Overview on the Antimicrobial Activity and Biocompatibility of Sputtered Carbon-Based Coatings
Abstract
:1. Introduction
2. Diamond-Like Carbon
2.1. Biomedical Applications
2.2. Preparation Method: Physical Vapor Deposition
3. Antimicrobial Response
4. DLC Biocompatibility
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Jumaili, A.; Alancherry, S.; Bazaka, K.; Jacob, M.V. Review on the Antimicrobial Properties of Carbon Nanostructures. Materials 2017, 10, 1066. [Google Scholar] [CrossRef]
- Raymundo-Pereira, P.A.; Baccarin, M.; Oliveira, O.N.; Janegitz, B.C. Thin Films and Composites Based on Graphene for Electrochemical Detection of Biologically-relevant Molecules. Electroanalysis 2018, 30, 1888–1896. [Google Scholar] [CrossRef]
- Alim, S.; Vejayan, J.; Yusoff, M.M.; Kafi, A. Recent uses of carbon nanotubes & gold nanoparticles in electrochemistry with application in biosensing: A review. Biosens. Bioelectron. 2018, 121, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Guz, A.N.; Rushchitsky, J.J. Establishing foundations of the mechanics of nanocomposites (Review). Int. Appl. Mech. 2011, 47, 2–44. [Google Scholar] [CrossRef]
- Zhai, W.; Srikanth, N.; Kong, L.B.; Zhou, K. Carbon nanomaterials in tribology. Carbon 2017, 119, 150–171. [Google Scholar] [CrossRef]
- Omprakash, P.; Viswesh, P.; Panemangalore, D.B. Review—A Review of 2D Perovskites and Carbon-Based Nanomaterials for Applications in Solar Cells and Photodetectors. ECS J. Solid State Sci. Technol. 2021, 10, 031009. [Google Scholar] [CrossRef]
- Lee, S.; Song, H.; Ahn, H.; Kim, S.; Choi, J.-R.; Kim, K. Fiber-Optic Localized Surface Plasmon Resonance Sensors Based on Nanomaterials. Sensors 2021, 21, 819. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Y.; Zhao, L.; Qi, Z.; Gou, J.; Zhang, S.; Zhang, J.Z. Recent advances in ultrathin two-dimensional materials and biomedical applications for reactive oxygen species generation and scavenging. Nanoscale 2020, 12, 19516–19535. [Google Scholar] [CrossRef] [PubMed]
- Knoblauch, R.; Geddes, C.D. Carbon Nanodots in Photodynamic Antimicrobial Therapy: A Review. Materials 2020, 13, 4004. [Google Scholar] [CrossRef]
- Ding, Q.; Cui, J.; Shen, H.; He, C.; Wang, X.; Shen, S.G.F.; Lin, K. Advances of nanomaterial applications in oral and maxillofacial tissue regeneration and disease treatment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1669. [Google Scholar] [CrossRef]
- Tahriri, M.; Del Monico, M.; Moghanian, A.; Yaraki, M.T.; Torres, R.; Yadegari, A.; Tayebi, L. Graphene and its derivatives: Opportunities and challenges in dentistry. Mater. Sci. Eng. C 2019, 102, 171–185. [Google Scholar] [CrossRef]
- Cruz, S.M.A.; Girão, A.F.; Gonçalves, G.; Marques, P.A.A.P. Graphene: The Missing Piece for Cancer Diagnosis? Sensors 2016, 16, 137. [Google Scholar] [CrossRef]
- Gao, X.; Cui, Y.; Levenson, R.M.; Chung, L.W.K.; Nie, S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat. Biotechnol. 2004, 22, 969–976. [Google Scholar] [CrossRef]
- Berlin, J.M.; Leonard, A.D.; Pham, T.T.; Sano, D.; Marcano, D.C.; Yan, S.; Fiorentino, S.; Milas, Z.L.; Kosynkin, D.V.; Price, B.K.; et al. Effective Drug Delivery, In Vitro and In Vivo, by Carbon-Based Nanovectors Noncovalently Loaded with Unmodified Paclitaxel. ACS Nano 2010, 4, 4621–4636. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-J.; Zhang, K.; Moore, L.; Ho, D. Diamond Nanogel-Embedded Contact Lenses Mediate Lysozyme-Dependent Therapeutic Release. ACS Nano 2014, 8, 2998–3005. [Google Scholar] [CrossRef]
- De Armentia, S.L.; Del Real, J.C.; Paz, E.; Dunne, N. Advances in Biodegradable 3D Printed Scaffolds with Carbon-Based Nanomaterials for Bone Regeneration. Materials 2020, 13, 5083. [Google Scholar] [CrossRef]
- Marches, R.; Mikoryak, C.; Wang, R.-H.; Pantano, P.; Draper, R.K.; Vitetta, E.S. The importance of cellular internalization of antibody-targeted carbon nanotubes in the photothermal ablation of breast cancer cells. Nanotechnology 2011, 22, 095101. [Google Scholar] [CrossRef]
- Feng, L.; Wu, L.; Qu, X. New Horizons for Diagnostics and Therapeutic Applications of Graphene and Graphene Oxide. Adv. Mater. 2013, 25, 168–186. [Google Scholar] [CrossRef] [PubMed]
- Kemp, J.A.; Shim, M.S.; Heo, C.Y.; Kwon, Y.J. “Combo” nanomedicine: Co-delivery of multi-modal therapeutics for efficient, targeted, and safe cancer therapy. Adv. Drug Deliv. Rev. 2016, 98, 3–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; He, X.; Liu, Y.; Huang, H.; Lian, S.; Lee, S.-T.; Kang, Z. One-step ultrasonic synthesis of water-soluble carbon nanoparticles with excellent photoluminescent properties. Carbon 2011, 49, 605–609. [Google Scholar] [CrossRef]
- Cao, Q.; Rogers, J.A. Ultrathin Films of Single-Walled Carbon Nanotubes for Electronics and Sensors: A Review of Fundamental and Applied Aspects. Adv. Mater. 2009, 21, 29–53. [Google Scholar] [CrossRef]
- Choi, W.; Lahiri, I.; Seelaboyina, R.; Kang, Y.S. Synthesis of Graphene and Its Applications: A Review. Crit. Rev. Solid State Mater. Sci. 2010, 35, 52–71. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef] [Green Version]
- Ohtake, N.; Hiratsuka, M.; Kanda, K.; Akasaka, H.; Tsujioka, M.; Hirakuri, K.; Hirata, A.; Ohana, T.; Inaba, H.; Kano, M.; et al. Properties and Classification of Diamond-Like Carbon Films. Materials 2021, 14, 315. [Google Scholar] [CrossRef] [PubMed]
- Harigai, T.; Degai, S.; Sugie, Y.; Takikawa, H.; Tanimoto, T.; Gonda, H.; Kaneko, S.; Kunitsugu, S.; Suzuki, K.; Kamiya, M.; et al. Improvement of drilling performance by overcoating diamond-like carbon films on diamond-coated drills for carbon fiber reinforced plastics processing. Vacuum 2021, 183, 109755. [Google Scholar] [CrossRef]
- Huang, B.; Zhang, E.-G.; Zhou, Q.; Lin, R.-C.; Du, H.-M. Research on the Performance of Diamond-Like Carbon Coatings on Cutting Aluminum Alloy: Cutting Experiments and First-Principles Calculations. Coatings 2021, 11, 63. [Google Scholar] [CrossRef]
- Claver, A.; Jiménez-Piqué, E.; Palacio, J.F.; Almandoz, E.; De Ara, J.F.; Fernández, I.; Santiago, J.A.; Barba, E.; García, J.A. Comparative Study of Tribomechanical Properties of HiPIMS with Positive Pulses DLC Coatings on Different Tools Steels. Coatings 2020, 11, 28. [Google Scholar] [CrossRef]
- Baragetti, S.; Božić, Ž.; Arcieri, E. Stress and fracture surface analysis of uncoated and coated 7075-T6 specimens under the rotating bending fatigue loading. Eng. Fail. Anal. 2020, 112, 104512. [Google Scholar] [CrossRef]
- Ferreira, F.; Cavaleiro, A.; Oliveira, J. Tribological performance of DLC coatings deposited by DOMS in mixed Ar-Ne discharges. Mater. Lett. 2021, 285, 129056. [Google Scholar] [CrossRef]
- Hakala, T.; Holmberg, K.; Laukkanen, A. Coupling Molecular Dynamics and Micromechanics for the Assessment of Friction and Damage Accumulation in Diamond-Like Carbon Thin Films under Lubricated Sliding Contacts. Lubricants 2021, 9, 30. [Google Scholar] [CrossRef]
- Laderou, A.; Mohammadpour, M.; Theodossiades, S.; Daubney, R.; Meeks, G. On the Effect of DLC and WCC Coatings on the Efficiency of Manual Transmission Gear Pairs. Appl. Sci. 2020, 10, 3102. [Google Scholar] [CrossRef]
- Park, Y.-P.; Kim, T.-G.; Cheon, M.-W. Analysis of Characteristics of DLC Coating Thin Film in Tungsten Carbide for Production of Medical Thermal-Infrared Lenses. Trans. Electr. Electron. Mater. 2014, 15, 344–347. [Google Scholar] [CrossRef]
- Bahre, H.; Behm, H.; Grochla, D.; Böke, M.; Dahlmann, R.; Hopmann, C.; Ludwig, A.; Winter, J. Film Stress of Amorphous Hydrogenated Carbon on Biaxially Oriented Polyethylene Terephthalate. Plasma Process. Polym. 2015, 12, 896–904. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, R.; Li, G.; Hu, G.; Sun, Y. Improving Barrier Properties of PET by Depositing a Layer of DLC Films on Surface. Adv. Mater. Sci. Eng. 2013, 2013, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Vetter, J. 60 years of DLC coatings: Historical highlights and technical review of cathodic arc processes to synthesize various DLC types, and their evolution for industrial applications. Surf. Coat. Technol. 2014, 257, 213–240. [Google Scholar] [CrossRef]
- Dwivedi, N.; Kumar, S.; Singh, S.; Malik, H.K. Oxygen modified diamond-like carbon as window layer for amorphous silicon solar cells. Sol. Energy 2012, 86, 220–230. [Google Scholar] [CrossRef]
- Liu, W.; Tu, H.; Gao, M.; Su, X.; Zhang, S.; Huo, C.; Yang, H. High performance DLC/BP and ZnS/YbF3 double-layer protective and antireflective coatings. J. Alloy. Compd. 2013, 581, 526–529. [Google Scholar] [CrossRef]
- Basman, N.; Uzun, R.; Gocer, E.; Bacaksiz, E.; Kolemen, U. Electrodeposition of Si–DLC nanocomposite film and its electronic application. Microsyst. Technol. 2017, 24, 2287–2294. [Google Scholar] [CrossRef]
- Nakajima, D.; Kuwabara, H.; Annaka, S.; Fujii, S.; Tanaka, Y.; Hirakuri, K. Diamond-like carbon coating for effective electrical insulation of Cu and Al wires. Diam. Relat. Mater. 2020, 103, 107731. [Google Scholar] [CrossRef]
- Silva, F.; Martinho, R.; Andrade, M.; Baptista, A.; Alexandre, R. Improving the Wear Resistance of Moulds for the Injection of Glass Fibre–Reinforced Plastics Using PVD Coatings: A Comparative Study. Coatings 2017, 7, 28. [Google Scholar] [CrossRef] [Green Version]
- Michalek, A.; Qi, S.; Batal, A.; Penchev, P.; Dong, H.; See, T.L.; Dimov, S. Sub-micron structuring/texturing of diamond-like carbon-coated replication masters with a femtosecond laser. Appl. Phys. A 2020, 126, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Jiang, B.; Zhou, M.; Chen, J.; Weng, C. Influence of Diamond-Like Carbon Coating on the Channel Deformation of Injection-Molded Microfluidic Chips during the Demolding Process. Polymers 2020, 12, 2914. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-Y.; Hwang, S.-J. Application of surface treatments on releasing adhesion force during thermoplastic polyurethane injection molding process. Polym. Eng. Sci. 2016, 57, 299–305. [Google Scholar] [CrossRef]
- Rahmati, M.; Mozafari, M. Biological Response to Carbon-Family Nanomaterials: Interactions at the Nano-Bio Interface. Front. Bioeng. Biotechnol. 2019, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Hauert, R. An overview on the tribological behavior of diamond-like carbon in technical and medical applications. Tribol. Int. 2004, 37, 991–1003. [Google Scholar] [CrossRef]
- Narayan, R.J. Laser processing of diamondlike carbon thin films for medical prostheses. Int. Mater. Rev. 2006, 51, 127–143. [Google Scholar] [CrossRef]
- Shim, J.W.; Bae, I.-H.; Jeong, M.H.; Park, D.S.; Lim, K.-S.; Kim, J.U.; Kim, M.-K.; Kim, J.H.; Sim, D.S. Effects of a Titanium Dioxide Thin Film for Improving the Biocompatibility of Diamond-Like Coated Coronary Stents. Met. Mater. Int. 2019, 26, 1–8. [Google Scholar] [CrossRef]
- Bito, K.; Hasebe, T.; Maegawa, S.; Maeda, T.; Matsumoto, T.; Suzuki, T.; Hotta, A. In vitro basic fibroblast growth factor (bFGF) delivery using an antithrombogenic 2-methacryloyloxyethyl phosphorylcholine (MPC) polymer coated with a micropatterned diamond-like carbon (DLC) film. J. Biomed. Mater. Res. Part A 2017, 105, 3384–3391. [Google Scholar] [CrossRef]
- Carvalho, I.; Curado, M.; Palacio, C.; Carvalho, S.; Cavaleiro, A. Ag release from sputtered Ag/a:C nanocomposite films after immersion in pure water and NaCl solution. Thin Solid Films 2018, 671, 85–94. [Google Scholar] [CrossRef]
- Manninen, N.; Calderon, S.; Carvalho, S.; Henriques, M.; Cavaleiro, A. Antibacterial Ag/a-C nanocomposite coatings: The influence of nano-galvanic a-C and Ag couples on Ag ionization rates. Appl. Surf. Sci. 2016, 377, 283–291. [Google Scholar] [CrossRef] [Green Version]
- McKenzie, D. Tetrahedral bonding in amorphous carbon. Rep. Prog. Phys. 1996, 59, 1611–1664. [Google Scholar] [CrossRef]
- Aisenberg, S.; Chabot, R. Ion-Beam Deposition of Thin Films of Diamondlike Carbon. J. Appl. Phys. 1971, 42, 2953–2958. [Google Scholar] [CrossRef]
- Hainsworth, S.; Uhure, N.J. Diamond like carbon coatings for tribology: Production techniques, characterisation methods and applications. Int. Mater. Rev. 2007, 52, 153–174. [Google Scholar] [CrossRef]
- Weiler, M.; Sattel, S.; Jung, K.; Ehrhardt, H.; Veerasamy, V.S.; Robertson, J. Highly tetrahedral, diamond-like amorphous hydrogenated carbon prepared from a plasma beam source. Appl. Phys. Lett. 1994, 64, 2797–2799. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Raman spectroscopy of amorphous, nanostructured, diamond–like carbon, and nanodiamond. Philos. Trans. R. Soc. A: Math. Phys. Eng. Sci. 2004, 362, 2477–2512. [Google Scholar] [CrossRef]
- Mayorga, J.L.C.; Randazzo, W.; Fabra, M.J.; Lagaron, J.; Aznar, R.; Sánchez, G. Antiviral properties of silver nanoparticles against norovirus surrogates and their efficacy in coated polyhydroxyalkanoates systems. LWT Food Sci. Technol. 2017, 79, 503–510. [Google Scholar] [CrossRef] [Green Version]
- Lv, X.; Wang, P.; Bai, R.; Cong, Y.; Suo, S.; Ren, X.; Chen, C. Inhibitory effect of silver nanomaterials on transmissible virus-induced host cell infections. Biomaterials 2014, 35, 4195–4203. [Google Scholar] [CrossRef]
- Ferreri, I.; Henriques, M.; De Hosson, J.; Cavaleiro, A.; Carvalho, S. Nano-galvanic coupling for enhanced Ag+ release in ZrCN-Ag films: Antibacterial application. Surf. Coat. Technol. 2016, 298, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, I.; Dias, N.; Henriques, M.; Ferreira, P.; Cavaleiro, A.; Carvalho, S. Antibacterial Effects of Bimetallic Clusters Incorporated in Amorphous Carbon for Stent Application. ACS Appl. Mater. Interfaces 2020, 12, 24555–24563. [Google Scholar] [CrossRef] [PubMed]
- Babaei, A.; Mousavi, S.M.; Ghasemi, M.; Pirbonyeh, N.; Soleimani, M.; Moattari, A. Gold nanoparticles show potential in vitro antiviral and anticancer activity. Life Sci. 2021, 119652, 119652. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Shabaan, M.T.; Hassan, L.; Morsi, H.H. Antiviral activity of algae biosynthesized silver and gold nanoparticles against Herps Simplex (HSV-1) virus in vitro using cell-line culture technique. Int. J. Environ. Health Res. 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mehranfar, A.; Izadyar, M. Theoretical Design of Functionalized Gold Nanoparticles as Antiviral Agents against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). J. Phys. Chem. Lett. 2020, 11, 10284–10289. [Google Scholar] [CrossRef]
- Zacheo, A.; Hodek, J.; Witt, D.; Mangiatordi, G.F.; Ong, Q.K.; Kocabiyik, O.; Depalo, N.; Fanizza, E.; Laquintana, V.; Denora, N.; et al. Multi-sulfonated ligands on gold nanoparticles as virucidal antiviral for Dengue virus. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lodeiro, A.; Djafari, J.; Fernández-Lodeiro, J.; Duarte, M.; Mauricio, E.M.; Capelo-Martínez, J.; Lodeiro, C. Synthesis of Mesoporous Silica Coated Gold Nanorods Loaded with Methylene Blue and Its Potentials in Antibacterial Applications. Nanomaterials 2021, 11, 1338. [Google Scholar] [CrossRef] [PubMed]
- Grisoli, P.; De Vita, L.; Milanese, C.; Taglietti, A.; Fernandez, Y.D.; Bouzin, M.; D’Alfonso, L.; Sironi, L.; Rossi, S.; Vigani, B.; et al. PVA Films with Mixed Silver Nanoparticles and Gold Nanostars for Intrinsic and Photothermal Antibacterial Action. Nanomaterials 2021, 11, 1387. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wu, Y.; Liu, L.; Xiao, X.; Cong, Z.; Shao, N.; Qiao, Z.; Chen, K.; Liu, S.; Zhang, H.; et al. The membrane-targeting mechanism of host defense peptides inspiring the design of polypeptide-conjugated gold nanoparticles exhibiting effective antibacterial activity against methicillin-resistant Staphylococcus aureus. J. Mater. Chem. B 2021, 9, 5092–5101. [Google Scholar] [CrossRef]
- Arif, Z.; Sethy, N.K.; Mishra, P.K.; Verma, B. Development of eco-friendly, self-cleaning, antibacterial membrane for the elimination of chromium (VI) from tannery wastewater. Int. J. Environ. Sci. Technol. 2020, 17, 4265–4280. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Shahid, S.; Hanif, S.; Almoallim, H.S.; Alharbi, S.A.; Sellami, H. Green Synthesis of Chromium Oxide Nanoparticles for Antibacterial, Antioxidant Anticancer, and Biocompatibility Activities. Int. J. Mol. Sci. 2021, 22, 502. [Google Scholar] [CrossRef] [PubMed]
- Terpiłowska, S.; Siwicki, A.K. Chromium(III) and iron(III) inhibits replication of DNA and RNA viruses. Biometals 2017, 30, 565–574. [Google Scholar] [CrossRef]
- Hauert, R. DLC Films in Biomedical Applications. In Tribology of Diamond-Like Carbon Films: Fundamentals and Applications; Donnet, C., Erdemir, A., Eds.; Springer: Boston, MA, USA, 2008; pp. 494–509. [Google Scholar]
- Taghizadeh, B.; Ghavami, L.; Derakhshankhah, H.; Zangene, E.; Razmi, M.; Jaymand, M.; Zarrintaj, P.; Zarghami, N.; Jaafari, M.R.; Shahri, M.M.; et al. Biomaterials in Valvular Heart Diseases. Front. Bioeng. Biotechnol. 2020, 8, 529244. [Google Scholar] [CrossRef]
- Dearnaley, G.; Lankford, J.J. Treatments to Reduce Thrombogeneticity in Heart Valves Made from Titanium and Its Alloys. U.S. Patent 5,605,714, 25 February 1997. [Google Scholar]
- Dearnaley, G.; Lankford, J.J. Medical Implants Made of Metal Alloys Bearing Cohesive Diamond Like Carbon. U.S. Patent 5,725,573, 10 March 1998. [Google Scholar]
- Beshchasna, N.; Saqib, M.; Kraskiewicz, H.; Wasyluk, Ł.; Kuzmin, O.; Duta, O.C.; Ficai, D.; Ghizdavet, Z.; Marin, A.; Ficai, A.; et al. Recent Advances in Manufacturing Innovative Stents. Pharmaceutics 2020, 12, 349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutensohn, K.; Beythien, C.; Bau, J.; Fenner, T.; Grewe, P.; Koester, R.; Padmanaban, K.; Kuehnl, P. In Vitro Analyses of Diamond-like Carbon Coated Stents: Reduction of Metal Ion Release, Platelet Activation, and Thrombogenicity. Thromb. Res. 2000, 99, 577–585. [Google Scholar] [CrossRef]
- Gillespie, W.; Frampton, C.; Henderson, R.; Ryan, P. The incidence of cancer following total hip-replacement. J. Bone Jt. Surg. Br. Vol. 1988, 70, 539–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauert, R.; Thorwarth, K. An overview on diamond-like carbon coatings in medical applications. Surf. Coat. Technol. 2013, 233, 119–130. [Google Scholar] [CrossRef]
- Pandiyaraj, K.N.; Heeg, J.; Lampka, A.; Junge, F.; Barfels, T.; Wienecke, M.; Rhee, Y.H.; Kim, H.W. In vitroCyto and Blood Compatibility of Titanium Containing Diamond-Like Carbon Prepared by Hybrid Sputtering Method. Plasma Sci. Technol. 2012, 14, 829–836. [Google Scholar] [CrossRef] [Green Version]
- Lackner, J.M.; Waldhauser, W.; Hartmann, P.; Bruckert, F.; Weidenhaupt, M.; Major, R.; Sanak, M.; Wiesinger, M.; Heim, D. Hemocompatibility of Inorganic Physical Vapor Deposition (PVD) Coatings on Thermoplastic Polyurethane Polymers. J. Funct. Biomater. 2012, 3, 283–297. [Google Scholar] [CrossRef] [Green Version]
- Salahas, A.; Vrahatis, A.; Karabinos, I.; Antonellis, I.; Ifantis, G.; Gavaliatsis, I.; Anthopoulos, P.; Tavernarakis, A. Success, Safety, and Efficacy of Implantation of Diamond-Like Carbon-Coated Stents. Angiology 2007, 58, 203–210. [Google Scholar] [CrossRef]
- O’Brien, B.; Carroll, W. The evolution of cardiovascular stent materials and surfaces in response to clinical drivers: A review. Acta Biomater. 2009, 5, 945–958. [Google Scholar] [CrossRef]
- Sydow-Plum, G.; Tabrizian, M. Review of stent coating strategies: Clinical insights. Mater. Sci. Technol. 2008, 24, 1127–1143. [Google Scholar] [CrossRef]
- Carvalho, I.; Faraji, M.; Ramalho, A.; Carvalho, A.P.; Carvalho, S.; Cavaleiro, A. Ex-vivo studies on friction behaviour of ureteral stent coated with Ag clusters incorporated in a:C matrix. Diam. Relat. Mater. 2018, 86, 1–7. [Google Scholar] [CrossRef]
- Dearnaley, G.; Arps, J.H. Biomedical applications of diamond-like carbon (DLC) coatings: A review. Surf. Coat. Technol. 2005, 200, 2518–2524. [Google Scholar] [CrossRef]
- Maguire, P.; McLaughlin, J.; Okpalugo, T.; Lemoine, P.; Papakonstantinou, P.; McAdams, E.; Needham, M.; Ogwu, A.; Ball, M.; Abbas, G. Mechanical stability, corrosion performance and bioresponse of amorphous diamond-like carbon for medical stents and guidewires. Diam. Relat. Mater. 2005, 14, 1277–1288. [Google Scholar] [CrossRef]
- Roy, R.K.; Lee, K.-R. Biomedical applications of diamond-like carbon coatings: A review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 83, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Endo, F.; Hotta, A. 11—Highly Functionalized Polyethylene Terephthalate for Food Packaging. In Poly(Ethylene Terephthalate) Based Blends, Composites and Nanocomposites; Visakh, P.M., Liang, M., Eds.; William Andrew Publishing: Oxford, UK, 2015; pp. 213–234. [Google Scholar]
- Dwivedi, N.; Dhand, C.; Kumar, R.; Lodhi, K.; Vishwakarma, J.; Gupta, R.K.; Kumar, P.; Hashmi, S.; Mishra, S.; Malik, H.K.; et al. Anomalous characteristics of nanostructured hydrogenated carbon thin films. Mater. Chem. Phys. 2021, 262, 124316. [Google Scholar] [CrossRef]
- Harigai, T.; Imai, T.; Takikawa, H.; Kaneko, S.; Kunitsugu, S.; Niibe, M.; Kanda, K.; Kamiya, M. Characterization of Hydrogen-Free and Hydrogenated DLC Films. In Carbon Related Materials; Satoru Kaneko, M.A., Pruna, A., Can, M., Mele, P., Ertugrul, M., Endo, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 55–69. [Google Scholar]
- Fedel, M. 4—Blood compatibility of diamond-like carbon (DLC) coatings. In Diamond-Based Materials for Biomedical Applications; Narayan, R., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 71–102. [Google Scholar]
- Wang, H.; Wang, L.; Wang, X. Structure characterization and antibacterial properties of Ag-DLC films fabricated by dual-targets HiPIMS. Surf. Coat. Technol. 2021, 410, 126967. [Google Scholar] [CrossRef]
- Moghadam, R.Z.; Ehsani, M.H.; Dizaji, H.R.; Kameli, P.; Jannesari, M. Oxygen doping effect on wettability of diamond-like carbon films. Mater. Res. Express 2021, 8, 035601. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, G.; Wang, S.; Xi, L.; Mi, C.; Wang, S.; Cheng, Y. Optical properties of diamond-like carbon films prepared by pulsed laser deposition onto 3D surface substrate. Surf. Eng. 2021, 37, 414–421. [Google Scholar] [CrossRef]
- Kim, J.-I.; Jang, Y.-J.; Kim, J.; Kim, J. Effects of silicon doping on low-friction and high-hardness diamond-like carbon coating via filtered cathodic vacuum arc deposition. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Zia, A.W. Chapter 11—New generation carbon particles embedded diamond-like carbon coatings for transportation industry. In Advances in Smart Coatings and Thin Films for Future Industrial and Biomedical Engineering Applications; Makhlouf, A.S.H., Abu-Thabit, N.Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 307–332. [Google Scholar]
- Bhushan, B. Nanotribology of Ultrathin and Hard Amorphous Carbon Films; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2008; pp. 843–899. [Google Scholar]
- Bociąga, D.; Sobczyk-Guzenda, A.; Szymanski, W.; Jedrzejczak, A.; Jastrzebska, A.; Olejnik, A.; Jastrzębski, K. Mechanical properties, chemical analysis and evaluation of antimicrobial response of Si-DLC coatings fabricated on AISI 316 LVM substrate by a multi-target DC-RF magnetron sputtering method for potential biomedical applications. Appl. Surf. Sci. 2017, 417, 23–33. [Google Scholar] [CrossRef]
- Bociaga, D.; Sobczyk-Guzenda, A.; Szymanski, W.; Jedrzejczak, A.; Jastrzebska, A.; Olejnik, A.; Swiatek, L.; Jastrzebski, K. Diamond like carbon coatings doped by Si fabricated by a multi-target DC-RF magnetron sputtering method—Mechanical properties, chemical analysis and biological evaluation. Vacuum 2017, 143, 395–406. [Google Scholar] [CrossRef]
- Liu, L.; Wang, T.; Huang, J.; He, Z.; Yi, Y.; Du, K. Diamond-like carbon thin films with high density and low internal stress deposited by coupling DC/RF magnetron sputtering. Diam. Relat. Mater. 2016, 70, 151–158. [Google Scholar] [CrossRef]
- Bociaga, D.; Kamińska, M.; Sobczyk-Guzenda, A.; Jastrzębski, K.; Swiatek, L.; Olejnik, A. Surface properties and biological behaviour of Si-DLC coatings fabricated by a multi-target DC–RF magnetron sputtering method for medical applications. Diam. Relat. Mater. 2016, 67, 41–50. [Google Scholar] [CrossRef]
- Khadem, M.; Kim, D.-E. Friction and wear behaviors of bare and diamond-like carbon/chromium bi-layer coated SKH51 steel at low temperatures. Surf. Coat. Technol. 2021, 412, 127018. [Google Scholar] [CrossRef]
- Manninen, N.K.; Ribeiro, F.; Escudeiro, A.; Polcar, T.; Carvalho, S.; Cavaleiro, A. Influence of Ag content on mechanical and tribological behavior of DLC coatings. Surf. Coat. Technol. 2013, 232, 440–446. [Google Scholar] [CrossRef] [Green Version]
- Manninen, N.K.; Escobar-Galindo, R.; Carvalho, S.; Cavaleiro, A. Silver surface segregation in Ag-DLC nanocomposite coatings. Surf. Coat. Technol. 2015, 267, 90–97. [Google Scholar] [CrossRef]
- Salehizadeh, S.; Carvalho, I.; Serra, R.; Ferreira, P.; Cavaleiro, A. Role of Au incorporation in the electrochemical behavior of Ag/a:C nanocomposite coatings. Surf. Coat. Technol. 2020, 401, 126240. [Google Scholar] [CrossRef]
- Balestra, R.; Castro, A.; Evaristo, M.; Escudeiro, A.; Mutafov, P.; Polcar, T.; Cavaleiro, A. Carbon-based coatings doped by copper: Tribological and mechanical behavior in olive oil lubrication. Surf. Coat. Technol. 2011, 205, S79–S83. [Google Scholar] [CrossRef]
- Evaristo, M.; Fernandes, F.; Cavaleiro, A. Room and High Temperature Tribological Behaviour of W-DLC Coatings Produced by DCMS and Hybrid DCMS-HiPIMS Configuration. Coatings 2020, 10, 319. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, F.; Serra, R.; Cavaleiro, A.; Oliveira, J.C. Diamond-like carbon coatings deposited by deep oscillation magnetron sputtering in Ar-Ne discharges. Diam. Relat. Mater. 2019, 98, 107521. [Google Scholar] [CrossRef]
- Pillaca, E.; Trava-Airoldi, V.; Ramírez, M. Axial distribution improvements of DLC film on the inner surface of a long stainless steel tube. Surf. Coat. Technol. 2021, 412, 126996. [Google Scholar] [CrossRef]
- Alaefour, I.; Shahgaldi, S.; Zhao, J.; Li, X. Synthesis and Ex-Situ characterizations of diamond-like carbon coatings for metallic bipolar plates in PEM fuel cells. Int. J. Hydrogen Energy 2021, 46, 11059–11070. [Google Scholar] [CrossRef]
- Padmanaban, D.B.; Mohan, L.; Giri, P.; Bera, P.; Anandan, C.; Barshilia, H.C. Effect of Molybdenum Content on Mechanical and Tribological Properties of Diamond-Like Carbon Coatings over Titanium β-21S Alloy. C J. Carbon Res. 2020, 7, 1. [Google Scholar] [CrossRef]
- Liu, J.; Xiong, J.; Zhou, L.; Guo, Z.; Wen, H.; You, Q.; Li, X.; Liu, J.; Zhao, W. Properties of TiN–Al 2 O 3 –TiCN–TiN, TiAlN, and DLC-coated Ti(C,N)-based cermets and their wear behaviors during dry cutting of 7075 aluminum alloys. Int. J. Appl. Ceram. Technol. 2020, 18, 792–802. [Google Scholar] [CrossRef]
- Wu, K.-Y.; Zhao, G.-R.; Li, Z.; Gong, Z.-B. Effects of electrode distance on mechanical and tribological properties of hydrogenated dlc films deposited by dc-pulse pecvd. Surf. Rev. Lett. 2021, 28, 1–10. [Google Scholar] [CrossRef]
- Jing, P.; Ma, D.; Gong, Y.; Luo, X.; Zhang, Y.; Weng, Y.; Leng, Y. Influence of Ag doping on the microstructure, mechanical properties, and adhesion stability of diamond-like carbon films. Surf. Coat. Technol. 2021, 405, 126542. [Google Scholar] [CrossRef]
- Xia, Y.; Hirai, Y.; Tsuchiya, T. Fracture behavior of single-crystal silicon microstructure coated with stepwise bias-graded a-C:H film. Surf. Coat. Technol. 2021, 405, 126559. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, G.; Wang, S.; Mi, C.; Wei, S.; Tian, F.; Li, W.; Cao, H.; Cheng, Y. A review on diamond-like carbon films grown by pulsed laser deposition. Appl. Surf. Sci. 2021, 541, 148573. [Google Scholar] [CrossRef]
- Yi-Min, L.; Guo-Jun, H.; Yong, C.; Sai, W.; Xu, L.; Shang-Fang, W.; Chao-Wei, M. Tribological and mechanical properties of non-hydrogenated W-doped diamond-like carbon film prepared by pulsed laser deposition. Acta Phys. Sin. 2021, 70, 046801. [Google Scholar] [CrossRef]
- Yoshinaka, H.; Inubushi, S.; Wakita, T.; Yokoya, T.; Muraoka, Y. Formation of Q-carbon by adjusting sp3 content in diamond-like carbon films and laser energy density of pulsed laser annealing. Carbon 2020, 167, 504–511. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, G.; Cheng, Y.; Xi, L.; Wang, S.; Guo, Y.; Mi, C.; Tian, F.; Wei, S. Optical and micro-structural properties of the uniform large-area carbon-based films prepared by pulsed laser deposition. Infrared Phys. Technol. 2019, 104, 103113. [Google Scholar] [CrossRef]
- Khan, M.I.; Adil, F.; Majeed, S.; Farooq, W.A.; Hasan, S.; Jabeen, R.; Al-Mutairi, M.A.; Bukhtiar, A.; Iqbal, M. Structural, morphological, electrical and optical properties of Cu doped DLC thin films. Mater. Res. Express 2019, 6, 126420. [Google Scholar] [CrossRef]
- Lu, Y.; Cheng, Y.; Huang, G.; Xi, L.; Wang, S.; Wei, S.; Mi, C. Effects of external magnetic field on the micro-structure of diamond-like carbon film prepared by pulsed laser deposition. Mater. Res. Express 2019, 6, 116433. [Google Scholar] [CrossRef]
- Lackner, J.; Stotter, C.; Waldhauser, W.; Ebner, R.; Lenz, W.; Beutl, M. Pulsed laser deposition of diamond-like carbon coatings for industrial tribological applications. Surf. Coat. Technol. 2003, 174–175, 402–407. [Google Scholar] [CrossRef]
- Shen, Y.; Luo, J.; Liao, B.; Zhang, X.; Zhao, Y.; Chen, L.; Pang, P.; Zeng, X. Microstructure, erosion and tribological behaviour of thick DLC coatings by the filtered cathodic vacuum arc combined with a high-voltage pulse power. Mater. Res. Express 2021, 8, 066405. [Google Scholar] [CrossRef]
- Orrit-Prat, J.; Bonet, R.; Rupérez, E.; Punset, M.; Ortiz-Hernández, M.; Guillem-Marti, J.; Lousa, A.; Cano, D.; Díaz, C.; Fuentes, G.G.; et al. Bactericidal silver-doped DLC coatings obtained by pulsed filtered cathodic arc co-deposition. Surf. Coat. Technol. 2021, 411, 126977. [Google Scholar] [CrossRef]
- Cao, H.; Ye, X.; Li, H.; Qi, F.; Wang, Q.; Ouyang, X.; Zhao, N.; Liao, B. Microstructure, mechanical and tribological properties of multilayer Ti-DLC thick films on Al alloys by filtered cathodic vacuum arc technology. Mater. Des. 2021, 198, 109320. [Google Scholar] [CrossRef]
- Lee, W.-Y.; Jang, Y.-J.; Tokoroyama, T.; Murashima, M.; Umehara, N. Effect of defects on wear behavior in ta-C coating prepared by filtered cathodic vacuum arc deposition. Diam. Relat. Mater. 2020, 105, 107789. [Google Scholar] [CrossRef]
- Shen, Y.; Zhou, H.; Wang, H.; Liao, B.; Wu, X.; Zhang, X. Tribological behavior of diamond-like carbon coatings with patterned structure deposited by the filtered cathodic vacuum arc. Thin Solid Films 2019, 685, 123–130. [Google Scholar] [CrossRef]
- Wei, R. Development of new technologies and practical applications of plasma immersion ion deposition (PIID). Surf. Coat. Technol. 2010, 204, 2869–2874. [Google Scholar] [CrossRef]
- Liu, X.; He, X.; Lin, Y.; Zhuang, J.; Hao, J. Enhanced adhesion strength and solid lubricity of graphite like amorphous carbon films by hydrogen implantation. Surf. Coat. Technol. 2021, 412, 127013. [Google Scholar] [CrossRef]
- Zhan, Z.; Ma, Y.; Ren, J.; Gao, X.; Li, L.; Xu, M. A new-structured nanocarbon cushion with highly impact-resistant properties. Carbon 2020, 170, 146–153. [Google Scholar] [CrossRef]
- Moghadam, R.Z.; Dizaji, H.R.; Ehsani, M.H. Modification of optical and mechanical properties of nitrogen doped diamond-like carbon layers. J. Mater. Sci. Mater. Electron. 2019, 30, 19770–19781. [Google Scholar] [CrossRef]
- Moghadam, R.Z.; Ehsani, M.; Dizaji, H.R.; Kameli, P.; Jannesari, M. Modification of hydrophobicity properties of diamond like carbon films using glancing angle deposition method. Mater. Lett. 2018, 220, 301–304. [Google Scholar] [CrossRef]
- Zhang, C.; Bhattacharya, S.; Li, Y.; Khatir, S.; Hu, Y.; Shiri, S.; Yang, Q. Effect of ion energy on microstructure and adhesion of diamond-like carbon on Ti6Al4V by ion beam deposition. Diam. Relat. Mater. 2016, 70, 12–17. [Google Scholar] [CrossRef]
- Deng, Q.; Wang, C.; Zhang, T.; Yang, W.; Li, X.; Huang, N.; Leng, Y. Regulating the uniformity of DLC films in ECR plasma with negative substrate biasing. Surf. Coat. Technol. 2019, 365, 15–23. [Google Scholar] [CrossRef]
- Akasaka, H.; Gawazawa, N.; Kishimoto, S.-I.; Ohshio, S.; Saitoh, H. Surface plasmon resonance detection using amorphous carbon/Au multilayer structure. Appl. Surf. Sci. 2009, 256, 1236–1239. [Google Scholar] [CrossRef]
- Cardoso, F.; Ferreira, F.; Cavaleiro, A.; Ramalho, A. Performance of diamond-like carbon coatings (produced by the innovative Ne-HiPIMS technology) under different lubrication regimes. Wear 2021, 203775. [Google Scholar] [CrossRef]
- Evaristo, M.; Azevedo, R.; Palacio, C.; Cavaleiro, A. Influence of the silicon and oxygen content on the properties of non-hydrogenated amorphous carbon coatings. Diam. Relat. Mater. 2016, 70, 201–210. [Google Scholar] [CrossRef]
- Hatem, A.; Lin, J.; Wei, R.; Torres, R.; Laurindo, C.; Soares, P. Tribocorrosion behavior of DLC-coated Ti-6Al-4V alloy deposited by PIID and PEMS + PIID techniques for biomedical applications. Surf. Coat. Technol. 2017, 332, 223–232. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Wu, B.; Zhang, T.; Leng, Y.; Huang, N. Tribocorrosion behavior of DLC-coated CoCrMo alloy in simulated biological environment. Vacuum 2013, 92, 39–43. [Google Scholar] [CrossRef]
- Pardo, A.; Ilic, E.; Thorwarth, K.; Stiefel, M.; Hauert, R. Corrosion fatigue in DLC-coated articulating implants: An accelerated methodology to predict realistic interface lifetime. Sci. Technol. Adv. Mater. 2019, 20, 173–186. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Zhang, M.; Shang, L.; Wang, Y.; Lu, Z.; Zhang, G. Enhancement in the corrosive and tribological properties of the inner wall of 6063Al and CI pipes by thick multilayer Si-DLC coatings. Mater. Res. Express 2019, 6, 085634. [Google Scholar] [CrossRef]
- Jastrzębski, K.; Białecki, J.; Jastrzębska, A.; Kaczmarek, A.; Para, M.; Niedzielski, P.; Bociaga, D. Induced Biological Response in Contact with Ag-and Cu-Doped Carbon Coatings for Potential Orthopedic Applications. Materials 2021, 14, 1861. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Lin, Y.; Zhang, D.; Ruan, Q.; Tang, K.; Li, M.; Liu, X.; Chu, P.K.; Zhang, Y. Corrosion Behavior and Biocompatibility of Diamond-like Carbon-Coated Zinc: An In Vitro Study. ACS Omega 2021, 6, 9843–9851. [Google Scholar] [CrossRef] [PubMed]
- Buchegger, S.; Kamenac, A.; Fuchs, S.; Herrmann, R.; Houdek, P.; Gorzelanny, C.; Obermeier, A.; Heller, S.; Burgkart, R.; Stritzker, B.; et al. Smart antimicrobial efficacy employing pH-sensitive ZnO-doped diamond-like carbon coatings. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Katouno, J.; Fujioka, K.; Kidera, S.; Mabuchi, Y.; Sato, K.; Ohgoe, Y.; Manome, Y.; Hiratsuka, M.; Nakamori, H.; Masuda, H.; et al. Evaluation of the enhancement of osteogenesis by Zn-releasing diamond-like carbon film. Diam. Relat. Mater. 2017, 77, 131–136. [Google Scholar] [CrossRef]
- Fialho, L.; Grenho, L.; Fernandes, M.H.; Carvalho, S. Porous tantalum oxide with osteoconductive elements and antibacterial core-shell nanoparticles: A new generation of materials for dental implants. Mater. Sci. Eng. C 2021, 120, 111761. [Google Scholar] [CrossRef]
- Costa, J.; de Lima, M.J.B.P.; Sampaio, M.J.; Neves, M.C.; Faria, J.; Torres, S.M.; Tavares, A.P.; Silva, C.G. Enhanced biocatalytic sustainability of laccase by immobilization on functionalized carbon nanotubes/polysulfone membranes. Chem. Eng. J. 2019, 355, 974–985. [Google Scholar] [CrossRef]
- Suzuki, K.; Hiyoshi, M.; Tada, H.; Bando, M.; Ichioka, T.; Kamemura, N.; Kido, H. Allergen diagnosis microarray with high-density immobilization capacity using diamond-like carbon-coated chips for profiling allergen-specific IgE and other immunoglobulins. Anal. Chim. Acta 2011, 706, 321–327. [Google Scholar] [CrossRef]
- Choudhury, D.; Morita, T.; Sawae, Y.; Lackner, J.M.; Towler, M.; Krupka, I. A novel functional layered diamond like carbon coating for orthopedics applications. Diam. Relat. Mater. 2016, 61, 56–69. [Google Scholar] [CrossRef]
- Donnet, C.; Erdemir, A. Diamond-like Carbon Films: A Historical Overview. In Tribology of Diamond-Like. Carbon Films: Fundamentals and Applications; Erdemir, C.D.A.A., Ed.; Springer: Boston, MA, USA, 2008; pp. 1–10. [Google Scholar]
- Wang, J.; Huang, N.; Pan, C.; Kwok, S.; Yang, P.; Leng, Y.; Chen, J.; Sun, H.; Wan, G.; Liu, Z.; et al. Bacterial repellence from polyethylene terephthalate surface modified by acetylene plasma immersion ion implantation–deposition. Surf. Coat. Technol. 2004, 186, 299–304. [Google Scholar] [CrossRef]
- Marciano, F.; Bonetti, L.; Mangolin, J.; Da-Silva, N.; Corat, E.J.; Trava-Airoldi, V. Investigation into the antibacterial property and bacterial adhesion of diamond-like carbon films. Vacuum 2011, 85, 662–666. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, F.; Fong, A.; Lai, K.; Shum, P.; Zhou, Z.; Gao, Z.; Fu, T. Effects of silver segregation on sputter deposited antibacterial silver-containing diamond-like carbon films. Thin Solid Films 2018, 650, 58–64. [Google Scholar] [CrossRef]
- Venkatesan, J.; Jayakumar, R.; Mohandas, A.; Bhatnagar, I.; Kim, S.-K. Antimicrobial Activity of Chitosan-Carbon Nanotube Hydrogels. Materials 2014, 7, 3946–3955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, D.; Dey, R.; Das, S.; Hussain, S.; Ghosh, A.K.; Pal, A.K. Nano-Ag/DLC/Cellulose Free-Standing Films Towards Anti-bacterial and Bio-compatible Futuristic Bandage Applications. J. Polym. Environ. 2020, 28, 284–294. [Google Scholar] [CrossRef]
- Chan, Y.-H.; Huang, C.-F.; Ou, K.-L.; Peng, P.-W. Mechanical properties and antibacterial activity of copper doped diamond-like carbon films. Surf. Coat. Technol. 2011, 206, 1037–1040. [Google Scholar] [CrossRef]
- Jung, W.K.; Koo, H.C.; Kim, K.W.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial Activity and Mechanism of Action of the Silver Ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2171–2178. [Google Scholar] [CrossRef] [Green Version]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Kung, M.-L.; Tai, M.-H.; Lin, P.-Y.; Wu, D.-C.; Wu, W.J.; Yeh, B.-W.; Hung, H.-S.; Kuo, C.-H.; Chen, Y.-W.; Hsieh, S.-L.; et al. Silver decorated copper oxide (Ag@CuO) nanocomposite enhances ROS-mediated bacterial architecture collapse. Colloids Surf. B Biointerfaces 2017, 155, 399–407. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, L.; Zhou, Y.; Li, Y.; Liu, Q.; Hu, J.; Yang, J. Light-induced ZnO/Ag/rGO bactericidal photocatalyst with synergistic effect of sustained release of silver ions and enhanced reactive oxygen species. Chin. J. Catal. 2019, 40, 691–702. [Google Scholar] [CrossRef]
- Liu, J.; Sonshine, D.A.; Shervani, S.; Hurt, R.H. Controlled Release of Biologically Active Silver from Nanosilver Surfaces. ACS Nano 2010, 4, 6903–6913. [Google Scholar] [CrossRef] [Green Version]
- Chekan, N.; Beliauski, N.; Akulich, V.; Pozdniak, L.; Sergeeva, E.; Chernov, A.; Kazbanov, V.; Kulchitsky, V. Biological activity of silver-doped DLC films. Diam. Relat. Mater. 2009, 18, 1006–1009. [Google Scholar] [CrossRef]
- Baba, K.; Hatada, R.; Flege, S.; Ensinger, W.; Shibata, Y.; Nakashima, J.; Sawase, T.; Morimura, T. Preparation and antibacterial properties of Ag-containing diamond-like carbon films prepared by a combination of magnetron sputtering and plasma source ion implantation. Vacuum 2013, 89, 179–184. [Google Scholar] [CrossRef]
- Lan, W.-C.; Ou, S.-F.; Lin, M.-H.; Ou, K.-L.; Tsai, M.-Y. Development of silver-containing diamond-like carbon for biomedical applications. Part I: Microstructure characteristics, mechanical properties and antibacterial mechanisms. Ceram. Int. 2013, 39, 4099–4104. [Google Scholar] [CrossRef]
- Almaguer-Flores, A.; Rodil, S.E.; Olivares-Navarrete, R. Oral Bacterial Adhesion and Biocompatibility of Silver-Amorphous Carbon Films: A Surface Modification for Dental Implants. Implant. Dent. Most Promis. Discip. Dent. 2011, 12, 263. [Google Scholar] [CrossRef]
- Mihailescu, I.N.; Bociąga, D.; Socol, G.; Stan, G.; Chifiriuc, M.-C.; Bleotu, C.; Husanu, M.-A.; Popescu-Pelin, G.; Duta, L.; Luculescu, C.R.; et al. Fabrication of antimicrobial silver-doped carbon structures by combinatorial pulsed laser deposition. Int. J. Pharm. 2016, 515, 592–606. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhao, Q.; Liu, Y.; Wang, S.; Abel, E. Reduction of bacterial adhesion on modified DLC coatings. Colloids Surf. B Biointerfaces 2008, 61, 182–187. [Google Scholar] [CrossRef]
- Zhang, X.; Williams, D. Definitions of Biomaterials for the Twenty-First Century, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 1, p. 290. [Google Scholar]
- Crawford, L.; Wyatt, M.; Bryers, J.; Ratner, B. Biocompatibility Evolves: Phenomenology to Toxicology to Regeneration. Adv. Health Mater. 2021, 10, 2002153. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, I.; Polcar, T.; Henriques, M.; Carvalho, S. Materials incompatibility as a major cause of hip prostheses rejection. Rev. Adv. Mater. Sci. 2015, 42, 36–49. [Google Scholar]
- Logothetidis, S.; Gioti, M.; Lousinian, S.; Fotiadou, S. Haemocompatibility studies on carbon-based thin films by ellipsometry. Thin Solid Films 2005, 482, 126–132. [Google Scholar] [CrossRef]
- Martino, S.; D’Angelo, F.A.; Armentano, I.; Tiribuzi, R.; Pennacchi, M.; Dottori, M.; Mattioli, S.; Caraffa, A.; Cerulli, G.G.; Kenny, J.M.; et al. Hydrogenated amorphous carbon nanopatterned film designs drive human bone marrow mesenchymal stem cell cytoskeleton architecture. Tissue Eng. Part A 2009, 15, 3139–3149. [Google Scholar] [CrossRef]
- Lantada, A.D.; Endrino, J.L.; Vaquero, V.S.; Mosquera, A.; Lafont, P.; García-Ruiz, J.P. Tissue Engineering Using Novel Rapid Prototyped Diamond-Like Carbon Coated Scaffolds. Plasma Process. Polym. 2012, 9, 98–107. [Google Scholar] [CrossRef]
- Wei, C.; Pan, W.-J.; Hung, M.-S. The effects of substrate roughness and associated surface properties on the biocompatibility of diamond-like carbon films. Surf. Coat. Technol. 2013, 224, 8–17. [Google Scholar] [CrossRef]
- Marczak, J.; Kusinski, J.; Major, R.; Rycyk, A.; Sarzynski, A.; Strzelec, M.; Czyz, K. Laser interference patterning of diamond-like carbon layers for directed migration and growth of smooth muscle cell depositions. Opt. Appl. 2014, 44, 575–586. [Google Scholar] [CrossRef]
- Czyz, K.; Marczak, J.; Major, R.; Mzyk, A.; Rycyk, A.; Sarzyński, A.; Strzelec, M. Selected laser methods for surface structuring of biocompatible diamond-like carbon layers. Diam. Relat. Mater. 2016, 67, 26–40. [Google Scholar] [CrossRef]
- Wei, C.; Peng, K.-S.; Hung, M.-S. The effect of hydrogen and acetylene mixing ratios on the surface, mechanical and biocompatible properties of diamond-like carbon films. Diam. Relat. Mater. 2016, 63, 108–114. [Google Scholar] [CrossRef]
- Gotzmann, G.; Beckmann, J.; Wetzel, C.; Scholz, B.; Herrmann, U.; Neunzehn, J. Electron-beam modification of DLC coatings for biomedical applications. Surf. Coat. Technol. 2017, 311, 248–256. [Google Scholar] [CrossRef]
- Ahmed, M.H.; Byrne, J.; Ahmed, W. Characteristic of silicon doped diamond like carbon thin films on surface properties and human serum albumin adsorption. Diam. Relat. Mater. 2015, 55, 108–116. [Google Scholar] [CrossRef]
- Schroeder, A.; Francz, G.; Bruinink, A.; Hauert, R.; Mayer, J.; Wintermantel, E. Titanium containing amorphous hydrogenated carbon films (a-C:H/Ti): Surface analysis and evaluation of cellular reactions using bone marrow cell cultures in vitro. Biomaterials 2000, 21, 449–456. [Google Scholar] [CrossRef]
- Bouabibsa, I.; Alhussein, A.; Lamri, S.; Sanchette, F.; Rtimi, S. Biological responses at the interface of Ti-doped diamond-like carbon surfaces for indoor environment application. Environ. Sci. Pollut. Res. 2020, 27, 31120–31129. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.-C.; Wang, C.-H.; Cheng, H.-C.; Chiou, S.-Y.; Chen, C.-S.; Ou, K.-L. Enhancement of hemocompatibility on titanium implant with titanium-doped diamond-like carbon film evaluated by cellular reactions using bone marrow cell cultures in vitro. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. 2009, 27, 1559. [Google Scholar] [CrossRef]
- Bharathy, P.V.; Nataraj, D.; Chu, P.K.; Wang, H.; Yang, Q.; Kiran, M.; Silvestre-Albero, J.; Mangalaraj, D. Effect of titanium incorporation on the structural, mechanical and biocompatible properties of DLC thin films prepared by reactive-biased target ion beam deposition method. Appl. Surf. Sci. 2010, 257, 143–150. [Google Scholar] [CrossRef]
- Popa, A.C.; Stan, G.E.; Husanu, M.-A.; Pasuk, I.; Popescu, I.D.; Popescu, A.C.; Mihailescu, I.N. Multi-layer haemocompatible diamond-like carbon coatings obtained by combined radio frequency plasma enhanced chemical vapor deposition and magnetron sputtering. J. Mater. Sci. Mater. Electron. 2013, 24, 2695–2707. [Google Scholar] [CrossRef] [PubMed]
- Hang, R.; Zhang, M.; Ma, S.; Chu, P. Biological response of endothelial cells to diamond-like carbon-coated NiTi alloy. J. Biomed. Mater. Res. Part A 2011, 100, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Xie, Y.; Zhang, J.; Wei, Q.; Zhou, B.; Luo, J.; Wang, Y.; Yu, Z.; Tang, Z. TiN coated stainless steel bracket: Tribological, corrosion resistance, biocompatibility and mechanical performance. Surf. Coat. Technol. 2015, 277, 227–233. [Google Scholar] [CrossRef]
- Li, L.; Bai, W.; Wang, X.; Gu, C.; Jin, G.; Tu, J. Mechanical Properties and in Vitro and in Vivo Biocompatibility of a-C/a-C:Ti Nanomultilayer Films on Ti6Al4V Alloy as Medical Implants. ACS Appl. Mater. Interfaces 2017, 9, 15933–15942. [Google Scholar] [CrossRef]
- Rubstein, A.; Makarova, E.; Trakhtenberg, I.; Kudryavtseva, I.; Bliznets, D.; Philippov, Y.; Shlykov, I. Osseointegration of porous titanium modified by diamond-like carbon and carbon nitride. Diam. Relat. Mater. 2012, 22, 128–135. [Google Scholar] [CrossRef]
- Jongwannasiri, C.; Moolsradoo, N.; Khantachawana, A.; Kaewtatip, P.; Watanabe, S. The Comparison of Biocompatibility Properties between Ti Alloys and Fluorinated Diamond-Like Carbon Films. Adv. Mater. Sci. Eng. 2012, 2012, 724126. [Google Scholar] [CrossRef] [Green Version]
- Lackner, J.M.; Meindl, C.; Wolf, C.; Fian, A.; Kittinger, C.; Kot, M.; Major, L.; Czibula, C.; Teichert, C.; Waldhauser, W.; et al. Gas Permeation, Mechanical Behavior and Cytocompatibility of Ultrathin Pure and Doped Diamond-Like Carbon and Silicon Oxide Films. Coatings 2013, 3, 268–300. [Google Scholar] [CrossRef]
- Rodil, S.; Olivares, R.; Arzate, H.; Muhl, S. Properties of carbon films and their biocompatibility using in-vitro tests. Diam. Relat. Mater. 2003, 12, 931–937. [Google Scholar] [CrossRef]
- Ali, N.; Kousar, Y.; Grácio, J.; Titus, E.; Okpalugo, T.I.; Singh, V.; Pease, M.; Ogwu, A.A.; Meletis, E.I.; Ahmed, W.; et al. Human Microvascular Endothelial Cell Seeding on Cr-DLC Thin Films for Heart Valve Applications. J. Mater. Eng. Perform. 2006, 15, 230–235. [Google Scholar] [CrossRef]
- Penkov, O.V.; Pukha, V.E.; Starikova, S.L.; Khadem, M.; Starikov, V.V.; Maleev, M.V.; Kim, D.-E. Highly wear-resistant and biocompatible carbon nanocomposite coatings for dental implants. Biomaterials 2016, 102, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Kopova, I.; Kronek, J.; Bacakova, L.; Fencl, J. A cytotoxicity and wear analysis of trapeziometacarpal total joint replacement implant consisting of DLC-coated Co-Cr-Mo alloy with the use of titanium gradient interlayer. Diam. Relat. Mater. 2019, 97, 107456. [Google Scholar] [CrossRef]
- Travnickova, M.; Vandrovcova, M.; Filova, E.; Steinerova, M.; Rackova, J.; Kocourek, T.; Bartova, J.; Suchy, T.; Zaloudkova, M.; Jelinek, M.; et al. Effect of diamond-like carbon doped with chromium on cell differentiation, immune activation and apoptosis. Eur. Cells Mater. 2020, 40, 276–302. [Google Scholar] [CrossRef]
- Endrino, J.; Escobar-Galindo, R.; Zhang, H.-S.; Allen, M.; Gago, R.; Espinosa, A.; Anders, A. Structure and properties of silver-containing a-C(H) films deposited by plasma immersion ion implantation. Surf. Coat. Technol. 2008, 202, 3675–3682. [Google Scholar] [CrossRef]
- Choi, H.W.; Dauskardt, R.H.; Lee, S.-C.; Lee, K.-R.; Oh, K.H. Characteristic of silver doped DLC films on surface properties and protein adsorption. Diam. Relat. Mater. 2008, 17, 252–257. [Google Scholar] [CrossRef]
- Pandiyaraj, K.N.; Heeg, J.; Mewes, C.; Wienecke, M.; Barfels, T.; Uthayakumar, V.; Su, P.G. Investigation on Surface and Biological Properties of Silver Containing Diamond Like Carbon Films on Polyethylene Terephthalate Film Surface by Hybrid Reactive Sputtering Method. Key Eng. Mater. 2012, 521, 191–205. [Google Scholar] [CrossRef]
- Švorčík, V.; Kubová, O.; Slepička, P.; Dvořánková, B.; Mackova, A.; Hnatowicz, V. Structural, chemical and biological properties of carbon layers sputtered on polyethyleneterephtalate. J. Mater. Sci. Mater. Electron. 2006, 17, 229–234. [Google Scholar] [CrossRef]
- Gorzelanny, C.; Kmeth, R.; Obermeier, A.; Bauer, A.T.; Halter, N.; Kümpel, K.; Schneider, M.F.; Wixforth, A.; Gollwitzer, H.; Burgkart, R.; et al. Silver nanoparticle-enriched diamond-like carbon implant modification as a mammalian cell compatible surface with antimicrobial properties. Sci. Rep. 2016, 6, 22849. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.-Y.; Huang, H.-L.; Chen, Y.-C.; Hsu, J.-T.; Shieh, T.-M.; Tsai, M.-T. Biological Characteristics of the MG-63 Human Osteosarcoma Cells on Composite Tantalum Carbide/Amorphous Carbon Films. PLoS ONE 2014, 9, e95590. [Google Scholar] [CrossRef] [Green Version]
- Tsai, M.-T.; Chang, Y.-Y.; Huang, H.-L.; Chen, Y.-C.; Wang, S.-P.; Lai, C.-H. Biological characteristics of human fetal skin fibroblasts and MG-63 human osteosarcoma cells on tantalum-doped carbon films. Surf. Coat. Technol. 2014, 245, 16–21. [Google Scholar] [CrossRef]
- Yate, L.; Coy, L.E.; Gregurec, D.; Aperador, W.; Moya, S.; Wang, G. Nb–C Nanocomposite Films with Enhanced Biocompatibility and Mechanical Properties for Hard-Tissue Implant Applications. ACS Appl. Mater. Interfaces 2015, 7, 6351–6358. [Google Scholar] [CrossRef] [PubMed]
- Bociaga, D.; Sobczyk-Guzenda, A.; Komorowski, P.; Balcerzak, J.; Jastrzebski, K.; Przybyszewska, K.; Kaczmarek, A. Surface Characteristics and Biological Evaluation of Si-DLC Coatings Fabricated Using Magnetron Sputtering Method on Ti6Al7Nb Substrate. Nanomaterials 2019, 9, 812. [Google Scholar] [CrossRef] [Green Version]
- Milan, P.B.; Khamseh, S.; Zarintaj, P.; Ramezanzadeh, B.; Badawi, M.; Morisset, S.; Vahabi, H.; Saeb, M.R.; Mozafari, M. Copper-enriched diamond-like carbon coatings promote regeneration at the bone–implant interface. Heliyon 2020, 6, e03798. [Google Scholar] [CrossRef]
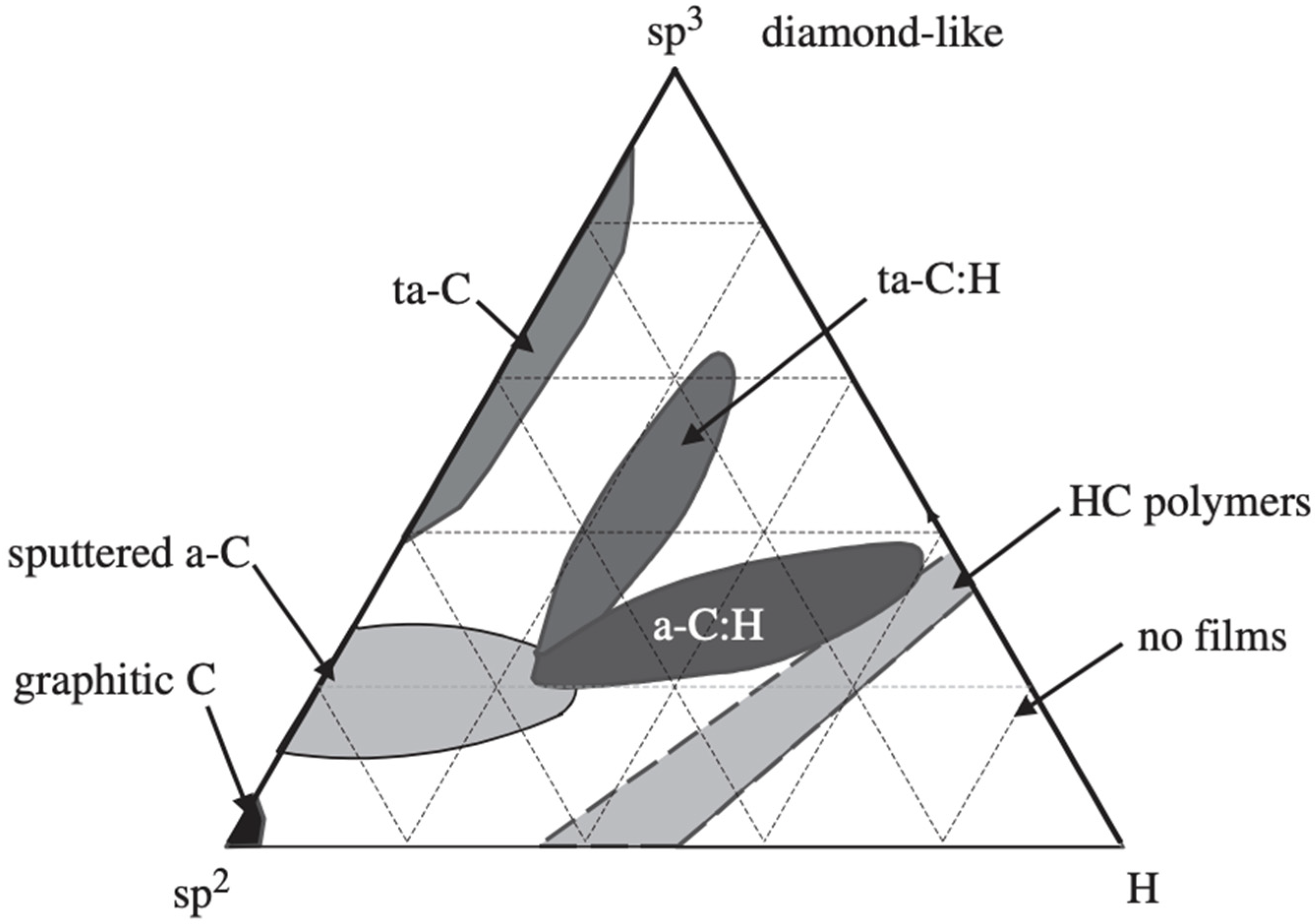

| Term | Hydrogen | sp2 | sp3 | Metal Doping |
|---|---|---|---|---|
| a-C | No | Main | ||
| a-C:H | Yes | Main | ||
| ta-C | No | Main | ||
| ta-C:H | Yes | Main | ||
| Me-DLC | No | Yes |
| Technique | Number of Publications | Deposition Rate (nm/s) | Reference |
|---|---|---|---|
| DC/RF sputtering | 898 | 1–10 | [29,49,50,58,59,83,96,97,98,99,100,101,102,103,104,105,106,107] |
| Plasma enhanced chemical vapor deposition (PECVD) | 610 | 1–10 | [88,96,108,109,110,111,112,113,114] |
| Pulsed laser deposition | 347 | 0.1–1 | [93,96,115,116,117,118,119,120,121] |
| Filtered cathodic arc (FCA) | 141 | 0.1–1 | [94,96,122,123,124,125,126] |
| Plasma immersion ion implantation | 121 | >0.28 | [127,128,129] |
| Direct ion beam (IB) | 36 | 0.1–1 | [92,96,130,131,132] |
| Electron cyclotron resonance plasma chemical vapor deposition (ECR-CVD) | 41 | 1–10 | [96,133,134] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, I.; Rodrigues, L.; Lima, M.J.; Carvalho, S.; Cruz, S.M.A. Overview on the Antimicrobial Activity and Biocompatibility of Sputtered Carbon-Based Coatings. Processes 2021, 9, 1428. https://doi.org/10.3390/pr9081428
Carvalho I, Rodrigues L, Lima MJ, Carvalho S, Cruz SMA. Overview on the Antimicrobial Activity and Biocompatibility of Sputtered Carbon-Based Coatings. Processes. 2021; 9(8):1428. https://doi.org/10.3390/pr9081428
Chicago/Turabian StyleCarvalho, Isabel, Lisa Rodrigues, Maria José Lima, Sandra Carvalho, and Sandra M. A. Cruz. 2021. "Overview on the Antimicrobial Activity and Biocompatibility of Sputtered Carbon-Based Coatings" Processes 9, no. 8: 1428. https://doi.org/10.3390/pr9081428







