Model-Based Evaluation of Hydrothermal Treatment for the Energy Efficient Dewatering and Drying of Sewage Sludge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
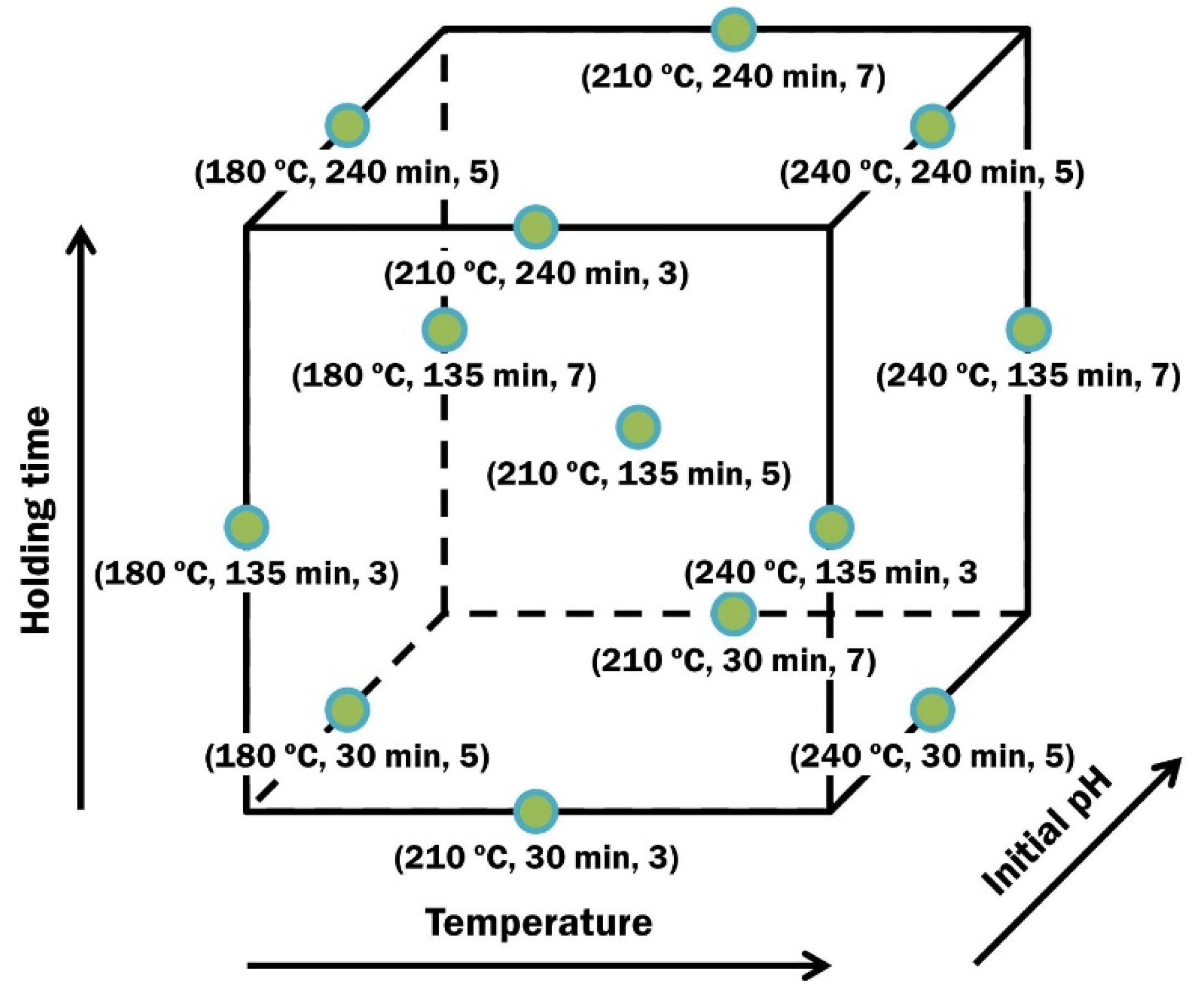
2.2. Conducting the Experiments
2.3. Analytical Methods
2.4. Calculations
2.5. Statistical Evaluation and Regression Modeling
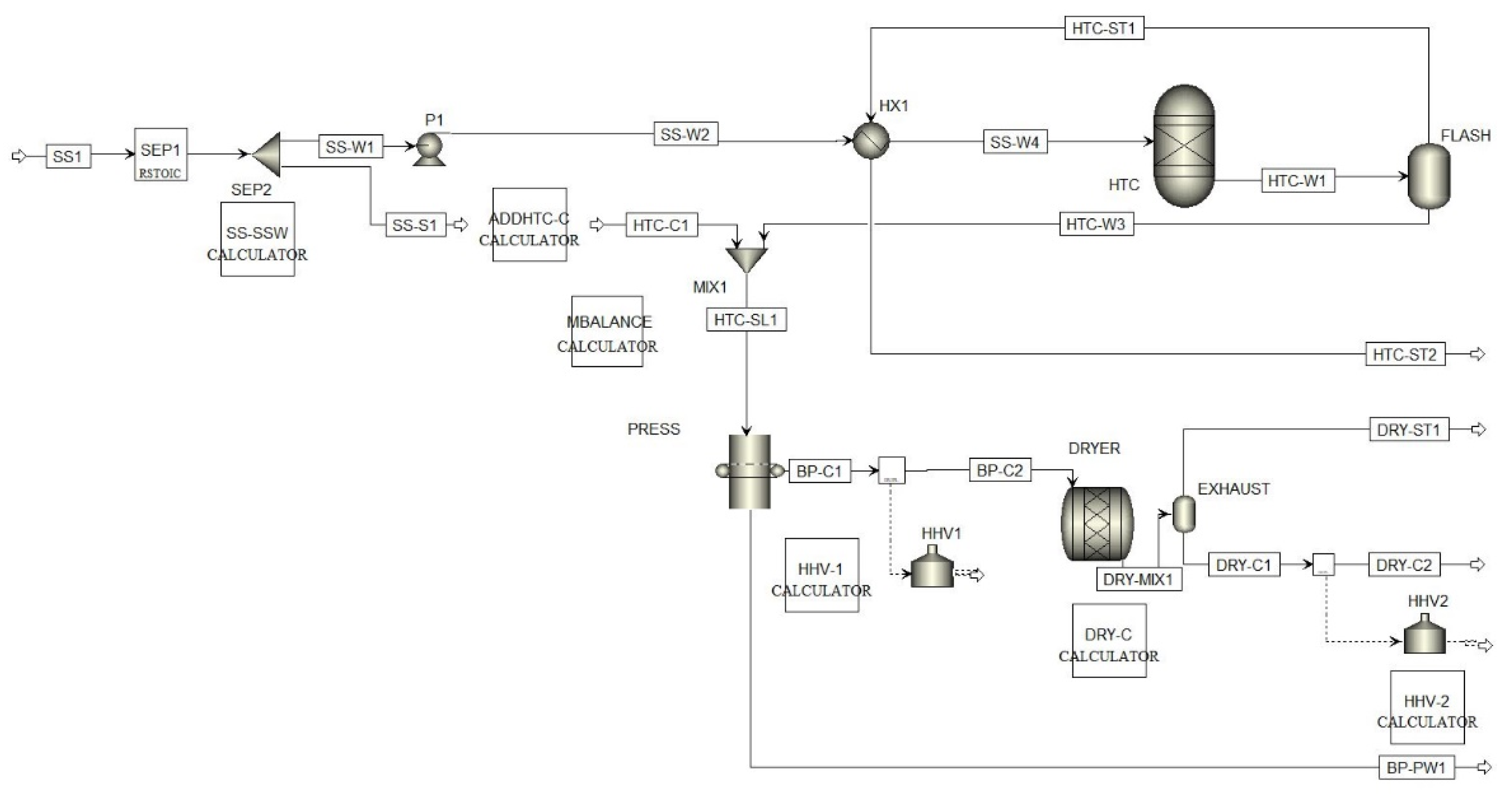
2.6. Aspen Plus Modeling
3. Results
3.1. Experimental Results
3.2. Regression Modeling
3.3. Numerical Optimization of Process Parameters
3.4. Flowsheeting Results
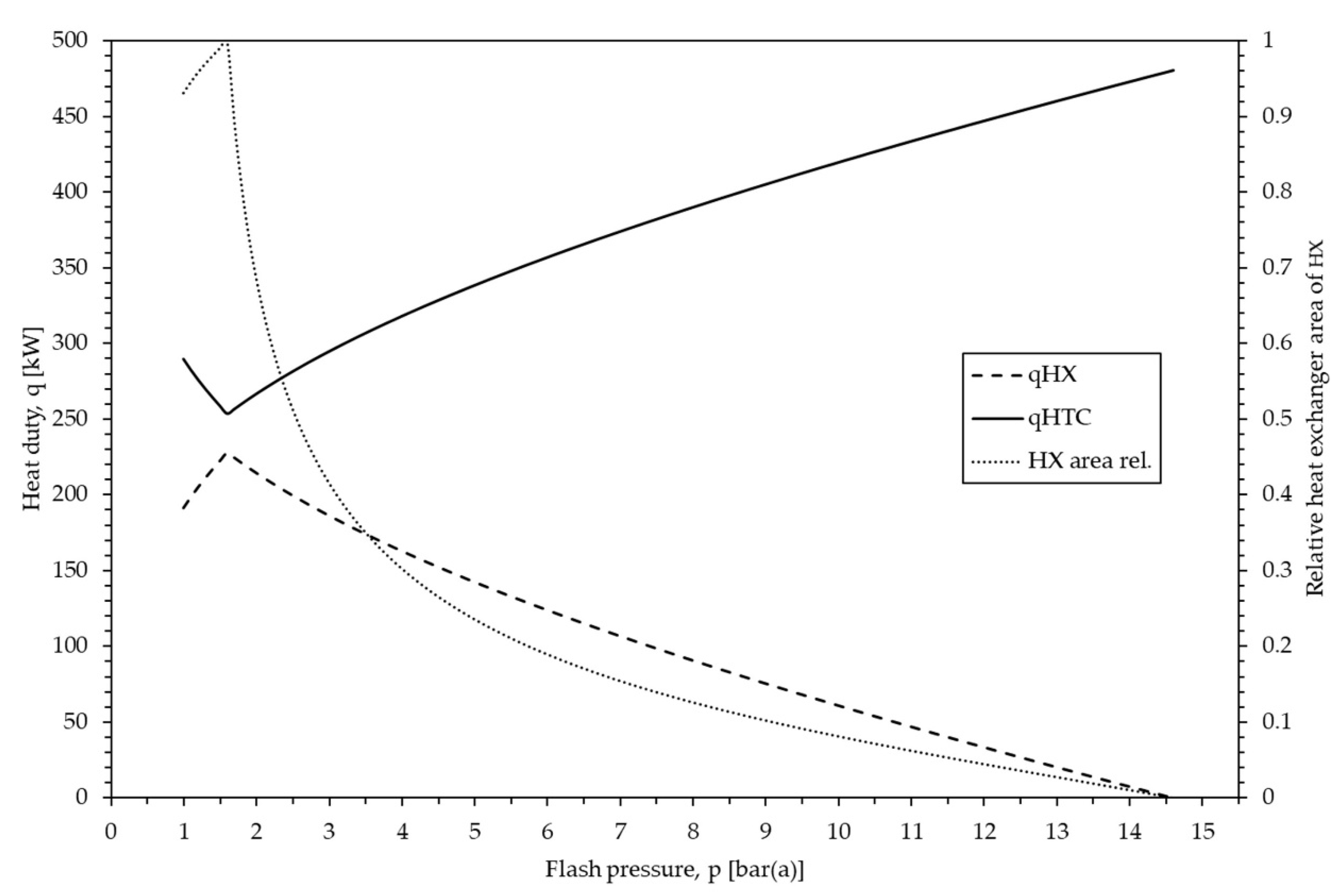
3.5. Sensitivity Analysis of the Flashing Pressure
3.6. Overall Energetic Considerations
3.7. Condsiderations Regarding Energy Generation by Incineration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Run No. | T (°C) | Tmax (°C) | T (min) | t140 (min) | Starting pH (-) |
|---|---|---|---|---|---|
| 1 | 210 | 227 | 30 | 113 | 3 |
| 2 | 240 | 250 | 30 | 146 | 5 |
| 3 | 240 | 249 | 135 | 248 | 3 |
| 4 | 210 | 229 | 240 | 315 | 7 |
| 5 | 210 | 218 | 135 | 223 | 5 |
| 6 | 210 | 229 | 135 | 233 | 5 |
| 7 | 240 | 250 | 135 | 240 | 7 |
| 8 | 180 | 208 | 240 | 292 | 5 |
| 9 | 180 | 205 | 135 | 189 | 3 |
| 10 | 240 | 249 | 240 | 349 | 5 |
| 11 | 210 | 223 | 240 | 322 | 3 |
| 12 | 210 | 226 | 135 | 216 | 5 |
| 13 | 180 | 197 | 30 | 85 | 5 |
| 14 | 210 | 228 | 30 | 125 | 7 |
| 15 | 180 | 201 | 135 | 193 | 7 |
Appendix A.1. Aspen Flowsheet and Modelling Assumptions
| Proximate Analysis | Ultimate Analysis | ||
|---|---|---|---|
| Moisture | 75.2 | Ash | 41.5 |
| FC | 7.2 | Carbon | 31.5 |
| VM | 54.7 | Hydrogen | 4.3 |
| Ash | 41.5 | Nitrogen | 4.8 |
| Chlorine | 0.0 | ||
| Sulfur | 1.7 | ||
| Oxygen | 16.2 | ||
| Specification Type | Stoichiometry |
|---|---|
| Frac. conversion | COAL --> 0.0555084 WATER(MIXED) |
Appendix A.2. Fortran Expressions of Underlying Calculator Blocks
| Variable | Information Flow | Definition |
|---|---|---|
| CONV | Export variable | Block-Var Block = SEP1 Variable = CONV Sentence = CONV ID1 = 1 |
| WSS | Import variable | Compattr-Var Stream = SS1 Substream = NC Component = COAL Attribute = PROXANAL Element = 1 |
| Variable | Information Flow | Definition |
|---|---|---|
| SS | Import variable | Stream-Var Stream = SS1 Substream = NC Variable = MASS-FLOW Units = kg/hr |
| W3 | Import variable | Stream-Var Stream = HTC-W3 Substream = MIXED Variable = MASS-FLOW Units = kg/hr |
| PW3 | Import variable | Stream-Var Stream = HTC-W3 Substream = MIXED Variable = PRES Units = bar |
| PC1 | Export variable | Stream-Var Stream = HTC-C1 Substream = NC Variable = PRES Units = bar |
| TW3 | Import variable | Stream-Var Stream = HTC-W3 Substream = MIXED Variable = TEMP Units = C |
| TC1 | Export variable | Stream-Var Stream = HTC-C1 Substream = MIXED Variable = TEMP Units = C |
| CC1 | Export variable | Stream-Var Stream = HTC-C1 Substream = NC Variable = MASS-FLOW Units = kg/hr |
| ST2 | Import variable | Stream-Var Stream = HTC-ST2 Substream = MIXED Variable = MASS-FLOW Units = kg/hr |
| CW1 | Export variable | Stream-Var Stream = HTC-C1 Substream = MIXED Variable = MASS-FLOW Units = kg/hr |
| SSS1 | Import variable | Stream-Var Stream = SS-S1 Substream = NC Variable = MASS-FLOW Units=kg/hr |
| Variable | Information Flow | Definition |
|---|---|---|
| MIXSL1 | Export variable | Stream-Var Stream = HTC-SL1 Substream = MIXED Variable = MASS-FLOW Units = kg/hr |
| NCSL1 | Export variable | Stream-Var Stream = HTC-SL1 Substream = NC Variable = MASS-FLOW Units = kg/hr |
| SS | Import variable | Stream-Var Stream = SS1 Substream = NC Variable = MASS-FLOW Units = kg/hr |
| ST2 | Import variable | Stream-Var Stream = HTC-ST2 Substream = MIXED Variable = MASS-FLOW Units = kg/hr |
| C1 | Import variable | Stream-Var Stream = HTC-C1 Substream = NC Variable = MASS-FLOW Units = kg/hr |
| C1W | Import variable | Compattr-Var Stream = HTC-C1 Substream = NC Component = COAL Attribute = PROXANAL Element = 1 |
| Variable | Information Flow | Definition |
|---|---|---|
| WIN | Import variable | Compattr-Var Stream = BP-C2 Substream = NC Component = COAL Attribute = PROXANAL Element = 1 |
| WOUT | Import variable | Block-Var Block = DRYER Variable = COMPATT Sentence = COMP-ATTR ID1 = NC ID2 = COAL ID3 = PROXANAL Element = 1 |
| CONV | Export variable | Block-Var Block = DRYER Variable = CONV Sentence = CONV ID1 = 1 |
| MF | Import variable | Stream-Var Stream = BP-C2 Substream = NC Variable = MASS-FLOW Units = kg/hr |
| Q | Export variable | Block-Var Block = DRYER Variable = DUTY Sentence = PARAM Units = kW |
References
- Umweltbundesamt (UBA). Klärschlammentsorgung in der Bundesrepublik Deutschland, Dessau-Roßlau. 2018. Available online: https://www.umweltbundesamt.de/publikationen/klaerschlammentsorgung-in-der-bundesrepublik (accessed on 8 July 2021).
- Statistisches Bundesamt (Destatis). Abwasserbehandlung–Klärschlamm, Berlin 2015/2016. Available online: https://www.destatis.de/DE/Themen/Gesellschaft-Umwelt/Umwelt/Wasserwirtschaft/Publikationen/Downloads-Wasserwirtschaft/klaerschlamm-5322101169004.html (accessed on 8 July 2021).
- Union of the Baltic Cities (UBC); Luft, J.-E.; Ojala, T.; Ruokanen, L.; Zinchuk, O. Bewährte Verfahren in der Schlammbehandlung. 2012. Available online: https://www.ubc-sustainable.net/library/publication/bew%C3%A4hrte-verfahren-der-schlammbehandlung (accessed on 8 July 2021).
- Verordnung über die Verwertung von Klärschlamm, Klärschlammgemisch und Klärschlammkompost (Klärschlammverordnung—AbfKlärV). 2017. Available online: https://www.gesetze-im-internet.de/abfkl_rv_2017/BJNR346510017.html (accessed on 8 July 2021).
- EUWID Wasser und Abwasser. Report Klärschlamm 2018: Die neue Klärschlammverordnung Paradigmenwechsel für die Abwasserentsorgung. 2017. Available online: https://www.euwid-wasser.de/klaerschlamm-report-2018 (accessed on 8 July 2021).
- Gerhardt, A.; Kabbe, C.; Rastetter, N.; Stemann, J.; Wilken, V. Sustainable Sewage Sludge Management Fostering Phosphorus Recovery and Energy Efficiency: Deliverable D 8.1 Quantification of Nutritional Value and Toxic Effects of Each P Recovery Product. 2015. Available online: https://zenodo.org/record/242550/files/P-REX%20D8.1%20Quantification%20of%20nutritional%20value%20and%20toxic%20effects%20of%20each%20P%20recovery%20product.pdf?download=1 (accessed on 8 July 2021).
- Escala, M.; Zumbühl, T.; Koller, C.; Junge, R.; Krebs, R. Hydrothermal Carbonization as an Energy-Efficient Alternative to Established Drying Technologies for Sewage Sludge: A Feasibility Study on a Laboratory Scale. Energy Fuels 2012, 27, 454–460. [Google Scholar] [CrossRef]
- Geyer, J. Klärschlammtrocknung in Deutschland: Stand und Perspektiven. Energie Abfall 2013, 2013, 927–948. [Google Scholar]
- Lühmann, T.; Wirth, B. Sewage Sludge Valorization via Hydrothermal Carbonization: Optimizing Dewaterability and Phosphorus Release. Energies 2020, 13, 4417. [Google Scholar] [CrossRef]
- Danso-Boateng, E.; Shama, G.; Wheatley, A.D.; Martin, S.J.; Holdich, R.G. Hydrothermal carbonisation of sewage sludge: Effect of process conditions on product characteristics and methane production. Bioresour. Technol. 2015, 177, 318–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reza, M.T.; Andert, J.; Wirth, B.; Busch, D.; Pielert, J.; Lynam, J.G.; Mumme, J. Hydrothermal Carbonization of Biomass for Energy and Crop Production. Appl. Bioenergy 2014, 1, 11–29. [Google Scholar] [CrossRef]
- Libra, J.A.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.-M.; Fühner, C.; Bens, O.; Kern, J.; et al. Hydrothermal carbonization of biomass residuals: A comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2011, 2, 71–106. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Luo, G.; Rao, Y.; Chen, H.; Zhang, S. Hydrothermal conversion of dewatered sewage sludge: Focusing on the transformation mechanism and recovery of phosphorus. Chemosphere 2019, 228, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhai, Y.; Zhu, Y.; Peng, C.; Wang, T.; Xu, B.; Li, C.; Zeng, G. Feedwater pH affects phosphorus transformation during hydrothermal carbonization of sewage sludge. Bioresour. Technol. 2017, 245, 182–187. [Google Scholar] [CrossRef]
- Channiwala, S.A.; Parikh, P.P. A unified correlation for estimating HHV of solid, liquid and gaseous fuels. Fuel 2002, 81, 1051–1063. [Google Scholar] [CrossRef]
- IAPWS Industrial Formulation 1997 for the Thermodynamic Properties of Water and Steam. In International Steam Tables; Wagner, W.; Kretzschmar, H.-J. (Eds.) Springer: Berlin, Heidelberg, Germany, 2008; pp. 7–150. ISBN 978-3-540-21419-9. [Google Scholar]
- Ramke, H.-G. Hydrothermal Carbonization of Organic Waste. In Proceedings of the Twelfth International Waste Management and Landfill Symposium, Cagliari, Italy, 5–9 October 2009. [Google Scholar]
- Wang, L.; Li, A. Hydrothermal treatment coupled with mechanical expression at increased temperature for excess sludge dewatering: The dewatering performance and the characteristics of products. Water Res. 2015, 68, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Jiang, Z.; Yoshikawa, K.; Mu, H. The effect of operation parameters on the hydrothermal drying treatment. Renew. Energy 2012, 42, 90–94. [Google Scholar] [CrossRef]
- Álvarez-Murillo, A.; Román, S.; Ledesma, B.; Sabio, E. Study of variables in energy densification of olive stone by hydrothermal carbonization. J. Anal. Appl. Pyrolysis 2015, 113, 307–314. [Google Scholar] [CrossRef]
- Reza, M.T. Hydrothermal Carbonization of Lignocellulosic Biomass. PhD’s Thesis, University of Nevada, Reno, NV, USA, 2013. [Google Scholar]
- Wiechmann, B.; Dienemann, C.; Kabbe, C. Sewage Sludge Management in German. Available online: https://www.umweltbundesamt.de/en/publikationen/sewage-sludge-management-in-germany (accessed on 18 May 2021).
- HTP-Fachforum 2019: Hydrothermale Prozesse zur Stofflichen und Energetischen Wertschöpfung. 5. HTP-Fachforum, Leipzig, 25–26 September 2019; DBFZ Leipzig: Leipzig, Germany. 2019. Available online: https://www.dbfz.de/pressemediathek/publikationsreihen-des-dbfz/tagungsreader/5-fachforum-hydrothermale-prozesse (accessed on 8 July 2021).
- Erlach, B.; Wirth, B.; Tsatsaronis, G. Co-production of electricity, heat and biocoal pellets from biomass: A techno-economic comparison with wood pelletizing. In Proceedings of the World Renewable Energy Congress 2011, Linkoping, Sweden, 8–13 May 2011. [Google Scholar]
- Köchermann, J.; Görsch, K.; Wirth, B.; Mühlenberg, J.; Klemm, M. Hydrothermal carbonization: Temperature influence on hydrochar and aqueous phase composition during process water recirculation. J. Environ. Chem. Eng. 2018, 6, 5481–5487. [Google Scholar] [CrossRef]
- Berge, N.D.; Ro, K.S.; Mao, J.; Flora, J.R.V.; Chappell, M.A.; Bae, S. Hydrothermal carbonization of municipal waste streams. Environ. Sci. Technol. 2011, 45, 5696–5703. [Google Scholar] [CrossRef] [PubMed]

| C | H | N | S | O | Ash | P | HHVar | HHVdaf |
|---|---|---|---|---|---|---|---|---|
| (%TS) | (g kgTS−1) | (MJ kgTS−1) | ||||||
| 31.5 | 4.3 | 4.8 | 1.7 | 16.2 | 41.5 | 47.0 | 13.6 | 23.3 |
| Run No. | TSHC (%FM) | SY (%) | C (%TS) | C (%TS,af) | HHV (MJ kgTS−1) | EY (%) | PHC (g kgTS−1) | PPW (mg L−1) |
|---|---|---|---|---|---|---|---|---|
| 1 | 40.0 | 77.2 | 29.0 | 58.4 | 12.9 | 73.2 | 34.4 | 2410 |
| 2 | 42.7 | 75.7 | 28.0 | 62.8 | 12.7 | 71.0 | 51.8 | 391 |
| 3 | 42.4 | 72.5 | 27.8 | 62.0 | 12.7 | 67.8 | 35.9 | 1110 |
| 4 | 41.5 | 61.7 | 29.7 | 68.0 | 13.6 | 61.6 | 57.6 | 134 |
| 5 | 42.0 | 80.0 | 28.8 | 61.2 | 12.9 | 76.2 | 49.2 | 300 |
| 6 | 40.6 | 70.4 | 28.3 | 62.5 | 12.8 | 66.1 | 49.5 | 375 |
| 7 | 41.2 | 52.4 | 29.6 | 71.1 | 13.5 | 52.3 | 61.7 | 146 |
| 8 | 39.8 | 76.6 | 29.3 | 60.5 | 13.3 | 75.0 | 48.6 | 598 |
| 9 | 40.9 | 76.8 | 28.8 | 58.1 | 12.7 | 72.0 | 35.1 | 2340 |
| 10 | 44.5 | 62.1 | 28.5 | 66.7 | 13.4 | 61.1 | 48.3 | 436 |
| 11 | 40.2 | 68.5 | 28.4 | 59.3 | 12.6 | 63.4 | 30.7 | 1660 |
| 12 | 42.0 | 70.6 | 28.8 | 63.4 | 13.3 | 69.2 | 46.7 | 484 |
| 13 | 39.7 | 84.9 | 29.5 | 58.3 | 13.2 | 82.2 | 44.2 | 927 |
| 14 | 41.9 | 72.3 | 30.0 | 65.0 | 13.8 | 73.3 | 54.2 | 171 |
| 15 | 38.6 | 69.5 | 30.2 | 61.7 | 13.7 | 69.9 | 52.3 | 202 |
| Run No. | TSHC | PPW | PHC at a. r. | PHC at 50%TS | PHC at 70%TS | PHC at 90%TS |
|---|---|---|---|---|---|---|
| (%FM] | (mg L−1) | (g kgTS−1) | ||||
| 1 | 40.0 | 2410 | 34.4 | 33.2 | 31.8 | 31.0 |
| 2 | 42.7 | 391 | 51.8 | 51.7 | 51.5 | 51.4 |
| 3 | 42.4 | 1110 | 35.9 | 35.5 | 34.8 | 34.5 |
| 4 | 41.5 | 134 | 57.6 | 57.5 | 57.5 | 57.4 |
| 5 | 42.0 | 300 | 49.2 | 49.1 | 48.9 | 48.8 |
| 6 | 40.6 | 375 | 49.5 | 49.4 | 49.1 | 49.0 |
| 7 | 41.2 | 146 | 61.7 | 61.7 | 61.6 | 61.5 |
| 8 | 39.8 | 598 | 48.6 | 48.3 | 48.0 | 47.8 |
| 9 | 40.9 | 2340 | 35.1 | 34.1 | 32.7 | 32.0 |
| 10 | 44.5 | 436 | 48.3 | 48.2 | 47.9 | 47.8 |
| 11 | 40.2 | 1660 | 30.7 | 29.9 | 29.0 | 28.5 |
| 12 | 42.0 | 484 | 46.7 | 46.5 | 46.2 | 46.0 |
| 13 | 39.7 | 927 | 44.2 | 43.7 | 43.2 | 42.9 |
| 14 | 41.9 | 171 | 54.2 | 54.1 | 54.0 | 54.0 |
| 15 | 38.6 | 202 | 52.3 | 52.2 | 52.1 | 52.0 |
| Target Value | Coded Regression Model Coefficients | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| xT | xt | xpH | xTxt | xTxpH | xtxpH | xT2 | xt2 | xpH2 | |
| TS (%) | 1.57 | −0.18 | 2.99 | 0.15 | −0.77 | ||||
| SY (%TS) | −6.37 | −4.92 | −2.66 | 4.98 | −1.04 | −4.90 | |||
| HHV (MJ kgTS−1) | −0.21 | −0.06 | 0.45 | −0.39 | 0.10 | ||||
| EY (%TS) | −6.90 | −4.58 | 0.02 | 4.43 | −0.78 | 2.26 | −4.10 | ||
| P in PW (mg L−1) | −0.73 | −0.37 | −2.48 | 0.13 | 0.55 | 0.04 | 0.83 | ||
| C (%TS) | −0.63 | 0.70 | 0.47 | ||||||
| H (%TS) | −0.07 | −0.01 | 0.07 | −0.06 | −0.08 | 0.11 | 0.16 | 0.21 | |
| N (%TS) | −0.44 | −0.20 | 0.02 | 0.12 | −0.41 | −0.14 | 0.09 | 0.11 | |
| S (%TS) | 0.41 | 0.07 | −1.71 | 0.27 | |||||
| O (%TS) | −3.22 | −0.30 | −0.78 | ||||||
| Ash (%TS) | 4.03 | 0.68 | 1.05 | 1.18 | 1.75 | −0.13 | −0.94 | −0.37 | |
| C | H | N | S | O | Ash | SY | HHVar | HHVdaf |
|---|---|---|---|---|---|---|---|---|
| (%TS) | (MJ kgTS−1) | |||||||
| 29.1 | 3.6 | 3.9 | 5.8 | 8.6 | 49.0 | 87.1 | 13.0 | 25.5 |
| SS | Condensate | Exhaust Air | HC | PW | |
|---|---|---|---|---|---|
| Mass flow (kg h−1) | 3000.00 | 369.58 | 188.18 | 720.03 | 1704.67 |
| ID | ss | htc-st2 | dry-st1 | dry-c2 | bp-pw1 |
| # | Scenario | QHTC | QDryer | QTotal |
|---|---|---|---|---|
| (kWh tSS−1) | ||||
| 1 | WWTP, no HTC, TS = 35% after MDW | 0.0 | 288.5 | 288.5 |
| 2 | HTC, no heat recovery, TS = 70% after 2nd MDW | 160.3 | 52.5 | 212.8 |
| 3 | HTC, flash to 1.6 bar(a), TS = 70% after 2nd MDW | 84.5 | 52.5 | 137.0 |
| Fuel | HHV (MJ kg−1) | Heat of Combustion (kW) | Qtotal * | Qthermal | Qelectrical |
|---|---|---|---|---|---|
| (kWh tSS−1) | |||||
| Sewage sludge (TS = 90%) | 12.2 | 2810.8 | 749.5 | 599.6 | 149.9 |
| Hydrochar (TS = 90%) | 11.7 | 2340.4 | 624.1 | 499.3 | 124.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knötig, P.; Etzold, H.; Wirth, B. Model-Based Evaluation of Hydrothermal Treatment for the Energy Efficient Dewatering and Drying of Sewage Sludge. Processes 2021, 9, 1346. https://doi.org/10.3390/pr9081346
Knötig P, Etzold H, Wirth B. Model-Based Evaluation of Hydrothermal Treatment for the Energy Efficient Dewatering and Drying of Sewage Sludge. Processes. 2021; 9(8):1346. https://doi.org/10.3390/pr9081346
Chicago/Turabian StyleKnötig, Philipp, Hendrik Etzold, and Benjamin Wirth. 2021. "Model-Based Evaluation of Hydrothermal Treatment for the Energy Efficient Dewatering and Drying of Sewage Sludge" Processes 9, no. 8: 1346. https://doi.org/10.3390/pr9081346




