CO2-Derived Carbon Capture Using Microalgae and Sodium Bicarbonate in a PhotoBioCREC Unit: Kinetic Modeling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalgae Strain and Medium
2.2. Experimental Setup
2.3. Analytical Methods
2.4. Inorganic Carbon Conversion Efficiency
2.5. Microalgae Characterization
3. Modeling Algae Growth
- (a)
- Algal growth takes places in a well-mixed PhotoBioCREC unit. This is considered adequate given the high mixing, which is the result of the important axial and circumferentially promoted fluid motion in this unit.
- (b)
- The incident irradiation passing through the flow media, containing the suspended alumina particles, remains steady during the entire algal growth period. This is achieved because of the self-cleaning walls promoted by the circumferential motion of the alumina particles in the region close to the wall.
- (a)
- Inorganic carbon consumption:
- (b)
- Organic carbon formation:
4. Results and Discussion
4.1. Cell Size and Biomass Composition
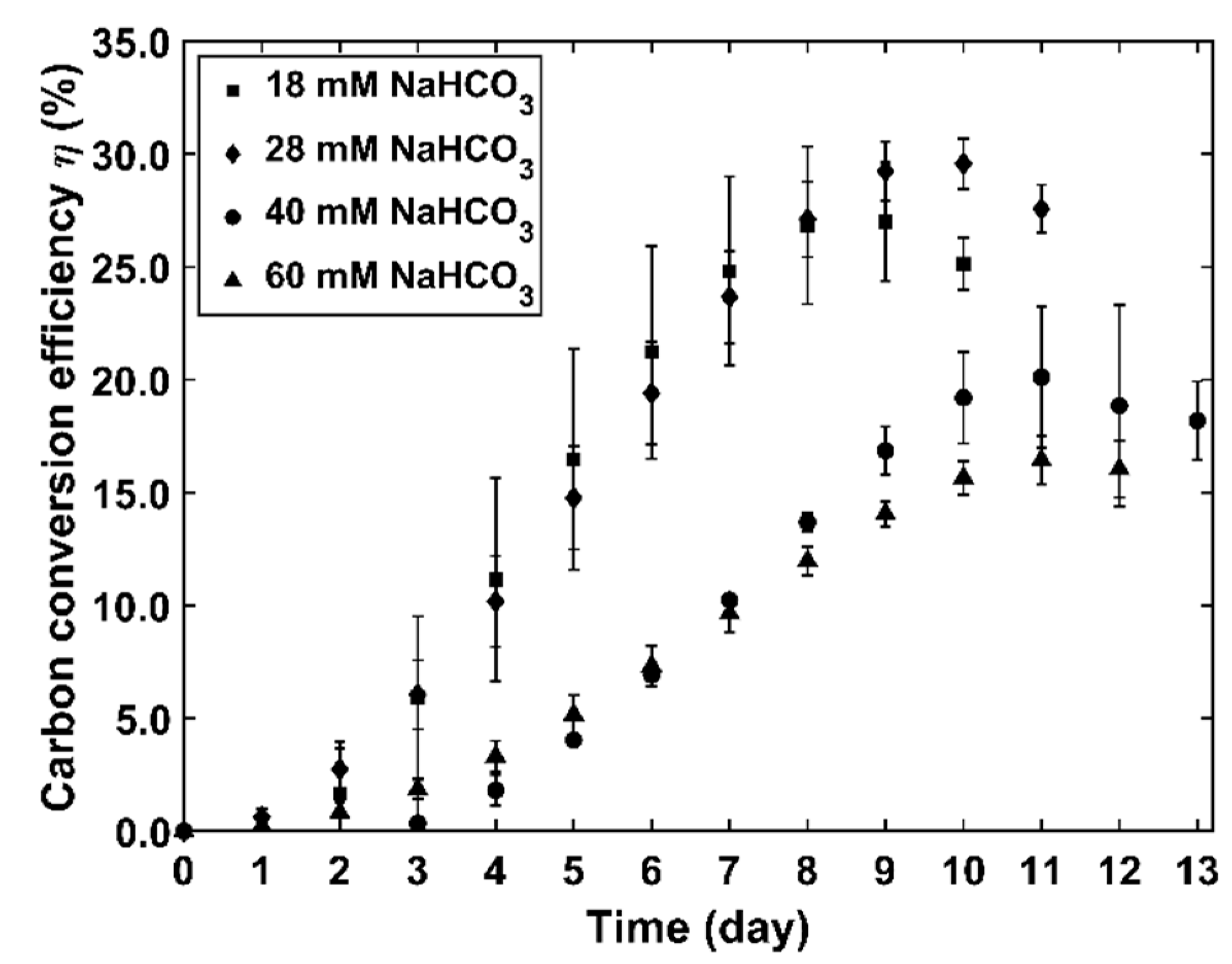
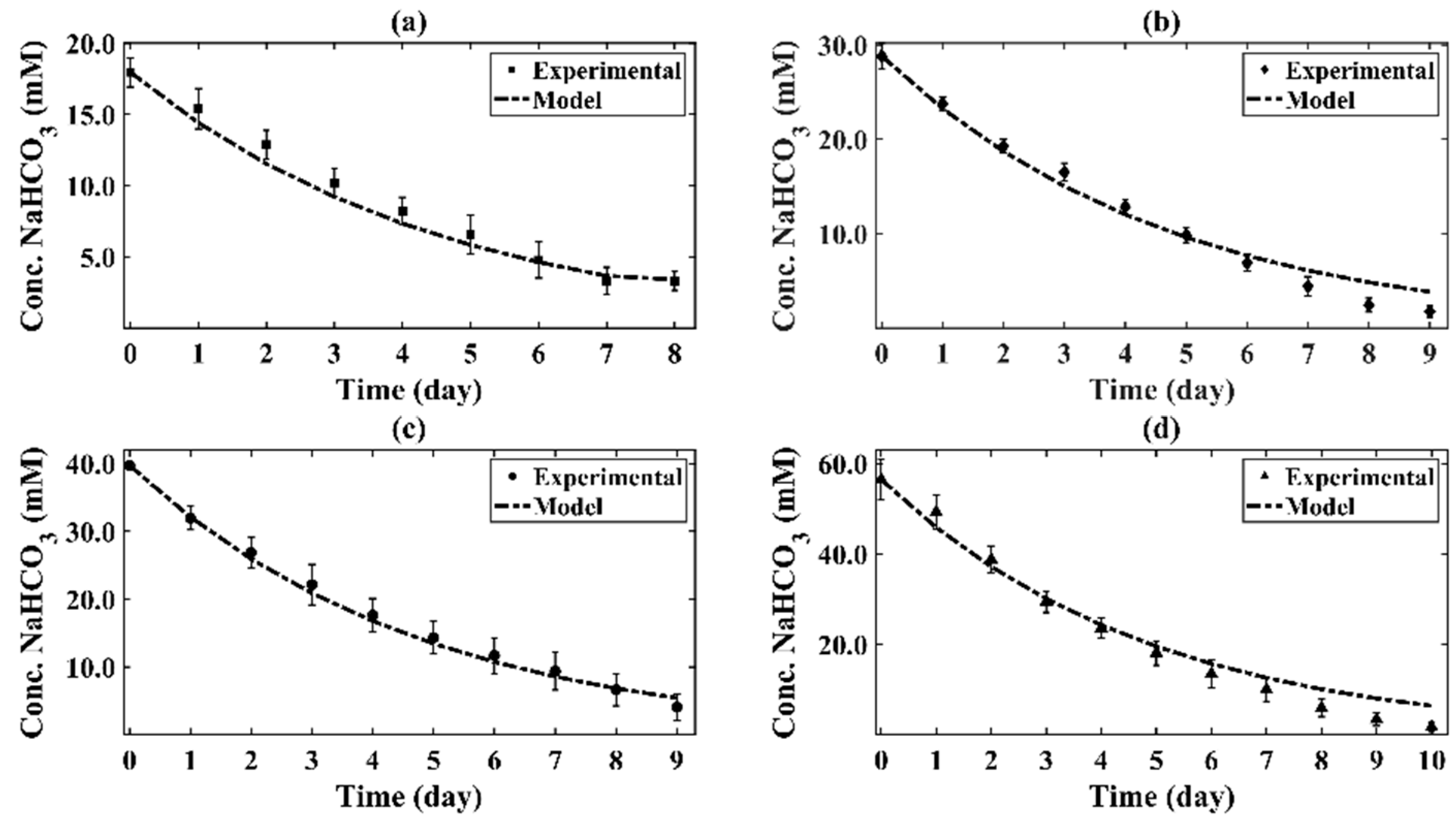
4.2. Inorganic Carbon Conversion and Kinetic Parameters
4.3. Kinetic Model
5. Conclusions
- (a)
- Sodium bicarbonate solutions are valuable vectors for CO2 capture by CPCC90 Chlorella vulgaris microalgae.
- (b)
- A PhotoBioCREC with controlled mixing and radiation conditions provides a suitable experimental prototype for the establishment of CPCC90 Chlorella vulgaris culture kinetics.
- (c)
- Measurements of bicarbonate and TOC changes with culture time show an up to 33.0% selective conversion of bicarbonates into microalgae, establishing Chlorella vulgaris photosynthesis in a PhotoBioCREC, as a promising process for carbon capture.
- (d)
- The developed experiments provide the needed data for the Chlorella vulgaris growth kinetic model.
- (e)
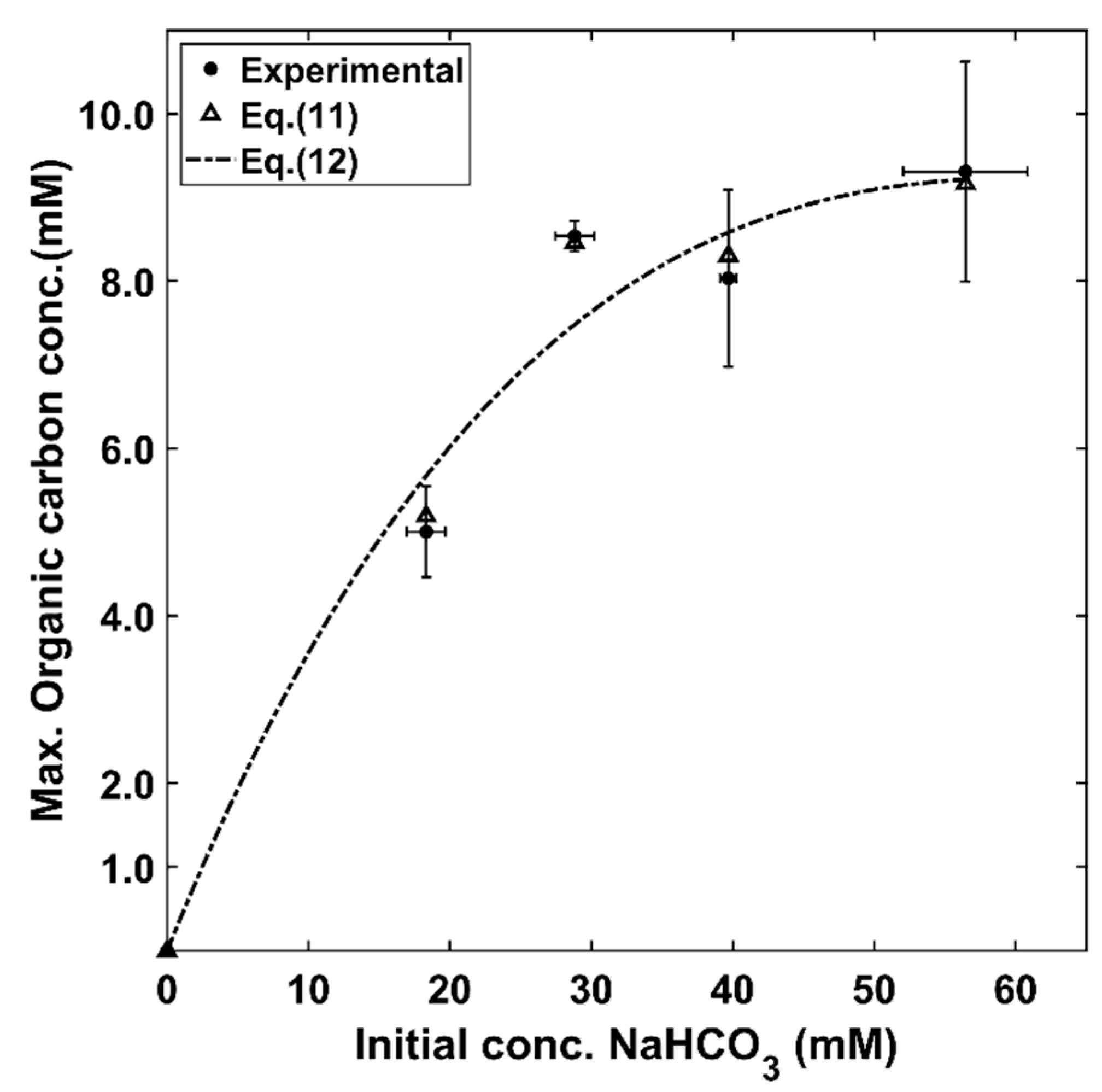
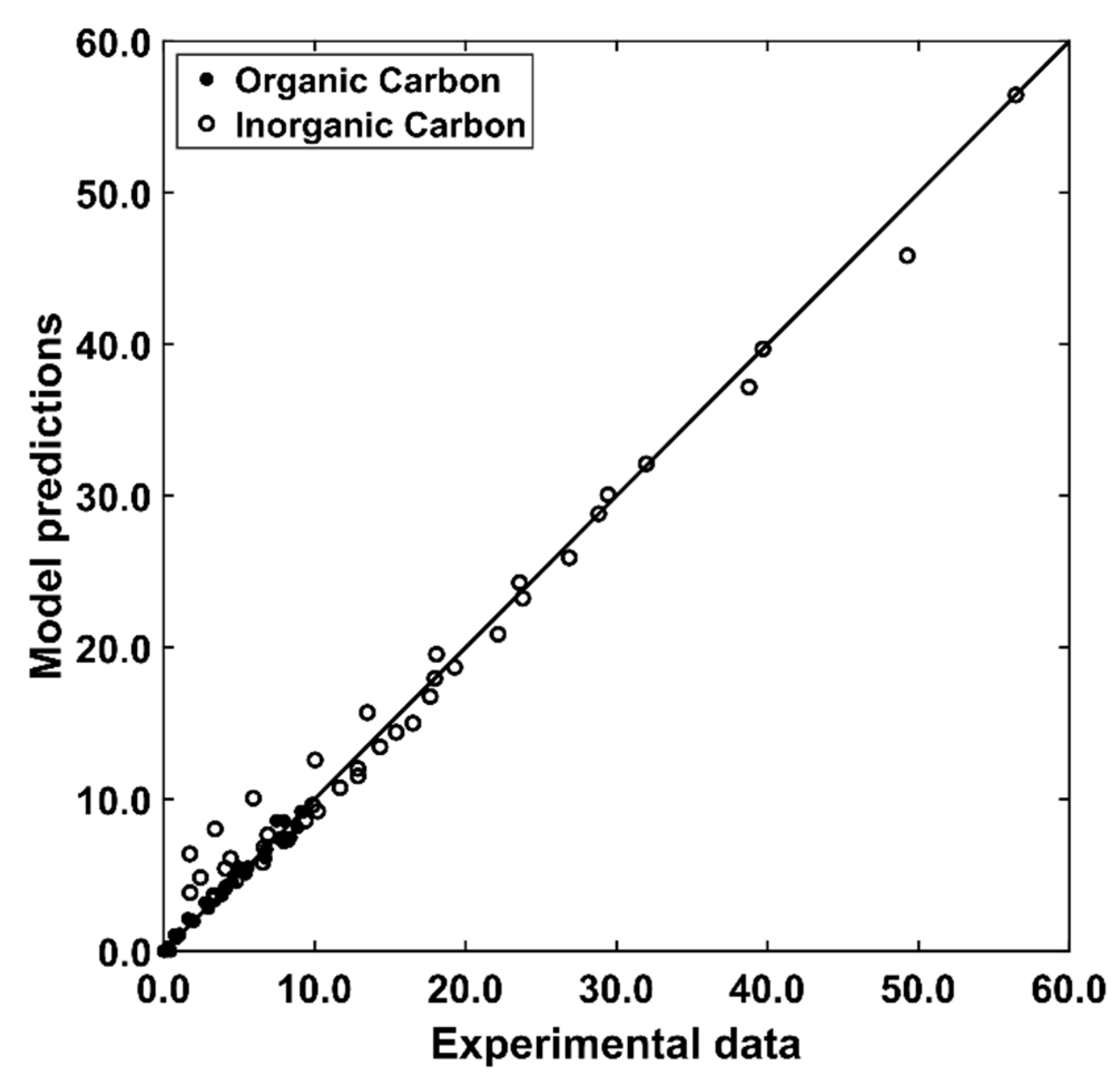
- The proposed kinetics allows one to predict both bicarbonate concentration changes and organic carbon concentration changes, during various CPCC90 Chlorella vulgaris growth phases, when using bicarbonate initial concentrations ranging from 18 to 60 mM.
- (f)
- The proposed model also reliably permits one to establish maximum CPCC90 Chlorella vulgaris concentrations values, for various initial bicarbonate concentrations.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| BBM | Bold Basal Medium |
| Inorganic carbon concentration | |
| Organic carbon concentration | |
| Initial inorganic carbon concentration | |
| Maximum organic carbon concentration in | |
| Reaction rate constant for inorganic carbon in | |
| Reaction rate constant for organic carbon for each initial concentration j in | |
| K | Constant |
| Moles of inorganic carbon | |
| Moles of organic carbon | |
| Molar rate of inorganic carbon consumption | |
| Molar rate of organic carbon formation | |
| Time | |
| Lag phase time | |
| Time representing the end of the growth phase (day) | |
| TOC | Total organic carbon |
| Two-value Heaviside function with for and for | |
| Liquid hold-up (mL) | |
| Stoichiometric coefficient | |
| Stoichiometric coefficient | |
| Stoichiometric coefficient | |
| Stoichiometric coefficient | |
| Model parameter | |
| Model parameter | |
| Growth phase duration time | |
| Carbon conversion efficiency (%) |
Appendix A. Kinetic Models Reported in the Literature
| Authors | Conditions of Runs | Quantum Yield Evaluation | Kinetics | ||
|---|---|---|---|---|---|
| Mixing Evaluation | Radiation Absorption Evaluation | TOC/Biomass | Substrate (CO2 or NaHCO3) | ||
| Novak and Brune [22] | ? | no | No | First order and Monod | none |
| de Morais and Costa [12] | No(intermittent aeration with air-CO2) | No | No | First order | None |
| Jacob-Lopes et al. [13] | No(bubble column PBR) | No | No | First order | First order |
| Yeh et al. [23] | Yes | No | No | First order and Monod model | None |
| Chun-Yen et al. [34] | Yes | No | No | First order and Monod model | None |
| Kumar and Das [19] | Yes | No | No | First order and logistic equation | None |
| Lam and Lee [14] | Yes | No | No | First order | None |
| Chang et al. [20] | ? | No | No | First order, Logistic equation | None |
| Adamczyk et al. [2] | ? | No | No | Logistic equation | None |
| This study | Yes | Yes | Yes | Zero order | First order |
Appendix B. Determination of Parameters for Equation (3)
Appendix C. Determination of Reaction Rate Constants
Appendix D. The Heaviside Step Function involved in Microalgae Growth Kinetics
- (a)
- For , the
- (b)
- For , the
- (a)
- During the lag phase: ,
- (b)
- During the growth phase: ,
- (c)
- During the stationary phase: ,
References
- Cordoba-Perez, M.; De Lasa, H. CO2-derived carbon capture and photon absorption efficiency by microalgae in novel photoBioCREC. Ind. Eng. Chem. Res. 2020, 59, 14710–14716. [Google Scholar] [CrossRef]
- Adamczyk, M.; Lasek, J.; Skawińska, A. CO2 Biofixation and Growth Kinetics of Chlorella vulgaris and Nannochloropsis gaditana. Appl. Biochem. Biotechnol. 2016, 179, 1248–1261. [Google Scholar] [CrossRef] [Green Version]
- Rawat, I.; Kumar, R.R.; Mutanda, T.; Bux, F. Biodiesel from microalgae: A critical evaluation from laboratory to large scale production. Appl. Energy 2013, 103, 444–467. [Google Scholar] [CrossRef]
- Zhao, B.; Su, Y. Process effect of microalgal-carbon dioxide fixation and biomass production: A review. Renew. Sustain. Energy Rev. 2014, 31, 121–132. [Google Scholar] [CrossRef]
- Perry, S.; Perry, R.H.; Green, D.W.; Maloney, J.O. Perry’s Chemical Engineers’ Handbook, 7th ed.; McGraw-Hill: New York, NY, USA, 1997; Volume 38, ISBN 0070498415. [Google Scholar]
- Chi, Z.; Fallon, J.V.O.; Chen, S. Bicarbonate produced from carbon capture for algae culture. Trends Biotechnol. 2011, 29, 537–541. [Google Scholar] [CrossRef]
- Merrett, M.J.; Nimer, N.A.; Dong, L.F. The utilization of bicarbonate ions by the marine microalga Nannochloropsis oculata (Droop) Hibberd. Plant, Cell Environ. 1996, 19, 478–484. [Google Scholar] [CrossRef]
- Vadlamani, A.; Viamajala, S.; Pendyala, B.; Varanasi, S. Cultivation of Microalgae at Extreme Alkaline pH Conditions: A Novel Approach for Biofuel Production. ACS Sustain. Chem. Eng. 2017, 5, 7284–7294. [Google Scholar] [CrossRef]
- Yadav, G.; Sen, R. Microalgal green refinery concept for biosequestration of carbon-dioxide vis-à-vis wastewater remediation and bioenergy production: Recent technological advances in climate research. J. CO2 Util. 2017, 17, 188–206. [Google Scholar] [CrossRef]
- White, D.; Pagarette, A.; Rooks, P.; Ali, S.T. The effect of sodium bicarbonate supplementation on growth and biochemical composition of marine microalgae cultures. J. Appl. Phycol. 2013, 25, 153–165. [Google Scholar] [CrossRef]
- Razzak, S.A.; Ali, S.A.M.; Hossain, M.M.; de Lasa, H. Biological CO2 fixation with production of microalgae in wastewater—A review. Renew. Sustain. Energy Rev. 2017, 76, 379–390. [Google Scholar] [CrossRef]
- de Morais, M.G.; Costa, J.A.V. Carbon dioxide fixation by Chlorella kessleri, C. vulgaris, Scenedesmus obliquus and Spirulina sp. cultivated in flasks and vertical tubular photobioreactors. Biotechnol. Lett. 2007, 29, 1349–1352. [Google Scholar] [CrossRef]
- Almomani, F.A.; Jacob-Lopes, E.; Gimenes Scoparo, C.H.; Teixeira Franco, T. Rates of CO2 removal by Aphanothece microscopica Nägeli in tubular photobioreactors. Chem. Eng. Process. Process Intensif. 2008, 47, 1365–1373. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T. Effect of carbon source towards the growth of Chlorella vulgaris for CO2 bio-mitigation and biodiesel production. Int. J. Greenh. Gas Control 2013, 14, 169–176. [Google Scholar] [CrossRef]
- Monod, J. The Growth of Bacterial Cultures. Annu. Rev. Microbiol. 1949, 3, 371–394. [Google Scholar] [CrossRef] [Green Version]
- Andrews, J.F. A mathematical model for the continuous culture of microorganisms utilizing inhibitory substrates. Biotechnol. Bioeng. 1968, 10, 707–723. [Google Scholar] [CrossRef]
- Blanch, H.W.W.; Clarck, D.S.; Clark, D.S.S.; Clarck, D.S. Biochemical Engineering; Marcel Dekker, Inc.: New York, NY, USA, 1996. [Google Scholar]
- Martínez, M.E.; Jiménez, J.M.; El Yousfi, F. Influence of phosphorus concentration and temperature on growth and phosphorus uptake by the microalga Scenedesmus obliquus. Bioresour. Technol. 1999, 67, 233–240. [Google Scholar] [CrossRef]
- Kumar, K.; Das, D. Growth characteristics of Chlorella sorokiniana in airlift and bubble column photobioreactors. Bioresour. Technol. 2012, 116, 307–313. [Google Scholar] [CrossRef]
- Chang, H.-X.; Huang, Y.; Fu, Q.; Liao, Q.; Zhu, X. Kinetic characteristics and modeling of microalgae Chlorella vulgaris growth and CO2 biofixation considering the coupled effects of light intensity and dissolved inorganic carbon. Bioresour. Technol. 2016, 206, 231–238. [Google Scholar] [CrossRef]
- Almomani, F.A. Assessment and modeling of microalgae growth considering the effects of CO2, nutrients, dissolved organic carbon and solar irradiation. J. Environ. Manag. 2019, 247, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Novak, J.T.; Brune, D.E. Inorganic carbon limited growth kinetics of some freshwater algae. Water Res. 1985, 19, 215–225. [Google Scholar] [CrossRef]
- Yeh, K.-L.; Chang, J.; Chen, W. Effect of light supply and carbon source on cell growth and cellular composition of a newly isolated microalga Chlorella vulgaris. Eng. Life Sci. 2010, 10, 201–208. [Google Scholar] [CrossRef]
- Babaei, A.; Mehrnia, M.R. Fouling in microalgal membrane bioreactor containing nitrate-enriched wastewater under different trophic conditions. Algal Res. 2018, 36, 167–174. [Google Scholar] [CrossRef]
- Hage, D.S.; Carr, J.D. Analytical Chemistry and Quantitative Analysis; Prentice Hall: Boston, MA, USA, 2011. [Google Scholar]
- Percopo, I.; Montresor, M.; Taxonomy, D.S.; Zoologica, S.; Dohrn, A. Microalgae Preparation for Scanning Electron Microscopy: Dehydration. Available online: https://www.assemblemarine.org/assets/Uploads/Documents/tool-box/Preparation-for-Scanning-Electron-Microscopy1.pdf (accessed on 19 May 2021).
- da Rosa, G.M.; Moraes, L.; Cardias, B.B.; Costa, J.A.V. Chemical absorption and CO2 biofixation via the cultivation of Spirulina in semicontinuous mode with nutrient recycle. Bioresour. Technol. 2015, 192, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Sa, C.; Zebib, B.; Merah, O.; Pontalier, P. Morphology, composition, production, processing and applications of Chlorella vulgaris: A review. Renew. Sustain. Energy Rev. 2014, 35, 265–278. [Google Scholar] [CrossRef] [Green Version]
- Raheem, A.; Sivasangar, S.; Wan Azlina, W.A.K.G.; Taufiq Yap, Y.H.; Danquah, M.K.; Harun, R. Thermogravimetric study of Chlorella vulgaris for syngas production. Algal Res. 2015, 12, 52–59. [Google Scholar] [CrossRef]
- Arif, M.; Li, Y.; El-Dalatony, M.M.; Zhang, C.; Li, X.; Salama, E.S. A complete characterization of microalgal biomass through FTIR/TGA/CHNS analysis: An approach for biofuel generation and nutrients removal. Renew. Energy 2021, 163, 1973–1982. [Google Scholar] [CrossRef]
- Gautam, R.; Varma, A.K.; Vinu, R. Apparent Kinetics of Fast Pyrolysis of Four Different Microalgae and Product Analyses Using Pyrolysis-FTIR and Pyrolysis-GC/MS. Energy Fuels 2017, 31, 12339–12349. [Google Scholar] [CrossRef]
- Moroney, J.V.; Somanchi, A. How Do Algae Concentrate CO2 to Increase the Efficiency of Photosynthetic Carbon Fixation? Plant Physiol. 2002, 119, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Yadav, N.; Gupta, N.; Singh, D.P. Ameliorating Effect of Bicarbonate on Salinity Induced Changes in the Growth, Nutrient Status, Cell Constituents and Photosynthetic Attributes of Microalga Chlorella vulgaris. Bull. Environ. Contam. Toxicol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Chun-Yen, C.; Yeh, K.-L.; Huei-Meei, S.; Yung-Chung, L.; Wen-Ming, C.; Jo-Shu, C. Strategies to Enhance Cell Growth and Achieve High-Level Oil Production of a Chlorella vulgaris Isolate. Biotechnol. Prog. 2010, 26, 679–686. [Google Scholar] [CrossRef]
- Farlow, S.J. Partial Differential Equations for Scientists and Engineers; Dover Publications, Inc.: New York, NY, USA, 1993. [Google Scholar]

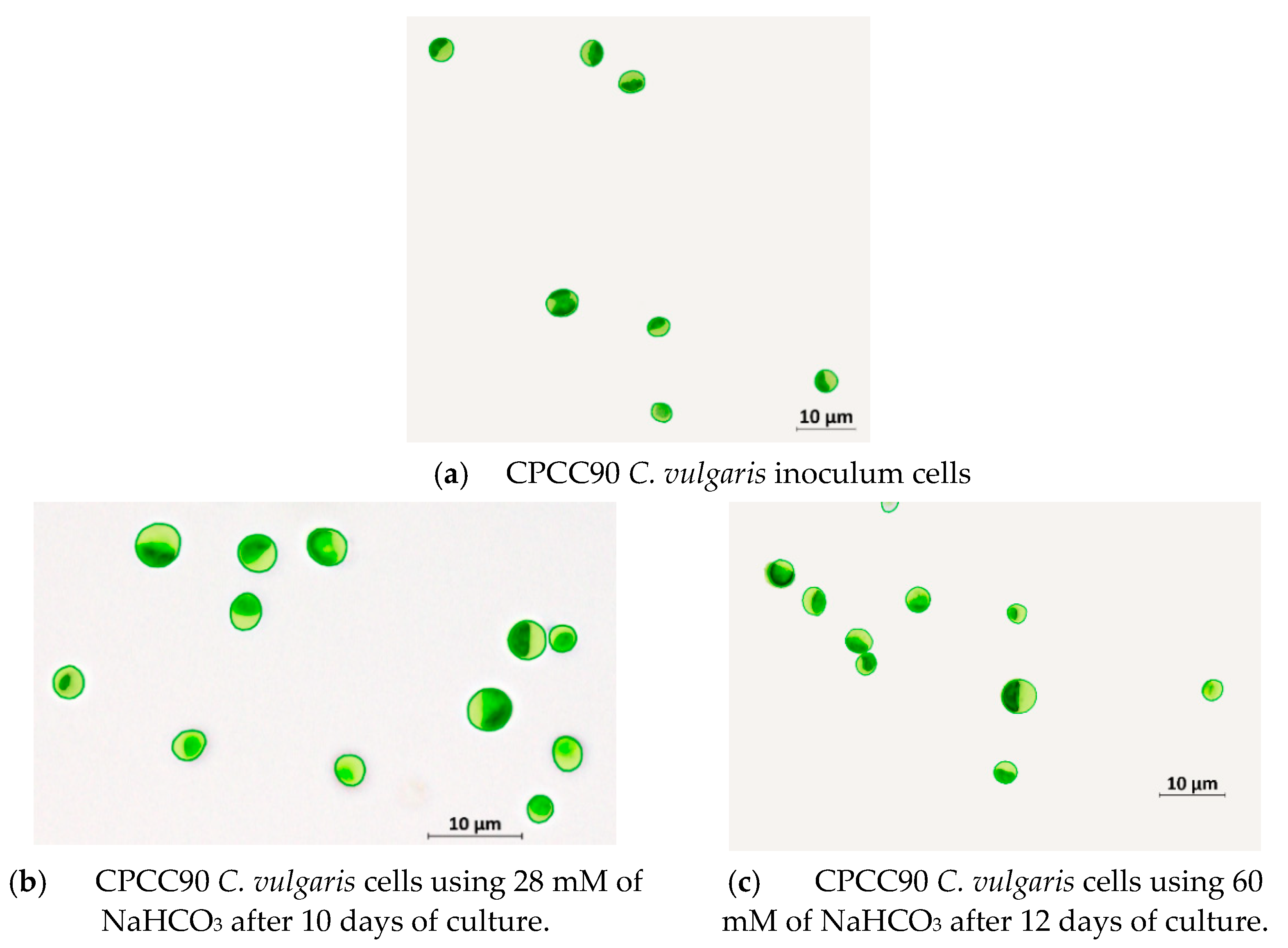

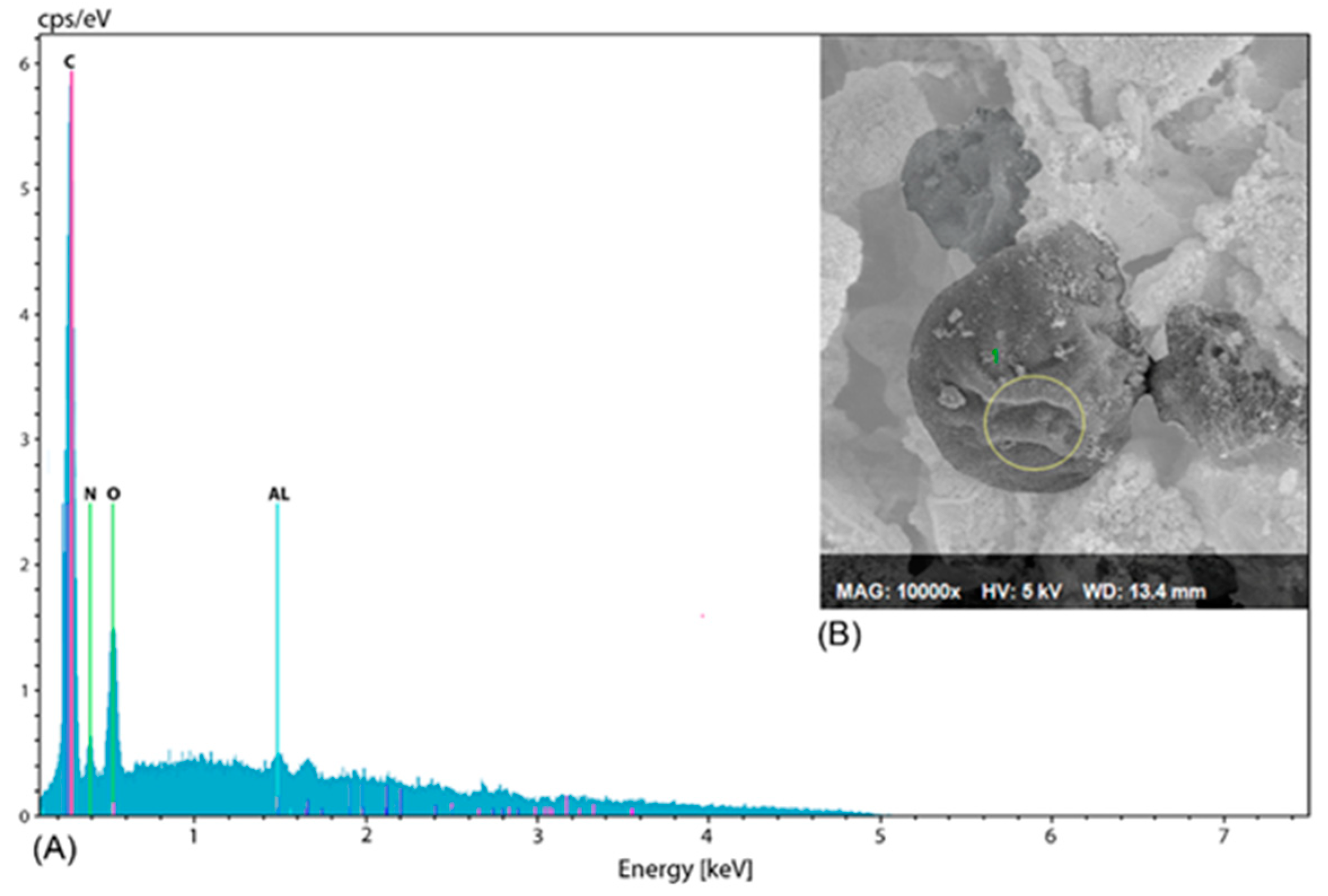

| Composition (%) | This Study | Literature [29] |
|---|---|---|
| CPCC Chlorella vulgaris | Chlorella vulgaris | |
| Carbon | 55.1 | 46.1–50.39 |
| Hydrogen | 8.2 | 6.01–6.41 |
| Oxygen 1 | 29.0 | 19.1–25.00 |
| Nitrogen | 7.1 | 9.01–14.77 |
| Sulfur | 0.6 | 0.4–6.05 |
| Molar ratios | ||
| H/C | 1.8 | 1.43 |
| C/N | 9.1 | |
| O/C | 0.39 | 0.339 |
| Parameter | Value |
|---|---|
| Nominal Conc. of NaHCO3 | ||
|---|---|---|
| 18 | 0.86 ± 0.13 | 6 |
| 28 | 1.18 ± 0.05 | 7.2 |
| 40 | 1.06 ± 0.08 | 8 |
| 60 | 1.02 ± 0.11 | 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordoba-Perez, M.; de Lasa, H. CO2-Derived Carbon Capture Using Microalgae and Sodium Bicarbonate in a PhotoBioCREC Unit: Kinetic Modeling. Processes 2021, 9, 1296. https://doi.org/10.3390/pr9081296
Cordoba-Perez M, de Lasa H. CO2-Derived Carbon Capture Using Microalgae and Sodium Bicarbonate in a PhotoBioCREC Unit: Kinetic Modeling. Processes. 2021; 9(8):1296. https://doi.org/10.3390/pr9081296
Chicago/Turabian StyleCordoba-Perez, Maureen, and Hugo de Lasa. 2021. "CO2-Derived Carbon Capture Using Microalgae and Sodium Bicarbonate in a PhotoBioCREC Unit: Kinetic Modeling" Processes 9, no. 8: 1296. https://doi.org/10.3390/pr9081296
APA StyleCordoba-Perez, M., & de Lasa, H. (2021). CO2-Derived Carbon Capture Using Microalgae and Sodium Bicarbonate in a PhotoBioCREC Unit: Kinetic Modeling. Processes, 9(8), 1296. https://doi.org/10.3390/pr9081296







