Synthesis and Physicochemical Characteristics of Chitosan-Based Polyurethane Flexible Foams
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
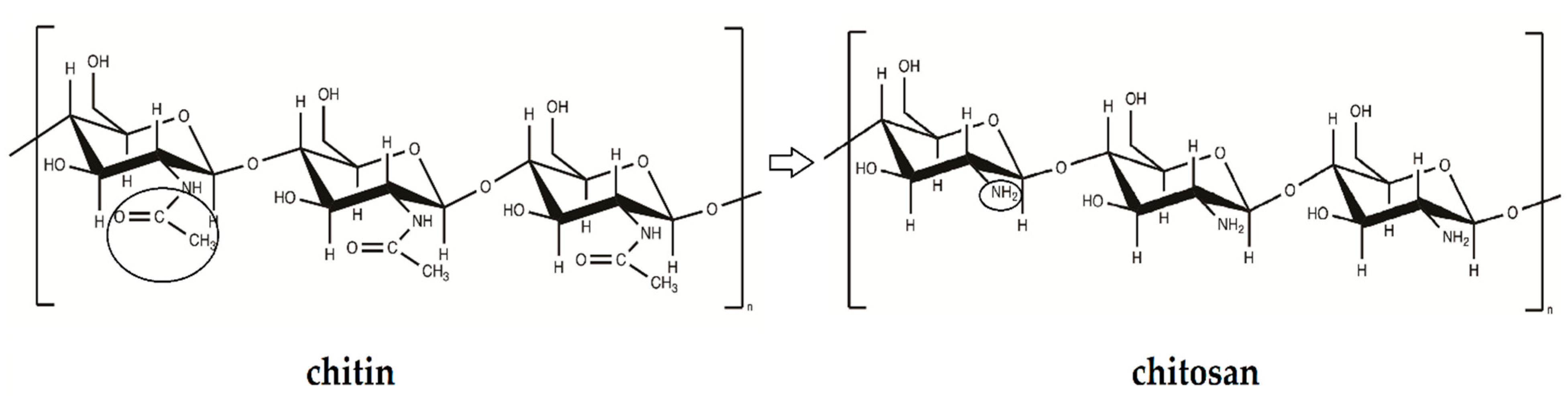
2.2.1. Chemical Structure
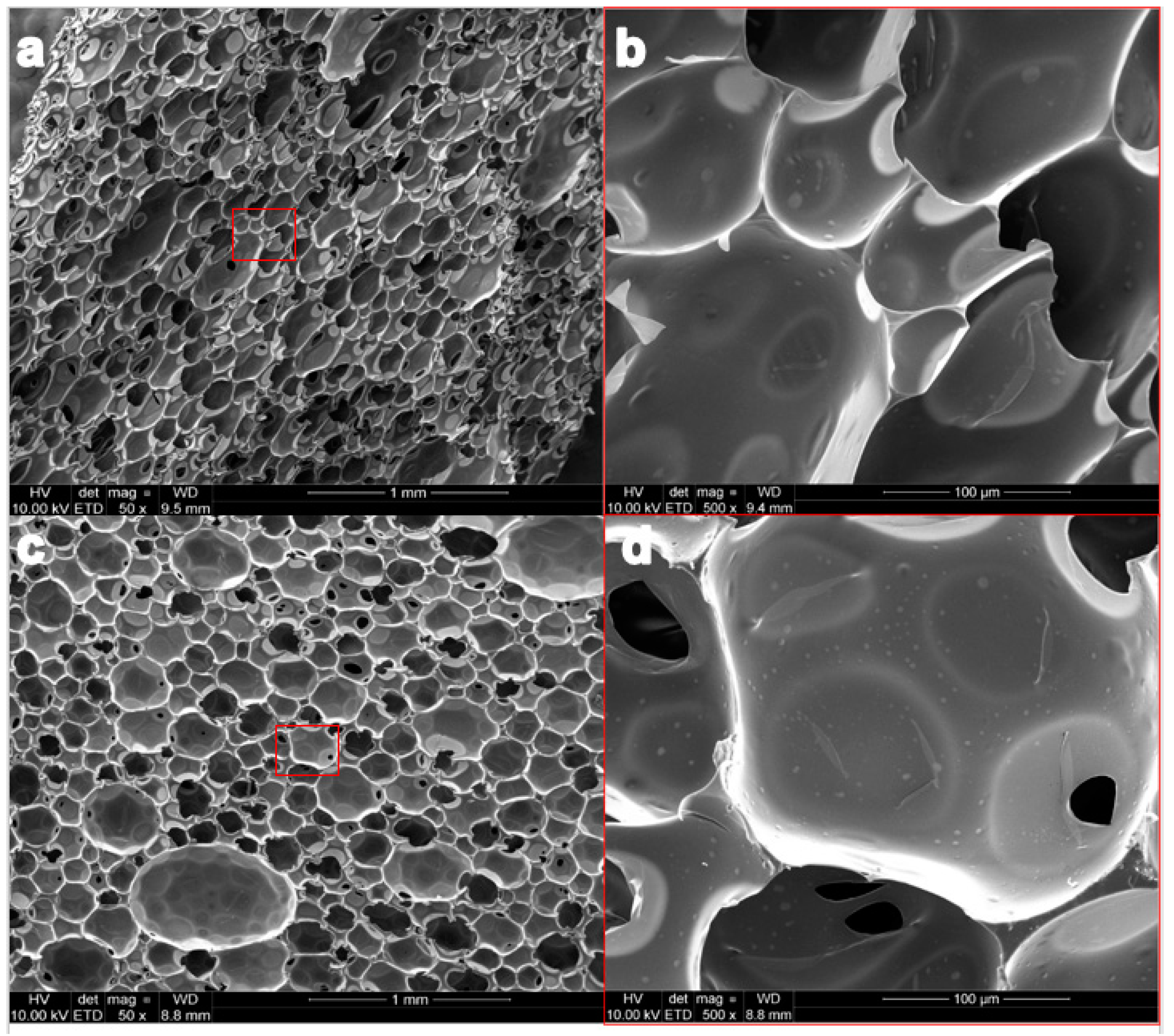
2.2.2. Structure Morphology and Characterization of Pore Structure
2.2.3. Hardness
2.2.4. Thermal Properties
2.2.5. Viscoelastic Properties
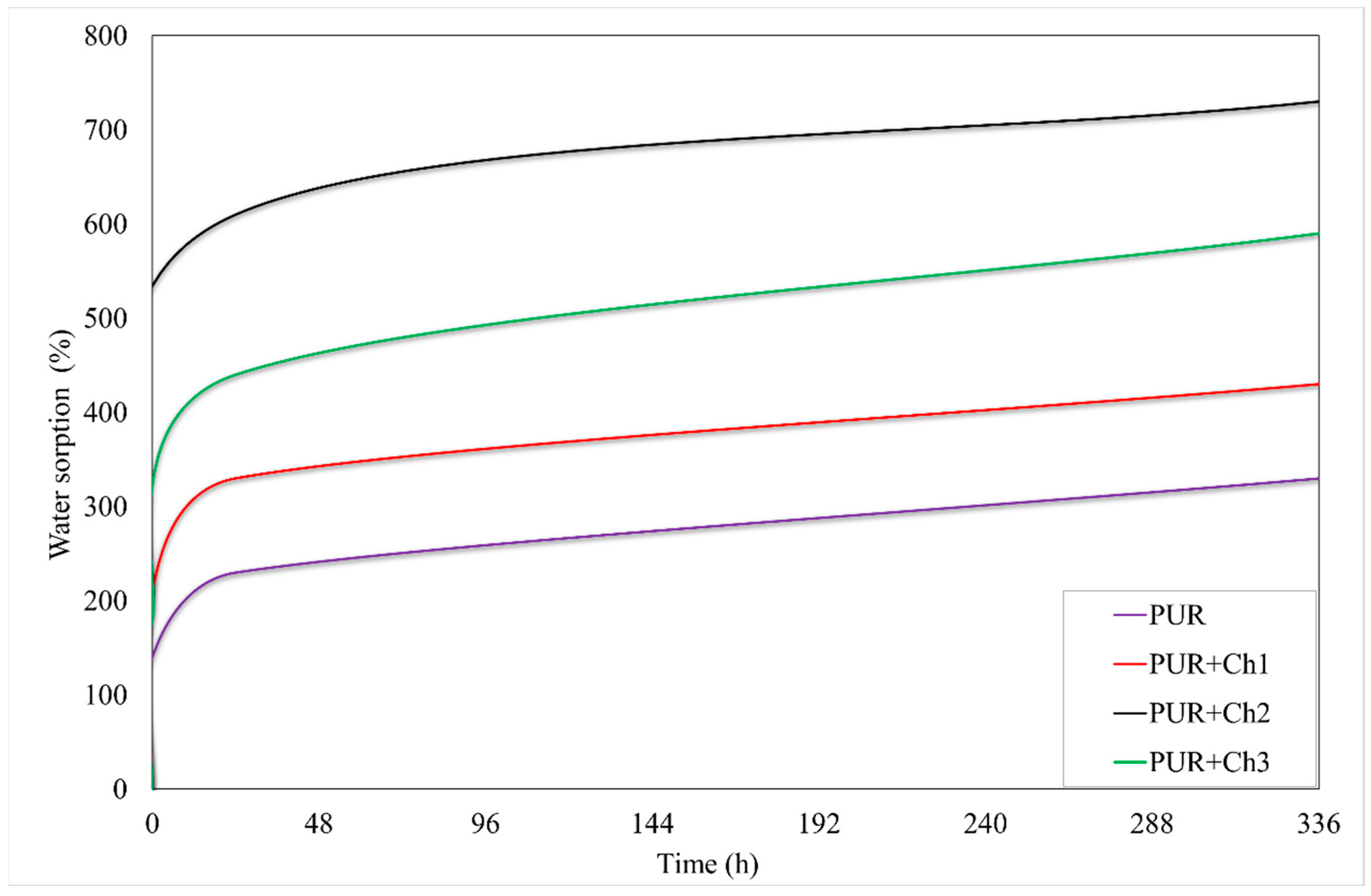
2.2.6. Sorption Properties
3. Results and Discussion
3.1. Chemical Structure
3.2. Structure Morphology and Characterization of Pore Structure
3.3. Hardness
3.4. Thermal and Viscoelastic Properties
3.5. Sorption Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNESCO—World Water Development Report 2020—Water and Climate Change. Available online: https://en.unesco.org/themes/water-security/wwap/wwdr/2020 (accessed on 26 May 2021).
- Piotrowska-Kirschling, A.; Brzeska, J. Environmental management through example of polysaccharide materials using in water treatment. Polish J. Commod. Sci. 2020, 4, 60–75. [Google Scholar]
- Amorim, F.V.; Padilha, R.J.R.; Vinhas, G.M.; Luiz, M.R.; de Souza, N.C.; de Almeida, Y.M.B. Development of hydrophobic polyurethane/castor oil biocomposites with agroindustrial residues for sorption of oils and organic solvents. J. Colloid Interface Sci. 2021, 581, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Prendergast, D.P.; MacFarlane, J.; Bagatin, R.; Stellacci, F.; Gschwend, P.M. Hydrophobic meshes for oil spill recovery devices. ASC Appl. Mater. Interfaces 2013, 5, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Ani, J.U.; Akpomie, K.G.; Okoro, U.C.; Aneke, L.E.; Onukwuli, O.D.; Ujam, O.T. Potentials of activated carbon produced from biomass, materials for the sequestration of dyes, heavy metals and crude oil components from aqueous environment. Appl. Water Sci. 2020, 10, 69. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Ma, H.; Chu, B.; Hsiao, B.S. Super-hydrophobic modification of porous natural polymer “luffa sponge” for oil absorption. Polymer 2017, 126, 470–476. [Google Scholar] [CrossRef]
- Beshkar, F.; Amiri, O.; Salehi, Z. Synthesis of ZnSnO3 nanostructures by using novel gelling agents and their application in degradation of textile dye. Sep. Purif. Technol. 2017, 184, 66–71. [Google Scholar] [CrossRef]
- Najafian, H.; Manteghi, F.; Beshkar, F.; Salavati-Niasari, M. Fabrication of nanocomposite photocatalyst CuBi2O4/Bi3ClO4 for removal of acid brown 14 as water pollutant under visible light irradiation. J. Hazard. Mater. 2019, 361, 210–220. [Google Scholar] [CrossRef]
- Speight, J.G.; El-Gendy, N.S. Chapter 11—Bioremediation of marine oil spills. In Introduction to Petroleum Biotechnology; Speight, J.G., El-Gendy, N.S., Eds.; Gulf Professional Publishing: Houston, TX, USA, 2018; Volume 1, pp. 419–470. [Google Scholar] [CrossRef]
- Muttin, F.; Campbell, R. 2—Oil spill containment in open areas: Four Atlantic and Mediterranean experiments. In Oil Spill Studies Healing the Ocean, Biomarking, and the Law; Muttin, F., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 1, pp. 19–43. [Google Scholar] [CrossRef]
- Shah, M.U.H.; Moniruzzaman, M.; Sivapragasam, M.; Talukder, M.M.R.; Yusup, S.B.; Goto, M. A binary mixture of a biosurfactant and an ionic liquid surfactant as a green dispersant for oil spill remediation. J. Mol. Liq. 2019, 280, 111–119. [Google Scholar] [CrossRef]
- Kong, D.; He, X.; Khan, F.; Chen, G.; Ping, P.; Yang, H.; Peng, R. Small scale experiment study on burning characteristics for in-situ burning of crude oil on open water. J. Loss Prev. Process Ind. 2019, 60, 46–52. [Google Scholar] [CrossRef]
- Lu, J.; Xu, Z.; Xu, S.; Xie, S.; Wu, H.; Yang, Z.; Liu, X. Experimental and numerical investigations on reliability of air barrier on oil containment in flowing water. Mar. Pollut. Bull. 2015, 95, 200–206. [Google Scholar] [CrossRef]
- Peng, D.; Li, H.; Li, W.J.; Zheng, L. Biosorbent with superhydrophobicity and superoleophilicity for spilled oil removal. Ecotoxicol. Environ. Saf. 2021, 209, 111803. [Google Scholar] [CrossRef]
- Dawodu, F.A.; Abonyi, C.J.; Akpomie, K.G. Feldspar-banana peel composite adsorbent for efficient crude oil removal from solution. Appl. Water Sci. 2021, 11, 3. [Google Scholar] [CrossRef]
- Sanguanwong, A.; Flood, A.E.; Ogawa, M.; Martin-Sampedro, R.; Darder, M.; Wicklein, B.; Aranda, P.; Ruiz-Hitzky, E. Hydrophobic composite foams based on nanocellulose-sepiolite for oil sorption applications. J. Hazard. Mater. 2021, 417, 126068. [Google Scholar] [CrossRef]
- Dong, Y.D.; Zhang, H.; Zhong, G.J.; Yao, G.; Lai, B. Cellulose/carbon composites and their applications in water treatment—A review. Chem. Eng. J. 2021, 405, 126980. [Google Scholar] [CrossRef]
- Gama, N.V.; Ferreira, A.; Barros-Timmons, A. Polyurethane foams: Past, present, and future. Materials 2018, 11, 1841. [Google Scholar] [CrossRef] [Green Version]
- Plastics Insight—Polyurethane Production, Pricing and Market Demand. Available online: https://www.plasticsinsight.com/resin-intelligence/resin-prices/polyurethane/ (accessed on 26 May 2021).
- Research and Markets—Polyurethane (PU) Foam Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2021–2026. Available online: https://www.researchandmarkets.com (accessed on 26 May 2021).
- BCC Publishing—Global Polyurethane Foam Market. Available online: https://www.bccresearch.com/market-research/plastics/polyurethane-foam-market-report.html#toc (accessed on 26 May 2021).
- Martins, L.S.; Zanini, N.C.; Maia, L.S.; Souza, A.G.; Barbosa, R.F.S.; Rosa, D.S.; Mulinari, D.R. Crude oil and S500 diesel removal from seawater by polyurethane composites reinforced with palm fiber residues. Chemosphere 2021, 267, 129288. [Google Scholar] [CrossRef]
- Piotrowska-Kirschling, A.; Szelągowska-Rudzka, K.; Karczewski, J.; Brzeska, J. Application of shrimp waste for the synthesis of polyurethane–chitosan materials with potential use in sorption of oil micro-spills in water treatment. Sustainability 2021, 13, 5098. [Google Scholar] [CrossRef]
- Jin, T.; Liu, T.; Lam, E.; Moores, A. Chitin and chitosan on the nanoscale. Nanoscale Horiz. 2021, 6, 505–542. [Google Scholar] [CrossRef] [PubMed]
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane types, synthesis and applications—A review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef] [Green Version]
- Brzeska, J.; Elert, A.M.; Morawska, M.; Sikorska, W.; Kowalczuk, M.; Rutkowska, M. Branched polyurethanes based on synthetic polyhydroxybutyrate with tunable structure and properties. Polymers 2018, 10, 826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piotrowska-Kirschling, A.; Brzeska, J. The effect of chitosan on the chemical structure, morphology, and selected properties of polyurethane/chitosan composites. Polymers 2020, 12, 1205. [Google Scholar] [CrossRef]
- Hejna, A.; Olszewski, A.; Zedler, Ł.; Kosmela, P.; Formela, K. The impact of ground tire rubber oxidation with H2O2 and KMnO4 on the structure and performance of flexible polyurethane/ground tire rubber composite foams. Materials 2021, 14, 499. [Google Scholar] [CrossRef]
- Li, H.; Liu, L.; Yang, F. Oleophilic polyurethane foams for oil spill cleanup. Procedia Environ. Sci. 2013, 18, 528–533. [Google Scholar] [CrossRef] [Green Version]
- Kwon, O.J.; Oh, S.T.; Lee, S.D.; Lee, N.R.; Shin, C.H.; Park, J.S. Hydrophilic and flexible polyurethane foams using sodium alginate as polyol: Effects of PEG molecular weight and cross-linking agent content on water absorbency. Fiber. Polym. 2007, 8, 347–355. [Google Scholar] [CrossRef]
- Queiroz, M.F.; Melo, K.R.T.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O. Does the use of chitosan contribute to oxalate kidney stone formation? Mar. Drugs 2015, 13, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Kumirska, J.; Czerwicka, M.; Kaczyński, Z.; Bychowska, A.; Brzozowski, K.; Thöming, J.; Stepnowski, P. Application of spectroscopic methods for structural analysis of chitin and chitosan. Mar. Drugs 2010, 8, 1567–1636. [Google Scholar] [CrossRef] [Green Version]
- Suchkova, G.G.; Maklakov, L.I. Amide bands in the IR spectra of urethanes. Vib. Spectrosc. 2009, 51, 333–339. [Google Scholar] [CrossRef]
- Zhang, C.; Ren, Z.; Yin, Z.; Qian, H.; Ma, D. Amide II and amide III bands in polyurethane model soft and hard segments. Polym. Bull. 2008, 60, 97–101. [Google Scholar] [CrossRef]
- Elliott, J.A.; Windle, A.H.; Hobdell, J.R.; Eeckhaut, G.; Oldman, R.J.; Ludwig, W.; Boller, E.; Cloetens, P.; Baruchel, J. In-situ deformation of an open-cell flexible polyurethane foam characterised by 3D computed microtomography. J. Mater. Sci. 2002, 37, 1547–1555. [Google Scholar] [CrossRef]
- Koumlis, S.; Lamberson, L. Strain rate dependent compressive response of open cell polyurethane foam. Exp. Mech. 2019, 59, 1087–1103. [Google Scholar] [CrossRef]
- Wu, J.W.; Sung, W.F.; Chu, H.S. Thermal conductivity of polyurethane foams. Int. J. Heat Mass Transf. 1999, 42, 2211–2217. [Google Scholar] [CrossRef]
- Husainie, S.M.; Khattak, S.U.; Robinson, J.; Naguib, H.E. A comparative study on the mechanical properties of different natural fiber reinforced free-rise polyurethane foam composites. Ind. Eng. Chem. Res. 2020, 59, 21745–21755. [Google Scholar] [CrossRef]
- Sakurai, K.; Maegawa, T.; Takahashi, T. Glass transition temperature of chitosan and miscibility of chitosan/poly(N-vinyl pyrrolidone) blends. Polymer 2000, 41, 7051–7056. [Google Scholar] [CrossRef]
- Ryszkowska, J.; Leszczyńska, M.; Auguścik, M.; Bryśkiewicz, A.; Półka, M.; Kukfisz, B.; Wierzbicki, Ł.; Aleksandrowicz, J.; Szczepkowski, L.; Oliwa, R. Rdzenie konstrukcji kompozytowych z pianek półsztywnych do zastosowań w tarczach ochronnych dla strażaków (in Polish). Polimery 2018, 63, 125–133. [Google Scholar] [CrossRef]
- Filip, D.; Macocinschi, D.; Vlad, S. Thermogravimetric study por polyurethane materials for biomedical applications. Compos. B Eng. 2011, 42, 1474–1479. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Webster, D.C. Thermal stability and flame retardancy of polyurethanes. Prog. Polym. Sci. 2009, 34, 1068–1133. [Google Scholar] [CrossRef]
- Hejna, A.; Kosmela, P.; Kirpluks, M.; Cabulis, U.; Klein, M.; Haponiuk, J.; Piszczyk, Ł. Structure, mechanical, thermal and fire behavior assessments of environmentally friendly crude glycerol-based rigid polyisocyanurate foams. J. Polym. Environ. 2018, 26, 1854–1868. [Google Scholar] [CrossRef] [Green Version]
- Olszewski, A.; Nowak, P.; Kosmela, P.; Piszczyk, Ł. Characterization of highly filled glass fiber/carbon fiber polyurethane composites with the addition of bio-polyol obtained through biomass liquefaction. Materials 2021, 14, 1391. [Google Scholar] [CrossRef]
- Santos, O.S.H.; da Silva, M.C.; Silva, V.R.; Mussel, W.N.; Yoshida, M.I. Polyurethane foam impregnated with lignin as a filler for the removal of crude oil from contaminated water. J. Hazard. Mater. 2017, 324, 406–413. [Google Scholar] [CrossRef]



| Foam | Quantitative Composition of Foams (wt%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Rokopol V700 | TEGOSTAB B8465 | DABCO K-15 | DABCO 33-LV | DBTDL | n-pentane | pMDI | Ch | |
| PUR | 49.7 | 1.6 | 1.3 | 1.2 | 0.8 | 10.7 | 34.7 | 0.0 |
| PUR+Ch1 | 49.8 | 1.7 | 1.3 | 1.2 | 0.8 | 10.6 | 33.6 | 1.0 |
| PUR+Ch2 | 49.2 | 1.9 | 1.3 | 1.3 | 0.8 | 10.6 | 32.8 | 2.1 |
| PUR+Ch3 | 51.1 | 1.9 | 1.2 | 1.2 | 0.8 | 10.2 | 30.6 | 3.0 |
| Foam | Open Cell Content ± SD (%) |
|---|---|
| PUR | 1.58 |
| PUR+Ch1 | 0.82 |
| PUR+Ch2 | 0.42 |
| PUR+Ch3 | 0.34 |
| Foam | Hardness ± SD (Shore H0) |
|---|---|
| PUR | 1.09 |
| PUR+Ch1 | 2.21 |
| PUR+Ch2 | 2.17 |
| PUR+Ch3 | 1.57 |
| Foam | wt% Ch | Tm1 (°C) | ΔH1 (J/g) | Tm2 (°C) | ΔH2 (J/g) | HDT (°C) |
|---|---|---|---|---|---|---|
| Ch | - | 80.3 | 145.3 | - | - | - |
| PUR | 0 | 44.9 | 73.9 | 159.7 | 62.4 | 185.8 |
| PUR+Ch1 | 1 | 50.5 | 29.4 | 221.3 | 10.5 | 166.5 |
| PUR+Ch2 | 2 | 50.2 | 9.6 | 160.6 | 86.4 | 172.5 |
| PUR+Ch3 | 3 | 47.8 | 16.2 | 161.3 | 86.1 | 177.8 |
| Sample | Different % Mass Loss in Temperature (°C) | Tmax (°C) | Char Residue after Test (%) | ||
|---|---|---|---|---|---|
| 2% | 5% | 50% | |||
| PUR | 211.5 | 241.5 | 353.5 | 346.4 | 19.34 |
| PUR+Ch1 | 208.0 | 250.7 | 353.9 | 350.6 | 21.20 |
| PUR+Ch2 | 208.4 | 242.1 | 351.6 | 346.9 | 20.72 |
| PUR+Ch3 | 213.6 | 249.4 | 352.4 | 345.7 | 18.95 |
| Temperature (°C) | Area under curve | Ratio of individual stagesof decomposition | |||
| Peak 1 | 302 | 242 | 1 | ||
| Peak 2 | 353 | 480 | 1.99 | ||
| Peak 3 | 547 | 55 | 0.23 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piotrowska-Kirschling, A.; Olszewski, A.; Karczewski, J.; Piszczyk, Ł.; Brzeska, J. Synthesis and Physicochemical Characteristics of Chitosan-Based Polyurethane Flexible Foams. Processes 2021, 9, 1394. https://doi.org/10.3390/pr9081394
Piotrowska-Kirschling A, Olszewski A, Karczewski J, Piszczyk Ł, Brzeska J. Synthesis and Physicochemical Characteristics of Chitosan-Based Polyurethane Flexible Foams. Processes. 2021; 9(8):1394. https://doi.org/10.3390/pr9081394
Chicago/Turabian StylePiotrowska-Kirschling, Agnieszka, Adam Olszewski, Jakub Karczewski, Łukasz Piszczyk, and Joanna Brzeska. 2021. "Synthesis and Physicochemical Characteristics of Chitosan-Based Polyurethane Flexible Foams" Processes 9, no. 8: 1394. https://doi.org/10.3390/pr9081394
APA StylePiotrowska-Kirschling, A., Olszewski, A., Karczewski, J., Piszczyk, Ł., & Brzeska, J. (2021). Synthesis and Physicochemical Characteristics of Chitosan-Based Polyurethane Flexible Foams. Processes, 9(8), 1394. https://doi.org/10.3390/pr9081394







