015, 36, 310–320. [Google Scholar]
- Soltana, H.; De Rosso, M.; Lazreg, H.; Vedova, A.D.; Hammami, M.; Flamini, R. LC-QTOF characterization of non-anthocyanic flavonoids in four Tunisian fig varieties. J. Mass Spectrom. 2018, 53, 817–823. [Google Scholar] [CrossRef]
- Suliman, S.; Yagi, S.; Elbashir, A.A.; Mohammed, A.; Hussein, A.; Ak, G.; Zengin, G.; Orlando, G.; Ferrante, C. Phenolic profile, enzyme inhibition and antioxidant activities and bioinformatics analysis of leaf and stem bark of Ficus sycomorus L. Process. Biochem. 2021, 101, 169–178. [Google Scholar] [CrossRef]
- Yunus, S.; Zolkefle, N.K.Z.; Abas, A.H.J.F. Metabolite identification in different fractions of Ficus auriculata Loureiro fruit using the 1H-NMR metabolomics approach and UHPLC-MS/MS. S. Afr. J. Bot. 2021, 138, 348–363. [Google Scholar] [CrossRef]
- Zingue, S.; Michel, T.; Nde, C.B.M.; Njuh, A.N.; Cisilotto, J.; Ndinteh, D.T.; Clyne, C.; Fernandez, X.; Creczynski-Pasa, T.B.; Njamen, D. Estrogen-like, and tissue-selective effects of 7-methoxycoumarin from Ficus umbellata (Moraceae): An in vitro and in vivo study. BMC Complement. Altern. Med. 2017, 17, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Micaelo, N.; González-Abuín, N.; Pinent, M.; Ardévol, A.; Blay, M. Procyanidin B2 inhibits inflammasome-mediated IL-1β production in lipopolysaccharide-stimulated macrophages. Mol. Nutr. Food Res. 2015, 59, 262–269. [Google Scholar] [CrossRef]
- He, J.; Sun, M.; Tian, S. Procyanidin B2 prevents lupus nephritis development in mice by inhibiting NLRP3 inflammasome activation. Innate Immun. 2018, 24, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Wienhoefer, N.; Bilitewski, U. Genistein induces morphology change and G2/M cell cycle arrest by inducing p38 MAPK activation in macrophages. Int. Immunopharmacol. 2014, 18, 142–150. [Google Scholar] [CrossRef]
- Mokdad-Bzeouich, I.; Maatouk, M.; Mustapha, N.; Mokdad-Bzeouich, I.; Chaaban, H.; Abed, B.; Iaonnou, I.; Ghedira, K.; Ghoul, M.; Ghedira, L.C. Investigation of immunomodulatory and anti-inflammatory effects of eriodictyol through its cellular antioxidant activity. Cell Stress Chaperones 2016, 21, 773–781. [Google Scholar] [CrossRef] [Green Version]
- Ganeshpurkar, A.; Saluja, A.K. Protective effect of catechin on humoral and cell mediated immunity in rat model. Int. Immunopharmacol. 2018, 54, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, Z.; Chadha, K.; Lieberman, A.; Drake, A.; Hojnacki, D.; Weinstock-Guttman, B.; Munschauer, F. Immunomodulatory responses of peripheral blood mononuclear cells from multiple sclerosis patients upon in vitro incubation with the flavonoid luteolin: Additive effects of IFN-β. J. Neuroinflammation 2009, 6, 28. [Google Scholar] [CrossRef] [Green Version]
- Sithisarn, P.; Michaelis, M.; Schubert-Zsilavecz, M.; Cinatl, J., Jr. Differential antiviral and anti-inflammatory mechanisms of the flavonoids biochanin A and baicalein in H5N1 influenza A virus-infected cells. Antivir. Res. 2013, 97, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Tang, X.; Guo, N.; An, Y.; Chen, X.; Shi, C.; Wang, C.; Li, Y.; Li, S.; Xu, H.; et al. Biochanin a enhances the defense against Salmonella enterica infection through AMPK/ULK1/mTOR-mediated autophagy and extracellular traps and reversing SPI-1-dependent macrophage (MΦ) M2 polarization. Front. Cell Infect. Microbiol. 2018, 8, 318. [Google Scholar] [CrossRef] [Green Version]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic acid: Recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef] [Green Version]
- El-Hashash, S.A. Effect of Ficus sycomorus L. Leaves on high fat diet-fed rats: Possible mechanisms behind the prevention of obesity and its related disorders. IOSR J. Environ. Sci. Toxicol. Food Technol. 2014, 8, 7–16. [Google Scholar] [CrossRef]
- Garb, S.; Prasad, J.; Sandabe, U.K. Histomorphological effect of the aqueous root -bark extract of Ficus Sycomorus (Linn) on the liver kidney of Albino rats. Int. J. Pharmacol. 2006, 2, 628–632. [Google Scholar]
- Paci, G.; Cecchi, F.; Preziuso, G.; Ciampolini, R.; D’Agata, M. Carcass traits and meat quality of two different rabbit genotypes. Ital. J. Anim. Sci. 2012, 11, 249–252. [Google Scholar] [CrossRef] [Green Version]
- Braide, W.; Dokubo, K.O.; Adeleye, S.A.; Uzoh, C.V.; Akobundu, C. Phytochemical properties, toxicological screening, and antibacterial qualities of various parts extracts of Ficus sycomorus. JMPHTR 2018, 6, 1–8. [Google Scholar]
- Sandabe, U.K.; Abdurrahaman, F.; Goniri, B.; Baba, U. The combined effects of aqueous extract of Ficus sycomorus l. (Moraceae) Stem bark and Nigella sativa l. (Ranunculaceae) seeds on hematological and biochemical parameters in rabbits. Anim. Res. Int. 2007, 4, 753–757. [Google Scholar] [CrossRef] [Green Version]
- Sukowati, Y.K.; Johan, A.; Murwani, R. Ethanol extracts of Ficus carica fruit and leaf normalize high serum lipid profile, TNF-α, and mda due to high fat diet in sprague dawley rat. Curr. Res. Nutr. Food Sci. 2019, 7, 772–782. [Google Scholar] [CrossRef]
- Oyewole, O.; Adanlawo, I.; Arise, R. Serum and tissue lipid profile in wistar rats administered leaf extract of Ficus exasperata. Ann. Biol. Res. 2013, 4, 288–291. [Google Scholar]
- Nurminen, M.-L.; Korpela, R.; Vapaatalo, H. Dietary factors in the pathogenesis and treatment of hypertension. Ann. Med. 1998, 30, 143–150. [Google Scholar] [CrossRef] [PubMed]

| Ingredients | Composition (%) |
|---|---|
| Maize | 40.00 |
| Soybean meal | 10.00 |
| Dried alfa alfa | 20.00 |
| Rice bran | 10.00 |
| White bran | 14.00 |
| Fish meal (72% CP) | 2.00 |
| Bone meal | 2.00 |
| Limestone | 1.00 |
| Premix (Growers) | 0.50 |
| Salt (NaCl) | 0.50 |
| Total | 100.00 |
| Calculated analysis | |
| Metabolizable energy (MJ/kg) | 10.91 |
| Crude protein (%) | 16.73 |
| Crude fiber (%) | 9.20 |
| Compound | MS | MF | Reference |
|---|---|---|---|
| Procyanidin B2 (1) | 579.642 | [C30H27O12]+ | [5,28] |
| Procyanidin A1 (2) | 577.281 | [C30H25O12]+ | [5,28] |
| Genistein (3) | 271.175 | [C15H11O5]+ | [29] |
| Dihydro-tetrahydroxy flavanone (4) Eriodictyol | 289.128 | [C15H13O6]+ | [30,31] |
| Catechin (5) | 291.297 | [C15H15O6]+ | [32] |
| Tetrahydroxy flavanone (6) Luteolin | 287.088 | [C15H11O6]+ | [33] |
| Biochanin_A (7) | 285.356 | [C16H13O5]+ | [34] |
| Chlorogenic acid (8) | 355.197 | [C16H19O9]+ | [33] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dawod, A.; Fathalla, S.I.; Elkhatam, A.; Osman, N.; Sheraiba, N.; Hammad, M.A.; El-Seedi, H.R.; Shehata, A.A.; Anis, A. UPLC-QToF Nanospray MS and NMR Analysis of Ficus sycomorus Stem Bark and Its Effects on Rabbit. Processes 2021, 9, 1201. https://doi.org/10.3390/pr9071201
Dawod A, Fathalla SI, Elkhatam A, Osman N, Sheraiba N, Hammad MA, El-Seedi HR, Shehata AA, Anis A. UPLC-QToF Nanospray MS and NMR Analysis of Ficus sycomorus Stem Bark and Its Effects on Rabbit. Processes. 2021; 9(7):1201. https://doi.org/10.3390/pr9071201
Chicago/Turabian StyleDawod, Ahmed, Said I. Fathalla, Ahmed Elkhatam, Noha Osman, Nagwa Sheraiba, Mohamed A. Hammad, Hesham R. El-Seedi, Awad A. Shehata, and Anis Anis. 2021. "UPLC-QToF Nanospray MS and NMR Analysis of Ficus sycomorus Stem Bark and Its Effects on Rabbit" Processes 9, no. 7: 1201. https://doi.org/10.3390/pr9071201
APA StyleDawod, A., Fathalla, S. I., Elkhatam, A., Osman, N., Sheraiba, N., Hammad, M. A., El-Seedi, H. R., Shehata, A. A., & Anis, A. (2021). UPLC-QToF Nanospray MS and NMR Analysis of Ficus sycomorus Stem Bark and Its Effects on Rabbit. Processes, 9(7), 1201. https://doi.org/10.3390/pr9071201








